Applied IrO2 Buffer Layer as a Great Promoter on Ti-Doping V2O5 Electrode to Enhance Electrochromic Device Properties
Abstract
:1. Introduction
2. Materials and Methods
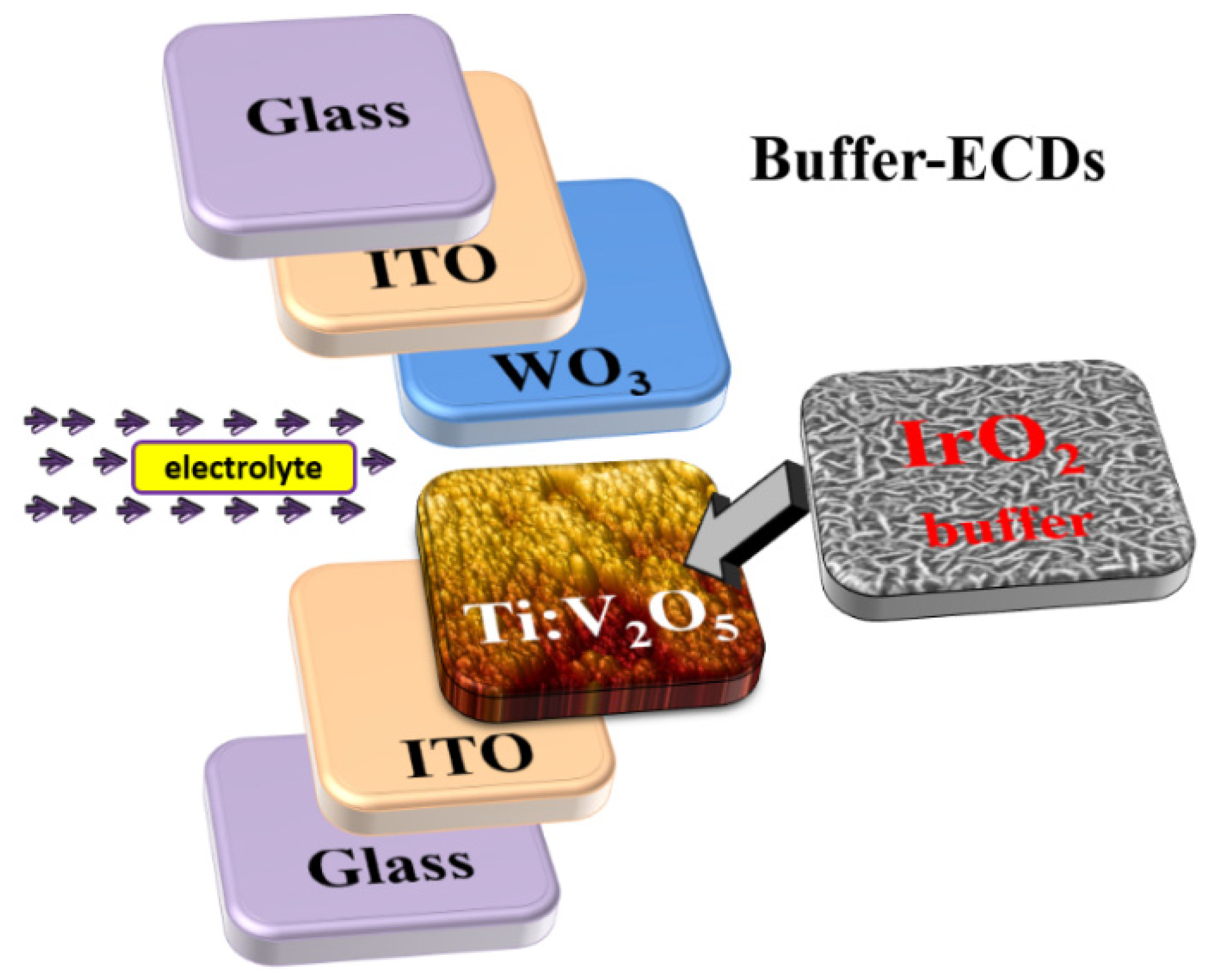
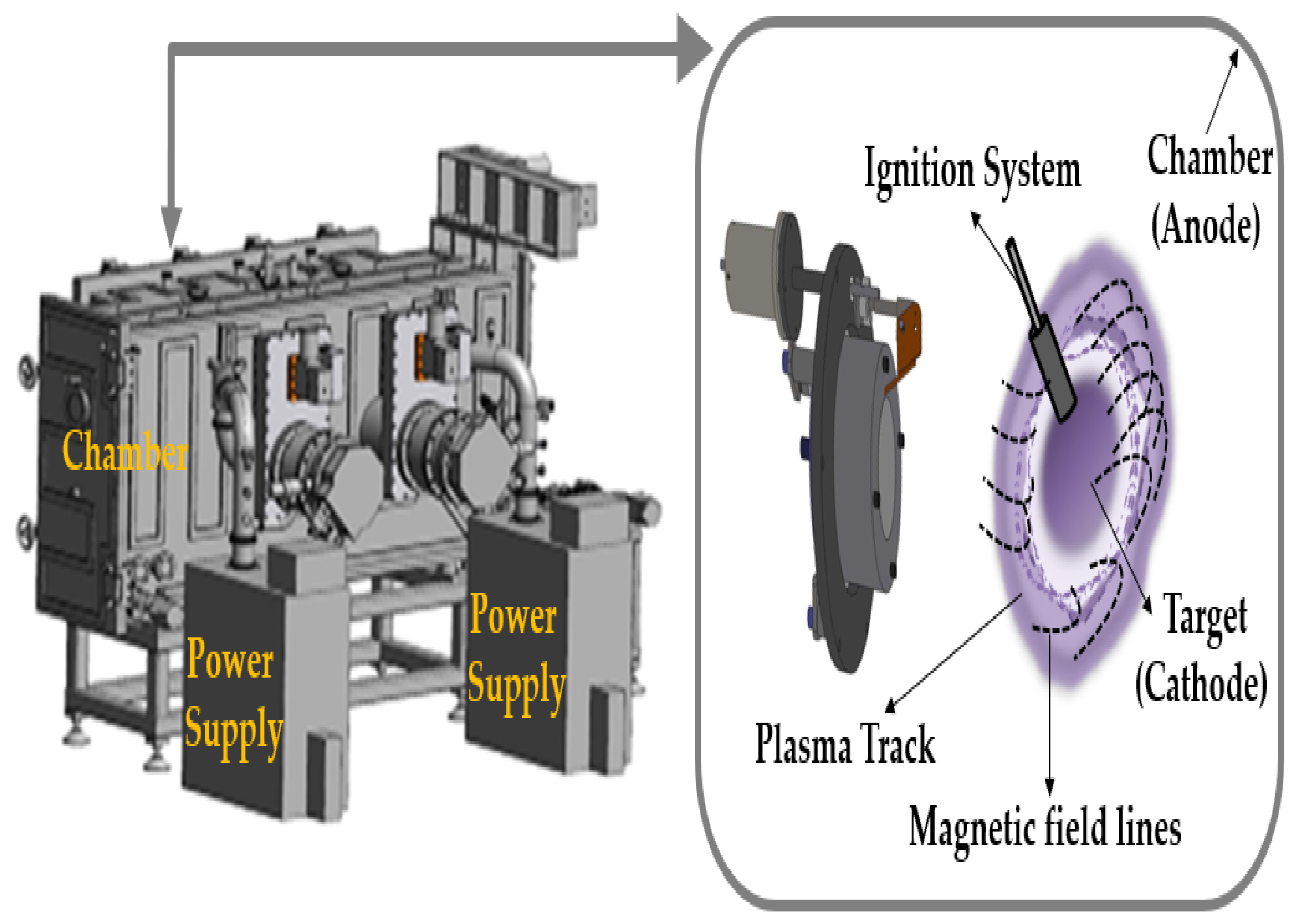
2.1. Deposition of Ti:V2O5 Counter Electrode and IrO2 Buffer Layer
2.2. Preparation of WO3 Working Electrochromic Electrode, Transparent and Electrolyte Layer
2.3. Experimental Details
3. Results
3.1. Ti:V2O5/ITO Films: Electrochemical and Optical Properties
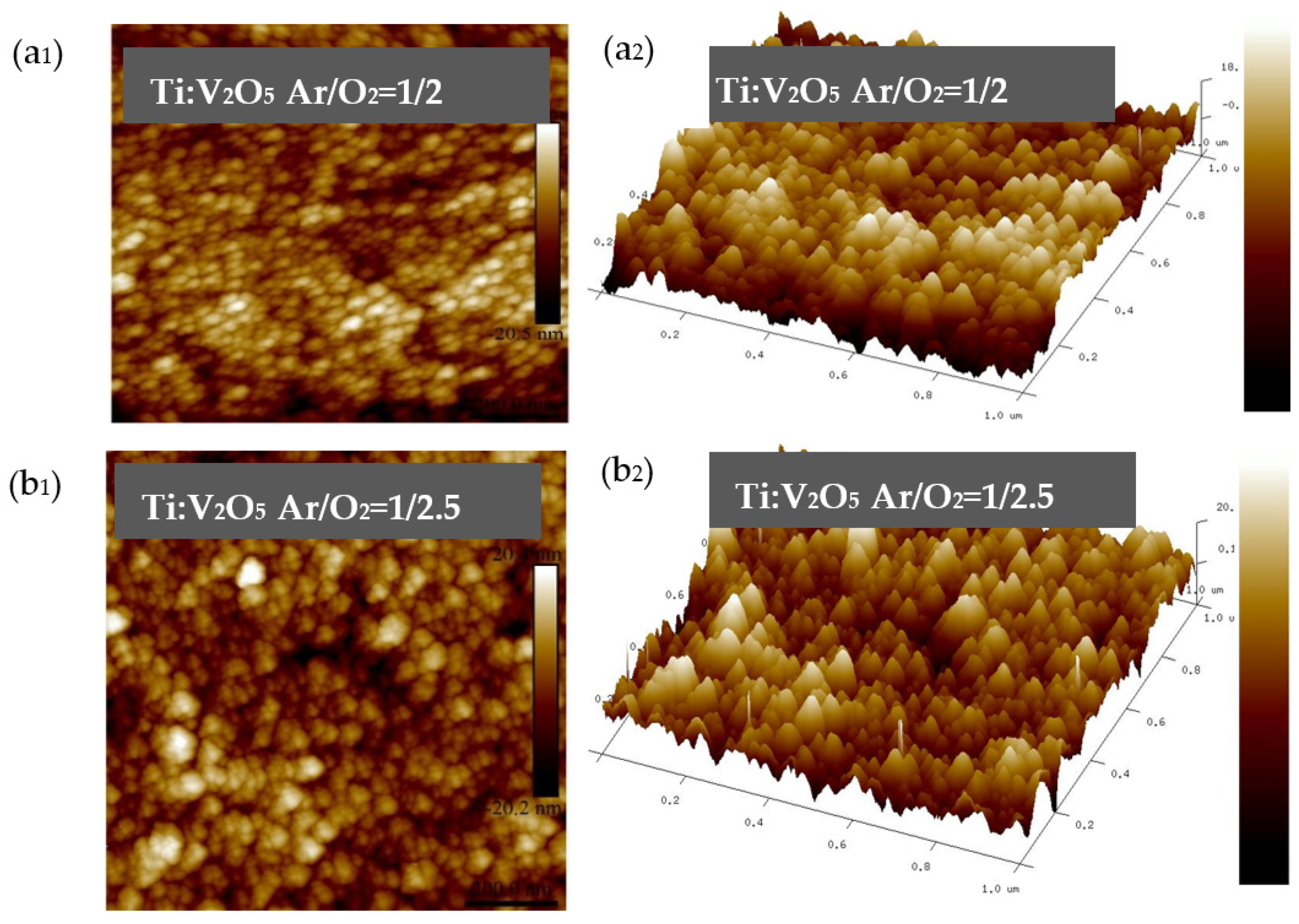
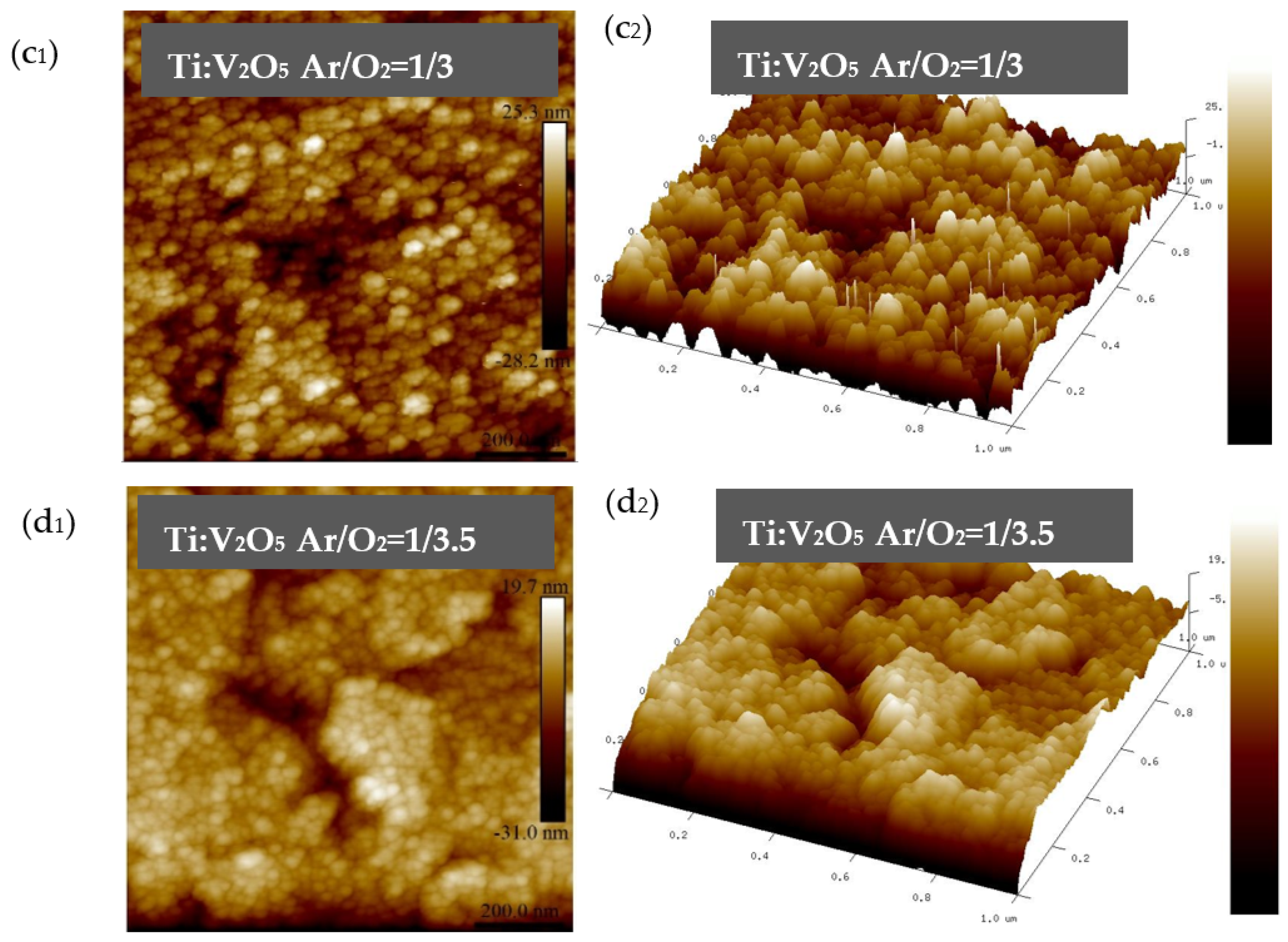
3.2. Ti:V2O5/ITO Films: Surface Roughness Properties
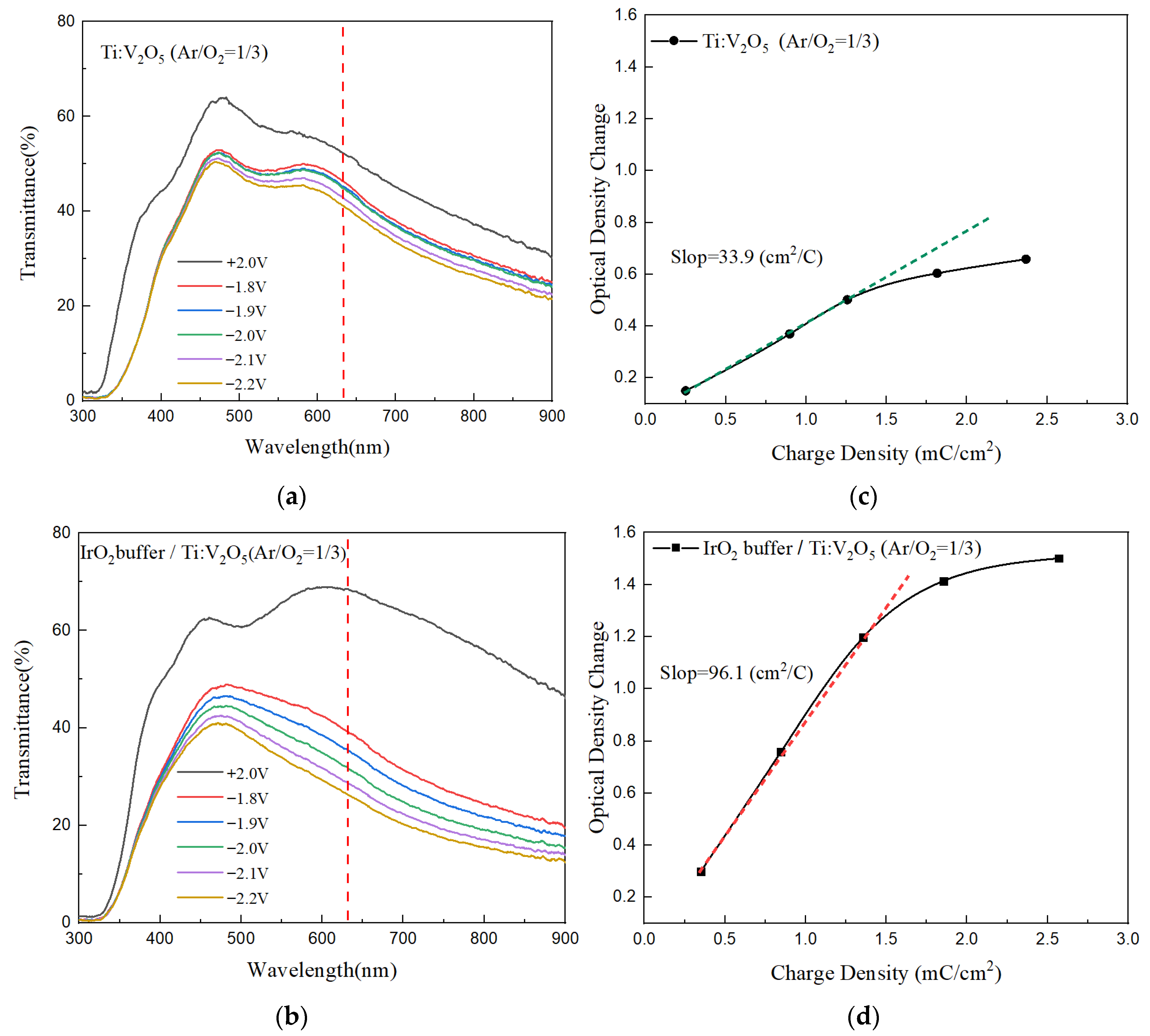
3.3. IrO2 Buffer/Ti:V2O5/ITO Films: Coloration Efficiency Analysis
3.4. IrO2 Buffer/Ti:V2O5/ITO Films: X-ray Diffraction and Raman Spectroscopy
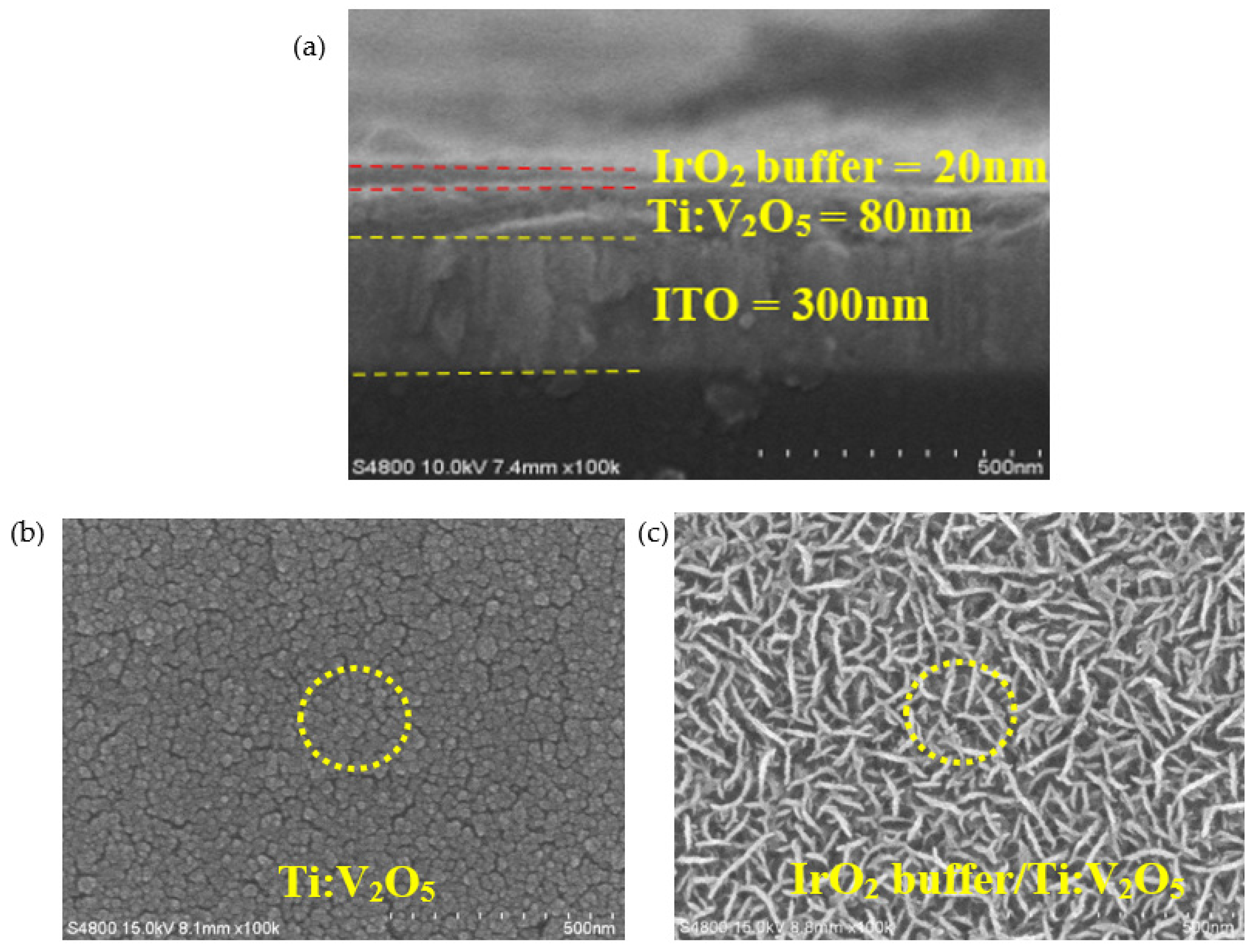
3.5. IrO2 Buffer/Ti:V2O5/ITO Films: Surface Morphology
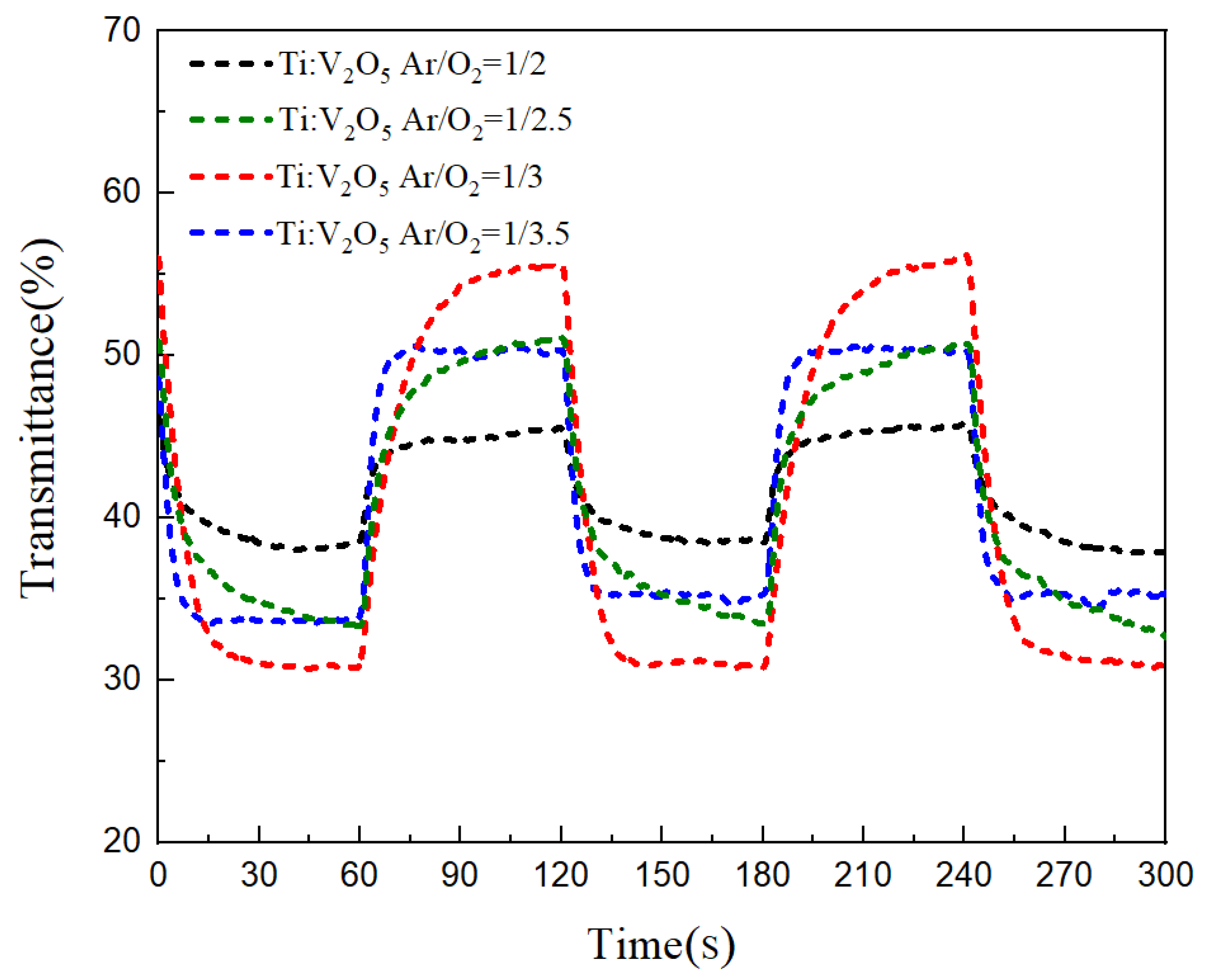
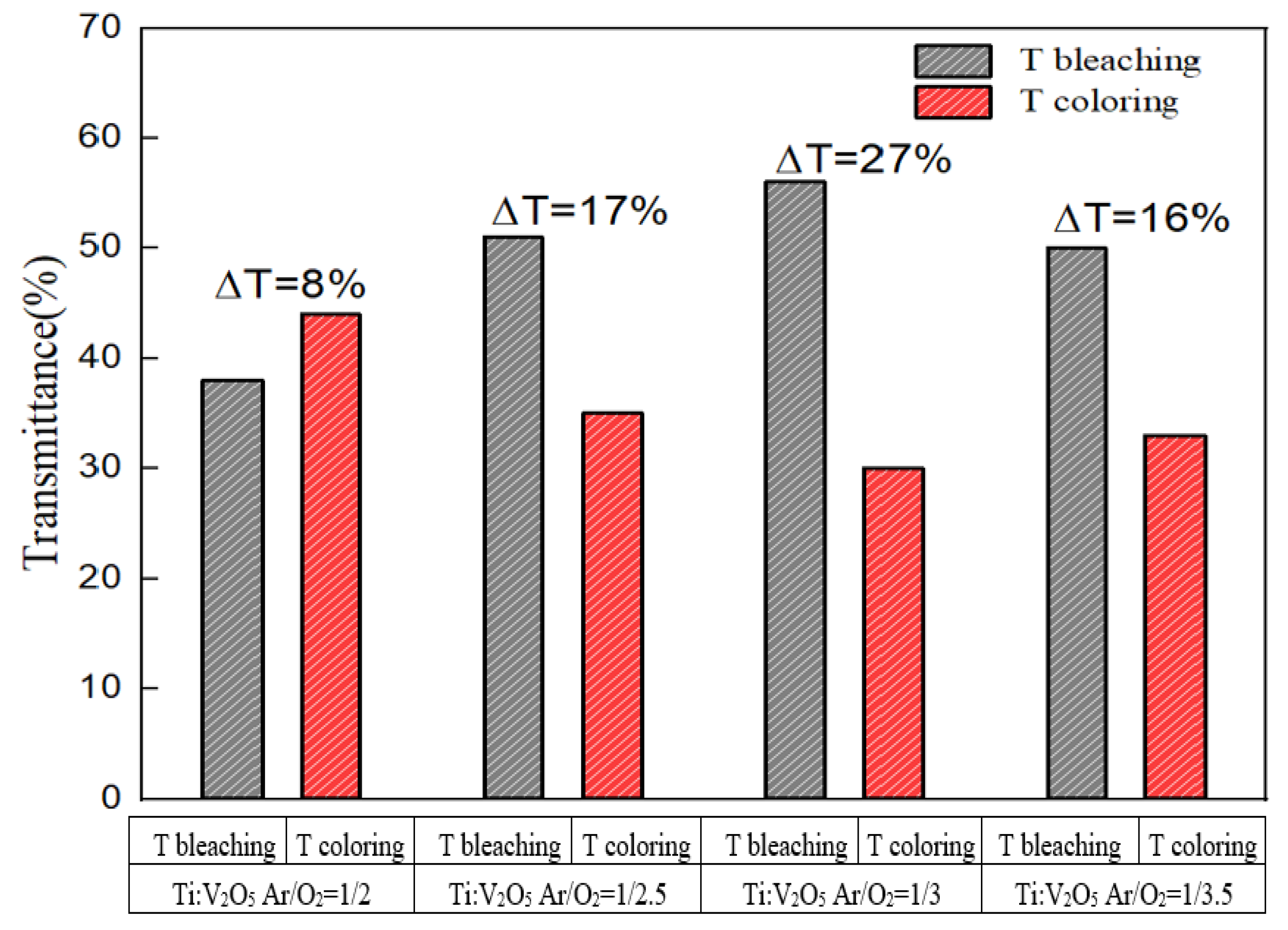
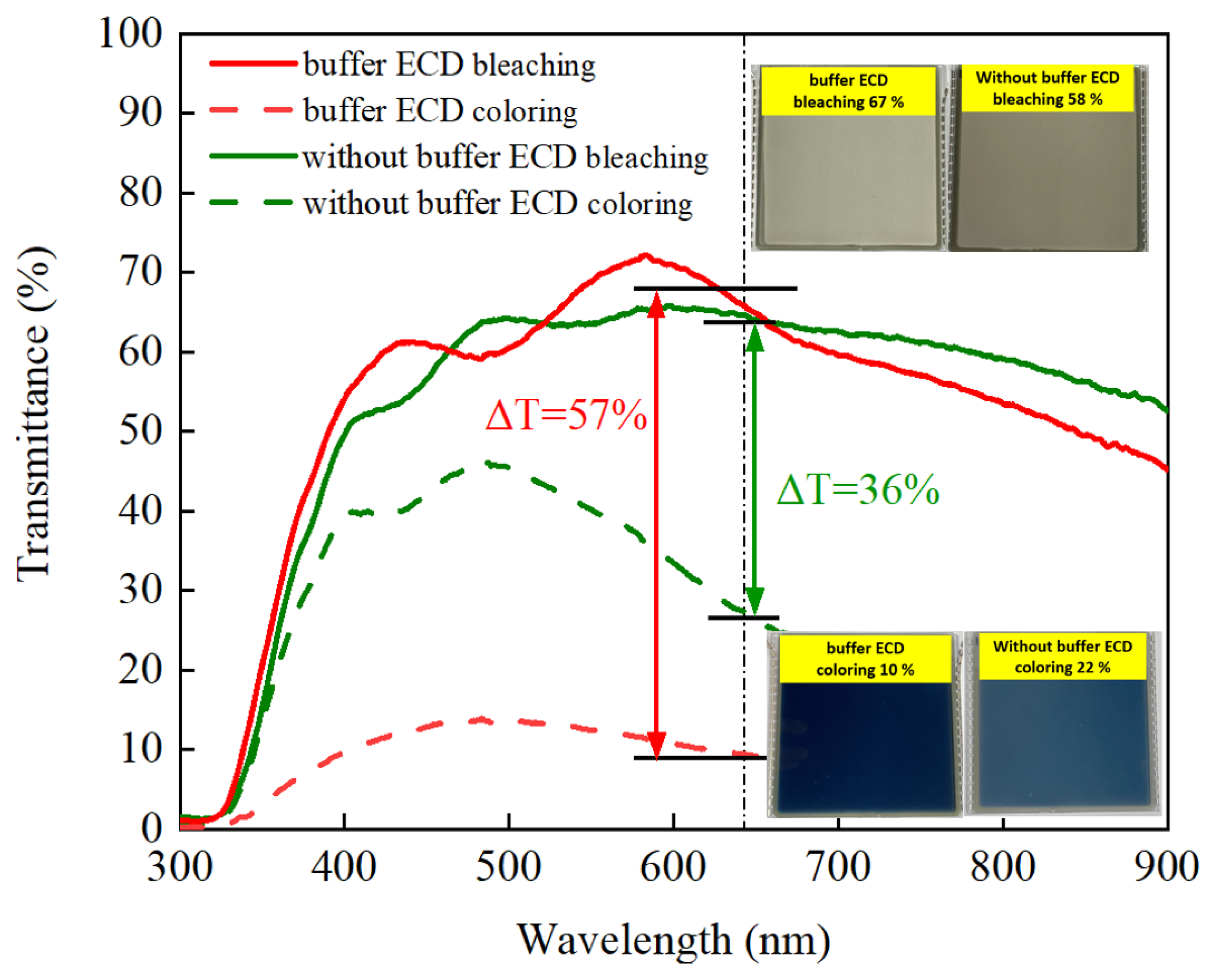
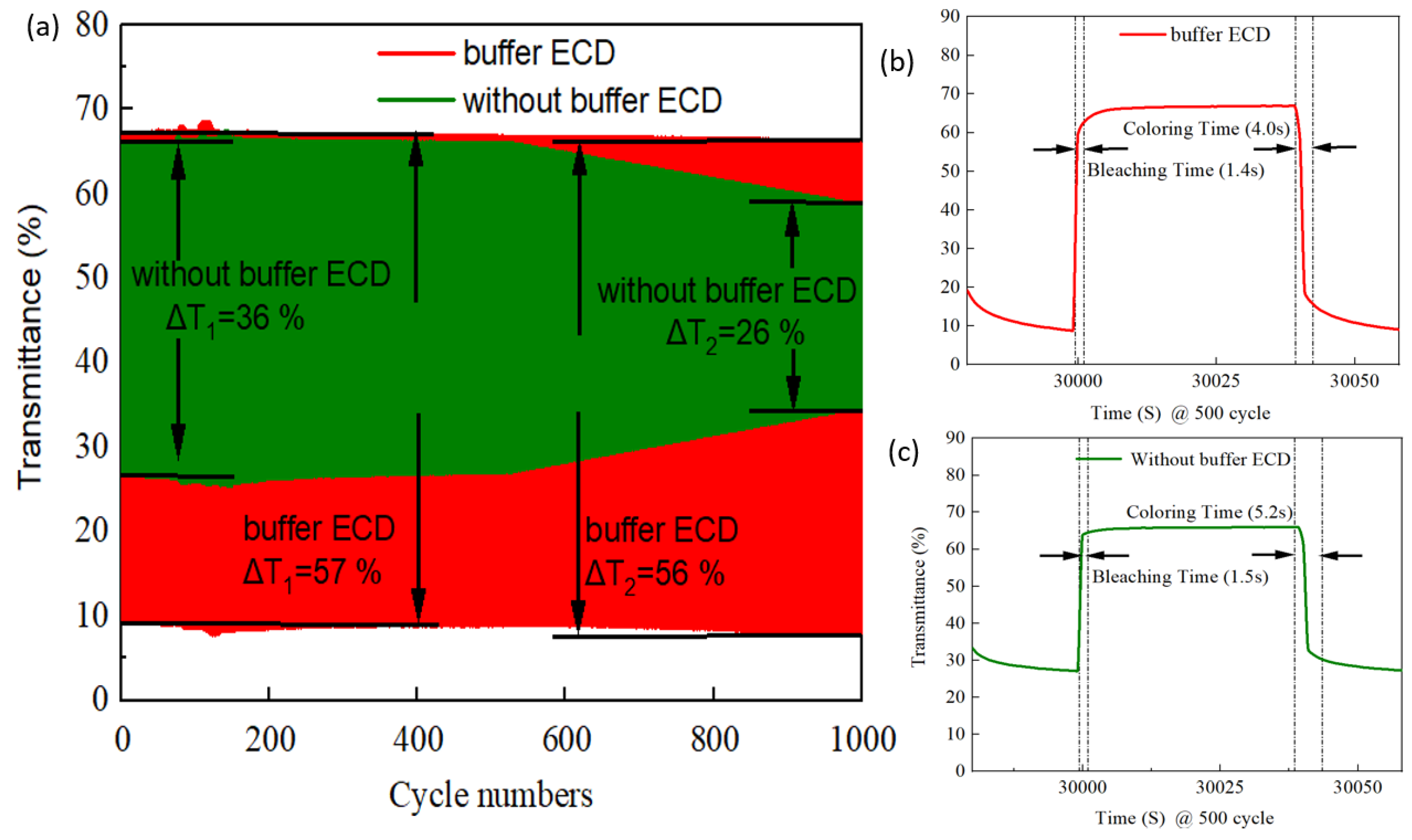
3.6. Buffer ECD: Optical Transmittance and Durability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Granqvist, C.G. Electrochromics for smart windows: Oxide-based thin films and devices. Thin Solid Films 2014, 564, 1–38. [Google Scholar] [CrossRef]
- Chang, J.Y.; Chen, Y.C.; Wang, C.M.; Wang, W.N.; Wen, C.Y.; Lin, J.M. Electrochromic properties of Lithium-doped tungsten oxide prepared by electron beam evaporation. Coatings 2019, 9, 191. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.H.; Koo, B.R.; Ahn, H.J. Title of the chapter Sheet resistance dependence of fluorine-doped tin oxide films for high-performance electrochromic devices. Ceram. Int. 2018, 44, 9408–9413. [Google Scholar] [CrossRef]
- Jiang, B.; Lou, B.; Li, J.; Peng, P.; Chen, J.W.; Chu, L.H.; Li, Y.F.; Li, M.C. Electrochemical effect of graphite fluoride modification on Li-rich cathode material in lithium ion battery. Ceram. Int. 2019, 45, 160–167. [Google Scholar] [CrossRef]
- Runnerstrom, E.L.; Llorde’s, A.; Lounisac, S.D.; Milliron, D.J. Nanostructured electrochromic smart windows: Traditional materials and NIR-selective plasmonic nanocrystals. Chem. Commun. 2014, 50, 10555–10572. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; McRaea, L.; Firby, C.J.; Hussein, M.A.; Elezzabi, A.Y. Nanohybridization of molybdenum oxide with tungsten molybdenum oxide nanowires for solution-processed fully reversible switching of energy storing smart windows. Nano Energy 2020, 47, 130–139. [Google Scholar] [CrossRef]
- Chen, P.W.; Chang, C.T.; Ko, T.F.; Hsu, S.C.; Li, K.F.; Wu, J.Y. Fast response of complementary electrochromic device based on WO3/NiO electrodes. Sci. Rep. 2020, 10, 8430. [Google Scholar] [CrossRef]
- Issam, M.; Gaudon, M.; Song, G.; Labrugere, C.; Rougier, A. Crystallized V2O5 as oxidized phase for unexpected multicolor electrochromism in V2O3 thick film. ACS Appl. Energy Mater. 2018, 1, 2721–2729. [Google Scholar]
- Niklasson, G.A.; Granqvist, C.G. Sol-gel fabrication of NiO and NiO/WO3 based electrochromic device on ITO and flexible substrate. Ceram. Int. 2020, 46, 8631–8639. [Google Scholar]
- Tritschler, U.; Beck, F.; Schlaad, H.; Cölfen, H. Electrochromic properties of self-organized multifunctional V2O5 polymer hybrid films. J. Mater. Chem. C 2015, 3, 950–954. [Google Scholar] [CrossRef] [Green Version]
- Salek, G.; Bellanger, B.; Mjejri, I.; Gaudon, M.; Rougier, A. Polyol Synthesis of Ti-V2O5 Nanoparticles and Their Use as Electrochromic Films. Inorg. Chem. 2016, 55, 9838–9847. [Google Scholar] [CrossRef]
- Xia, X.H.; Tu, J.P.; Zhang, J.; Wang, X.L.; Zhang, W.K.; Huang, H. Electrochromic properties of porous NiO thin flms prepared by a chemical bath deposition. Sol. Energy Mater. Sol. Cells 2008, 92, 628–633. [Google Scholar] [CrossRef]
- Subrahmanyam, A.; Kumar, C.S.; Karuppasamy, K.M. A note on fast protonic solid state electrochromic device: NiOx/Ta2O5/WO3−x. Sol. Energy Mater. Sol. Cells 2007, 91, 62–66. [Google Scholar] [CrossRef]
- McRae, L.; Li, H.; Elezzabi, A.Y. Solution-processed interfacial PEDOT: PSS assembly into porous tungsten molybdenum oxide nanocomposite flms for electrochromic applications. ACS Appl. Mater. Interfaces 2018, 10, 10520–10527. [Google Scholar]
- Lang, F.; Liu, J.; Wang, H.; Yan, H. NiO nanocrystalline/reduced graphene oxide composite film with enhanced electrochromic properties. Nano 2017, 12, 1750058. [Google Scholar] [CrossRef]
- Cai, G.; Darmawan, P.; Cui, M.; Chen, J.; Wang, X.; Eh, A.L.S.; Magdassi, S.; Lee, P.S. Inkjet-printed all solid-state electrochromic devices based on NiO/WO3. Nanoscale 2016, 8, 348–357. [Google Scholar] [CrossRef]
- Chang, C.C.; Chi, P.W.; Chandan, P.; Lin, C.K. Electrochemistry and Rapid ElectrochromismControl of MoO3/V2O5 Hybrid Nano bilayers. Nat. Mater. 2019, 12, 2475. [Google Scholar] [CrossRef] [Green Version]
- Mouratis, K.; Tudose, V.; Romanitan, C.; Pachiu, C.; Tutunaru, O.; Suchea, M.; Couris, S.; Vernardou, D.; Emmanouel, K. Electrochromic Performance of V2O5 Thin Films Grown by Spray Pyrolysis. Materials 2020, 13, 3859. [Google Scholar] [CrossRef]
- Wei, Y.; Zhou, J.; Xu, C. Improved stability of electrochromic devices using Ti-doped V2O5 film. Electrochim. Acta 2015, 166, 277–284. [Google Scholar] [CrossRef]
- Zeng, H.; Liu, D.; Zhang, Y.; See, K.A.; Jun, Y.S.; Wu, G.; Gerbec, J.A.; Ji, X.; Stucky, G.D. Nanostructured Mn-Doped V2O5 Cathode Material Fabricated from Layered Vanadium Jarosite. Chem. Mater. 2015, 27, 7331–7336. [Google Scholar] [CrossRef] [Green Version]
- Vernardou, D.; Marathianou, I.; Katsarakis, N.; Koudoumas, E.; Kazadojev, I.I.; O’Brien, S.; Pemble, M.E.; Povey, I.M. Capacitive behavior of Ag doped V2O5 grown by aerosol assisted chemical vapour deposition. Electrochim. Acta 2016, 196, 294–299. [Google Scholar] [CrossRef]
- Venkatesan, A.; Chandar, N.R.K.; Pradeeswari, K.; Pandi, P.; Kandasamy, A.; Kumar, R.M.; Jayavel, R. Influence of Al doping on structural, luminescence and electrochemical properties of V2O5 nanostructures synthesized via non-hydrolytic sol-gel technique. Mater. Res. Express 2019, 6, 015017. [Google Scholar] [CrossRef]
- Anil, K.; Sahay, P.P. Influence of Ti doping on the microstructural and electrochromic properties of dip-coated nanocrystalline V2O5 thin films. J. Solgel Sci. Technol. 2020, 95, 34–51. [Google Scholar]
- Tian, S.; Xing, A.; Tang, H.; Bao, Z.H.; Wu, G.M. Enhanced cycling stability of TiO2-coated V2O5 nanorods through a surface sol–gel process for lithium ion battery application. J. Mater. Chem. A 2014, 2, 2896. [Google Scholar] [CrossRef]
- Madhavi, V.; Kumar, P.J.; Kondaiah, P.; Hussain, O.M.; Uthanna, S. Efect of molybdenum doping on the electrochromic properties of tungsten oxide thin fms by RF magnetron sputtering. Ionics 2014, 20, 1737–1745. [Google Scholar] [CrossRef]
- Pattarapon, P.; Son, Y.H.; Sung, Y.M.; Ok, J.W. The effect of sputtering Ar gas pressure on optical and electrical properties of flexible ECD device with WO3 electrode deposited by RF magnetron sputtering on ITO/PET substrate. Opt. Mater. 2022, 123, 111829. [Google Scholar]
- Panagopoulou, M.; Vernardou, D.; Koudoumas, E.; Tsoukalas, D.; Raptis, Y.S. Oxygen and temperature effects on the electrochemical and electrochromic properties of rf-sputtered V2O5 thin films. Electrochim. Acta 2017, 232, 54–63. [Google Scholar] [CrossRef]
- Liu, Y.; Jia, C.; Wan, Z.; Weng, X.; Xie, J.; Deng, L. Electrochemical and electrochromic properties of novel nanoporous NiO/V2O5 hybrid film. Sol. Energy Mater. Sol. Cells 2015, 132, 467–475. [Google Scholar] [CrossRef]
- Najdosk, M.; Koleva, V.; Samet, A. Effect of deposition conditions on the electrochromic properties of nanostructured thin films of ammonium intercalated vanadium pentoxide xerogel. J. Phys. Chem. C 2014, 118, 9636–9646. [Google Scholar] [CrossRef]
- Yu, D.M.; Zhang, S.T.; Liu, D.W.; Zhou, X.Y.; Xie, S.H.; Zhang, Q.F.; Liu, Y.Y.; Cao, G.Z. Effect of manganese doping on Li-ion intercalation properties of V2O5 films. J. Mater. Chem. 2010, 20, 10841–10846. [Google Scholar] [CrossRef]
- Loi, M.R.; Moura, E.A.; Westphal, T.M.; Balboni, R.D.C.; Gündelc, A.; Flores, W.H.; Pereira, M.B.; Santos, M.J.L.; Santos, J.F.L.; Pawlicka, A.; et al. Impact of Zr precursor on the electrochemical properties of V2O5 sol-gel films. J. Electroanal. Chem. 2019, 839, 67–74. [Google Scholar] [CrossRef]
- Moura, E.A.; Cholan, C.M.; Balboni, R.D.C.; Westphal, T.M.; Lemos, R.M.J.; Azevedo, C.F.; Gündel, A.; Flores, W.H.; Gomez, J.A.; Ely, F.; et al. Electrochemical properties of thin films of V2O5 doped with TiO2. J. Phys. Chem. Solids 2018, 119, 1–8. [Google Scholar] [CrossRef]
- Vasanth, R.D.; Ponpandian, N.; Mangalaraj; Viswanathan, C. Effect of annealing and electrochemical properties of sol–gel dip coated nanocrystalline V2O5 thin films. Mater. Sci. Semicond. Process. 2013, 16, 256–262. [Google Scholar] [CrossRef]
- Sinha, S.K. Effect of temperature on structural, optical and electrical properties of pulsed-laser deposited W-doped V2O5 thin films. Superlattices Microstruct. 2019, 25, 88–94. [Google Scholar] [CrossRef]
- Qiu, D.; Wu, J.; Liang, L.; Zhang, H.; Cao, H.; Yong, W.; Tian, T.; Gao, J.; Zhuge, F. Structural and electrochromic properties of undoped and Mo-doped V2O5 thin films by a two-electrode electrodeposition. J. Nanosci. Nanotechnol. 2018, 18, 7502–7507. [Google Scholar] [CrossRef]
- Wang, C.; Shi, Z.; Li, W.; Song, X.; Jia, S.; Wang, L. IEEE Transactions on Plasma Science. IEEE 2015, 43, 2267–2274. [Google Scholar]
- Thornton, J.A. Influence of apparatus geometry and deposition conditions on the structure and topography of thick sputtered coatings. J. Vac. Sci. Technol. 1974, 11, 666. [Google Scholar] [CrossRef]
- Ko, T.F.; Chen, P.W.; Li, K.M.; Young, H.T.; Chang, C.T.; Shu, S.C. High-Performance Complementary Electrochromic Device Based on Iridium Oxide as a Counter Electrode. Materials 2012, 14, 1591. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Q.; Zhang, Q.; Dong, G.; Zhong, X.; Xiao, Y.; Delplancke-Ogletree, M.P.; Reniers, F.; Diao, X. Dynamic behaviors of inorganic all-solid-state electrochromic device: Role of potential. Electrochim. Acta 2018, 269, 617–623. [Google Scholar] [CrossRef]
- Chen, P.W.; Chang, C.T.; Ali, M.; Wu, J.-Y.; Li, Y.-C.; Chen, M.-H.; Jan, D.-J.; Yuan, C.-T. Tantalum oxide film deposited by vacuum cathodic arc plasma with improved electrochromic performance. Sol. Energy Mater. Sol. Cells 2018, 182, 188–195. [Google Scholar] [CrossRef]
- Atak, G.; Coşkun, Ö.D. Annealing effects of NiO thin films for all-solid-state electrochromic devices. Solid State Ion. 2017, 305, 43–51. [Google Scholar] [CrossRef]



| No. | Film | Ar/O2 (Ar = 20 sccm) | W.P. (Torr) | DC Power (W) | Deposition Temp. (°C) | Deposition Time (s) | Thickness (nm) |
|---|---|---|---|---|---|---|---|
| Sample 1 | Ti:V2O5 | 1/2 | 2.1 × 10−3 | 1200 | 100 | 80 | 80 |
| Sample 2 | Ti:V2O5 | 1/2.5 | 2.3 × 10−3 | 1200 | 100 | 80 | 80 |
| Sample 3 | Ti:V2O5 | 1/3 | 2.5 × 10−3 | 1200 | 100 | 80 | 80 |
| Sample 4 | Ti:V2O5 | 1/3.5 | 2.8 × 10−3 | 1200 | 100 | 80 | 80 |
| Sample 5 | IrO2 buffer | 1/3 | 1.8 × 10−3 | 1250 | 100 | 10 | 20 |
| Target | Ar/O2 (sccm) | W.P. (Torr) | DC Power (W) | Deposition Time (min) | Deposition Rate (nm/min) | Deposition Temp. °C | Thickness (nm) |
|---|---|---|---|---|---|---|---|
| W Metal | 1/3 (Ar = 100) | 8 × 10−3 | 1500 | 15 | 13 | 50 | 200 |
| ITO | 1/3 (Ar = 100) | 3 × 10−3 | 500 | 60 | 5 | 200 | 300 |
| No. | Film | Ar/O2 Ratio | Image Surface Area (µm2) | Image Projected Surface Area (µm2) | Root Mean Square Roughness (nm) | Average Roughness (nm) |
|---|---|---|---|---|---|---|
| Sample 1 | Ti:V2O5 | 1/2 | 1.33 | 1 | 7.22 | 5.63 |
| Sample 2 | Ti:V2O5 | 1/2.5 | 1.22 | 1 | 6.54 | 4.83 |
| Sample 3 | Ti:V2O5 | 1/3 | 1.10 | 1 | 5.01 | 3.91 |
| Sample 4 | Ti:V2O5 | 1/3.5 | 1.27 | 1 | 6.62 | 4.94 |
| Counter Electrode | Method | ∆T (%) | CE (cm2/C) | Switching Time (tc/tb) | Ref. |
|---|---|---|---|---|---|
| IrO2 buffer/Ti:V2O5 | CAP | 57 | 96.1 | 4.0/1.4 s | This work |
| IrO2 | CAP | 50 | - | 4.8/1.5 s | [38] |
| V2O5 | polyol-mediated synthesis | 50 | - | - | [8] |
| V2O5 | polymer synthesis | 33 | 89 | 20/23 s | [10] |
| Ti:V2O5 | polyol synthesis | 10 | - | - | [11] |
| NiO | spray pyrolysis | 37.4 | 42 | 8/10 s | [12] |
| V2O5 | spin coating | 31 | - | 8.2/6.3 s | [17] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, T.-F.; Chen, P.-W.; Li, K.-M.; Young, H.-T. Applied IrO2 Buffer Layer as a Great Promoter on Ti-Doping V2O5 Electrode to Enhance Electrochromic Device Properties. Materials 2022, 15, 5179. https://doi.org/10.3390/ma15155179
Ko T-F, Chen P-W, Li K-M, Young H-T. Applied IrO2 Buffer Layer as a Great Promoter on Ti-Doping V2O5 Electrode to Enhance Electrochromic Device Properties. Materials. 2022; 15(15):5179. https://doi.org/10.3390/ma15155179
Chicago/Turabian StyleKo, Tien-Fu, Po-Wen Chen, Kuan-Ming Li, and Hong-Tsu Young. 2022. "Applied IrO2 Buffer Layer as a Great Promoter on Ti-Doping V2O5 Electrode to Enhance Electrochromic Device Properties" Materials 15, no. 15: 5179. https://doi.org/10.3390/ma15155179
APA StyleKo, T.-F., Chen, P.-W., Li, K.-M., & Young, H.-T. (2022). Applied IrO2 Buffer Layer as a Great Promoter on Ti-Doping V2O5 Electrode to Enhance Electrochromic Device Properties. Materials, 15(15), 5179. https://doi.org/10.3390/ma15155179







