Microstructural Evolution of Diamond-Based Composites at High Temperature and High Pressure
Abstract
1. Introduction
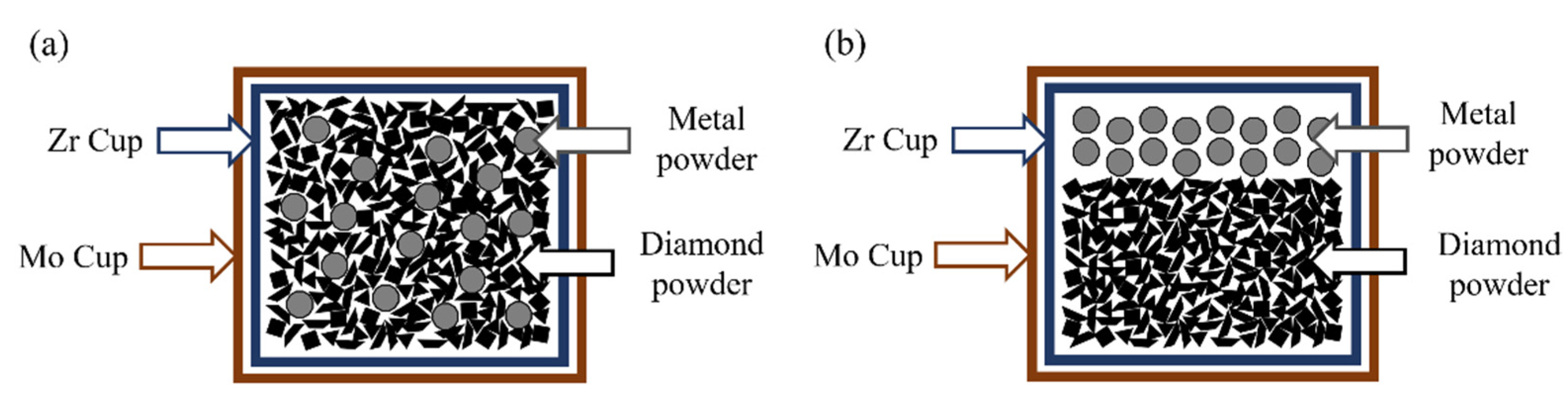
2. Materials and Methods
3. Results and Discussion
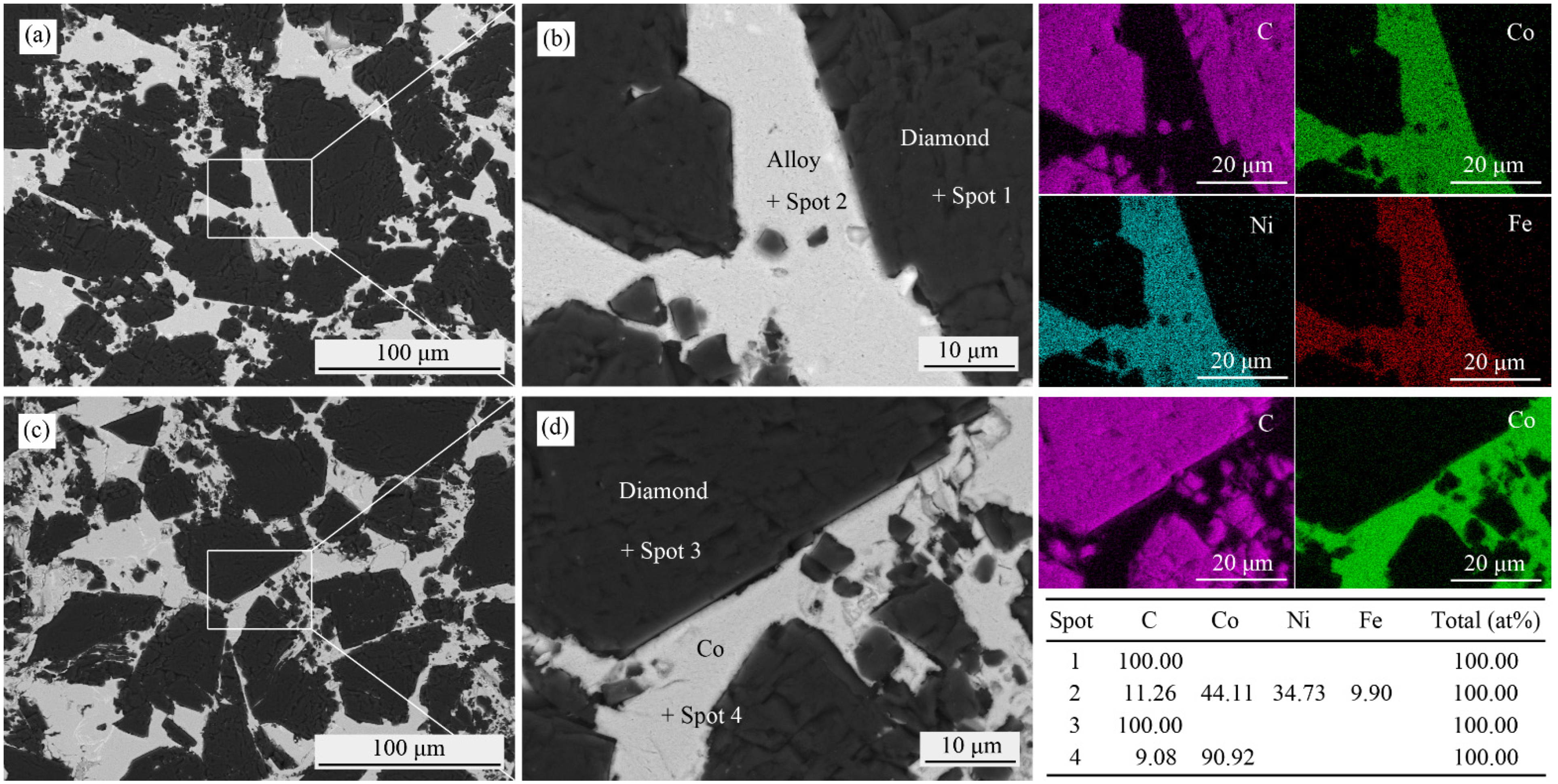
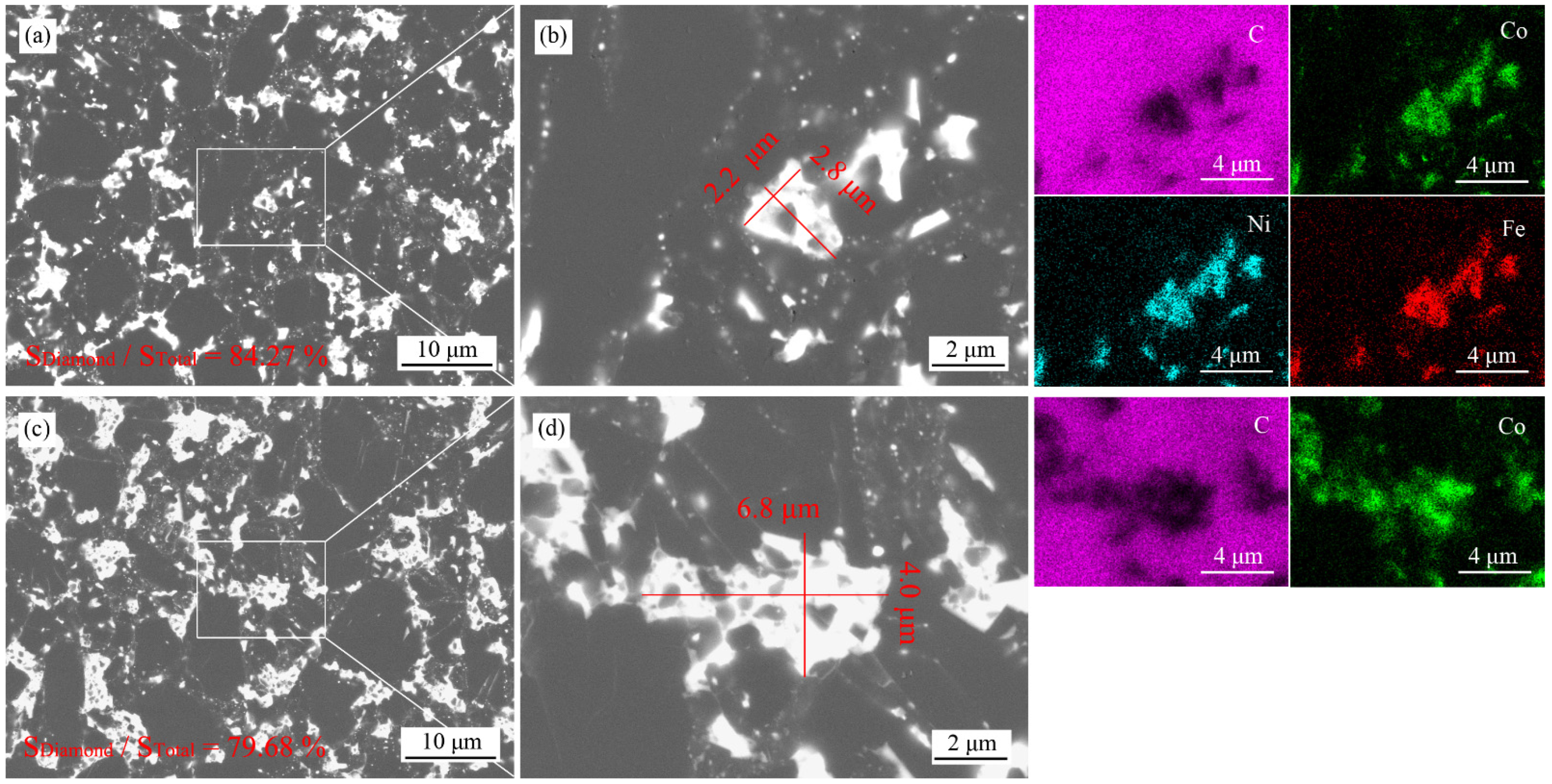
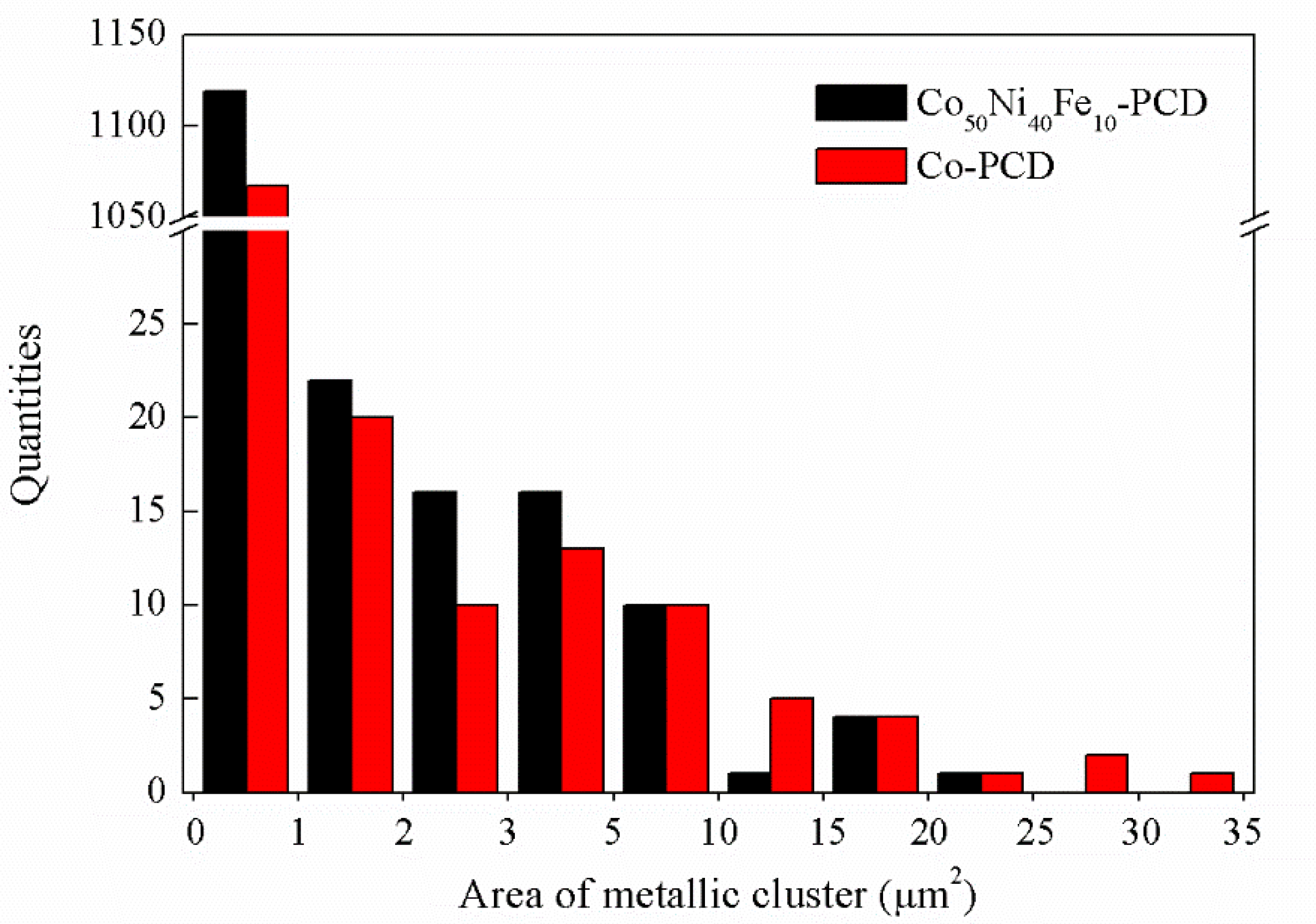
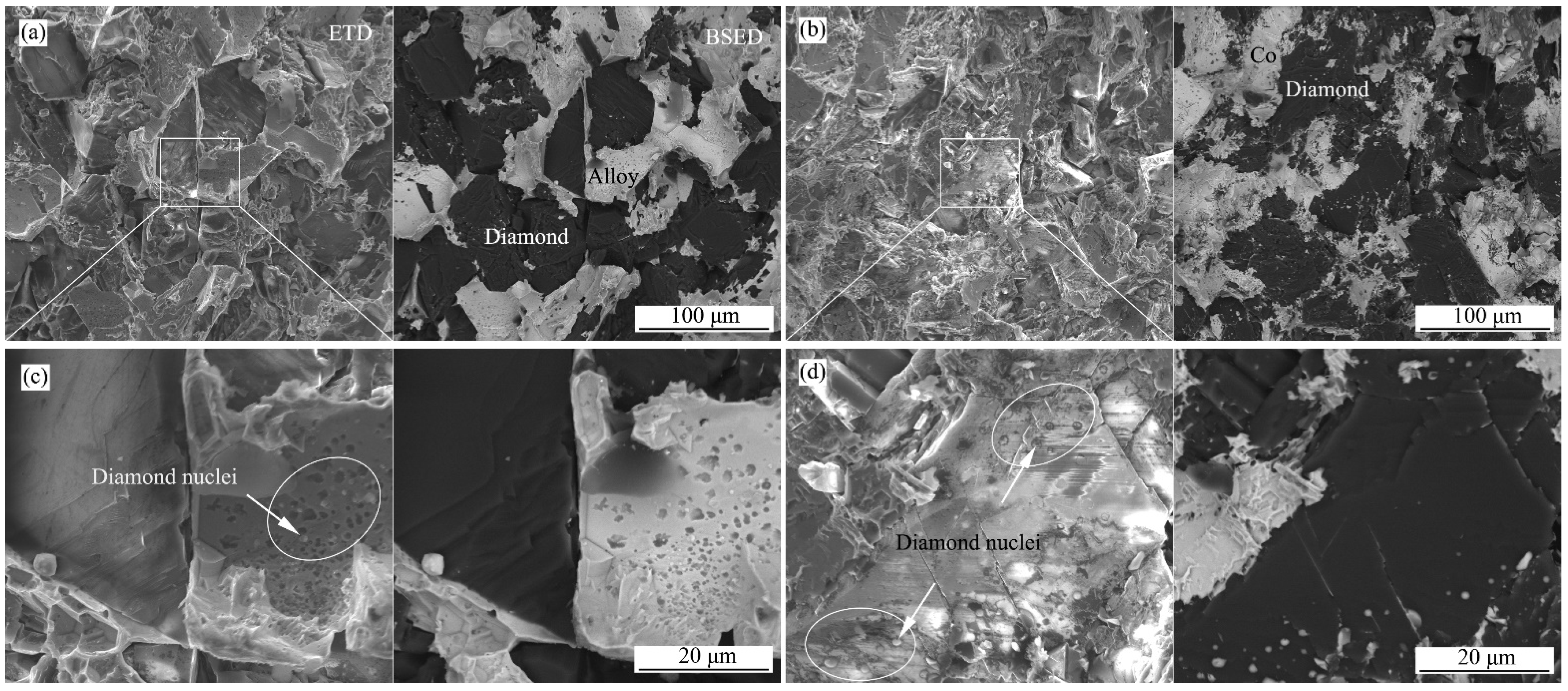
3.1. Microstructures
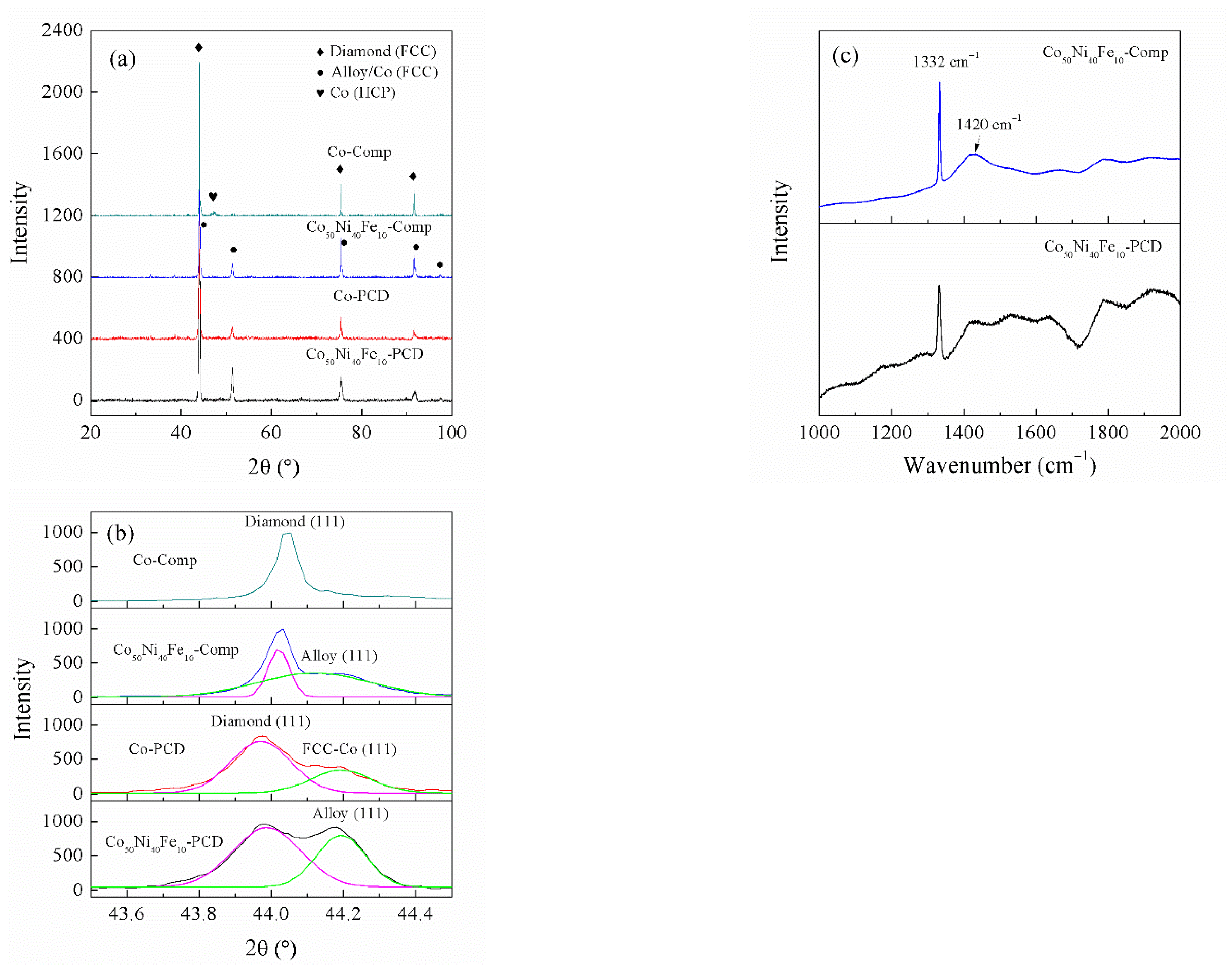
3.2. Phase Analyses
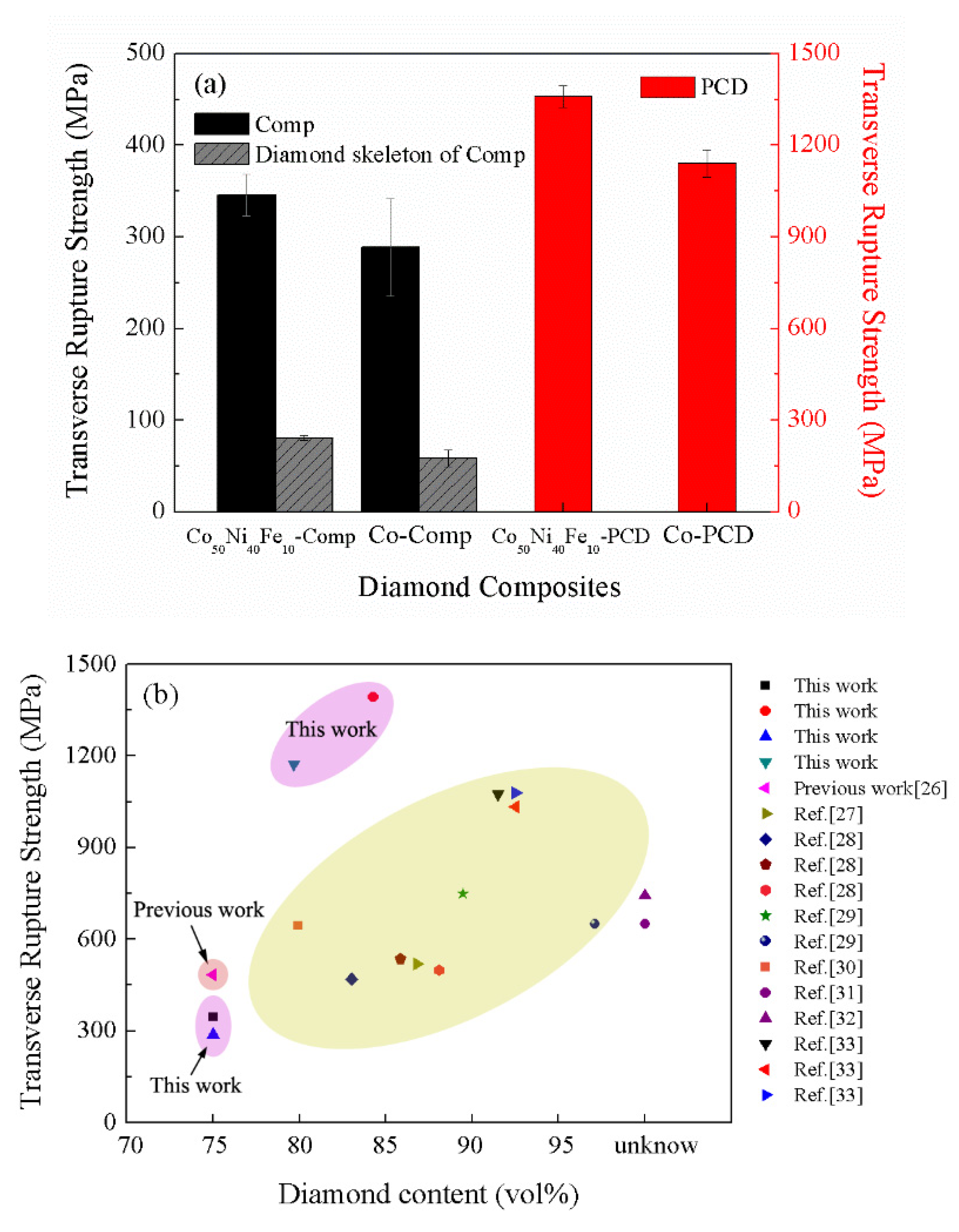
3.3. Mechanical Properties
3.4. Fracture Morphologies
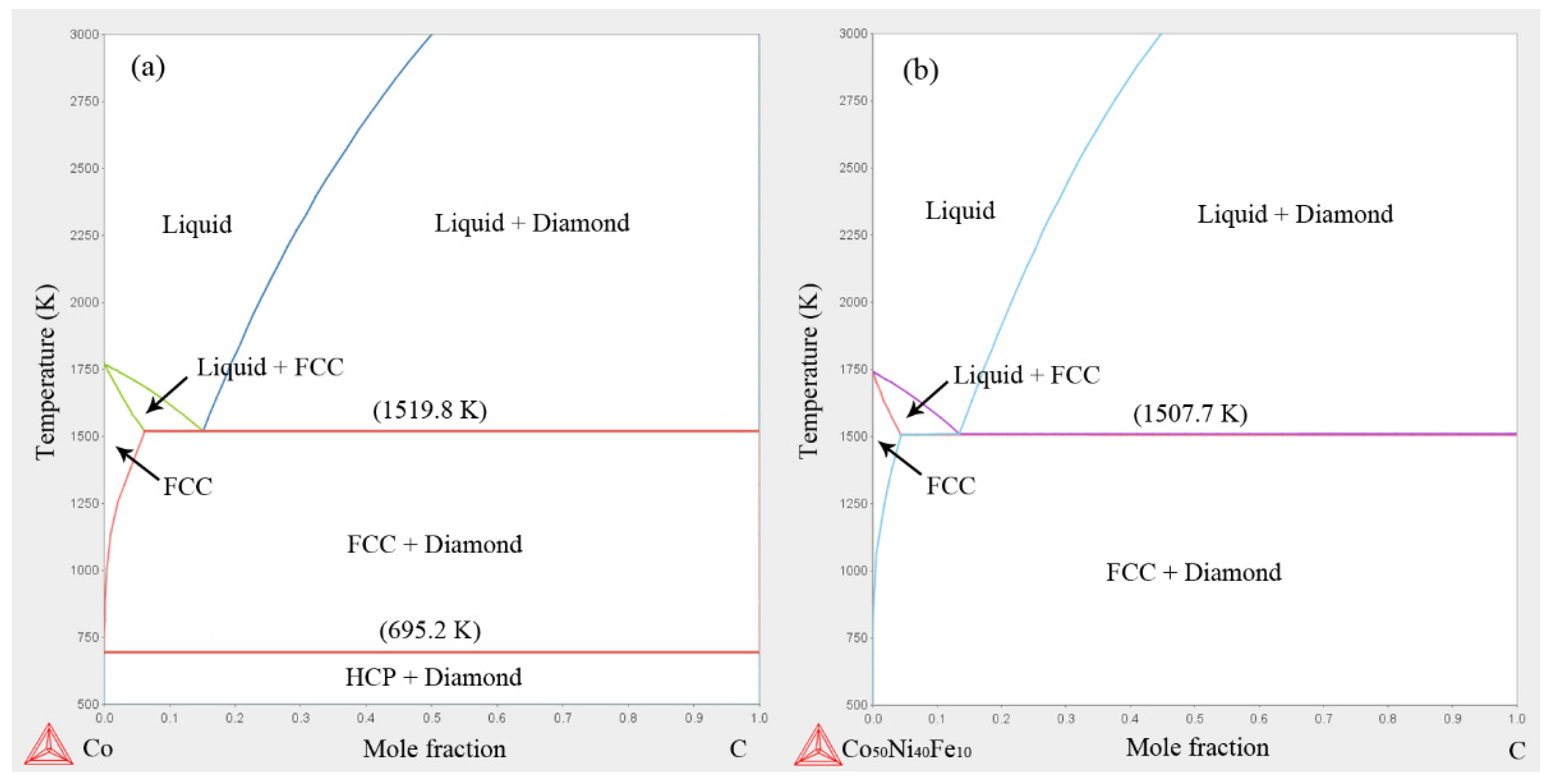
3.5. Thermodynamics at HTHP
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tanigaki, K.; Ogi, H.; Sumiya, H.; Kusakabe, K.; Nakamura, N.; Hirao, M.; Ledbetter, H. Observation of higher stiffness in nanopolycrystal diamond than monocrystal diamond. Nat. Commun. 2013, 4, 2343. [Google Scholar] [CrossRef]
- Anaya, J.; Bai, T.; Wang, Y.; Li, C.; Goorsky, M.; Bougher, T.L.; Yates, L.; Cheng, Z.; Graham, S.; Hobart, K.D.; et al. Simultaneous determination of the lattice thermal conductivity and grain/grain thermal resistance in polycrystalline diamond. Acta Mater. 2017, 139, 215–225. [Google Scholar] [CrossRef]
- Tang, H.; Yuan, X.; Cheng, Y.; Fei, H.; Liu, F.; Liang, T.; Zeng, Z.; Ishii, T.; Wang, M.-S.; Katsura, T.; et al. Synthesis of paracrystalline diamond. Nature 2021, 599, 605–610. [Google Scholar] [CrossRef]
- Huang, Q.; Yu, D.; Xu, B.; Hu, W.; Ma, Y.; Wang, Y.; Zhao, Z.; Wen, B.; He, J.; Liu, Z.; et al. Nanotwinned diamond with unprecedented hardness and stability. Nature 2014, 510, 250–253. [Google Scholar] [CrossRef]
- Wen, B.; Xu, B.; Wang, Y.; Gao, G.; Zhou, X.-F.; Zhao, Z.; Tian, Y. Continuous strengthening in nanotwinned diamond. npj Comput. Mater. 2019, 5, 117. [Google Scholar] [CrossRef]
- Xiao, J.; Wen, B.; Xu, B.; Zhang, X.; Wang, Y.; Tian, Y. Intersectional nanotwinned diamond-the hardest polycrystalline diamond by design. npj Comput. Mater. 2020, 6, 119. [Google Scholar] [CrossRef]
- Shang, Y.; Liu, Z.; Dong, J.; Yao, M.; Yang, Z.; Li, Q.; Zhai, C.; Shen, F.; Hou, X.; Wang, L.; et al. Ultrahard bulk amorphous carbon from collapsed fullerene. Nature 2021, 599, 599–604. [Google Scholar] [CrossRef]
- Furberg, A.; Fransson, K.; Zackrisson, M.; Larsson, M.; Arvidsson, R. Environmental and resource aspects of substituting cemented carbide with polycrystalline diamond: The case of machining tools. J. Clean. Prod. 2020, 277, 123577. [Google Scholar] [CrossRef]
- Kunuku, S.; Sankaran, K.J.; Tsai, C.-Y.; Chang, W.-H.; Tai, N.-H.; Leou, K.C.; Lin, I.-N. Investigations on Diamond Nanostructuring of Different Morphologies by the Reactive-Ion Etching Process and Their Potential Applications. ACS Appl. Mater. Interfaces 2013, 5, 7439–7449. [Google Scholar] [CrossRef]
- Sánchez Egea, A.J.; Martynenko, V.; Martínez Krahmer, D.; López de Lacalle, L.N.; Benítez, A.; Genovese, G. On the Cutting Performance of Segmented Diamond Blades when Dry-Cutting Concrete. Materials 2018, 11, 264. [Google Scholar] [CrossRef]
- Ternero, F.; Amaral, P.M.; Fernandes, J.C.; Rosa, L.G. Evaluation of Wear Behaviour in Metallic Binders Employed in Diamond Tools for Cutting Stone. Materials 2021, 14, 3988. [Google Scholar] [CrossRef] [PubMed]
- McNamara, D.; Alveen, P.; Carolan, D.; Murphy, N.; Ivanković, A. Fracture toughness evaluation of polycrystalline diamond as a function of microstructure. Eng. Fract. Mech. 2015, 143, 1–16. [Google Scholar] [CrossRef]
- Hu, Q.; Jia, X.-P.; Li, S.-S.; Su, T.-C.; Hu, M.-H.; Fang, C.; Zhang, Y.-W.; Li, G.; Liu, H.-Q.; Ma, H.-A. Research on mechanism of carbon transformation in the preparation of polycrystalline diamond by melt infiltration and growth method under high pressures. Acta Phys. Sin. 2016, 65, 068101. (In Chinese) [Google Scholar] [CrossRef]
- Li, Q.; Zhan, G.; Li, D.; He, D.W.; Moellendick, T.E.; Gooneratne, C.P.; Alalsayednassir, A.G. Ultrastrong catalyst-free polycrystalline diamond. Sci. Rep. 2020, 10, 22020. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Liu, B.; Hu, W.; Dong, X.; Wang, Y.; Huang, Q.; Gao, Y.; Sun, L.; Zhao, Z.; Wu, Y.; et al. Coherent interfaces govern direct transformation from graphite to diamond. Nature 2022, 607, 486–491. [Google Scholar] [CrossRef]
- Xie, H.; Yin, F.; Yu, T.; Wang, J.-T.; Liang, C. Mechanism for direct graphite-to-diamond phase transition. Sci. Rep. 2014, 4, 5930. [Google Scholar] [CrossRef]
- Mallika, K.; DeVries, R.C.; Komanduri, R. On the low pressure transformation of graphite to diamond in the presence of a ‘catalyst-solvent’. Thin Solid Films 1999, 339, 19–33. [Google Scholar] [CrossRef]
- Chen, N.; Ma, H.; Fang, C.; Li, Y.; Liu, X.; Zhou, Z.; Jia, X. Synthesis and characterization of IIa-type S-doped diamond in FeNi catalyst under high pressure and high temperature conditions. Int. J. Refract. Met. Hard Mater. 2017, 66, 122–126. [Google Scholar] [CrossRef]
- Bai, Q.; Wang, Z.; Guo, Y.; Chen, J.; Shang, Y. Graphitization Behavior of Single Crystal Diamond for the Application in Nano-Metric Cutting. Curr. Nanosci. 2018, 14, 377–383. [Google Scholar] [CrossRef]
- de Oliveira, L.J.; Cabral, S.C.; Filgueira, M. Study hot pressed Fe-diamond composites graphitization. Int. J. Refract. Met. Hard Mater. 2012, 35, 228–234. [Google Scholar] [CrossRef]
- Guo, Z.; Deng, F.; Zhang, L.; Zhang, Z.; Wang, H.; Zheng, K.; Gao, Y.; Fang, Y. The novel and facile electrolysis method for removing the cobalt binder phase from large diameter polycrystalline diamond compacts. Ceram. Int. 2021, 48, 3125–3132. [Google Scholar] [CrossRef]
- Jia, H.; Jia, X.; Ma, H.; Li, H. Synthesis of growth-type polycrystalline diamond compact (PDC) using the solvent Fe55Ni29Co16 alloy under HPHT. Sci. China Phys. Mech. Astron. 2012, 55, 1394–1398. [Google Scholar] [CrossRef]
- Chen, F.; Xu, G.; Ma, C.-D.; Xu, G.-P. Thermal residual stress of polycrystalline diamond compacts. Trans. Nonferrous Met. Soc. China 2010, 20, 227–232. [Google Scholar] [CrossRef]
- Huang, H.; Zhao, B.; Wei, W.; Si, Z.; Huang, K. Effect of cobalt content on the performance of polycrystalline diamond compacts. Int. J. Refract. Met. Hard Mater. 2020, 92, 105312. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, Y.K.; Wu, W.Q.; Song, M.; Zhang, W.; Liu, B. Progress of powder metallurgical high entropy alloys. Chin. J. Nonferrous Met. 2019, 29, 2155–2184. (In Chinese) [Google Scholar] [CrossRef]
- Qiu, T.X.; Zhang, W.; Liu, Y. Effect of multi-element alloy-carbide bonding phase on the microstructure of diamond composites. Acta Mater. Compos. Sin. 2022. (In Chinese) [Google Scholar] [CrossRef]
- Lv, X.; Jian, Q.; Li, Z.; Sun, K.; Ji, H.; Zhu, Y. Effect of controllable decomposition of MAX phase (Ti3SiC2) on mechanical properties of rapidly sintered polycrystalline diamond by HPHT. Ceram. Int. 2019, 45, 16564–16568. [Google Scholar] [CrossRef]
- Lv, X. Study on Synthetize and Properties of Ti Based Polycrystalline Diamond. Master’s Thesis, Tianjin University, Tianjin, China, 2019. (In Chinese). [Google Scholar] [CrossRef]
- Petrovic, M.; Ivankovic, A.; Murphy, N. The mechanical properties of polycrystalline diamond as a function of strain rate and temperature. J. Eur. Ceram. Soc. 2012, 32, 3021–3027. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, L.; Ji, H.; Sun, K.; Li, Z. Effect of SiC whiskers on mechanical properties of thermally stable polycrystalline diamond prepared by HPHT sintering. Diam. Relat. Mater. 2018, 90, 54–61. [Google Scholar] [CrossRef]
- Jian, Q.; Jiang, Z.; Han, Y.; Zhu, Y.; Li, Z. Fabrication and evaluation of mechanical properties of polycrystalline diamond reinforced with carbon-nanotubes by HPHT sintering. Ceram. Int. 2020, 46, 21527–21532. [Google Scholar] [CrossRef]
- Huang, P.; Wang, W.; Wang, S.; Zhang, X.; Wei, X.; Zhu, Y.; Li, Z. Effect of transition metal carbides on mechanical properties of polycrystalline diamond by HPHT sintering. Ceram. Int. 2022, 48, 15959–15965. [Google Scholar] [CrossRef]
- McNamara, D.; Carolan, D.; Alveen, P.; Murphy, N.; Ivankovic, A. The influence of microstructure on the fracture statistics of polycrystalline diamond and polycrystalline cubic boron nitride. Ceram. Int. 2014, 40, 11543–11549. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, T.; Feng, J.; Cai, B.; Fan, G.; Zhang, W.; Liu, Y. Microstructural Evolution of Diamond-Based Composites at High Temperature and High Pressure. Materials 2022, 15, 8753. https://doi.org/10.3390/ma15248753
Qiu T, Feng J, Cai B, Fan G, Zhang W, Liu Y. Microstructural Evolution of Diamond-Based Composites at High Temperature and High Pressure. Materials. 2022; 15(24):8753. https://doi.org/10.3390/ma15248753
Chicago/Turabian StyleQiu, Tianxu, Jianwei Feng, Bo Cai, Guojiang Fan, Wei Zhang, and Yong Liu. 2022. "Microstructural Evolution of Diamond-Based Composites at High Temperature and High Pressure" Materials 15, no. 24: 8753. https://doi.org/10.3390/ma15248753
APA StyleQiu, T., Feng, J., Cai, B., Fan, G., Zhang, W., & Liu, Y. (2022). Microstructural Evolution of Diamond-Based Composites at High Temperature and High Pressure. Materials, 15(24), 8753. https://doi.org/10.3390/ma15248753






