Densification and Surface Carbon Transformation of Diamond Powders under High Pressure and High Temperature
Abstract
1. Introduction
2. Experimental Procedures
2.1. Materials
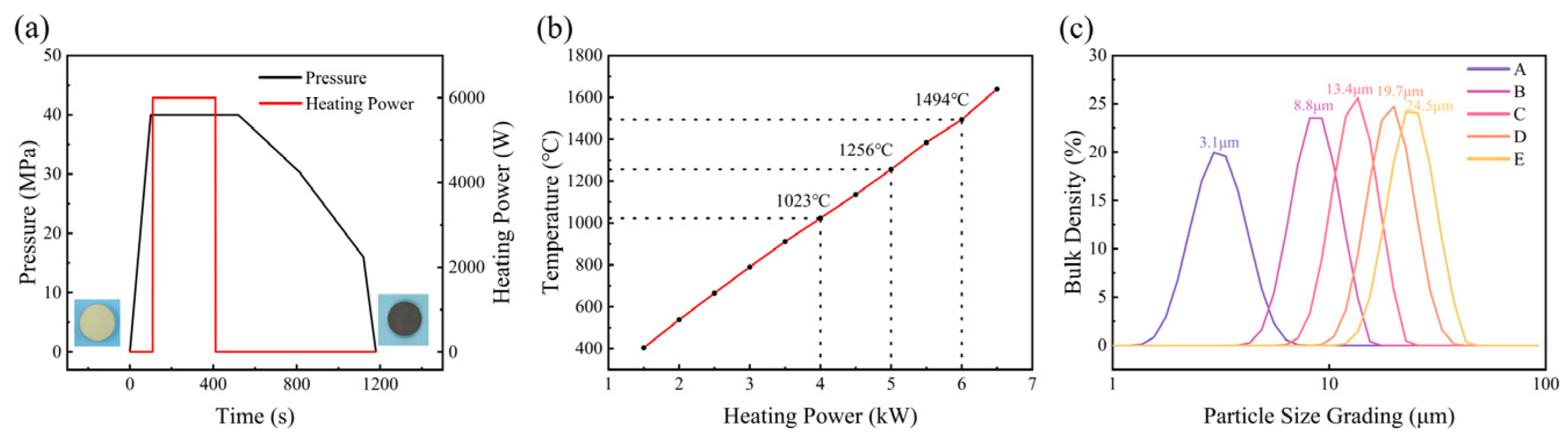
2.2. HPHT Experiment
2.3. Characterization Methods
3. Results
3.1. Diamond Particle Size and Hot-Pressed Conditions
3.2. Densities and Microstructure of Hot-Pressed Diamond Samples
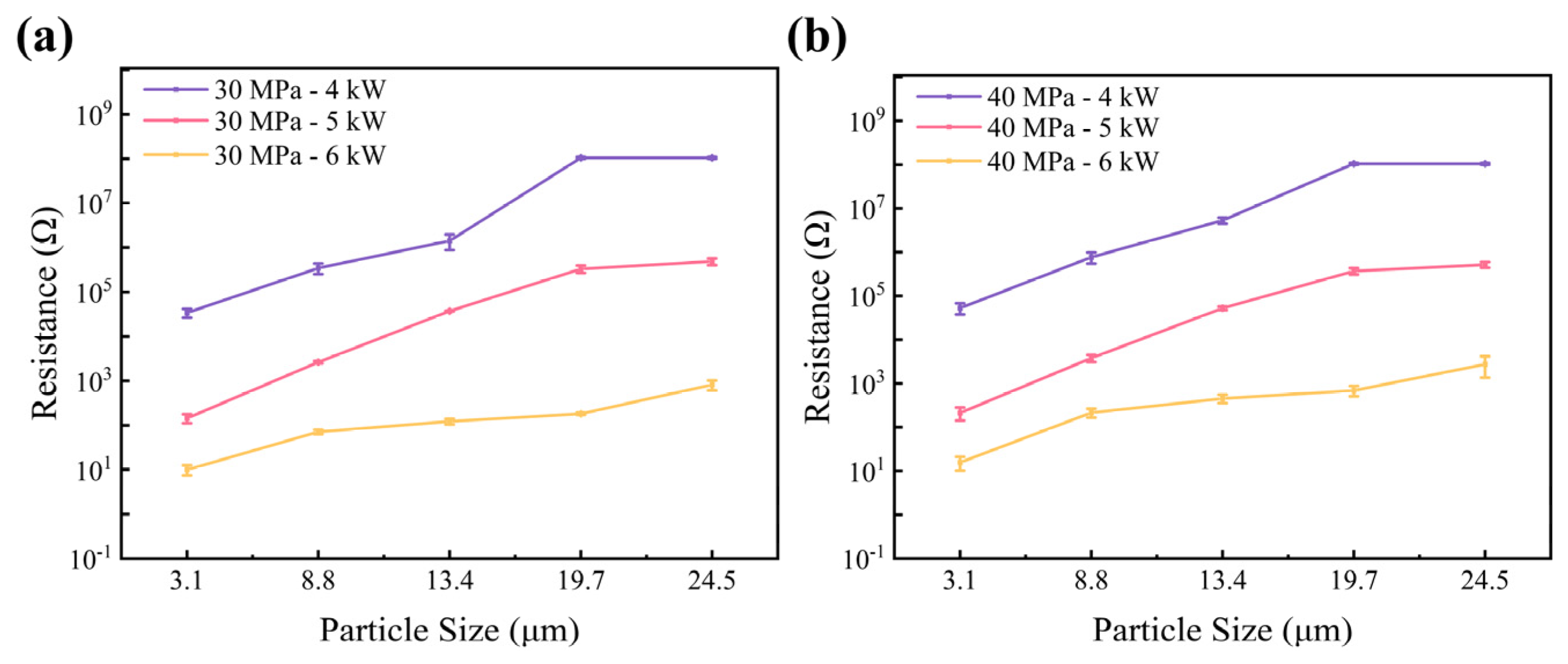
3.3. Electrical Resistance of Hot-Pressed Samples
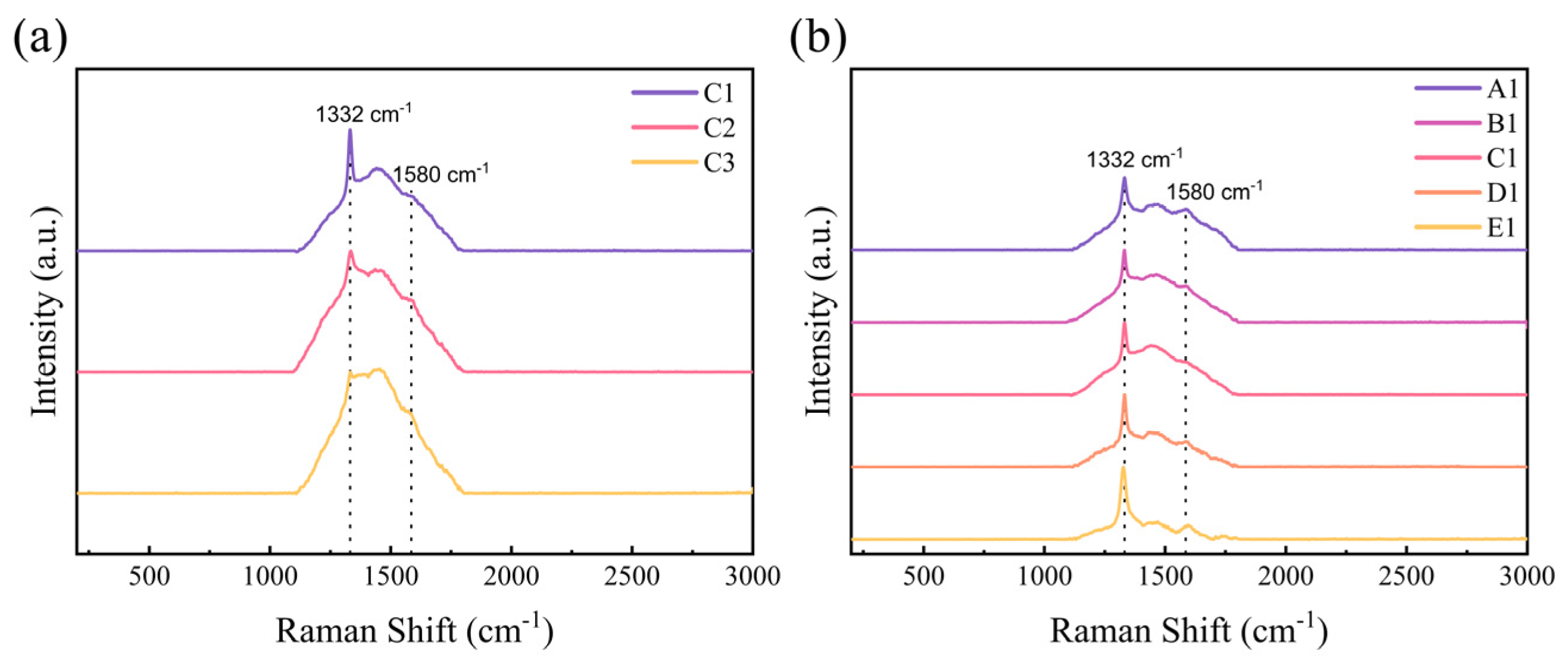
3.4. Raman Spectra
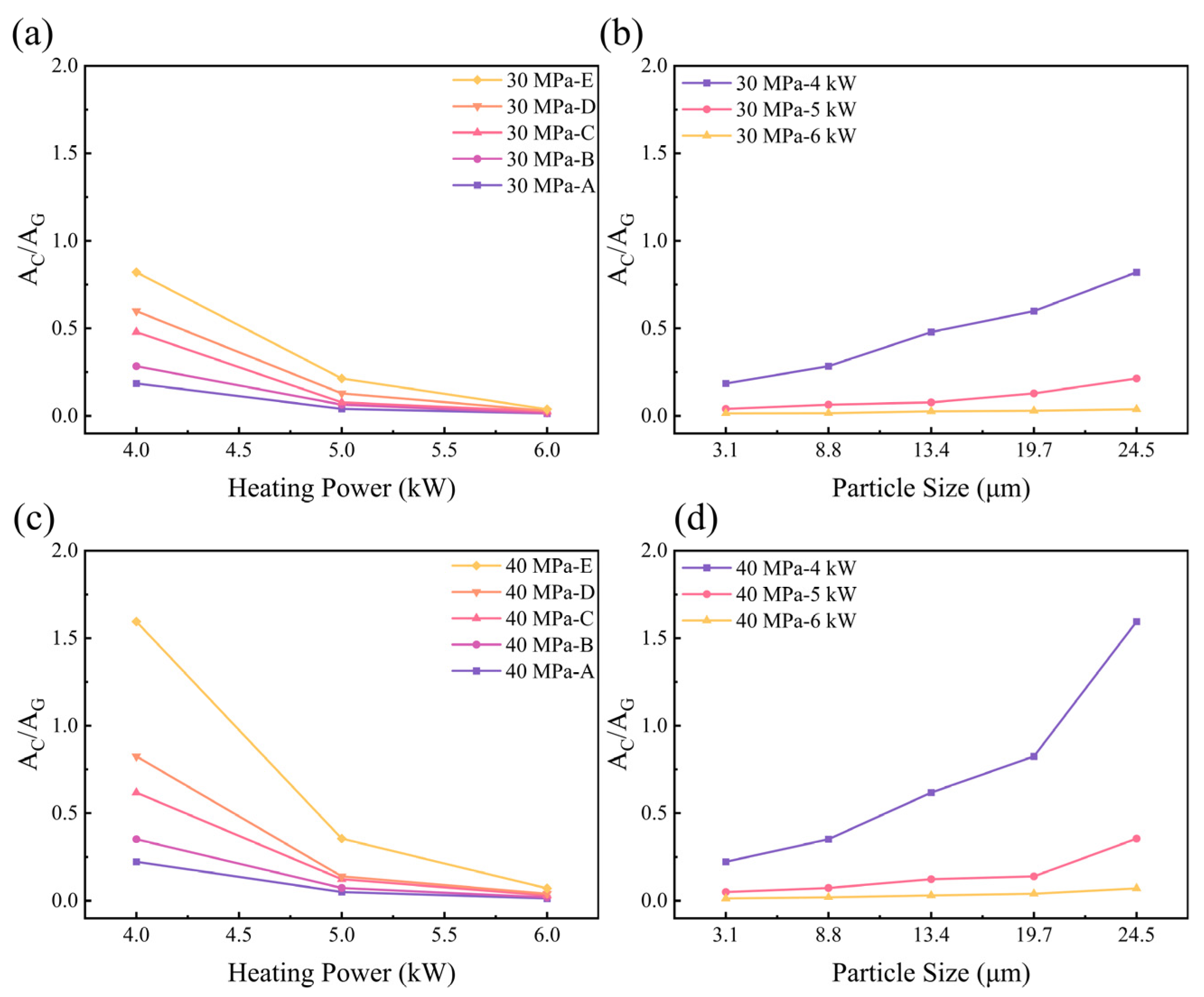
3.5. Influence of Particle Sizes and Heating Powers on the Residual Stresses in Hot-Pressed Samples
4. Conclusions and Prospects
- (1)
- After HPHT treatment, the diamond particles were observed to bond together in certain areas, resulting in the formation of diamond plate samples exhibiting both strength and some electrical conductivity.
- (2)
- During the HPHT hot-compressing stage, diamond powders were further densified via the elastic and non-elastic deformation of diamond powders, and the density can reach up to 3.4 g/cm3, 97.1% of the diamond itself, approaching a full dense diamond plate. The density increases with the diamond particle size, pressure and temperature.
- (3)
- Diamond powders with different particle sizes undergo varying degrees of sp3 to sp2 or amorphous carbon transformation on the surface. The graphite content on diamond surfaces increases as the particle size decreases. There are two primary reasons for this phenomenon. Firstly, smaller particles have a higher surface energy, resulting in a lower activation energy for graphite transformation and making the diamond more prone to graphitize. Secondly, smaller particles possess a higher number of contact areas, which can reduce the local stress on the diamond under the same pressure. Conversely, coarser diamond particles have a lower surface energy, higher activation energy for graphite and fewer contact areas. This can result in higher residual stresses and a reduced graphitization under the same pressure.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeren, M.; Karagöz, Ş. Sintering of polycrystalline diamond cutting tools. Mater. Des. 2007, 28, 1055–1058. [Google Scholar] [CrossRef]
- Hall, H.T. Sintered diamond: A synthetic carbonado. Science 1970, 169, 868–869. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.; Sun, H.; Guo, H.; Dai, B.; Zhu, J. Thermal characteristics of GaN-on-diamond HEMTs: Impact of anisotropic and inhomogeneous thermal conductivity of polycrystalline diamond. Diam. Relat. Mater. 2019, 95, 28–35. [Google Scholar] [CrossRef]
- Li, J.; Yue, W.; Qin, W.; Mao, Q.; Gao, B.; Li, Y. Effect of quenching processes on microstructures and tribological behaviors of polycrystalline diamond compact (PCD/WC-Co) in annealing treatment. Diam. Relat. Mater. 2017, 79, 79–87. [Google Scholar] [CrossRef]
- Knuteson, C.W.; Sexton, T.N.; Cooley, C.H. Wear-in behaviour of polycrystalline diamond thrust bearings. Wear 2011, 271, 2106–2110. [Google Scholar] [CrossRef]
- Belnap, D.; Griffo, A. Homogeneous and structured PCD/WC-Co materials for drilling. Diam. Relat. Mater. 2004, 13, 1914–1922. [Google Scholar] [CrossRef]
- Yahiaoui, M.; Gerbaud, L.; Paris, J.Y.; Denape, J.; Dourfaye, A. A study on PDC drill bits quality. Wear 2013, 298–299, 32–41. [Google Scholar] [CrossRef]
- Li, Z.; Jia, H.; Ma, H.; Guo, W.; Liu, X.; Huang, G.; Li, R.; Jia, X. FEM analysis on the effect of cobalt content on thermal residual stress in polycrystalline diamond compact (PDC). Sci. China Phys. Mech. Astron. 2012, 55, 639–643. [Google Scholar] [CrossRef]
- Sexton, T.N.; Cooley, C.H. Polycrystalline diamond thrust bearings for down-hole oil and gas drilling tools. Wear 2009, 267, 1041–1045. [Google Scholar] [CrossRef]
- Wentorf, R.H.; Devries, R.C.; Bundy, F.P. Sintered superhard materials. Science 1980, 208, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Scott, T.A. The influence of microstructure on the mechanical properties of polycrystalline diamond: A literature review. Adv. Appl. Ceram. 2017, 117, 161–176. [Google Scholar] [CrossRef]
- Schnell, E.; Rein, C.; Rhede, D.; Zahn, G.; Stiller, H. To the synthesis of polycrystalline diamond. High Press. Res. 2006, 5, 917–919. [Google Scholar] [CrossRef]
- Yang, X.; Deng, F. Synthesis and characterisation of Φ62 mm polycrystalline diamond compact. Diam. Relat. Mater. 2019, 100, 107594. [Google Scholar] [CrossRef]
- Sha, X.; Feng, B.; Yue, W.; Wang, C. Comparison of tribological behaviors of polycrystalline diamonds synthesized by titanium- and boron-coated diamond particles. Diam. Relat. Mater. 2022, 128, 109242. [Google Scholar] [CrossRef]
- Cui, X.; Qin, Y.; Mao, R.; Hao, J.; Zhao, S.; Lin, Z.; Deng, L.; Jiang, N.; Cui, P. Particle Cold Pressing Fragmentation in the Process of Synthesizing Polycrystalline Diamond. Diam. Abras. Eng. 2023, 43, 440–446. [Google Scholar] [CrossRef]
- Hickey, D.P.; Jones, K.S.; Elliman, R.G. Amorphization and graphitization of single-crystal diamond—A transmission electron microscopy study. Diam. Relat. Mater. 2009, 18, 1353–1359. [Google Scholar] [CrossRef]
- Nikhar, T.; Rechenberg, R.; Becker, M.F.; Baryshev, S.V. Dynamic graphitization of ultra-nano-crystalline diamond and its effects on material resistivity. J. Appl. Phys. 2020, 128, 235305. [Google Scholar] [CrossRef]
- Bokhonov, B.B.; Dudina, D.V.; Sharafutdinov, M.R. Graphitization of synthetic diamond crystals: A morphological study. Diam. Relat. Mater. 2021, 118, 108563. [Google Scholar] [CrossRef]
- Chen, L.; Yang, X.; Fang, C.; Shi, A.; Liu, R.; Huang, Q. Improved Thermal Conductivity and Ablation Resistance of Microdiamond-Modified C/C Composites after Diamond Graphitization. Adv. Eng. Mater. 2019, 22, 1900934. [Google Scholar] [CrossRef]
- Yang, Z.-L.; Wang, L.-G.; Wang, L.-M.; He, X.-B.; Qu, X.-H.; Liu, R.-J.; Hu, H.-F. Microstructure and graphitization behavior of diamond/SiC composites fabricated by vacuum vapor reactive infiltration. Rare Met. 2014, 34, 400–406. [Google Scholar] [CrossRef]
- Yan, X.; Wei, J.; An, K.; Liu, J.; Chen, L.; Zheng, Y.; Zhang, X.; Li, C. High temperature surface graphitization of CVD diamond films and analysis of the kinetics mechanism. Diam. Relat. Mater. 2021, 120, 108647. [Google Scholar] [CrossRef]
- Davies, G.; Evans, T. Graphitization of Diamond at Zero Pressure and at a High Pressure. Proc. R. Soc. A Math. Phys. Eng. Sci. 1972, 328, 413–427. [Google Scholar]
- Qian, J.; Pantea, C.; Huang, J.; Zerda, T.W.; Zhao, Y. Graphitization of diamond powders of different sizes at high pressure-high temperature. Carbon 2004, 42, 2691–2697. [Google Scholar] [CrossRef]
- Iwanaga, K.; Tada, K.-i.; Chiba, H.; Yamamoto, T.; Maniwa, A.; Yotsuya, T.; Oshima, N. Development of Novel Silicon Precursors for Low-Temperature CVD/ALD Processes. ECS Trans. 2011, 41, 211–218. [Google Scholar] [CrossRef]
- Carcione, R.; Guglielmotti, V.; Mura, F.; Orlanducci, S.; Tamburri, E. Monitoring of Carbonated Hydroxyapatite Growth on Modified Polycrystalline CVD-Diamond Coatings on Titanium Substrates. Crystals 2024, 14, 66. [Google Scholar] [CrossRef]
- Haikuo, W.; Ying, R.; Duanwei, H.; Chao, X. Force analysis and pressure quantitative measurement for the high pressure cubic cell. Acta Phys. Sin. 2017, 66, 090702. [Google Scholar]
- Merlen, A.; Buijnsters, J.; Pardanaud, C. A Guide to and Review of the Use of Multiwavelength Raman Spectroscopy for Characterizing Defective Aromatic Carbon Solids: From Graphene to Amorphous Carbons. Coatings 2017, 7, 153. [Google Scholar] [CrossRef]
- Pardanaud, C.; Cartry, G.; Lajaunie, L.; Arenal, R.; Buijnsters, J.G. Investigating the Possible Origin of Raman Bands in Defective sp2/sp3 Carbons below 900 cm−1: Phonon Density of States or Double Resonance Mechanism at Play? J. Carbon Res. 2019, 5, 79. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Raman spectroscopy in carbons: From nanotubes to diamond. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2004, 362, 2269–2270. [Google Scholar] [CrossRef]
- Brubaker, Z.E.; Langford, J.J.; Kapsimalis, R.J.; Niedziela, J.L. Quantitative analysis of Raman spectral parameters for carbon fibers: Practical considerations and connection to mechanical properties. J. Mater. Sci. 2021, 56, 15087–15121. [Google Scholar] [CrossRef]
- Moseenkov, S.I.; Kuznetsov, V.L.; Zolotarev, N.A.; Kolesov, B.A.; Prosvirin, I.P.; Ishchenko, A.V.; Zavorin, A.V. Investigation of Amorphous Carbon in Nanostructured Carbon Materials (A Comparative Study by TEM, XPS, Raman Spectroscopy and XRD). Materials 2023, 16, 1112. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Robertson, J. Raman spectroscopy of amorphous, nanostructured, diamond-like carbon, and nanodiamond. Philos. Trans. A Math. Phys. Eng. Sci. 2004, 362, 2477–2512. [Google Scholar] [CrossRef]
- Abdu, Y.A. Raman micro-spectroscopy of nanodiamonds from the Kapoeta meteorite. Diam. Relat. Mater. 2021, 118, 108536. [Google Scholar] [CrossRef]
- Orwa, J.O.; Andrienko, I.; Peng, J.L.; Prawer, S.; Zhang, Y.B.; Lau, S.P. Thermally induced sp2 clustering in tetrahedral amorphous carbon (ta-C) films. J. Appl. Phys. 2004, 96, 6286–6297. [Google Scholar] [CrossRef]
- Knight, D.S.; White, W.B. Characterization of diamond films by Raman spectroscopy. J. Mater. Res. 2011, 4, 385–393. [Google Scholar] [CrossRef]
- Conway, N.M.J.; Ferrari, A.C.; Flewitt, A.J.; Robertson, J.; Milne, W.I.; Tagliaferro, A.; Beyer, W. Defect and disorder reduction by annealing in hydrogenated tetrahedral amorphous carbon. Diam. Relat. Mater. 2000, 9, 765–770. [Google Scholar] [CrossRef]
- Rodil, S.E.; Ferrari, A.C.; Robertson, J.; Muhl, S. Infrared spectra of carbon nitride films. Thin Solid Film. 2002, 420–421, 122–131. [Google Scholar] [CrossRef]
- Gou, R.; Luo, X.; Li, K.; Kang, C.; Chen, J. PCD after cobalt leaching reinforced by high temperature annealing: Tribological properties and graphitization evolution. Diam. Relat. Mater. 2022, 125, 108988. [Google Scholar] [CrossRef]
- Zhang, Y.; Polychronopoulou, K.; Humood, M.; Polycarpou, A.A. High temperature nanotribology of ultra-thin hydrogenated amorphous carbon coatings. Carbon 2017, 123, 112–121. [Google Scholar] [CrossRef]
- Li, J.; Yue, W.; Qin, W.; Wang, C. Approach to controllable tribological properties of sintered polycrystalline diamond compact through annealing treatment. Carbon 2017, 116, 103–112. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, J.; Liu, J.; Tian, Y.; Liang, W.; Zheng, L.; Zhou, L.; He, D. Effect of stress state on graphitization behavior of diamond under high pressure and high temperature. Diam. Relat. Mater. 2022, 128, 109241. [Google Scholar] [CrossRef]
- Osswald, S.; Mochalin, V.N.; Havel, M.; Yushin, G.; Gogotsi, Y. Phonon confinement effects in the Raman spectrum of nanodiamond. Phys. Rev. B 2009, 80, 075419. [Google Scholar] [CrossRef]
- Jia, H.; Ma, H.; Guo, W.; Jia, X. HPHT preparation and Micro-Raman characterization of polycrystalline diamond compact with low residual stress. Sci. China Phys. Mech. Astron. 2010, 53, 1445–1448. [Google Scholar] [CrossRef]
- Catledge, S.A.; Vohra, Y.K.; Ladi, R.; Rai, G. Micro-raman stress investigations and X-ray diffraction analysis of polycrystalline diamond (PCD) tools. Diam. Relat. Mater. 1996, 5, 1159–1165. [Google Scholar] [CrossRef]


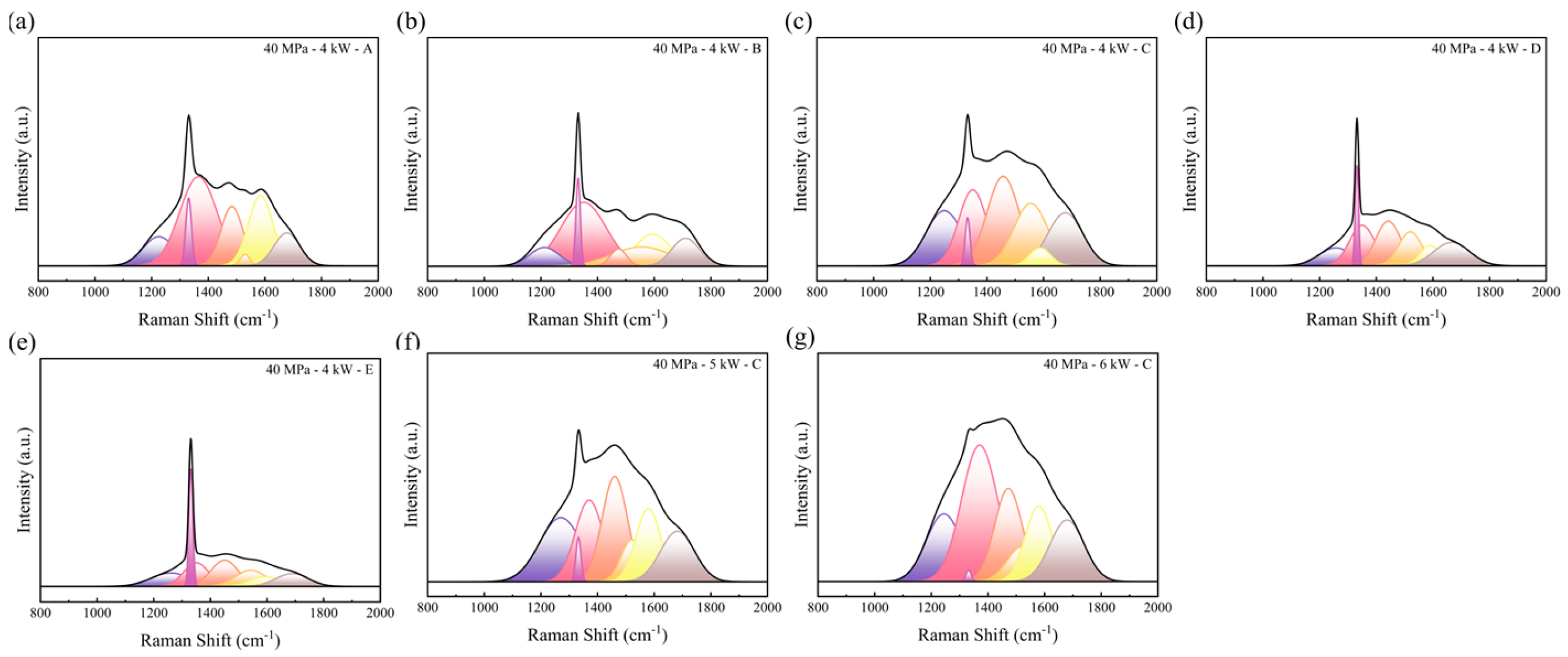


| Peak Number | Source of Characteristic Peaks [27,32] | Characteristic Peak Boundary (cm−1) |
|---|---|---|
| D4 | Carbon black and multi walled carbon nanotubes | 1210~1290 |
| C | Diamond | 1330~1340 |
| D1 | Lattice vibration of disordered graphite | 1350~1390 |
| D3′ | Amorphous | 1450~1490 |
| D3″ | Amorphous | 1520~1555 |
| G | Ordered graphitization | 1575~1590 |
| D2/D′ | Defects due to the breaking of the crystal symmetry | 1615~1650 |
| Hydraulic Pressure (MPa) | Power (kW) | Particle Size (μm) | Sample | Diamond Peak (cm−1) | Reference Peak (cm−1) | Peak Shift (cm−1) | Stress (GPa) |
|---|---|---|---|---|---|---|---|
| 30 MPa | 4 kW | G2–4 | A1 | 1332.01 | 1331.91 | −0.10 | −0.04 |
| G6–12 | B1 | 1332.11 | 1331.99 | −0.12 | −0.04 | ||
| G8–16 | C1 | 1332.16 | 1332.01 | −0.15 | −0.05 | ||
| G15–25 | D1 | 1332.29 | 1332.08 | −0.21 | −0.07 | ||
| G20–30 | E1 | 1332.36 | 1332.13 | −0.23 | −0.08 | ||
| 40 MPa | 4 kW | G2–4 | A4 | 1332.10 | 1331.91 | −0.19 | −0.07 |
| G6–12 | B4 | 1332.21 | 1331.99 | −0.22 | −0.08 | ||
| G8–16 | C4 | 1332.26 | 1332.01 | −0.25 | −0.09 | ||
| G15–25 | D4 | 1332.35 | 1332.08 | −0.27 | −0.09 | ||
| G20–30 | E4 | 1332.46 | 1332.13 | −0.33 | −0.12 | ||
| 30 MPa | 5 kW | G2–4 | A2 | 1332.84 | 1331.91 | −0.93 | −0.32 |
| G6–12 | B2 | 1332.94 | 1331.99 | −0.95 | -0.33 | ||
| G8–16 | C2 | 1332.99 | 1332.01 | −0.98 | −0.34 | ||
| G15–25 | D2 | 1333.10 | 1332.08 | −1.02 | −0.35 | ||
| G20–30 | E2 | 1333.19 | 1332.13 | −1.06 | −0.37 | ||
| 40 MPa | 5 kW | G2–4 | A5 | 1332.92 | 1331.91 | −1.01 | −0.35 |
| G6–12 | B5 | 1333.02 | 1331.99 | −1.03 | −0.36 | ||
| G8–16 | C5 | 1333.14 | 1332.01 | −1.13 | −0.39 | ||
| G15–25 | D5 | 1333.25 | 1332.08 | −1.17 | −0.41 | ||
| G20–30 | E5 | 1333.36 | 1332.13 | −1.23 | −0.43 | ||
| 30 MPa | 6 kW | G2–4 | A3 | 1334.00 | 1331.91 | −2.09 | −0.73 |
| G6–12 | B3 | 1334.10 | 1331.99 | −2.11 | −0.73 | ||
| G8–16 | C3 | 1334.18 | 1332.01 | −2.17 | −0.75 | ||
| G15–25 | D3 | 1334.30 | 1332.08 | −2.22 | −0.77 | ||
| G20–30 | E3 | 1334.46 | 1332.13 | −2.33 | −0.81 | ||
| 40 MPa | 6 kW | G2–4 | A6 | 1334.05 | 1331.91 | −2.14 | −0.74 |
| G6–12 | B6 | 1334.17 | 1331.99 | −2.17 | −0.76 | ||
| G8–16 | C6 | 1334.25 | 1332.01 | −2.24 | −0.78 | ||
| G15–25 | D6 | 1334.38 | 1332.08 | −2.30 | −0.80 | ||
| G20–30 | E6 | 1334.50 | 1332.13 | −2.37 | −0.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, R.; Cui, X.; Hao, J.; Zhao, S.; Hou, S.; Lan, F.; Li, Y.; Deng, L.; Li, H. Densification and Surface Carbon Transformation of Diamond Powders under High Pressure and High Temperature. Materials 2024, 17, 603. https://doi.org/10.3390/ma17030603
Mao R, Cui X, Hao J, Zhao S, Hou S, Lan F, Li Y, Deng L, Li H. Densification and Surface Carbon Transformation of Diamond Powders under High Pressure and High Temperature. Materials. 2024; 17(3):603. https://doi.org/10.3390/ma17030603
Chicago/Turabian StyleMao, Rongqi, Xiwei Cui, Jinglin Hao, Sizhuang Zhao, Shuai Hou, Fuli Lan, Yanbiao Li, Lifen Deng, and He Li. 2024. "Densification and Surface Carbon Transformation of Diamond Powders under High Pressure and High Temperature" Materials 17, no. 3: 603. https://doi.org/10.3390/ma17030603
APA StyleMao, R., Cui, X., Hao, J., Zhao, S., Hou, S., Lan, F., Li, Y., Deng, L., & Li, H. (2024). Densification and Surface Carbon Transformation of Diamond Powders under High Pressure and High Temperature. Materials, 17(3), 603. https://doi.org/10.3390/ma17030603






