Abstract
To address the environmental pollution caused by nitrogen oxides, V2O5-WO3/TiO2 is widely used as a catalyst based on selective catalytic reduction (SCR) technology. However, spent SCR catalysts pose a potential hazard to the environment due to the presence of heavy metals. This problem continues to plague countries with predominantly thermal power generation, and landfills as the dominant disposal method wastes significant metal resources. Previous research into the recovery of these metal resources has received considerable attention. Here, we summarise the methods of recovery and find that research trends are beginning to move towards improving the added value of recovered products. One very promising application is photocatalysts; however, the atomic efficiency of current methods is not satisfactory. Therefore, this review first focuses on the regeneration of spent SCR catalysts and the processes used for elemental extraction to clarify what forms of V, W and Ti can be obtained from existing processes. This is followed by providing directions for the conversion of spent SCR catalysts into photocatalysts with improvements based on such processes. From a different perspective, this also provides a new resource for photocatalysts and is expected to significantly reduce the cost of photocatalyst production.
1. Introduction
Industrial gas emission has been a concern for decades, and nitrogen oxides (NOX) are one of the most important toxic industrial gases [1,2]. A selective catalytic reduction catalyst (SCR catalyst) is widely used for coal-fired boilers as a flue gas denitrification catalyst to convert NOX into harmless N2 emission [3]. Furthermore, V2O5-WO3/TiO2-based SCR catalysts are the most common type. At high temperatures, the presence of H2O, SO2, alkali metals, heavy metals and halogens causes the SCR catalyst to be poisoned and, after deactivation, become a spent SCR catalyst [4]. Spent SCR catalysts have several characteristics: (i) increasing waste generation [5], (ii) containing large amounts of metal resources [6], and (iii) causing serious threats to soil and water bodies [7]. The severity and urgency of the problem is reflected in the increase in patents for the recycling of spent SCR catalysts in China since 2013 [8].
The treatment process must prevent toxic elements (e.g., V, As, Pb, etc.) from entering soil and water bodies to avoid secondary pollution. Conventional treatment is landfill for spent SCR catalysts as hazardous solid waste [9]. According to the policy guidelines, such wastes are required to be roasted above 1000 °C with flux to prevent the leaching of toxic elements [10]. Recent research has tended to add spent catalysts to production lines that require significant use of SCR catalyst for denitrification, thus avoiding transportation. For instance, spent SCR catalysts can be blended into the iron-ore sintering process [11], with heavy metals being recovered from the sintering dust [12]. However, the addition of spent SCR catalysts will reduce several indicators [13]. Alternatively, the spent SCR catalyst can also be mixed into cement and the leaching of As and Pb is significantly inhibited after sintering [14].
However, spent SCR catalysts have a high recovery value with components of over 80wt% consisting of V2O5, WO3 and TiO2 and should, therefore, be regenerated or recovered in order to recycle the resource [15,16,17]. Although spent SCR catalysts may contain oxides such as SiO2, Al2O3 and CaO that constitute the ceramics, as well as compounds of elements including As and Pb deposited from the flue gas, the extraction of these materials is not reviewed in this article. Regeneration methods can extend the life of SCR catalysts by focusing on detoxification and reactivation. The major problems to be addressed during regeneration are the deactivation and loss of active sites as well as blocking of pores [4,18,19,20]. Specific regeneration methods for different types of deactivations can achieve promising results [21]. However, current regeneration processes are unable to achieve the expected results when the catalyst is severely deactivated or has undergone several regenerations. In this case, the SCR catalyst will reach the end of its life and be recycled by recovery [22]. Conventional recovery methods focused on the extraction and purification of metals (V, W and Ti) [23]. However, recent research has increasingly turned to the added value of the product with a view to increasing the economic benefits of recycling. For instance, after separation, NH4VO3, ammonium paratungstate and anatase TiO2 are produced, respectively [24].
Under light conditions, photocatalysts produce photogenerated electrons and holes and, further, form active species such as hydroxyl (·OH) and oxygen (O2−) radicals. Due to the strong redox properties of the active species, photocatalysts can address pollutions caused by heavy metals, organics and other substances [25]. Anatase TiO2 is a classical photocatalyst with excellent photocatalytic activity. Studies have often used doping to build heterostructures to extend the wavelength range of light and to suppress the separation of photogenerated carriers [26,27]. On the other hand, to inhibit the agglomeration of nano TiO2 and to assist recycling, the preference is to use carrier-loaded nano TiO2 [28]. Spent SCR catalysts contain a large amount of anatase TiO2 and are cost-effective; therefore, recovery as photocatalysts not only increases the recovery benefit, but also overcomes the high cost of conventional photocatalysts. In addition, V [29,30] and W [31,32] in spent SCR catalysts have the potential to build heterostructures with TiO2.
This paper will first review the research on the recycling of spent SCR catalysts carried out between 2013 and 2022. Thereafter, it will continue with a discussion of the studies on the recycling of spent SCR catalysts between 2019 and 2022, including both the regeneration and recovery of spent SCR catalysts. The discussion will focus on the use of reagents with acid, base, complexing, oxidising, or reducing properties in these studies and will summarise the different effects of the different reagents. For the recovery methods, the extraction process of the elements is discussed. This is followed by an introduction to the idea of converting spent SCR catalysts into photocatalysts. Finally, the developments in the recycling of spent SCR catalysts are concluded.
2. Progress in Research on Spent SCR Catalysts
2.1. Methodology
Articles were retrieved on 24 July 2022 from Web of Science (www.webofscience.com) database. The articles were found using the following search formula: TS = (selective-catalytic-reduction OR NH3-scr or deNO(x)-catalysts OR scr) AND TS = (spent OR waste) AND TS = (recovery OR recycling OR leaching OR extraction OR management) AND TS = (titanium OR vanadium OR tungsten). Patents were found through the European Patent Office (www.epo.org) on 24 July 2022. The keywords used are selective-catalytic-reduction, NH3 scr, deNO, scr, spent, recovery, recycling, leaching, extraction, management, titanium, vanadium, tungsten. Patents and articles between 2013 and 2022 were counted using the search method described above to give a general trend of research on recycling spent SCR catalysts. Other sections review articles for 2019 to 2022.
2.2. Research Process
The data on articles and patents described in 2.1 are plotted in Figure 1, where Figure 1a shows the number of articles and patents issued with the year, and Figure 1b shows the proportion of patent disclosures in different countries or organisations. From 2013 onwards, the number of studies addressing the recovery and regeneration of spent SCR catalysts continues to rise and reaches a peak in 2020. The proportion of patents is higher than that of articles, reflecting the huge demand for practical applications in this field. In Figure 1b, significant interest in the disposal of waste SCR catalysts is shown, since the energy structure in China, Korea and Japan is dominated by thermal power generation. Take China as an example: the Emission standard of air pollutants for thermal power plants (GB 13223-2011) limits NOX emissions to <100 mg/m3. With increasingly stringent restrictions on NOX emissions, the use of SCR catalysts will also increase. In view of the limited effect of regeneration on spent SCR catalysts, the cycle time of regeneration has been limited to ensure that gas emissions comply with standards [22]. As a result, there is a growing interest in the development of regeneration processes and the reuse of resources through recycling.
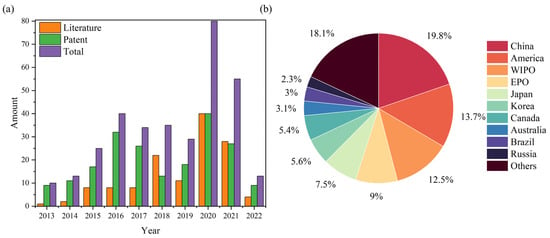
Figure 1.
(a) Annual number of publications of papers and patents. (b) Patent issuance by countries and organizations between 2013 and 2022.
The principles of green chemistry and green engineering by Ziemmerman et al. [33] in 2020 also emphasise the importance of this idea of recycling. The principles point to a shift in future industry from mostly linear processes to circular processes and a shift from “waste” treatment to “waste” utilisation. In this field, the main idea is to avoid landfills in favour of efficient regeneration of spent SCR catalysts and recycling those that cannot be regenerated. Current research has explored several methods for recycling spent SCR catalysts, but these methods have not been replicated in industry, so there is still much scope for research into recycling methods. Factors limiting their application include the process, waste generation, material consumption, and equipment requirements and, in the case of recycled production products, the value of the product is also important [22].
3. Regeneration of Spent SCR Catalysts
SCR catalysts under a high-temperature flue gas condition are subjected to deactivation due to multiple factors including pore blocking, catalytic-site poison and catalytic-component loss [4,18,19,20]. In recent years, most regeneration processes have employed ultrasonic instead of stirring to obtain better results (Table 1). Regeneration processes have been designed to address the poisoning of spent SCR catalysts and to attempt to restore the structure of spent SCR catalysts. Following this effort, some studies have supplemented the regenerated catalysts with active sites by impregnation. NH4VO3 solutions are usually used to reload V2O5 [34], and, relatively, Ce(NO3)3 solutions can be used instead to load CeO2 avoiding the use of V [35].

Table 1.
Regeneration process for SCR catalysts.
Improvement of the pore landscape of regenerated SCR catalysts can effectively increase catalytic activity without reloading the active site. Various data can be used to characterise the pore landscape, such as: mesopore volume, mesopore surface area, BET surface area, acidic sites etc. Here, the mesopore volume data obtained with thermoporometry measurements (TPM) provides a reliable indication of the effect of the immersing method [36]. In addition to regenerating the activity by improving the pore landscape, pore size and specific surface area can also be enhanced by loading the regenerated SCR catalyst powder onto the cloth surface. Shi et al. [34] regenerated the spent SCR catalyst in H2C2O4 and NH4VO3 solutions after sequential blowing, grinding, deionised water washing and 0.5 M sulphuric acid washing. The regenerated catalyst was then loaded onto a P84 filter cloth by impregnation and used as a catalytic filter for low-temperature flue gas denitrification achieving 91.6% of the catalytic effect of the fresh catalyst.
The regeneration process attempts to achieve better results for catalysts that are severely deactivated as well as repeatedly experiencing deactivation–regeneration [22]. Complex processes can effectively extend the life cycle of SCR catalysts. However, the complexity of the process also results in higher costs. Therefore, despite the fact that complex processes can be used for longer cycle times, the difficult regenerated spent SCR catalysts need to be further recovered as metal (V, W, and Ti) resources to reduce costs.
4. Extraction of Elements from Spent SCR Catalysts
Two common methods can be used to effectively extract V and W from spent SCR catalysts: the leaching method [39,40] and the roasting method [16,41]. The leaching method is a process dissolving certain components from the catalyst. Leaching reagents are typically employed to selectively separate specific components with acid [42], base [43], complexation [44], reduction [44], oxidation [45] and other properties. In most cases, TiO2 will be retained in the residue to be recycled [46,47]. The roasting method is a process in which reagents eutectic with spent SCR catalyst under high-temperature conditions form salts. This process generally forms soluble salts which are subsequently separated from the solid phase in a leaching step [48,49]. Table 2 summarises the studies of extracting by roasting or leaching methods for V, W and lists the final form of Ti.

Table 2.
Extraction process for spent SCR catalysts.
4.1. Leaching Method
Leaching is a process of separating V2O5, WO3 from the catalyst carrier using a solvent to break the V-O, W-O and Ti-O bonds [52]. The separation can be achieved by selectively dissolving V2O5, WO3 and TiO2 into different solvents based on the difference in solubility. Whereas V2O5 is usually well soluble in acids and bases, WO3 is only soluble in bases and stable to acids, while TiO2 is always retained in residues. Therefore, leaching with normal acid (e.g., H2SO4 [52]) allows a highly selective separation of V from the catalyst, and, conversely, extracts both V and W.
In the case of V extraction, V(III) is not easily soluble and can be oxidised by adding an oxidant or V(V) under an acidic condition. H2O2 is commonly used as an oxidant to convert low-valent V to V(V) under alkaline conditions, contributing to the leaching efficiency in both leaching [45] and precipitation [54] processes. In contrast, the reaction between V(III) and V(V) will take place at 5wt% H2SO4 at 95 °C and will eventually be converted to VOSO4 in the presence of the reducing agent Na2SO3 (leaching efficiency is nearly 100%) [57]. Theoretical calculations show [52] that (i) with alkaline reagents, OH- reacts directly with V and W atoms; and (ii) with acidic reagents, H+ reacts with O atoms. Organic acids such as H2C2O4 exhibit complexation reaction activity as well, e.g., V can be separated out as VOC2O4 using 1.0 mol/L H2C2O4 at 90 °C in a reaction of 3 h at a liquid-to-solid ratio of 20 mL g−1 (leaching efficiency of 84.22%) [44]. Among the organic acids, H2C2O4 is more effective than citric and tartaric acids for the extraction of V [58].
The extraction of W was also facilitated by the addition of the oxidising agent H2O2 but the leaching efficiency remained at a low level [54]. Additionally, at high V content, the leaching efficiency of W is limited due to the strong V-W-Ti interaction [50]. WO3 has good stability in acid and requires alkaline reagents for dissolution. Leaching of W generally uses NaOH as the leaching agent and requires heating. For instance, heating a 1.5 mol L−1 NaOH solution to 100 °C at atmospheric pressure for a 4 h leaching (liquid-to-solid ratio = 15) resulted in leaching efficiencies ranging from 38.0% to 57.3% for W [50]. In contrast, applying a pressure condition to heat a 4.75 mol L−1 NaOH solution to 190 °C for 44.5 min leaching reaction (liquid-to-solid ratio = 10) could achieve 98.63% leaching efficiency [51]. When the temperature was increased to 300 °C, 96% W (liquid-to-solid ratio = 10) was leached after 2 h of immersing using a 2 mol L−1 NaOH solution [39]. Further studies have found that mixing NaOH with spent SCR catalysts using a ball-mill premix promotes leaching at lower temperatures [59]. After premixing NaOH to a catalyst at a mass ratio of 0.9, leaching with the addition of water for 20 min at 25 °C (liquid-to-solid ratio = 15) could achieve the same result as a 4 h reaction at 100 °C under the same conditions (see Figure 2 for SEM images). The final leaching efficiencies for V and W were 67.7% and 56.3%, respectively.
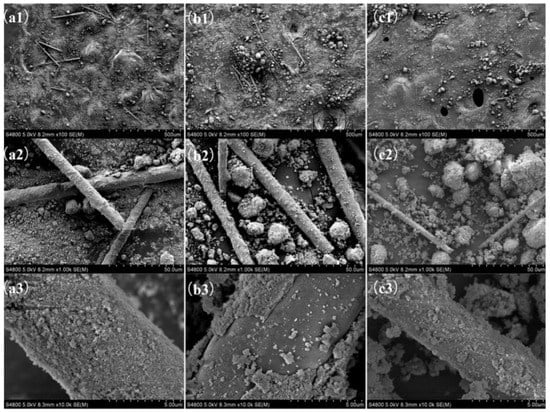
Figure 2.
SEM images of (a) the original catalyst, (b) the catalyst treated by the premixing method and (c) the catalyst treated by the common method (from 1 to 3 at 100×, 1000× and 10,000×, respectively). Reproduced with permission [59]. Copyright 2018, Elsevier.
Ammonium salt can form water-soluble NH4VO3 and (NH4)2WO4 with V and W, respectively. Cao et al. [45] leached V and W using a solution of 2 mol L−1 (NH4)2CO3 and 1.5 mol L−1 H2O2. After 0.5 h leaching at 70 °C, the leaching efficiencies of V and W reached 98% and 99% (liquid-to-solid ratio = 25). The addition of H2O2 has a promoting effect on V but is not sensitive to the concentration. The use of alkaline leaching agents requires the further separation of V and W. For example, diethylhydroxydodecanoneoxime (LIX 63) can selectively separate V from W [45]. Similar extractants are trioctylphosphine oxide (TOPO), triisobutylphosphine sulphide (TIBPS), trioctylmethylammonium chloride (Aliquat 336) and di-2-ethylhexylphosphoric acid (HDEHP) [60]. Aliquat 336 can simultaneously extract V and W [24]. After separation, W can be converted to ammonium paratungstate, while V can be converted to NH4VO3.
4.2. Roasting Method
Roasting is a method of converting oxides of V and W into soluble salts with the assistant of alkaline reagents by high-temperature solid-state reactions. The main reagents used are NaOH, Na2CO3 and NaCl. NaOH and Na2CO3 are the major alkaline reagents, which can react with W more effectively than NaCl [53]. The Na2CO3 will decompose above the melting temperature, accompanied by the occurrence of alkali fusion. When the CaO content is low and the Na2O content is high, V and W tend to form soluble NaVO3 and Na2WO4, thereby avoiding the formation of insoluble CaV2O6 and CaWO4 during the process [48]. Due to the different proportions of Na2O and metal oxides, the catalyst will exhibit different states after the reaction (Figure 3). Studies have shown that mixing NaCl with Na2CO3 [49] (NaCl:Na2CO3 = 8.8:16) and NaOH [53] (NaCl:NaOH = 3:2) can effectively promote leaching efficiency. NaCl will produce Cl2 in the process of high-temperature calcination, acting as catalyst and oxidant, thus reducing the reaction temperature (from 1000 °C to 750 °C) [53].

Figure 3.
Actual appearance of samples after alkali fusion reactions for 20 min at 950 °C in various molar ratios (MOX/Na2O+MOX, M = Ti, Si, W): (a) 0.3, (b) 0.4, (c) 0.5, (d) 0.6, (e) 0.7. Reproduced with permission [48]. Copyright 2019, Springer Nature.
After roasting with an alkaline reagent, the obtained solid can be selectively extracted using the leaching method. In this condition, water is able to dissolve the sodium salts formed during roasting, including NaVO3, Na2WO4, etc. [49]. Thus, V and W are transferred to water. Since Si affects the leaching efficiency of V and W, Si needs to be removed first in the case of high Si content. At room temperature, 85% of Si can be removed in the form of silicate precipitation by reducing the pH to 9.5 by HCl, avoiding the loss of W and V. Water leaching can reach more than 99% leaching of V and W, and is less affected by leaching conditions [55]. Afterwards, V and W can be precipitated from water with Ca2+ ions [54]. Alternatively, highly selective extraction can be achieved with the help of the selective complexation of V and W by organic ammonium salts (e.g., [R3NCH3]+Cl− [24]). Following a stripping process using a solution of NaOH and NaCl, the stripping step has a leaching efficiency of around 70% for V and W.
Leaching processes are limited for the efficient leaching of W due to the stability of the W-O bond. In contrast, roasting methods are highly efficient in breaking such stable bonds at high temperatures, thus reducing the requirement for a leaching reagent. However, roasting reagents require NaOH or Na2CO3 for alkali fusion at high temperatures, raising the equipment requirements and, therefore, making it difficult to promote. To solve this problem, the leaching process can be adjusted to reduce the reliance on alkaline reagents during roasting. In the case of leaching with HCl instead, for example, CaO can be added to the spent SCR catalyst instead of the alkaline reagent [17]. Such acid leaching takes place by the selective dissolution of the calcium salts (e.g., CaV2O6 and CaWO4) formed from roasting step. The use of a high concentration (4 mol L−1) of HCl promotes the dissolution of V and inhibits the dissolution of W, thus achieving the separation of V and W. The undissolved W will be deposited on the surface of CaWO4 in the form of H2WO4. The H2WO4 precipitation should be dissolved in a low-concentration (1 mol L−1) NaOH solution to inhibit the dissolution of Fe, Al, and V. Subsequently CaCl2 is added to once again precipitate W from the solution as CaWO4 (CaWO4 content was 96.1%). Using H2C2O4 (0.5 mol L−1) instead of HCl (1 mol L−1) was found to increase the dissolution efficiency of W as well as reduce the dissolution of V [56]. In contrast to HCl, the use of H2C2O4 does not produce H2WO4, but is further converted to soluble H2[WO3(C2O4)H2O]. This dissolution process is accompanied by a precipitation of CaC2O4. A secondary roasting of the leach residue converts this precipitate into CaO. Further H2C2O4 leaching after the secondary roasting leads to an increase in the leaching of W to 87% and directly obtaining a H2WO4 product.
4.3. Separation of Ti
The content of Ti is the highest in spent SCR catalysts and Ti will be retained in the residue after the extraction of V and W. With similar leaching efficiencies of V and W, differences in the form of Ti are presented due to the different processes. During roasting above 500 °C, anatase TiO2 tends to change to rutile TiO2 [7] and a portion of TiO2 is converted to titanate [54], yet the leaching process is relatively gentle and does not affect the structure of TiO2 [45]. The form of Ti varies depending on the recovery method (Figure 4): (i) roasting with Na2CO3 and NaCl at 750 °C gives a mixture of rutile TiO2, anatase TiO2 and sodium titanate [49]; (ii) roasting with K2CO3 gives potassium titanate [54]; (iii) electrolysis of Ti2CO (obtaining by a carbothermal reduction) gives Ti metal [6]; and (iv) adding hot concentrated HCl to the roasting residue gives a TiOCl2 solution [55], etc.
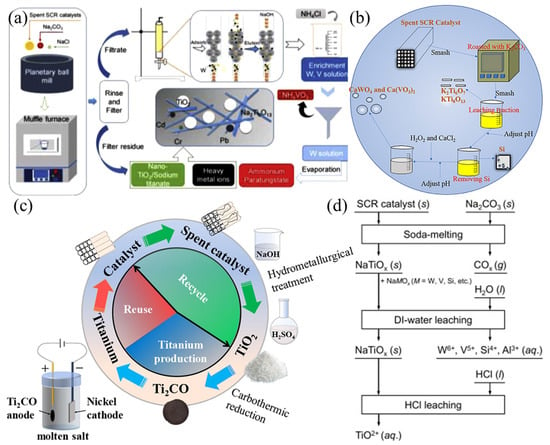
Figure 4.
The different recovery products of Ti: (a) mixture of rutile TiO2, anatase TiO2 and sodium titanate. Reproduced with permission [49]. Copyright 2020, Elsevier. (b) Potassium titanate. Reproduced with permission [54]. Copyright 2020, Springer Nature. (c) Ti metal [6]. Reproduced with permission [6]. Copyright 2021, Elsevier. (d) TiOCl2 solution. Reproduced with permission [55]. Copyright 2019, Elsevier.
5. Conversion of Spent SCR Catalysts into Photocatalysts
Recent research has explored new recycling methods to convert spent SCR catalysts into products rather than raw materials, such as a pigment [61] and ceramic opacifier [62]. The idea of recycling spent SCR catalysts as photocatalysts for pollution treatment is in-line with the concept of “treating waste with waste” [63].
5.1. Catalyst Carriers
Photocatalytic degradation is a promising technology to address environmental pollution with economic and environmental efficiency [64]. However, as most photocatalysts are nanoparticles, they are difficult to recycle and, therefore, present the potential risk of secondary pollution [65]. On the other hand, the tendency of nanoparticles to agglomerate can also limit their catalytic activity [66]. To solve these problems, photocatalysts can be loaded onto carriers, such as fly ash [67] and activated carbon [68]. The carriers not only provide dispersion and easier recycling, but specific carriers can also improve the catalytic activity of the catalyst by forming a specific heterostructure [69].
Spent SCR catalysts contain a variety of oxides with catalytic activity, which can be used as carriers to enhance catalytic activity. However, it is important to ensure that components such as V2O5 and As2O5 do not enter the environment during utilization and cause secondary contamination. Jin et al. [70] investigated spent SCR catalysts by grinding into powder and adding Al2O3, diatomite and agglomerant to calcine at 1000 °C to obtain ceramics. This was followed by impregnation and sintering in an 8wt% Ni(NO3)2 solution to obtain NiO loading (Figure 5). The prepared NiO-based catalysts were used for the reforming of formaldehyde and water vapour for hydrogen production with a selectivity of 100% for H2, 31.9% for CO and 53.2% for CO2 at 500 °C, and a conversion of formaldehyde above 93.0%. The analysis of the XPS data and the mechanism of the reforming reaction revealed that the presence of oxides on the surface had a positive effect on the performance of the catalysis. Based on the work of Jin et al., it can be demonstrated that spent SCR catalysts have potential as catalyst carriers. Particularly, TiO2 shows typical photocatalytic activity and research on recycling spent SCR catalysts as photocatalyst carriers for this feature awaits further exploration.
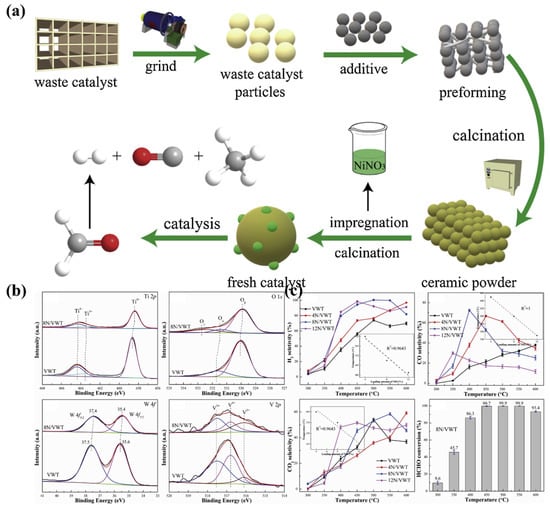
Figure 5.
(a) Process of converting spent SCR catalysts into NiO-based catalysts, (b) XPS test results of spent SCR catalysts after sintering into ceramics, (c) catalytic performance test results of NiO-based catalysts. Reproduced with permission [70]. Copyright 2020, Elsevier.
5.2. Photocatalysts
V2O5, WO3 and TiO2 from spent SCR catalysts can be used for the preparation of photocatalysts. V2O5 and WO3 can form crystals with cations such as Bi3+ in the form of VO43− and WO66−, respectively [71], while TiO2 exists as anatase TiO2 mostly [72]. Zhang et al. [57] used a solution of H2SO4 and Na2SO3 to leach out V from spent SCR catalyst, followed by dissolving the Ti-containing residue in HF solution to obtain WO3-TiO2 photocatalyst after a hydrothermal reaction. In another case, V and W were leached out using a NaOH solution, followed by the addition of Bi(NO3)3 for a hydrothermal reaction to obtain BiVO4/Bi2WO6 photocatalysts [71]. Wang et al. [73] also used NaOH to retain Ti in the leach residue for separation. Subsequently, Na3PO4 and Mg(NO3)2 were used to precipitate Ca2+, SiO44−, and PO43−, respectively. This was followed by the precipitation of Al3+ and Mg2+ using HNO3 and NaOH to adjust the pH. The solution retains VO3− and WO42−, but additional NH4VO3 is required due to the low V. The addition of Zn(NO3)2 eventually leads to a visible light responsive Zn3(VO4)2/ZnWO4 photocatalyst by hydrothermal reaction.
Qian et al. [74] used NaOH to leach the V element from the catalyst, followed by a hydrothermal reaction using 96% H2SO4 to convert the TiO2 to TiOSO4 after roasting at 200 °C (Figure 6). V is removed after reaction with NaOH, while W remains in the residue. This method overcomes the difficulty of separating Ti and W and makes effective use of Ti. The prepared photocatalyst exhibits similar catalytic activity to P25 at a lower Ti content and demonstrates the feasibility of dissolving and converting Ti to nano TiO2. Furthermore, loading TiO2 onto the fly-ash surface also overcomes the shortcomings of nano TiO2, which is difficult to recover and prone to agglomeration.
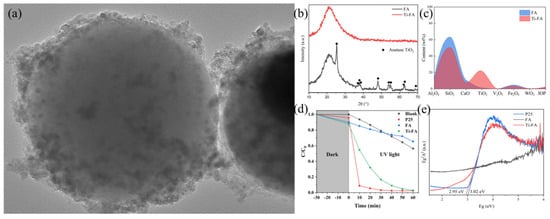
Figure 6.
(a) Microscopic view of Ti-FA surface by TEM, (b) XRD pattern, (c) XRF test results and (d) UV degradation of rhodamine B catalytic performance test results; (e) UV-DRS test results. Reproduced with permission [74]. Copyright 2022, Elsevier.
Current research has focused on the separation of TiO2 from V2O5 and WO3 with some progress being achieved. Figure 7 illustrates the transformation relationships of substances in these works. Since the goal is no longer to separate and purify V, W and Ti, the process can be simplified based on current separation processes. New extraction processes can also be developed, such as the dissolution of TiO2 with sulphuric acid under hydrothermal conditions [75]. Mechanochemical methods also have potential applications in the preparation of TiO2 [76]. Ball milling as a pre-treatment has proven to be effective in the recycling of spent SCR catalysts [59]. On the other hand, both BiVO4 [77] and Bi2WO6 [78] can form corresponding heterostructures with TiO2. Moreover, V and W can be extracted directly into solution as NH4VO3 and (NH4)2WO4 [45], which would assist in the further synthesis of bismuth salt.
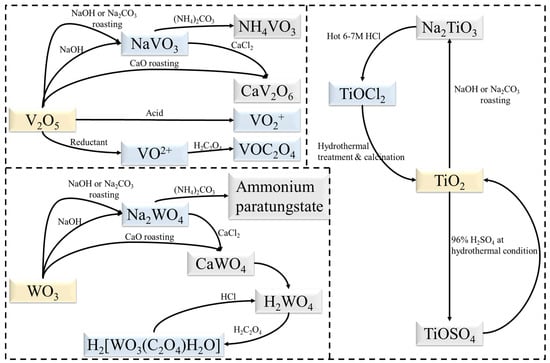
Figure 7.
Transformation relationships of substances during extraction.
6. Conclusions
To recycle spent SCR catalysts as a metal resource, it is vitally important for future research to explore environmentally friendly and economically feasible recycling methods. Regeneration processes are currently employed in industry to extend the life of SCR catalysts, but catalysts that have undergone three to four regenerations are eventually abandoned as they are difficult to regenerate. Landfill is the main disposal method for spent SCR catalysts at present; although wasteful of resources, it is much easier to process. Consequently, even though the recycling process is more in-line with the principles of green chemistry, it is difficult to promote because of its complexity, high cost, low economic efficiency, and the risk of secondary pollution. Nevertheless, further exploration of the recycling process is necessary to address these shortcomings to achieve broader application.
This review of research progress in the last 3 years shows that an increasing number of studies are focusing on the added value of the product obtained after extraction. The common elemental extraction processes include leaching and roasting methods. The leaching method can achieve the extraction of elemental V at lower temperatures, and efficiency can be improved by combining with a reducing agent (e.g., Na2SO3) under acidic conditions and oxidizing agent (e.g., H2O2) under alkaline conditions. The roasting method can overcome the difficulty of extracting W by the leaching method as it converts insoluble oxides into soluble salts. Improvements to the roasting method include (i) adaptation of the roasting condition with reference to the phase diagram, (ii) addition of a Cl− containing catalyst (e.g., NaCl) to reduce the temperature and time for the reaction, and (iii) application of acid leaching rather than water leaching to avoid the use of an alkaline reagent in the roasting process. With the above method, V2O5 can be recovered as CaV2O6, NH4VO3, BiVO4, and Zn3(VO4)2, while WO3 is recovered as CaWO4, ammonium tungstate, Bi2WO6, and ZnWO4. Additionally, both BiVO4/Bi2WO6, and Zn3(VO4)2/ZnWO4 and TiO2 can be utilized as photocatalysts for environmental management, respectively. Further research should explore photocatalyst conversion processes with higher atomic efficiency. The selective conversion of V, W and Ti into their salt or oxide can be applied in the treatment of spent SCR catalysts, and attempts can be made to combine these substances into a heterogeneous photocatalyst. In addition, new treatment technologies such as mechanochemical processes to reduce contamination during the recycling of spent SCR catalysts need to be introduced. The process should avoid high-temperature and pressure-reaction conditions and reduce the production of wastewater, waste gases and residues.
Author Contributions
Conceptualization, X.Q., W.A., H.D., X.W. and S.S.; methodology, X.Q., W.A., H.D., X.W. and S.S.; software, X.Q.; validation, X.Q., W.A. and H.D.; formal analysis, X.Q., X.W. and S.S.; investigation, X.Q.; resources, X.Q., W.A. and H.D.; data curation, X.Q.; writing—original draft preparation, X.Q.; writing—review and editing, X.Q.; visualization, X.Q.; supervision, W.A.; project administration, H.D.; funding acquisition, W.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program (No. 2022YFC3702301) and the Fundamental Research Funds for the Central Universities (No. 37532020015 and 2652022412).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used to support the findings of this study are included in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Busca, G.; Lietti, L.; Ramis, G.; Berti, F. Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts: A review. Appl. Catal. B-Environ. 1998, 18, 1–36. [Google Scholar] [CrossRef]
- Han, L.; Cai, S.; Gao, M.; Hasegawa, J.-y.; Wang, P.; Zhang, J.; Shi, L.; Zhang, D. Selective Catalytic Reduction of NOx with NH3 by Using Novel Catalysts: State of the Art and Future Prospects. Chem. Rev. 2019, 119, 10916–10976. [Google Scholar] [CrossRef]
- Wang, D.; Chen, Q.; Zhang, X.; Gao, C.; Wang, B.; Huang, X.; Peng, Y.; Li, J.; Lu, C.; Crittenden, J. Multipollutant Control (MPC) of Flue Gas from Stationary Sources Using SCR Technology: A Critical Review. Environ. Sci. Technol. 2021, 55, 2743–2766. [Google Scholar] [CrossRef] [PubMed]
- Szymaszek, A.; Samojeden, B.; Motak, M. The Deactivation of Industrial SCR Catalysts-A Short Review. Energies 2020, 13, 3870. [Google Scholar] [CrossRef]
- Yuan, L.; Qiu, Z.; Yang, J.; Ma, B.; Cao, L.; Zhang, W. Research progress of alkali(alkaline earth) metal poisoning and modified regeneration of scr catalyst. Environ. Eng. 2018, 36, 117–121. [Google Scholar]
- Bai, X.; Shang, X.; Wan, H.; Che, Y.; Yang, B.; He, J.; Song, J. Sustainable recycling of titanium from TiO2 in spent SCR denitration catalyst via molten salt electrolysis. J. Energy Chem. 2021, 58, 557–563. [Google Scholar] [CrossRef]
- Chen, H.-J.; Wang, R.; Yang, Y.-L.; Shi, X.-L.; Lu, S.; Chen, Z.-G. Environmentally-friendly harvesting TiO2 nanospheres and V2O5 microrods from spent selective catalytic reduction catalysts. Prog. Nat. Sci. Mater. Int. 2021, 31, 858–864. [Google Scholar] [CrossRef]
- Ferella, F. A review on management and recycling of spent selective catalytic reduction catalysts. J. Clean. Prod. 2020, 246, 118990. [Google Scholar] [CrossRef]
- Choi, I.-H.; Cho, Y.-C.; Moon, G.; Kang, H.-N.; Oh, Y.; Lee, J.-Y.; Kang, J. Recent Developments in the Recycling of Spent Selective Catalytic Reduction Catalyst in South Korea. Catalysts 2020, 10, 182. [Google Scholar] [CrossRef]
- Zhou, H.; Xing, Y.-j.; Xu, J.-n.; Zhou, M.-x. In-situ investigation of melting characteristics of waste selective catalytic reduction catalysts during harmless melting treatment. J. Zhejiang Univ. -Sci. A 2021, 22, 207–221. [Google Scholar] [CrossRef]
- Long, H.-m.; Zhang, Y.-d.; Yang, T.; Qian, L.-x.; Yu, Z.-w. A promising method to recover spent V2O5–WO3/TiO2 catalyst: Treatment by vanadium–titanium magnetite sintering process. J. Iron Steel Res. Int. 2021, 29, 1176–1184. [Google Scholar] [CrossRef]
- Qian, L.; Yang, T.; Long, H.; Ding, L.; Xu, C.C. Recycling of Waste V2O5–WO3/TiO2 Catalysts in the Iron Ore Sintering Process Via a Preballing Approach. ACS Sustain. Chem. Eng. 2021, 9, 16373–16383. [Google Scholar] [CrossRef]
- Zhou, H.; Ma, P.; Lai, Z.; Zuo, Y.; Xing, Y.; Shi, H.; Cen, K. Harmless treatment of waste selective catalytic reduction catalysts during iron ore sintering process. J. Clean. Prod. 2020, 275, 122954. [Google Scholar] [CrossRef]
- He, C.; Kong, F.; Bao, Q.; Zhang, F.; Wang, L.; Ma, Y.; Yao, Y. Cement solidification/stabilization of the toxicants from spent commercial SCR catalyst. J. Chem. Technol. Biotechnol. 2021, 96, 514–520. [Google Scholar] [CrossRef]
- Niu, T.; Wang, J.; Chu, H.; Qian, C.; Duan, N.; Michael Gadd, G.; Shi, W.; Xin, B. Deep removal of arsenic from regenerated products of spent V2O5-WO3/TiO2 SCR catalysts and its concurrent activation by bioleaching through a novel mechanism. Chem. Eng. J. 2021, 420, 127722. [Google Scholar] [CrossRef]
- Choi, I.-H.; Kim, H.-R.; Moon, G.; Jyothi, R.K.; Lee, J.-Y. Spent V2O5-WO3/TiO2 catalyst processing for valuable metals by soda roasting-water leaching. Hydrometallurgy 2018, 175, 292–299. [Google Scholar] [CrossRef]
- Choi, I.-h.; Moon, G.; Lee, J.-Y.; Jyothi, R.K. Hydrometallurgical processing of spent selective catalytic reduction (SCR) catalyst for recovery of tungsten. Hydrometallurgy 2018, 178, 137–145. [Google Scholar] [CrossRef]
- Lisi, L.; Cimino, S. Poisoning of SCR Catalysts by Alkali and Alkaline Earth Metals. Catalysts 2020, 10, 1475. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Q. Research on the deactivation mechanism of a denitration catalyst WO3–V2O5/TiO2 at a coal-fired power plant. RSC Adv. 2020, 10, 44025–44033. [Google Scholar] [CrossRef]
- Zyrkowski, M.; Motak, M.; Samojeden, B.; Szczepanek, K. Deactivation of V2O5−WO3/TiO2 DeNOx Catalyst under Commercial Conditions in Power Production Plant. Energies 2020, 13, 6200. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, Y.; Yuan, H. Recycling strategies of spent V2O5-WO3/TiO2 catalyst: A review. Resour. Conserv. Recycl. 2020, 161, 104983. [Google Scholar] [CrossRef]
- Wang, B.; Liu, Z.; Lin, D.; Cao, Z.; He, F.; Lu, G.; Xiao, Y. A Review on Recovery and Utilization of Spent V2O5-WO3/TiO2 Catalyst. Mater. Rev. 2021, 35, 15001–15010. [Google Scholar]
- Kim, J.W.; Hwang, I.-J. Separation of valuables from spent selective catalytic reduction catalyst leaching solution by fabricated anion extraction resins. J. Environ. Chem. Eng. 2018, 6, 1100–1108. [Google Scholar] [CrossRef]
- Cueva Sola, A.B.; Parhi, P.K.; Lee, J.-Y.; Kang, H.N.; Jyothi, R.K. Environmentally friendly approach to recover vanadium and tungsten from spent SCR catalyst leach liquors using Aliquat 336. RSC Adv. 2020, 10, 19736–19746. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.Y.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Meng, A.; Zhang, L.; Cheng, B.; Yu, J. Dual Cocatalysts in TiO2 Photocatalysis. Adv. Mater. 2019, 31, 1807660. [Google Scholar] [CrossRef]
- Nair, R.V.; Gummaluri, V.S.; Matham, M.V.; Vijayan, C. A review on optical bandgap engineering in TiO2 nanostructures via doping and intrinsic vacancy modulation towards visible light applications. J. Phys. D Appl. Phys. 2022, 55, 313003. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, S.; Gu, P.; Zhang, T.; Chen, D.; Li, N.; Xu, Q.; Lu, J. Conjugate Polymer-clothed TiO2@V2O5 nanobelts and their enhanced visible light photocatalytic performance in water remediation. J. Colloid Interface Sci. 2020, 578, 402–411. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Li, Z.; Liu, Y.; Peng, Z.; Zhou, M.; Zhang, C.; Jin, W. Synthesis and photocatalytic property of V2O5@TiO2 core-shell microspheres towards gaseous benzene. Catal. Today 2019, 321, 164–171. [Google Scholar] [CrossRef]
- Cai, Z.; Hao, X.; Sun, X.; Du, P.; Liu, W.; Fu, J. Highly active WO3@anatase-SiO2 aerogel for solar-light-driven phenanthrene degradation: Mechanism insight and toxicity assessment. Water Res. 2019, 162, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Anwer, H.; Mahmood, A.; Lee, J.; Kim, K.-H.; Park, J.-W.; Yip, A.C.K. Photocatalysts for degradation of dyes in industrial effluents: Opportunities and challenges. Nano Res. 2019, 12, 955–972. [Google Scholar] [CrossRef]
- Zimmerman, J.B.; Anastas, P.T.; Erythropel, H.C.; Leitner, W. Designing for a green chemistry future. Science 2020, 367, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Du, X.; Wang, X.; Yang, G.; Wan, Y.; Chen, Y.; Song, L.; Xue, Z.; Zhang, L. Recycling of Waste SCR Catalysts Using a Catalytic Filter: A Study on the Catalytic Performance for NOx Abatement. Ind. Eng. Chem. Res. 2021, 60, 4622–4629. [Google Scholar] [CrossRef]
- Zhang, Y.; Teng, Y.; Lu, B.; Zhuang, K.; Wang, S.; Liu, Y.; Wang, J. Regeneration Treatment Technology of Deactivated Vanadium Tungsten and Titanium Denitration Catalysts. J. Chin. Ceram. Soc. 2019, 47, 440–449. [Google Scholar]
- Kapkowski, M.; Siudyga, T.; Sitko, R.; Niemczyk-Wojdyla, A.; Zelenka, T.; Zelenková, G.; Golba, S.; Smolinski, A.; Polanski, J. Toward a viable ecological method for regenerating a commercial SCR catalyst—Selectively leaching surface deposits and reconstructing a pore landscape. J. Clean. Prod. 2021, 316, 128291. [Google Scholar] [CrossRef]
- Huang, X.; Wang, D.; Zhao, H.; Yang, Q.; Peng, Y.; Li, J. Severe deactivation and artificial enrichment of thallium on commercial SCR catalysts installed in cement kiln. Appl. Catal. B Environ. 2020, 277, 119194. [Google Scholar] [CrossRef]
- Zhou, K.; Li, G.; Lu, B.; Wang, S.; Zhang, Y.; Teng, Y.; Li, J. Regeneration of Deactivated V2O5-WO3/TiO2 Selective Catalytic Reduction Denitration Catalyst. J. Chin. Ceram. Soc. 2019, 47, 916–923. [Google Scholar]
- Kim, J.W.; Lee, W.G.; Hwang, I.S.; Lee, J.Y.; Han, C. Recovery of tungsten from spent selective catalytic reduction catalysts by pressure leaching. J. Ind. Eng. Chem. 2015, 28, 73–77. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Z.; Liu, Q. Kinetics of Vanadium Leaching from a Spent Industrial V2O5/TiO2 Catalyst by Sulfuric Acid. Ind. Eng. Chem. Res. 2014, 53, 2956–2962. [Google Scholar] [CrossRef]
- Kim, H.-R.; Lee, J.; Kim, J. Leaching of Vanadium and Tungsten from Spent SCR Catalysts for De-NOx by Soda Roasting and Water Leaching Method. J. Korean Inst. Resour. Recycl. 2012, 21, 65–73. [Google Scholar] [CrossRef]
- Qi, C.; Bao, W.; Wang, L.; Li, H.; Wu, W. Study of the V2O5-WO3/TiO2 Catalyst Synthesized from Waste Catalyst on Selective Catalytic Reduction of NOx by NH3. Catalysts 2017, 7, 110. [Google Scholar] [CrossRef]
- Wu, W.-C.; Tsai, T.-Y.; Shen, Y.-H. Tungsten Recovery from Spent SCR Catalyst Using Alkaline Leaching and Ion Exchange. Minerals 2016, 6, 107. [Google Scholar] [CrossRef]
- Wu, W.; Wang, C.; Bao, W.; Li, H. Selective reduction leaching of vanadium and iron by oxalic acid from spent V2O5-WO3/TiO2 catalyst. Hydrometallurgy 2018, 179, 52–59. [Google Scholar] [CrossRef]
- Cao, Y.; Yuan, J.; Du, H.; Dreisinger, D.; Li, M. A clean and efficient approach for recovery of vanadium and tungsten from spent SCR catalyst. Miner. Eng. 2021, 165, 106857. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, Z.; Wang, C. Study on the TiO2 Recovery from SCR Catalyst Waste in Coal-Fired Power Plants. Electr. Power 2016, 49, 151–156,180. [Google Scholar]
- Wang, S.; Xie, Y.; Yan, W.; Wu, X.; Wang, C.-T.; Zhao, F. Leaching of vanadium from waste V2O5-WO3/TiO2 catalyst catalyzed by functional microorganisms. Sci. Total Environ. 2018, 639, 497–503. [Google Scholar] [CrossRef]
- Choi, I.-H.; Moon, G.; Lee, J.-Y.; Jyothi, R.K. Alkali fusion using sodium carbonate for extraction of vanadium and tungsten for the preparation of synthetic sodium titanate from spent SCR catalyst. Sci. Rep. 2019, 9, 12316. [Google Scholar] [CrossRef]
- Yang, B.; Zhou, J.; Wang, W.; Liu, C.; Zhou, D.; Yang, L. Extraction and separation of tungsten and vanadium from spent V2O5–WO3/TiO2 SCR catalysts and recovery of TiO2 and sodium titanate nanorods as adsorbent for heavy metal ions. Colloids Surf. A Physicochem. Eng. Asp. 2020, 601, 124963. [Google Scholar] [CrossRef]
- Su, Q.; Yi, X.; Miao, J.; Chen, Y.; Chen, J.; Wang, J. A Comparative Study in Vanadium and Tungsten Leaching from Various Sources of SCR Catalysts with Local Difference. Sustainability 2020, 12, 1499. [Google Scholar] [CrossRef]
- Liu, N.; Xu, X.; Liu, Y. Recovery of vanadium and tungsten from spent selective catalytic reduction catalyst by alkaline pressure leaching. Physicochem. Probl. Miner. Processing 2020, 56, 407–420. [Google Scholar] [CrossRef]
- Nie, Z.; Ma, L.; Xi, X.; Guo, F.; Nie, Z. Studying the leaching mechanism of spent SCR catalyst with different leaching agents (NaOH, H2SO4, HCl and HNO3) using DFT calculations. Appl. Surf. Sci. 2022, 584, 152577. [Google Scholar] [CrossRef]
- Wang, B.; Yang, Q. Optimization of Roasting Parameters for Recovery of Vanadium and Tungsten from Spent SCR Catalyst with Composite Roasting. Processes 2021, 9, 1923. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Q. Recovery of vanadium and tungsten from waste selective catalytic reduction catalysts by K2CO3 roasting and water leaching followed by CaCl2 precipitation. Int. J. Coal Sci. Technol. 2021, 8, 727–736. [Google Scholar] [CrossRef]
- Moon, G.; Kim, J.H.; Lee, J.-Y.; Kang, J. Leaching of spent selective catalytic reduction catalyst using alkaline melting for recovery of titanium, tungsten, and vanadium. Hydrometallurgy 2019, 189, 105132. [Google Scholar] [CrossRef]
- Yao, J.; Cao, Y.; Wang, J.; Zhang, C.; Wang, W.; Bao, W.; Chang, L. Successive calcination-oxalate acid leaching treatment of spent SCR catalyst: A highly efficient and selective method for recycling tungsten element. Hydrometallurgy 2021, 201, 105576. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, Y.; Li, L.; Zuo, T. Sustainable Approach for Spent V2O5–WO3/TiO2 Catalysts Management: Selective Recovery of Heavy Metal Vanadium and Production of Value-Added WO3–TiO2 Photocatalysts. ACS Sustain. Chem. Eng. 2018, 6, 12502–12510. [Google Scholar] [CrossRef]
- Chen, F.; Cui, C.; Qi, L. Utilizing organic acids for V2O5 recovery from denitration catalyst alkaline inactivation. CIESC J. 2017, 68, 4717–4722. [Google Scholar]
- Su, Q.; Miao, J.; Li, H.; Chen, Y.; Chen, J.; Wang, J. Optimizing vanadium and tungsten leaching with lowered silicon from spent SCR catalyst by pre-mixing treatment. Hydrometallurgy 2018, 181, 230–239. [Google Scholar] [CrossRef]
- Foulon, C.; Pareau, D.; Durand, G. Thermodynamic and kinetic studies of palladium (II) extraction by extractant mixtures containing LIX 63: Part I. Thermodynamic study. Hydrometallurgy 1999, 51, 139–153. [Google Scholar] [CrossRef]
- Moon, G.; Cho, Y.-C.; Lee, J.-Y.; Kang, J. Desilication of Highly Acidic Titanyl Chloride Solution for the Production of High-Purity Titania Pigment from a Spent Selective Catalytic Reduction Catalyst. Mater. Trans. 2019, 60, 988–996. [Google Scholar] [CrossRef]
- Ao, W.; Ding, H.; Sun, S.; Lin, C.; Zhang, X. Processing of Waste Selective Catalytic Reduction Denitration Catalyst Used for Preparing Composite Opacifier for, e.g. Ceramics by Mixing e.g. Pre-Treated Waste Selective Catalytic Reduction Catalyst and Wollastonite, and Crushing. CN Patent CN110981199-A; CN110981199-B, 10 April 2020. [Google Scholar]
- Son, B.T.; Long, N.V.; Nhat Hang, N.T. Fly ash-, foundry sand-, clay-, and pumice-based metal oxide nanocomposites as green photocatalysts. RSC Adv. 2021, 11, 30805–30826. [Google Scholar] [CrossRef] [PubMed]
- Mohan, H.; Vadivel, S.; Rajendran, S. Removal of harmful algae in natural water by semiconductor photocatalysis- A critical review. Chemosphere 2022, 302, 134827. [Google Scholar] [CrossRef] [PubMed]
- Shabir, M.; Yasin, M.; Hussain, M.; Shafiq, I.; Akhter, P.; Nizami, A.-S.; Jeon, B.-H.; Park, Y.-K. A review on recent advances in the treatment of dye-polluted wastewater. J. Ind. Eng. Chem. 2022, 112, 1–19. [Google Scholar] [CrossRef]
- Li, X.; Simon, U.; Bekheet, M.F.; Gurlo, A. Mineral-Supported Photocatalysts: A Review of Materials, Mechanisms and Environmental Applications. Energies 2022, 15, 5607. [Google Scholar] [CrossRef]
- Gajera, R.; Patel, R.V.; Yadav, A.; Labhasetwar, P.K. Adsorption of cationic and anionic dyes on photocatalytic flyash/TiO2 modified chitosan biopolymer composite. J. Water Process Eng. 2022, 49, 102993. [Google Scholar] [CrossRef]
- Ao, W.; Qu, J.; Yu, H.; Liu, Y.; Liu, C.; Fu, J.; Dai, J.; Bi, X.; Yuan, Y.; Jin, Y. TiO2/activated carbon synthesized by microwave-assisted heating for tetracycline photodegradation. Environ. Res. 2022, 214, 113837. [Google Scholar] [CrossRef]
- Mishra, A.; Mehta, A.; Basu, S. Clay supported TiO2 nanoparticles for photocatalytic degradation of environmental pollutants: A review. J. Environ. Chem. Eng. 2018, 6, 6088–6107. [Google Scholar] [CrossRef]
- Jin, Q.; Shen, Y.; Cai, Y.; Chu, L.; Zeng, Y. Resource utilization of waste V2O5-based deNO(x) catalysts for hydrogen production from formaldehyde and water via steam reforming. J. Hazard. Mater. 2020, 381, 120934. [Google Scholar] [CrossRef]
- Huo, Y.; Chang, Z.; Li, W.; Liu, S.; Dong, B. Reuse and Valorization of Vanadium and Tungsten from Waste V2O5-WO3/TiO2 SCR Catalyst. Waste Biomass Valorization 2015, 6, 159–165. [Google Scholar] [CrossRef]
- Kim, J.B.; Seol, D.-H.; Shon, H.K.; Kim, G.-J.; Kim, J.-H. Preparation and Characterization of Titania Nanoparticles from Titanium Tetrachloride and Titanium Sulfate Flocculation of Dye Wastewater. J. Jpn. Pet. Inst. 2010, 53, 167–172. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.Z.; Chang, Z.D.; Blamo, B.J.; Wu, X.; Liu, S.X.; Li, W.J. Preparation of Photocatalytic Zn-3(VO4)(2)/ZnWO4 from Waste V2O5-WO3/TiO2 SCR Catalyst. Waste Biomass Valorization 2017, 8, 2423–2430. [Google Scholar] [CrossRef]
- Qian, X.; Ao, W.; Wang, X.; Sun, S.; Zhang, J.; Ding, H. Preparation of fly ash based titanium dioxide composite photocatalysts using spent SCR catalyst carriers. J. Environ. Chem. Eng. 2022, 10, 107980. [Google Scholar] [CrossRef]
- Ao, W.; Ding, H.; Sun, S.; Qian, X.; Jiang, C.; Jiang, J. Use of Waste Selective Catalytic Reduction Catalyst in Preparing Silicon Dioxide-Titanium Dioxide Composite Photocatalyst. CN Patent CN113145093-A, 23 July 2021. [Google Scholar]
- Giannakoudakis, D.A.; Chatel, G.; Colmenares, J.C. Mechanochemical Forces as a Synthetic Tool for Zero- and One-Dimensional Titanium Oxide-Based Nano-photocatalysts. Top. Curr. Chem. 2019, 378, 2. [Google Scholar] [CrossRef]
- Sajid, M.M.; Alomayri, T. Synthesis of TiO2/BiVO4 Composite and Cogitation the Interfacial Charge Transportation for Evaluation of Photocatalytic Activity. Arab. J. Sci. Eng. 2022. [Google Scholar] [CrossRef]
- Zhang, L.; Meng, G.; Liu, B.; Ge, X. Heterogeneous photocatalytic ozonation of sulfamethoxazole by Z-scheme Bi2WO6/TiO2 heterojunction: Performance, mechanism and degradation pathway. J. Mol. Liq. 2022, 360, 119427. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).