Discoloration Improvement by Mechanically-Milled Binary Oxides as Radiopacifier for Mineral Trioxide Aggregates
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of Mechanically-Milled Radiopacifier
2.2. Preparation and Characterization of MTA-Like Cements
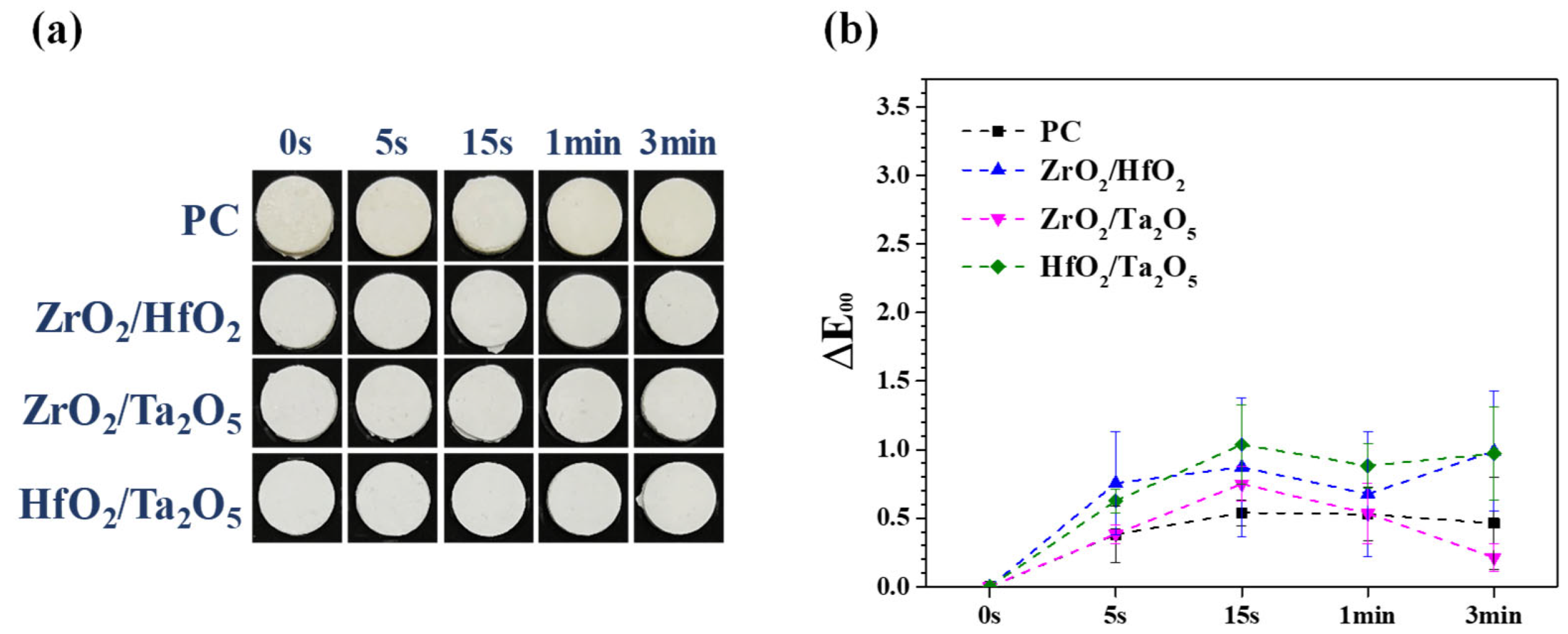
2.3. Discoloration Examination of MTA-Like Cements
2.4. Statistical Analysis
3. Results and Discussion
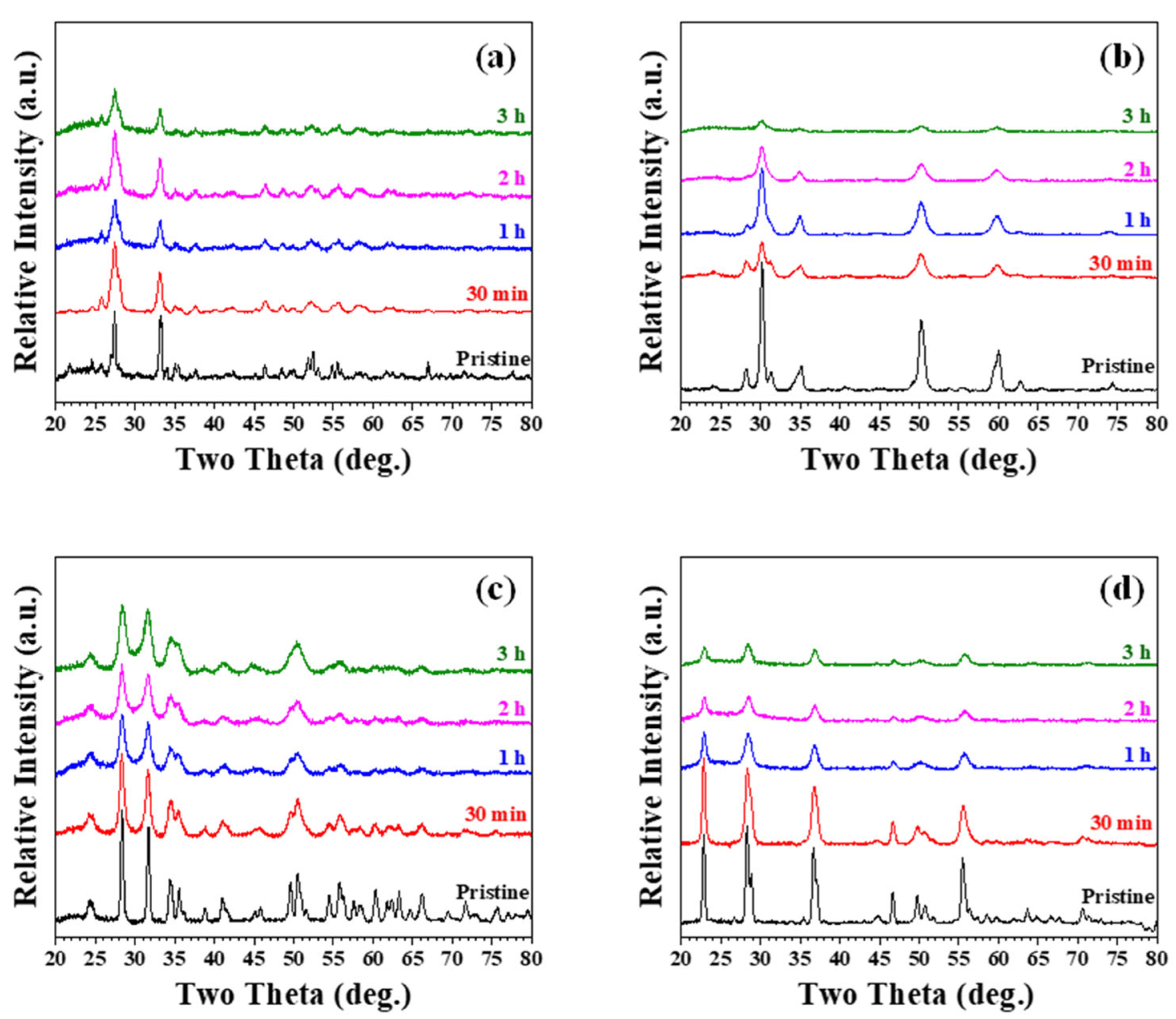
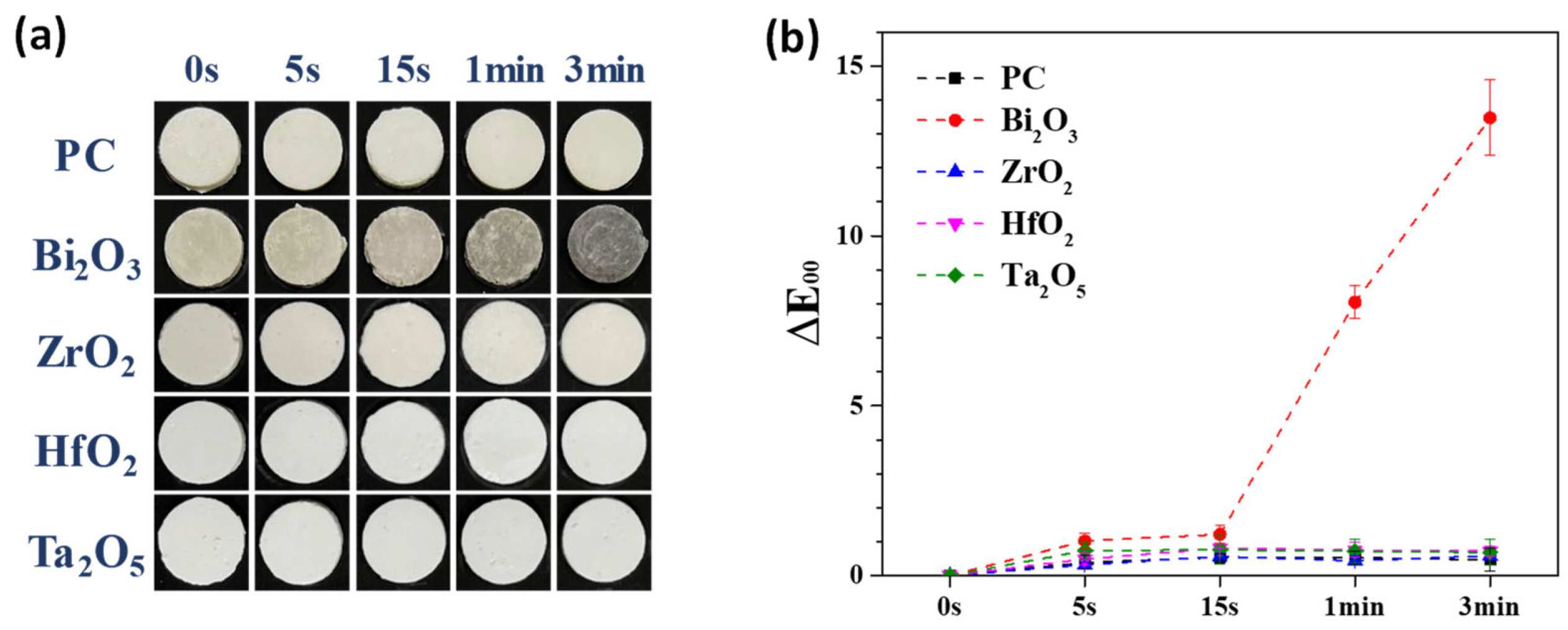
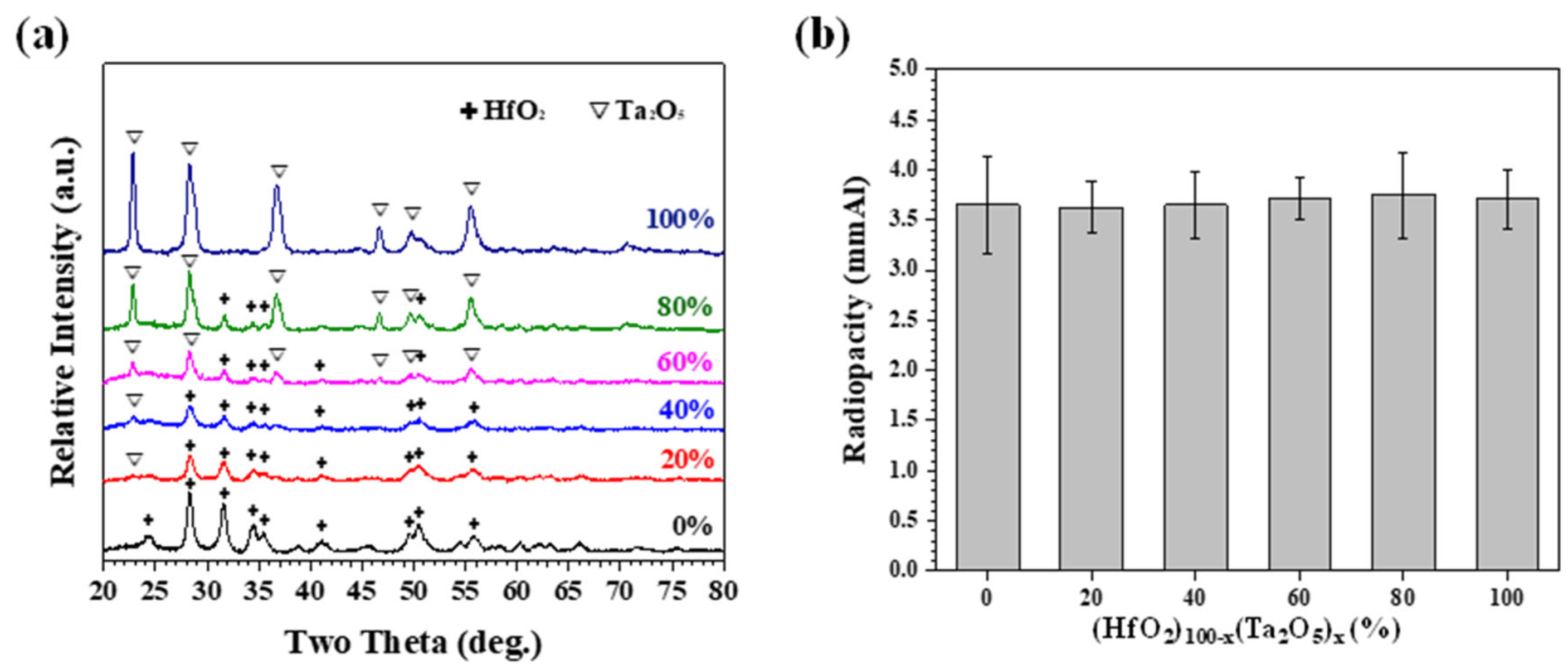
3.1. Mechanically Milled Single Oxide as Radiopacifier
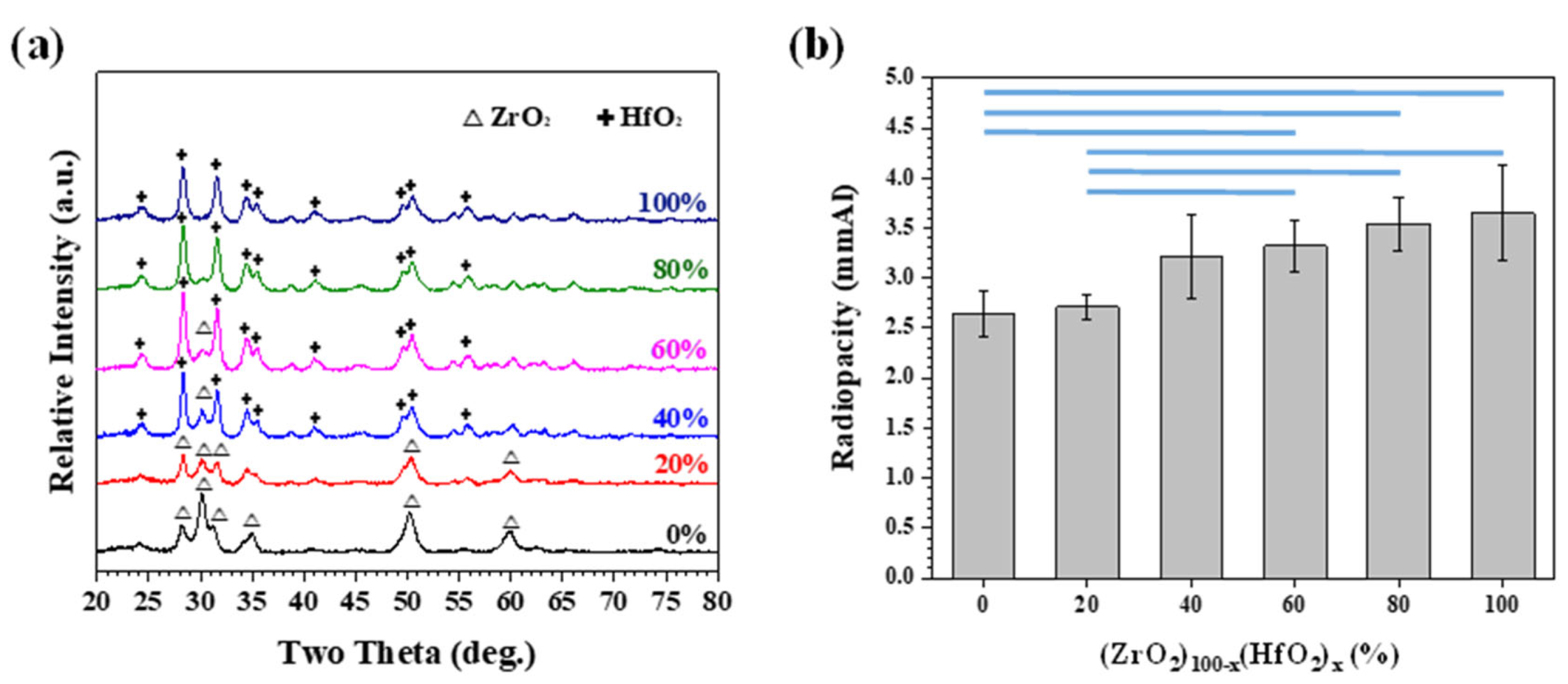
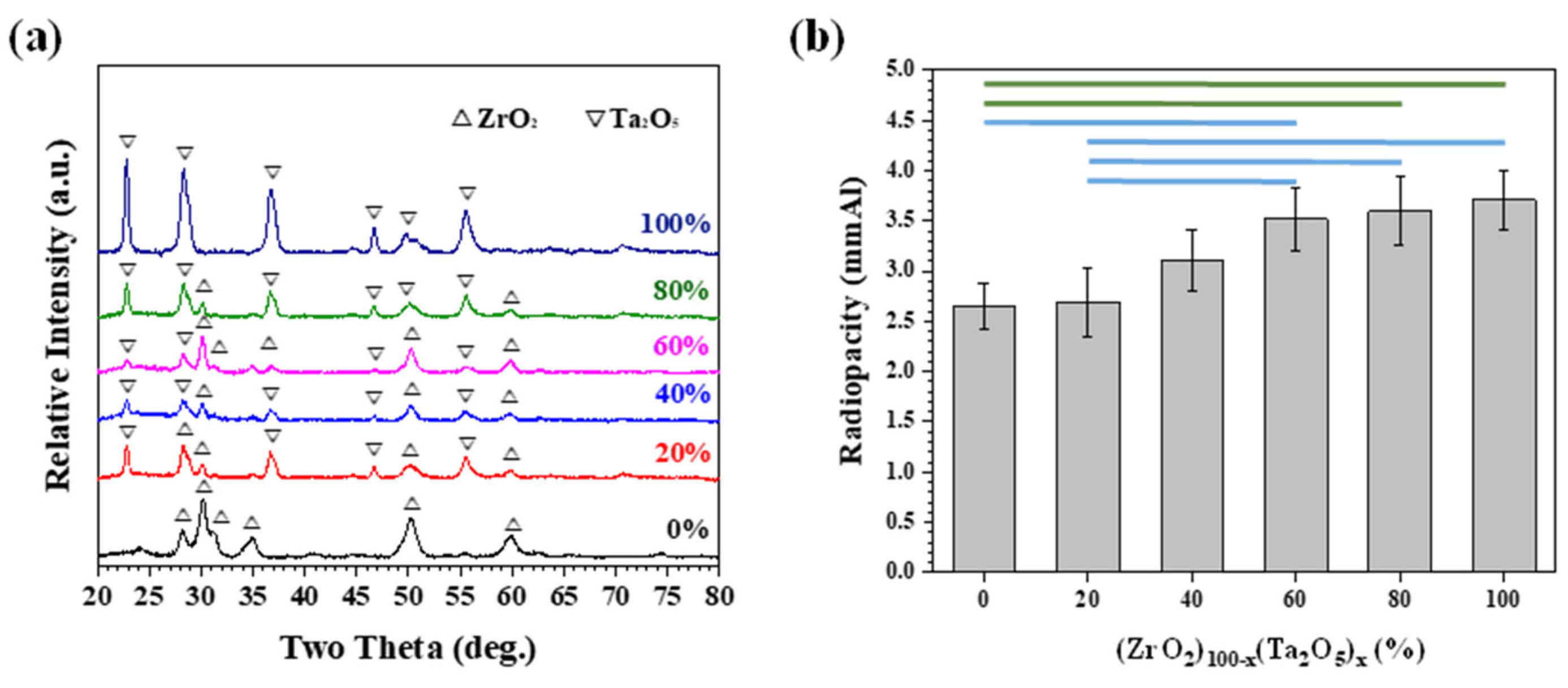
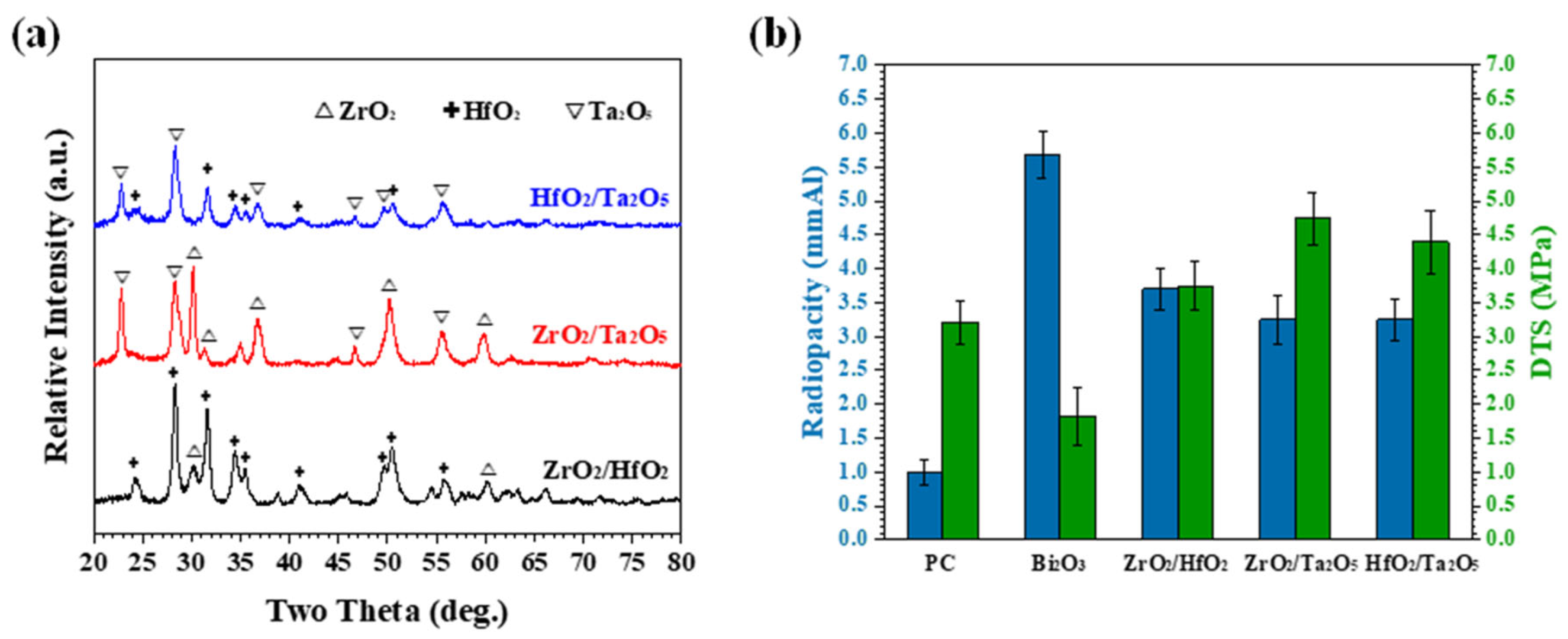
3.2. Mechanically Milled Binary Oxides as Radiopacifier
3.3. Performance of Equivalent Binary Oxide Radiopacifier
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Camilleri, J. Mineral trioxide aggregate: Present and future developments. Endod. Top. 2015, 32, 31–46. [Google Scholar] [CrossRef]
- Parirokh, M.; Torabinejad, M.; Dummer, P. Mineral trioxide aggregate and other bioactive endodontic cements: An updated overview—Part I: Vital pulp therapy. Int. Endod. J. 2018, 51, 177–205. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Parirokh, M.; Dummer, P.M. Mineral trioxide aggregate and other bioactive endodontic cements: An updated overview—Part II: Other clinical applications and complications. Int. Endod. J. 2018, 51, 284–317. [Google Scholar] [CrossRef] [PubMed]
- Kahler, B.; Rossi-Fedele, G. A review of tooth discoloration after regenerative endodontic therapy. J. Endod. 2016, 42, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Sheykhrezae, M.S.; Meraji, N.; Ghanbari, F.; Nekoofar, M.H.; Bolhari, B.; Dummer, P.M. Effect of blood contamination on the compressive strength of three calcium silicate-based cements. Aust. Endod. J. 2018, 44, 255–259. [Google Scholar] [CrossRef]
- Meraji, N.; Bolhari, B.; Sefideh, M.R.; Niavarzi, S. Prevention of tooth discoloration due to calcium-silicate cements: A review. Dent. Hypotheses 2019, 10, 4. [Google Scholar]
- Prati, C.; Gandolfi, M.G. Calcium silicate bioactive cements: Biological perspectives and clinical applications. Dent. Mater. 2015, 31, 351–370. [Google Scholar] [CrossRef]
- de Oliveira, N.G.; de Souza Araújo, P.R.; da Silveira, M.T.; Sobral, A.P.V.; de Vasconcelos Carvalho, M. Comparison of the biocompatibility of calcium silicate-based materials to mineral trioxide aggregate: Systematic review. Eur. J. Dent. 2018, 12, 317–326. [Google Scholar] [CrossRef]
- Palczewska-Komsa, M.; Kaczor-Wiankowska, K.; Nowicka, A. New Bioactive Calcium Silicate Cement Mineral Trioxide Aggregate Repair High Plasticity (MTA HP)—A Systematic Review. Materials 2021, 14, 4573. [Google Scholar] [CrossRef]
- Kharouf, N.; Zghal, J.; Addiego, F.; Gabelout, M.; Jmal, H.; Haïkel, Y.; Bahlouli, N.; Ball, V. Tannic acid speeds up the setting of mineral trioxide aggregate cements and improves its surface and bulk properties. J. Colloid Interface Sci. 2021, 589, 318–326. [Google Scholar] [CrossRef]
- Chen, S.; Shi, L.; Luo, J.; Engqvist, H. Novel fast-setting mineral trioxide aggregate: Its formulation, chemical–physical properties, and Cytocompatibility. ACS Appl. Mater. Interfaces 2018, 10, 20334–20341. [Google Scholar] [CrossRef]
- Pednekar, A.A.; De Ataide, I.D.N.; Fernandes, M.; Lambor, R.; Soares, R. Spectrophotometric Analysis of Coronal Discolouration Induced by ProRoot MTA, Biodentine and MTA Repair HP Used for Pulpotomy Procedures. Eur. Endod. J. 2021, 6, 189–196. [Google Scholar]
- Vallés, M.; Roig, M.; Duran-Sindreu, F.; Martínez, S.; Mercadé, M. Color stability of teeth restored with Biodentine: A 6-month in vitro study. J. Endod. 2015, 41, 1157–1160. [Google Scholar] [CrossRef]
- Yoldaş, S.E.; Bani, M.; Atabek, D.; Bodur, H. Comparison of the potential discoloration effect of bioaggregate, biodentine, and white mineral trioxide aggregate on bovine teeth: In vitro research. J. Endod. 2016, 42, 1815–1818. [Google Scholar] [CrossRef]
- Chen, M.-S.; Lin, H.-N.; Cheng, Y.-C.; Fang, A.; Chen, C.-Y.; Lee, P.-Y.; Lin, C.-K. Effects of milling time, zirconia addition, and storage environment on the radiopacity performance of mechanically milled Bi2O3/ZrO2 composite powders. Materials 2020, 13, 563. [Google Scholar] [CrossRef]
- Lin, H.-N.; Lin, C.-K.; Chang, P.-J.; Chang, W.-M.; Fang, A.; Chen, C.-Y.; Yu, C.-C.; Lee, P.-Y. Effect of Tantalum Pentoxide Addition on the Radiopacity Performance of Bi2O3/Ta2O5 Composite Powders Prepared by Mechanical Milling. Materials 2021, 14, 7447. [Google Scholar] [CrossRef]
- Chen, M.-S.; Yang, J.-C.; Lai, F.-C.; Chen, C.-Y.; Hsieh, M.-Y.; Fang, A.; Chen, S.-H.; Lin, C.-K. Radiopacity performances of precipitated ZrO2-doped Bi2O3 powders and the influences of dopant concentrations and sintering temperatures. Ceram. Int. 2017, 43, 14008–14014. [Google Scholar] [CrossRef]
- Chen, M.S.; Chen, S.H.; Lai, F.C.; Chen, C.Y.; Hsieh, M.Y.; Chang, W.J.; Yang, J.C.; Lin, C.K. Sintering Temperature-Dependence on Radiopacity of Bi(2−x)ZrxO(3+x/2) Powders Prepared by Sol-Gel Process. Materials 2018, 11, 1685. [Google Scholar] [CrossRef]
- Yang, T.-S.; Chen, M.-S.; Huang, C.-J.; Chen, C.-Y.; Brangule, A.; Zarkov, A.; Kareiva, A.; Lin, C.-K.; Yang, J.-C. A novel sol-gel Bi2-xHfxO3+x/2 radiopacifier for mineral trioxide aggregates (MTA) as dental filling materials. Appl. Sci. 2021, 11, 7292. [Google Scholar] [CrossRef]
- Benjamin, J. Mechanical alloying. Sci. Am. 1976, 234, 40–49. [Google Scholar] [CrossRef]
- Murty, B.; Ranganathan, S. Novel materials synthesis by mechanical alloying/milling. Int. Mater. Rev. 1998, 43, 101–141. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Lee, P.; Koch, C. Formation of amorphous Ni-Zr alloy powder by mechanical alloying of intermetallic powder mixtures and mixtures of nickel or zirconium with intermetallics. J. Mater. Sci. 1988, 23, 2837–2845. [Google Scholar] [CrossRef]
- Koch, C.C.; Whittenberger, J. Mechanical milling/alloying of intermetallics. Intermetallics 1996, 4, 339–355. [Google Scholar] [CrossRef]
- Lin, C.K.; Liu, S.W.; Lee, P.Y. Preparation and thermal stability of Zr-Ti-Al-Ni-Cu amorphous powders by mechanical alloying technique. Metall. Mater. Trans. A 2001, 32, 1777–1786. [Google Scholar] [CrossRef]
- Zenou, V.Y.; Bakardjieva, S. Microstructural analysis of undoped and moderately Sc-doped TiO2 anatase nanoparticles using Scherrer equation and Debye function analysis. Mater. Charact. 2018, 144, 287–296. [Google Scholar] [CrossRef]
- Kang, S.-H.; Shin, Y.-S.; Lee, H.-S.; Kim, S.-O.; Shin, Y.; Jung, I.-Y.; Song, J.S. Color changes of teeth after treatment with various mineral trioxide aggregate–based materials: An ex vivo study. J. Endod. 2015, 41, 737–741. [Google Scholar] [CrossRef]
- CIE2000 Calculator. Available online: http://colormine.org/delta-e-calculator/Cie2000 (accessed on 26 July 2022).
- Gorodylova, N.; Dohnalová, Ž.; Šulcová, P. High Energy Milling of Zirconia: A Systematic Critical Review on the Phase Transformation. Adv. Sci. Technol. 2014, 87, 6–11. [Google Scholar]
- Johnston, W.M.; Kao, E.C. Assessment of appearance match by visual observation and clinical colorimetry. J. Dent. Res. 1989, 68, 819–822. [Google Scholar] [CrossRef]
- Camilleri, J. Staining Potential of Neo MTA Plus, MTA Plus, and Biodentine Used for Pulpotomy Procedures. J. Endod. 2015, 41, 1139–1145. [Google Scholar] [CrossRef]
- Shokouhinejad, N.; Alikhasi, M.; Khoshkhounejad, M.; Pirmoazen, A. Effect of irrigation solutions on the coronal discoloration induced by mineral trioxide aggregate cements containing different radiopacifiers. Dent. Res. J. 2020, 17, 447–451. [Google Scholar]

| Milling Time | Pristine | 30 min | 1 h | 2 h | 3 h | |
|---|---|---|---|---|---|---|
| Material | ||||||
| Portland cement | 0.91 ± 0.14 | 1.00 ± 0.18 | N.A. | N.A. | N.A. | |
| Bi2O3 | 5.10 ± 0.36 | 5.69 ± 0.35 | 5.52 ± 0.48 | 5.59 ± 0.35 | 5.57 ± 0.24 | |
| ZrO2 | 2.31 ± 0.36 | 2.65 ± 0.23 | 2.61 ± 0.26 | 2.65 ± 0.26 | 2.69 ± 0.29 | |
| HfO2 | 3.13 ± 0.19 | 3.65 ± 0.48 | 3.58 ± 0.16 | 3.61 ± 0.36 | 3.59 ± 0.44 | |
| Ta2O5 | 3.28 ± 0.42 | 3.71 ± 0.30 | 3.65 ± 0.27 | 3.70 ± 0.34 | 3.75 ± 0.46 | |
| UV Exposure | 0 s | 5 s | 15 s | 1 min | 3 min | |
|---|---|---|---|---|---|---|
| Material | ||||||
| Portland cement | 0.00 | 0.38 ± 0.21 | 0.54 ± 0.09 | 0.53 ± 0.20 | 0.46 ± 0.33 | |
| Bi2O3 | 0.00 | 1.02 ± 0.22 | 1.21 ± 0.26 | 8.05 ± 0.48 | 13.48 ± 1.11 | |
| ZrO2 | 0.00 | 0.32 ± 0.08 | 0.56 ± 0.09 | 0.44 ± 0.12 | 0.58 ± 0.29 | |
| HfO2 | 0.00 | 0.49 ± 0.18 | 0.81 ± 0.01 | 0.75 ± 0.22 | 0.71 ± 0.15 | |
| Ta2O5 | 0.00 | 0.73 ± 0.25 | 0.76 ± 0.41 | 0.72 ± 0.36 | 0.68 ± 0.39 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.-N.; Wang, L.-C.; Chen, M.-S.; Chang, P.-J.; Lin, P.-Y.; Fang, A.; Chen, C.-Y.; Lee, P.-Y.; Lin, C.-K. Discoloration Improvement by Mechanically-Milled Binary Oxides as Radiopacifier for Mineral Trioxide Aggregates. Materials 2022, 15, 7934. https://doi.org/10.3390/ma15227934
Lin H-N, Wang L-C, Chen M-S, Chang P-J, Lin P-Y, Fang A, Chen C-Y, Lee P-Y, Lin C-K. Discoloration Improvement by Mechanically-Milled Binary Oxides as Radiopacifier for Mineral Trioxide Aggregates. Materials. 2022; 15(22):7934. https://doi.org/10.3390/ma15227934
Chicago/Turabian StyleLin, Hsiu-Na, Ling-Chi Wang, May-Show Chen, Pei-Jung Chang, Pin-Yu Lin, Alex Fang, Chin-Yi Chen, Pee-Yew Lee, and Chung-Kwei Lin. 2022. "Discoloration Improvement by Mechanically-Milled Binary Oxides as Radiopacifier for Mineral Trioxide Aggregates" Materials 15, no. 22: 7934. https://doi.org/10.3390/ma15227934
APA StyleLin, H.-N., Wang, L.-C., Chen, M.-S., Chang, P.-J., Lin, P.-Y., Fang, A., Chen, C.-Y., Lee, P.-Y., & Lin, C.-K. (2022). Discoloration Improvement by Mechanically-Milled Binary Oxides as Radiopacifier for Mineral Trioxide Aggregates. Materials, 15(22), 7934. https://doi.org/10.3390/ma15227934









