Mechanosynthesis of High-Nitrogen Steels Strengthened by Secondary Titanium Nitrides
Abstract
1. Introduction
2. Experimental Procedure
- “A”: = Fe–2Ti + CrN;
- “B”: = Fe + CrN;
- “C”: = Fe–6Ni + CrN;
- “D”: = Fe–5Ti + Mn2N.
| Formula of Composition | PBM 10 h | Annealing at 500 °C, 4 h after PBM | ||||
|---|---|---|---|---|---|---|
| Amount of Austenite vol.% | Content of Nitrogen in Austenite, cN, at.% | Content of Alloying Metal in Ferrite, cMe, at.% | Amount of Austenite vol.% | Content of Nitrogen in Austenite, cN, at.% | Content of Alloying Metal in Ferrite, cMe, at.% | |
| Fe–2Ti + CrN | 0 | – | cTi + Cr = 6.2 | 0 | – | cCr = 5.0 |
| Fe + CrN | 7 | 9 | cCr = 3.9 | 0 | – | cCr = 1.7 |
| Fe–6Ni + CrN | 10 | 7 | cCr = 4.6 | 9 | 2 | cCr = 0.8 |
| Fe–5Ti + Mn2N | >70 | 1.3 | cTi + Mn = 12 | >70 | 0.5 | cMn = 4.1 |
| cMn = 10 | ||||||
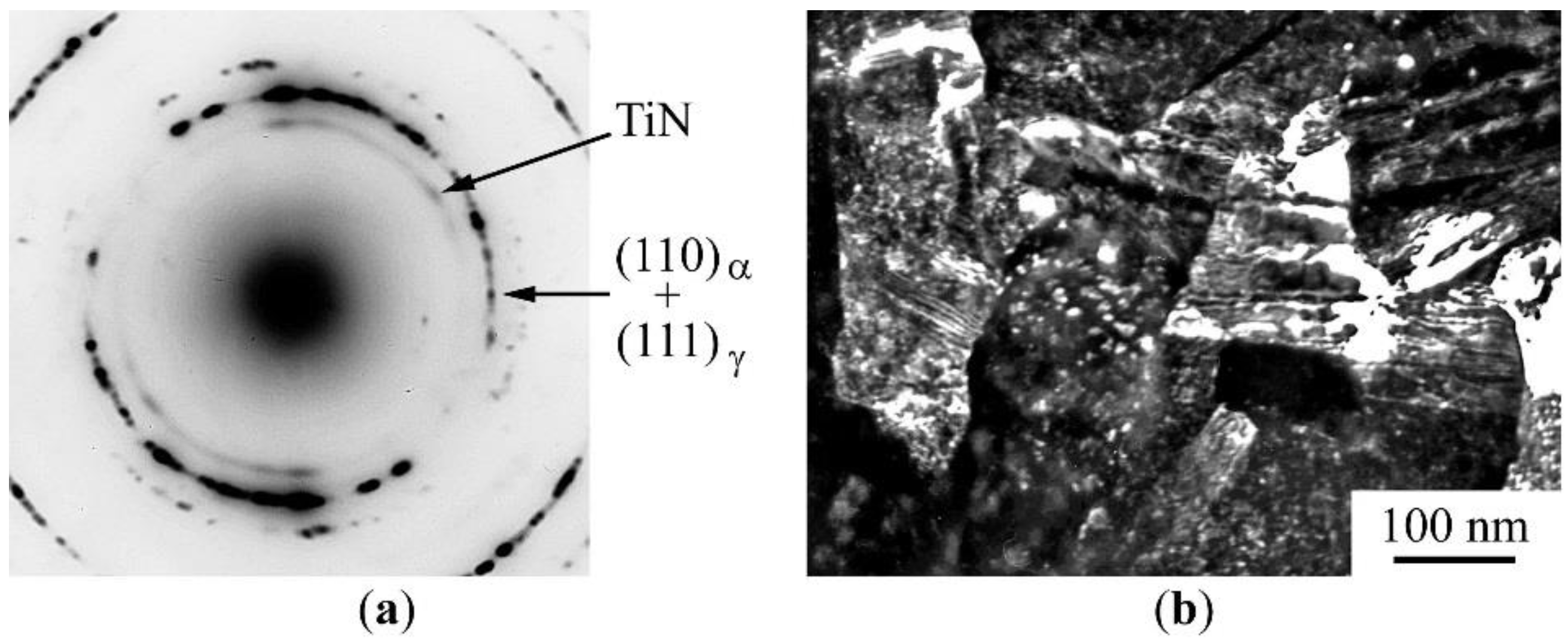
3. Results of the Experiments
3.1. Mössbauer Analysis of the Phase and Concentration Composition of the Mechanically Synthesized Steels
3.2. Mechanical Synthesis to Produce Fe–Cr–Ti–N Ferrite-Martensitic Steel
3.3. Mechanical Synthesis of Fe–Mn–Ti–N Austenitic Steel
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berns, H.; Gavljuk, V.; Riedner, S. High Interstitial Stainless Austenitic Steels; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Rashev, T.V. High Nitrogen Steels and Metallurgy under Pressure; Bulgarien Academy of Science: Sofia, Bulgaria, 1995. [Google Scholar]
- Simmons, J.W. Overview: High-nitrogen alloying of stainless steels. Mater. Sci. Eng. A 1996, 207, 159–169. [Google Scholar] [CrossRef]
- Goldschmidt, H.J. Interstitial Alloys; Butterworth-Heinemann: London, UK, 1967. [Google Scholar]
- Menthe, E.; Rie, K.-T.; Schultze, J.W.; Simson, S. Structure and properties of plasma nitrided stainless steel. Surf. Coat. Technol. 1995, 74–75, 412–416. [Google Scholar] [CrossRef]
- Shabashov, V.A.; Gavrilov, N.V.; Kozlov, K.A.; Makarov, A.V.; Titova, S.G.; Voronin, V.I. Structure of the surface layers of metastable austenitic stainless steel nitrided in electron beam plasma. Phys. Met. Metallograph. 2018, 119, 755–763. [Google Scholar] [CrossRef]
- Biro, A.S. Trends of nitriding processes. Prod. Processes Syst. 2013, 6, 57–66. [Google Scholar]
- Narkevich, N.A.; Deryugin, E.E.; Perevalova, O.B.; Vlasov, I.V. Effect of ultrasonic forging strain processing on the surface layer microstructure and temperature-dependent mechanical properties of high nitrogen austenitic steel. Mater. Sci. Eng. A 2022, 834, 142590. [Google Scholar] [CrossRef]
- Bae, J.W.; Asghari-Rad, P.; Amanov, A.; Kim, H.S. Gradient-structured ferrous medium-entropy alloys with enhanced strength-ductility synergy by ultrasonic nanocrystalline surface modification. Mater. Sci. Eng. A 2021, 826, 141966. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Estrin, Y.; Horita, Z.; Langdon, T.G.; Zechetbauer, M.J.; Zhu, Y.T. Producing bulk ultrafine-grained materials by severe plastic deformation. JOM 2006, 58, 33–39. [Google Scholar] [CrossRef]
- Meyers, M.A.; Mishra, A.; Benson, D.J. Mechanical properties of nanocrystalline materials. Progr. Mat. Sci. 2006, 51, 427–556. [Google Scholar] [CrossRef]
- Estrin, Y.; Vinogradov, A. Extreme grain refinement by severe plastic deformation: A wealth of challenging science. Acta Mater. 2013, 61, 782–817. [Google Scholar] [CrossRef]
- Li, J.G.; Umemoto, M.; Todaka, Y.; Fujisaku, K.; Tsuchiya, K. The dynamic phase transformation and formation of nanocrystalline structure in SUS304 austenitic stainless steel subjected to high pressure torsion. Rev. Adv. Mater. Sci. 2008, 18, 577–582. [Google Scholar]
- Tsuchiyama, T.; Uchida, H.; Kataoka, K.; Takaki, S. Fabrication of fine-grained high nitrogen austenitic steels through mechanical alloying treatment. ISIJ Int. 2002, 42, 1438–1443. [Google Scholar] [CrossRef][Green Version]
- Shabashov, V.A.; Kozlov, K.A.; Lyashkov, K.A.; Litvinov, A.V.; Dorofeev, G.A.; Titova, S.G. Solid-phase mechanical alloying of BCC iron alloys by nitrogen in ball mills. Defect Diffus. Forum 2012, 330, 25–37. [Google Scholar] [CrossRef]
- Dorofeev, G.A.; Lubnin, A.N.; Ulyanov, A.L.; Mukhgalin, V.V. Accelerated mechanosynthesis of high-nitrogen stainless steel: Mössbauer and X-ray diffraction studies. Bull. Russ. Acad. Sci. Phys. 2017, 81, 803–806. [Google Scholar] [CrossRef]
- Shabashov, V.A.; Korshunov, L.G.; Sagaradze, V.V.; Kataeva, N.V.; Zamatovsky, A.E.; Litvinov, A.V.; Lyashkov, K.A. Mössbauer analysis of deformation dissolution of the products of cellular decomposition in high-nitrogen chromium manganese austenite steel. Philos. Mag. 2014, 94, 668–682. [Google Scholar] [CrossRef]
- Shabashov, V.A.; Borisov, S.V.; Zamatovsky, A.E.; Vildanova, N.F.; Mukoseev, A.G.; Litvinov, A.V.; Shepatkovsky, O.P. Deformation induced transformations in nitride layers formed in bcc iron. Mater. Sci. Eng. A 2007, 452–453, 575–583. [Google Scholar] [CrossRef]
- Shabashov, V.A.; Kozlov, K.; Lyashkov, K.A.; Kataeva, N.V.; Litvinov, A.V.; Sagaradze, V.V.; Zamatovskii, A.E. Solid-state mechanical synthesis of austenitic Fe–Ni–Cr–N alloys. Phys. Met. Metallograph. 2014, 115, 392–402. [Google Scholar] [CrossRef]
- Shabashov, V.; Lyashkov, K.; Kozlov, K.; Zavalishin, V.; Kataeva, N.; Sagaradze, V.; Ustyugov, Y. Critical redistribution of nitrogen in the austenitic Cr–Mn steel under severe plastic deformation. Materials 2021, 14, 7116. [Google Scholar] [CrossRef]
- Lyashkov, K.; Shabashov, V.; Zamatovskii, A.; Kozlov, K.; Kataeva, N.; Novikov, E.; Ustyugov, Y. Structure-phase transformations in the course of solid-state mechanical alloying of high-nitrogen chromium-manganese steels. Metals 2021, 11, 301. [Google Scholar] [CrossRef]
- Shabashov, V.A.; Sagaradze, V.V.; Litvinov, A.V.; Mukoseev, A.G.; Vildanova, N.F. Mechanical synthesis in the iron oxide-metal system. Mat. Sci. Eng. A 2005, 392, 62–72. [Google Scholar] [CrossRef]
- Shabashov, V.A.; Litvinov, A.V.; Sagaradze, V.V.; Kozlov, K.A.; Vil’danova, N.F. Mechanosynthesis of ODS alloys with an FCC lattice on the basis of the Fe–Ni system. Phys. Met. Metallograph. 2008, 105, 157–167. [Google Scholar] [CrossRef]
- Sagaradze, V.V.; Litvinov, A.V.; Shabashov, V.A.; Vil’danova, N.F.; Mukoseev, A.G.; Kozlov, K.A. New method of mechanical alloying of ODS steels using iron oxides. Phys. Met. Metallograph. 2006, 101, 566–576. [Google Scholar] [CrossRef]
- Kozlov, K.; Sagaradze, V.; Kataeva, N.; Afanasyev, S.; Shabashov, V.; Chernov, I. Oxide strengthening of iron oxidized with air. J. Mat. Eng. Perform. 2020, 29, 7722–7727. [Google Scholar] [CrossRef]
- Feichtinger, H.K.; Plazza, D.; Lustenberg, M.; Schmid, F. Nitrogen steel as a functional material for electrical and magnetic applications. High Nitrogen Steels 93. Proc. 3rd Inter. Conf. Kiev. 1993, 2, 586. [Google Scholar]
- Yuan, Z.; Pan, L.; Jiang, S.; Wang, L.; Zuo, M.; Zhao, D. TiN-Al composite coatings on A356 alloy by mechanical alloying. Mater. Sci. Forum 2017, 898, 1359–1368. [Google Scholar] [CrossRef]
- Wang, R.; Wang, W.; Zhu, G.; Pan, W.; Zhou, W.; Wang, D.; Li, F.; Huang, H.; Jia, Y.; Du, D.; et al. Microstructure and mechanical properties of the TiN particles reinforced IN718C composite. J. Alloys Compd. 2018, 762, 237–245. [Google Scholar] [CrossRef]
- Gavriljuk, V.G.; Berns, H. High Nitrogen Steel: Structure, Properties, Manufacture, Applications; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Kirchner, G.; Uhrenins, B. Experimental study of the ferrite/austenite equilibrium in the Fe–Cr–Mn system and the optimization of thermodynamic parameters by means of a general mathematic method. Acta Metall. 1974, 22, 523–532. [Google Scholar] [CrossRef]
- Dorofeev, G.A.; Karev, V.; Goncharov, O.; Kuzminykh, E.; Sapegina, I.; Lubnin, A.; Mokrushina, M.; Lad’Yanov, V. Aluminothermic reduction process under nitrogen gas pressure for preparing high nitrogen austenitic steels. Met. Mater. Trans. B 2019, 50, 632–640. [Google Scholar] [CrossRef]
- Dorofeev, G.A.; Sapegina, I.V.; Lad’Yanov, V.I.; Pushkarev, B.E.; Pechina, E.A.; Prokhorov, D.V. Mechanical alloying and severe plastic deformation of nanocrystalline high-nitrogen stainless steels. Phys. Met. Metallograph. 2012, 113, 963–973. [Google Scholar] [CrossRef]
- Rusakov, V.S.; Kadyrzhanov, K.K. Mössbauer Spectroscopy of locally inhomogeneous systems. Hyperfine Interact. 2005, 164, 87–97. [Google Scholar] [CrossRef]
- Van der Woude, F.; Sawatzky, G.A. Mössbauer effect in iron and dilute iron based alloys. Phys. Rep. 1974, 12, 335–374. [Google Scholar] [CrossRef]
- Vincze, I.; Campbell, I.A. Mössbauer measurements in iron based alloys with transition metals. J. Phys. F Met. Phys. 1973, 3, 647–663. [Google Scholar] [CrossRef]
- Dubiel, S.M.; Zukrowski, J. Distribution of Cr atoms in a strained and strain-relaxed Fe89.15Cr10.75 alloy: A Mössbauer effect study. Philos. Mag. Lett. 2017, 97, 386–392. [Google Scholar] [CrossRef]
- Moriya, T.; Sumitomo, Y.; Ino, H.; Fujita, F.E.; Maeda, Y. Mossbauer effect in iron-nitrogen alloys and compounds. J. Phys. Soc. Jpn. 1973, 35, 1378–1385. [Google Scholar] [CrossRef]
- Renot, R.C.; Swartzendruber, L.J. Origin of Mössbauer linewidth in stainless steel. AIP Conf. Proc. 1973, 10, 1350–1353. [Google Scholar] [CrossRef]
- Srivastava, B.P.; Sarma, H.N.K.; Bhattacharya, D.L. Quadrupole splitting in deformed stainless steel. Phys. Status Solidi A 1972, 10, K117–K118. [Google Scholar] [CrossRef]
- Oda, K.; Umezu, K.; Ino, H. Interaction and arrangement of nitrogen atoms in FCC γ-iron. J. Phys. Condens. Matter. 1990, 2, 10147–10158. [Google Scholar] [CrossRef]
- Ino, H.; Umezu, K.; Kajiwara, S.; Uehara, S. Interstitial solute atom configuration in Fe–N and Fe–C based austenite and relation to the abnormal tetragonality of fresh martensite. In Proceedings of the International Conference on Martensitic Transformations (ICOMAT-86), Nara, Japan, 26–30 August 1986; The Japan Institute of Metals: Sendai, Japan, 1986; pp. 313–318. [Google Scholar]
- Eickel, K.H.; Pitsch, W. Magnetic properties of the hexagonal iron nitride ε-Fe3.2N. Phys. Stat. Sol. 1970, 39, 121–129. [Google Scholar] [CrossRef]
- Kurian, S.; Gajbhiye, N.S. Magnetic and Mossbauer study of ε-FeyN (2 < y < 3) nanoparticles. J. Nanoparticle Res. 2010, 12, 1197–1209. [Google Scholar] [CrossRef]
- Kim, K.-J.; Sumiyama, K.; Onodera, H.; Suzuki, K. Structure and magnetic properties of mechanically ground ε-Fe2.3N. Jpn. J. Appl. Phys. 1994, 33, 6539–6541. [Google Scholar] [CrossRef]
- Shabashov, V.A.; Sagaradze, V.V.; Kozlov, K.A.; Ustyugov, Y.M. Atomic order and submicrostructure in iron alloys at megaplastic deformation. Metals 2018, 8, 995. [Google Scholar] [CrossRef]
- Shelekhov, E.V.; Tcherdyntsev, V.V.; Pustov, L.Y.; Kaloshkin, S.D.; Tomilin, I.A. Calculation of energy intensity and temperature of mechanoactivation process in planetary ball mill by computer simulation. Investig. Appl. Sev. Plast. Deformation. NATO Sci. 2000, 80, 139–146. [Google Scholar] [CrossRef]
- Shabashov, V.A.; Afanasiev, S.V.; Zavalishin, V.A.; Korshunov, L.G.; Borisov, S.V.; Litvinov, A.V.; Zamatovsky, A.E.; Semionkin, V.A. Implementation of megaplastic deformation for control of the gradient composition of pseudo-layers in the nitride surface of Fe–Ni–Cr steel—Production of quasi-bimetallic plate. Defect Diffus. Forum. 2016, 371, 56–59. [Google Scholar] [CrossRef]
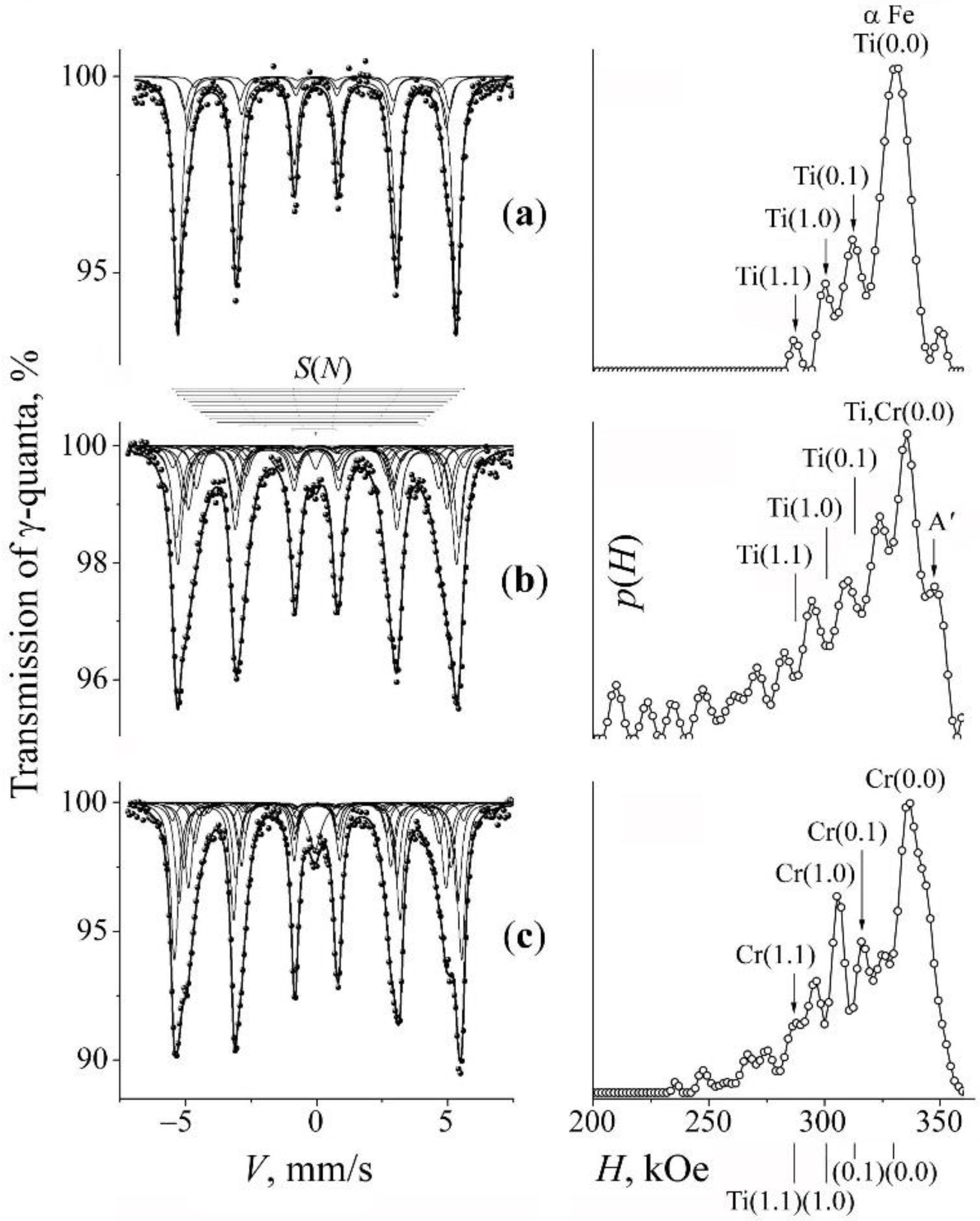



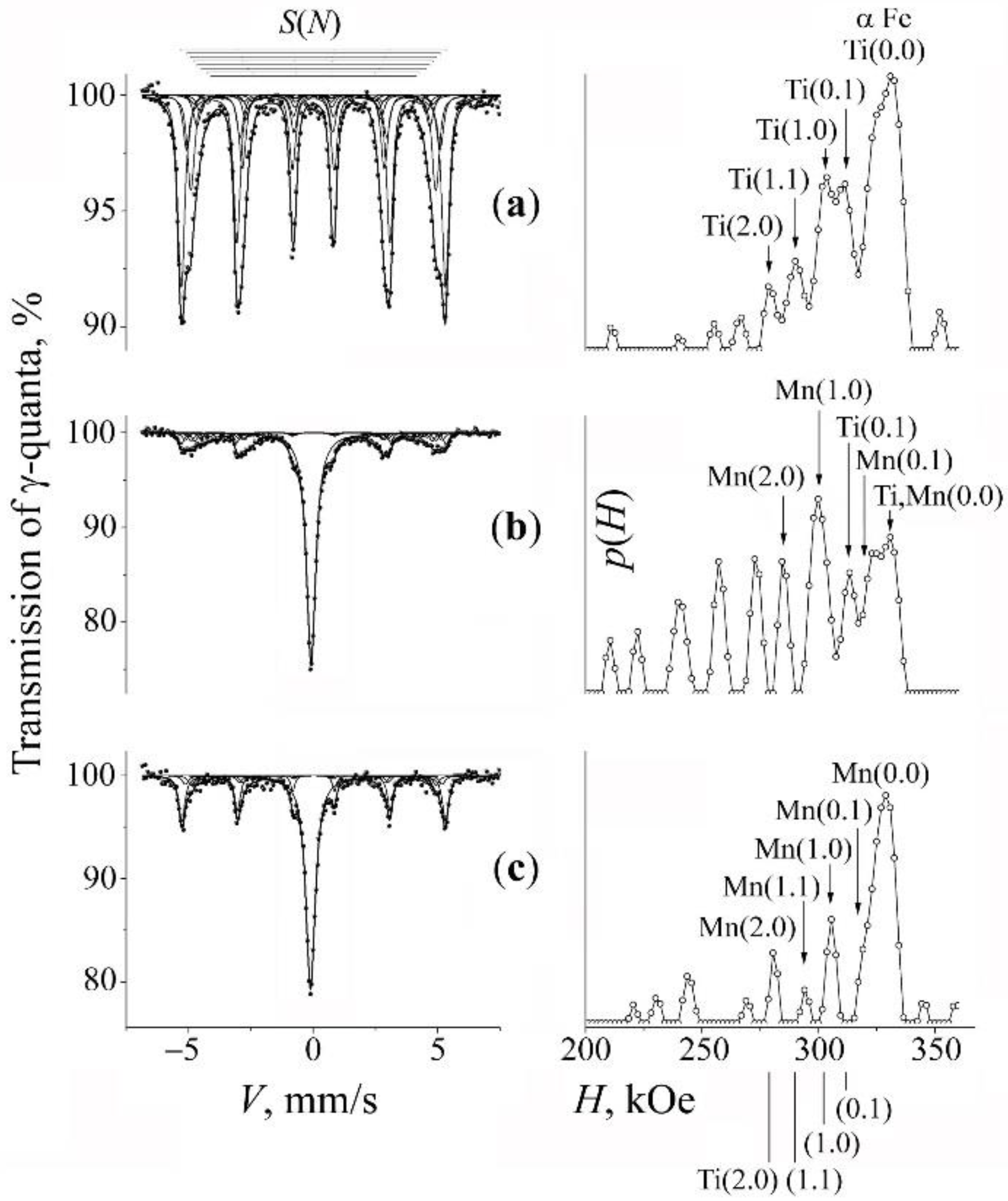

| Formula of Binary Alloys in Mixtures | Hyperfine Effective Fields H(m,n) | Formula of Nitride | Heat of Formation, kcal/g·atom | ||
|---|---|---|---|---|---|
| H(0,0) | H(1,0) | H(0,1) | |||
| Armco-Fe | 330 | – | Fe4N | –2.6 | |
| Fe–Ni | 331 | 325 | 325 | Ni3N | 0.2 |
| Fe–Mn | 330 | 305 | 320 | Mn2N | –48.2 |
| Fe–Cr | 335 | 304 | 311 | CrN/Cr2N | –30/–31 |
| Fe–Ti | 330 | 306 | 314 | TiN | −80.4 |
| Treatment | Expanded Singlet D(0) | Doublet D(1) | Doublet D(2) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IS, mm/s | G, mm/s | S, % | IS, mm/s | QS, mm/s | G, mm/s | S, % | IS, mm/s | G, mm/s | S, % | |
| PBM, 10 h | –0.07 | 0.38 | 77.4 | 0.02 | 0.37 | 0.21 | 6.0 | 0.22 | 0.78 | 3.0 |
| PBM, 10 h + anneal 500 °C | –0.08 | 0.38 | 82.6 | 0.02 | 0.36 | 0.20 | 2.5 | 0.20 | 0.76 | 1.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shabashov, V.; Lyashkov, K.; Zamatovskii, A.; Kozlov, K.; Kataeva, N.; Novikov, E.; Ustyugov, Y. Mechanosynthesis of High-Nitrogen Steels Strengthened by Secondary Titanium Nitrides. Materials 2022, 15, 5038. https://doi.org/10.3390/ma15145038
Shabashov V, Lyashkov K, Zamatovskii A, Kozlov K, Kataeva N, Novikov E, Ustyugov Y. Mechanosynthesis of High-Nitrogen Steels Strengthened by Secondary Titanium Nitrides. Materials. 2022; 15(14):5038. https://doi.org/10.3390/ma15145038
Chicago/Turabian StyleShabashov, Valery, Kirill Lyashkov, Andrey Zamatovskii, Kirill Kozlov, Natalya Kataeva, Evgenii Novikov, and Yurii Ustyugov. 2022. "Mechanosynthesis of High-Nitrogen Steels Strengthened by Secondary Titanium Nitrides" Materials 15, no. 14: 5038. https://doi.org/10.3390/ma15145038
APA StyleShabashov, V., Lyashkov, K., Zamatovskii, A., Kozlov, K., Kataeva, N., Novikov, E., & Ustyugov, Y. (2022). Mechanosynthesis of High-Nitrogen Steels Strengthened by Secondary Titanium Nitrides. Materials, 15(14), 5038. https://doi.org/10.3390/ma15145038







