Secondary Hardening of a High-N Ni-Free Stainless Steel
Abstract
1. Introduction
1.1. Deformation Mechanisms
1.2. Effect of Temperature on Mechanical Strength
1.3. Cr2N Precipitation
1.4. Aim of the Paper
2. Materials and Methods
2.1. Material and Treatment
2.2. Investigation Methods
3. Results
3.1. As-Received Material
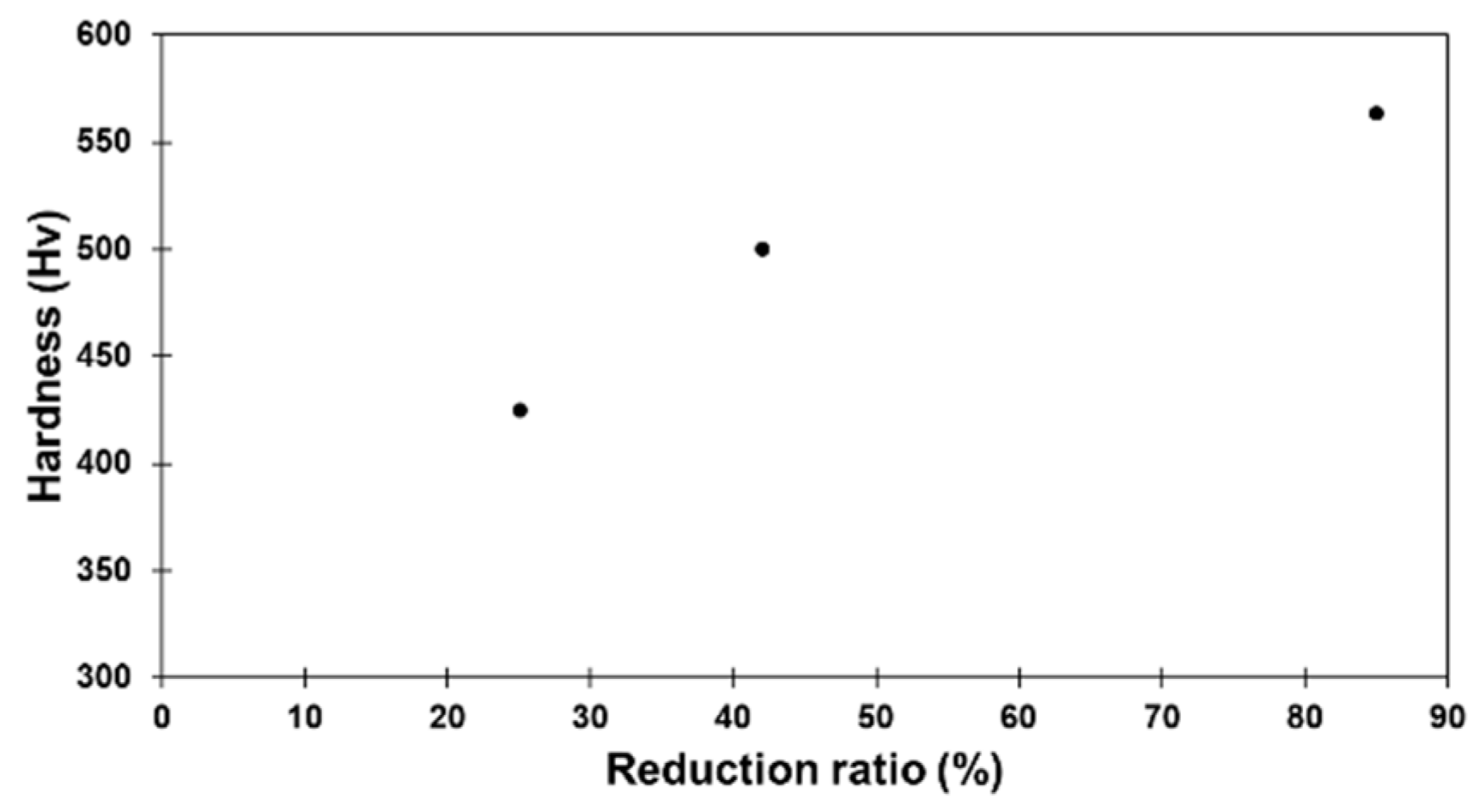
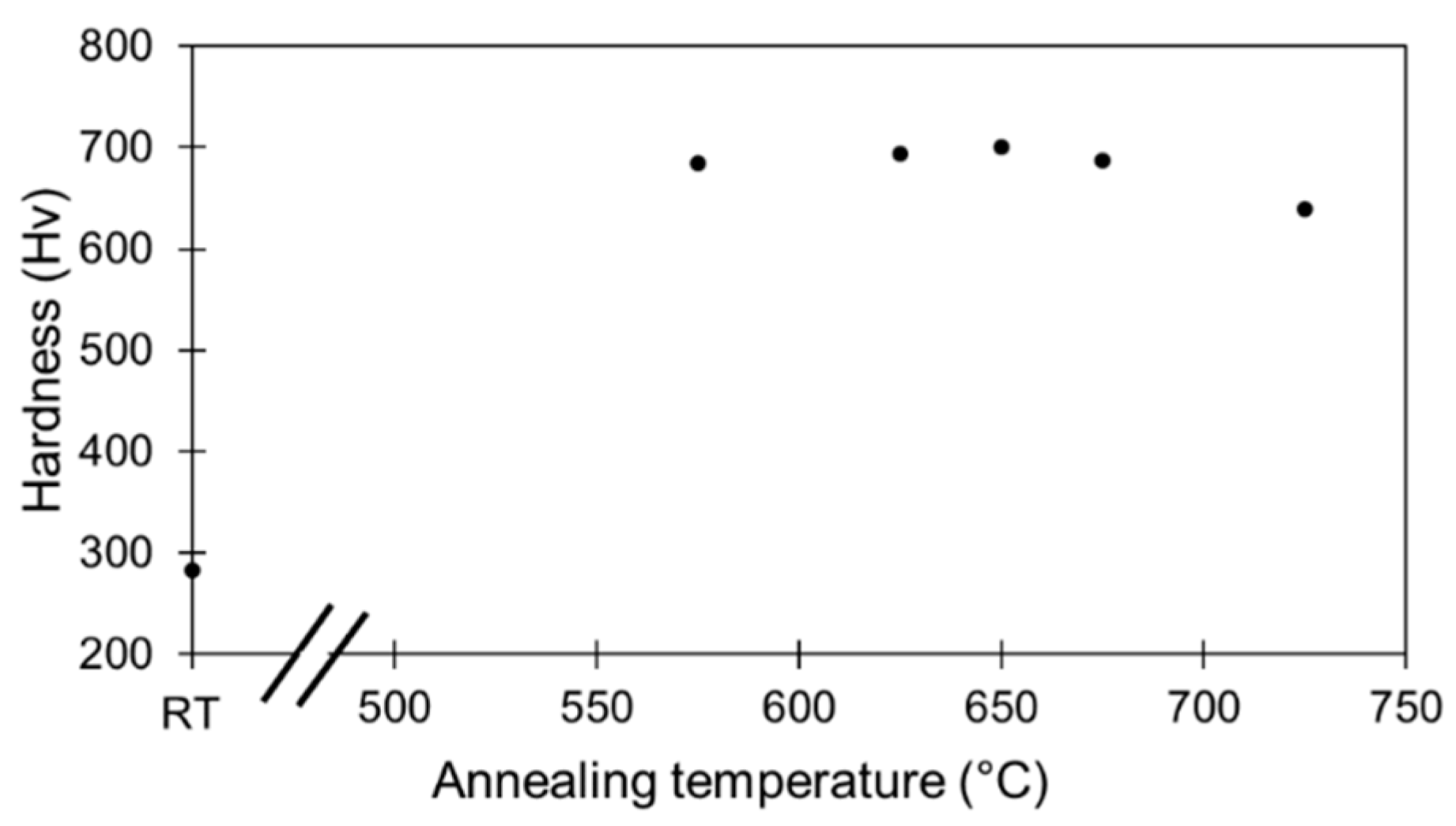
3.2. Evolution of Hardness with Deformation and Annealing Time
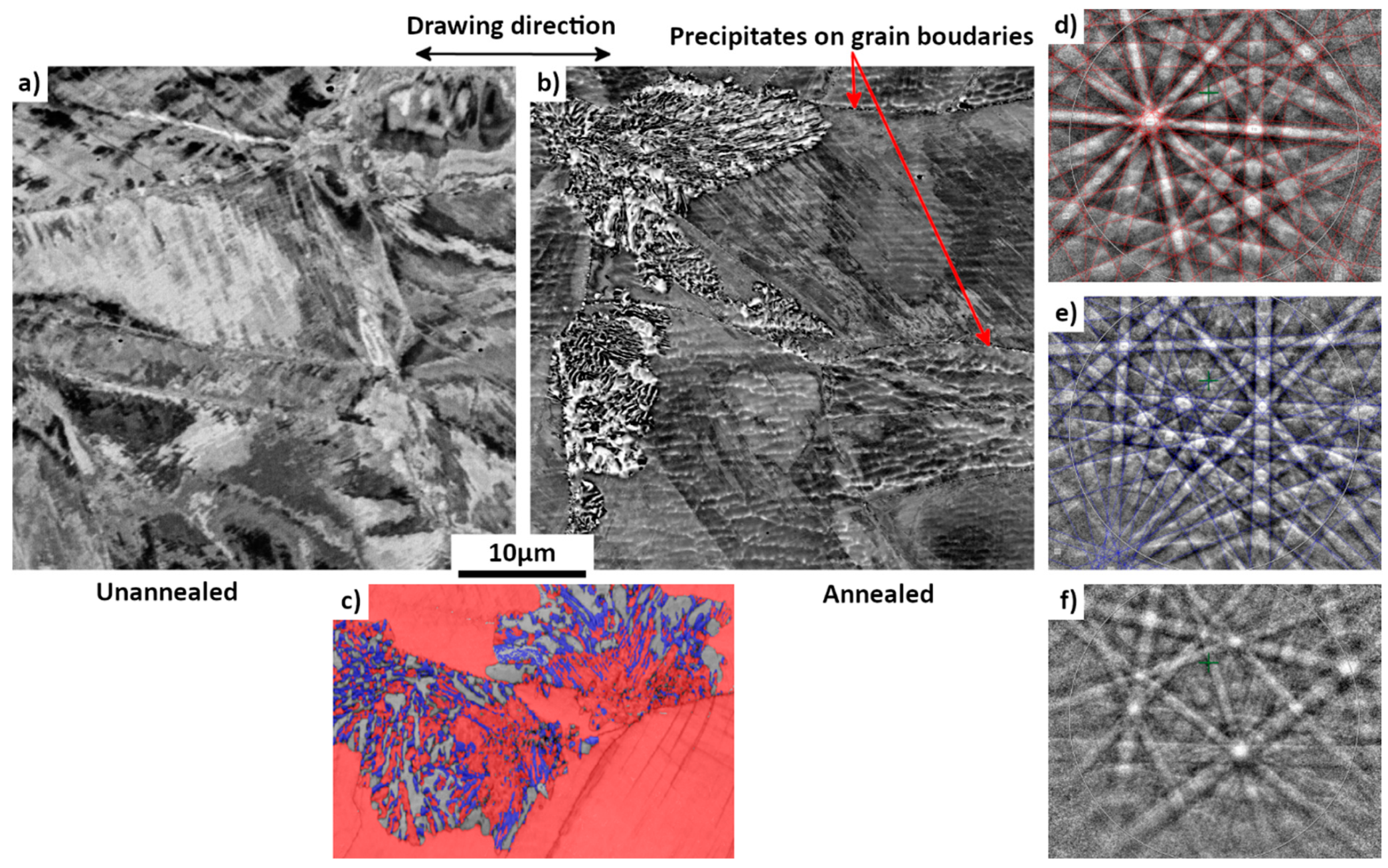
3.3. Microstructure: Evolution of As-Received Sample with Annealing Time
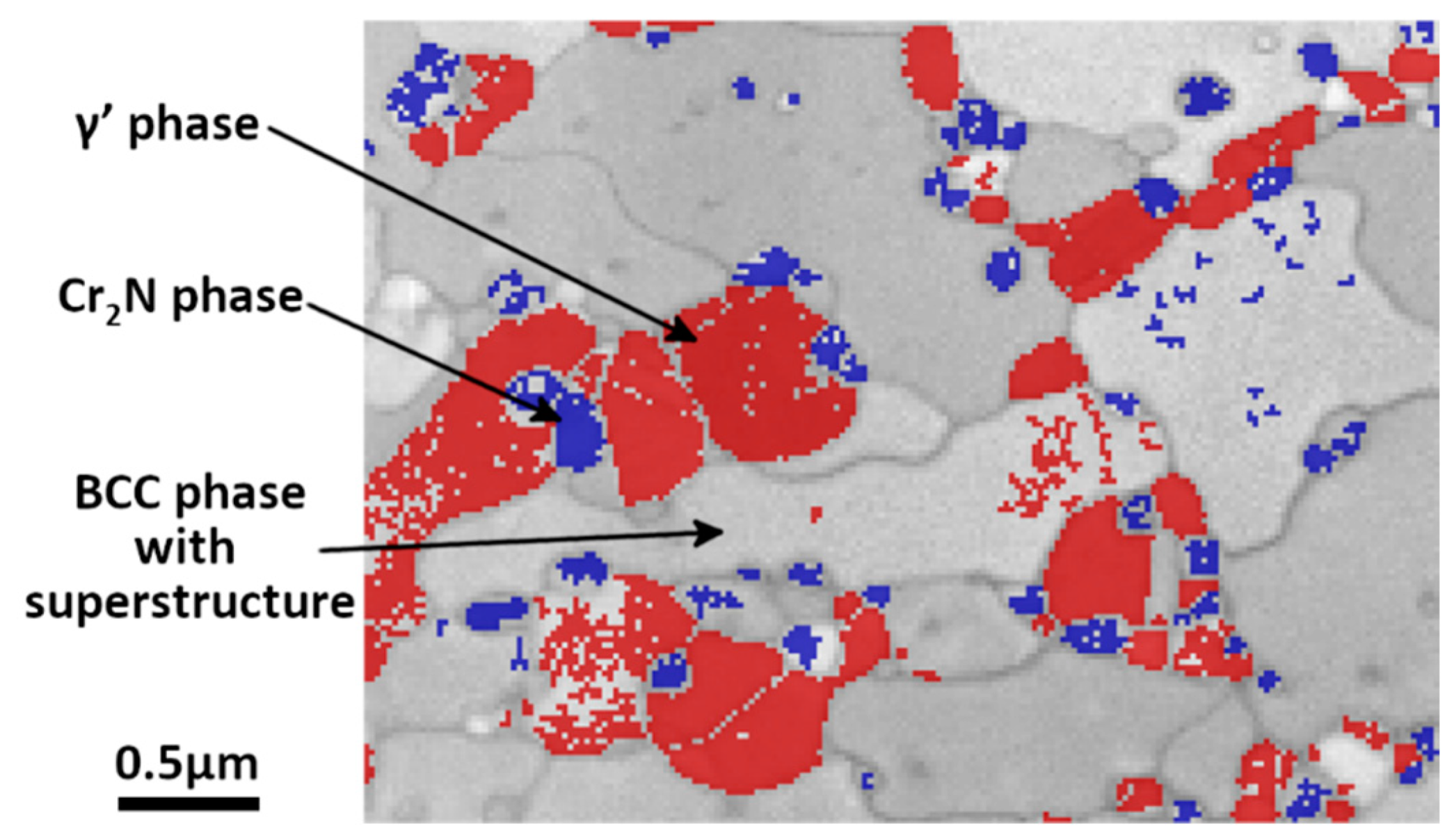
3.4. Microstructure of Highly Deformed Sample after Annealing
- (i)
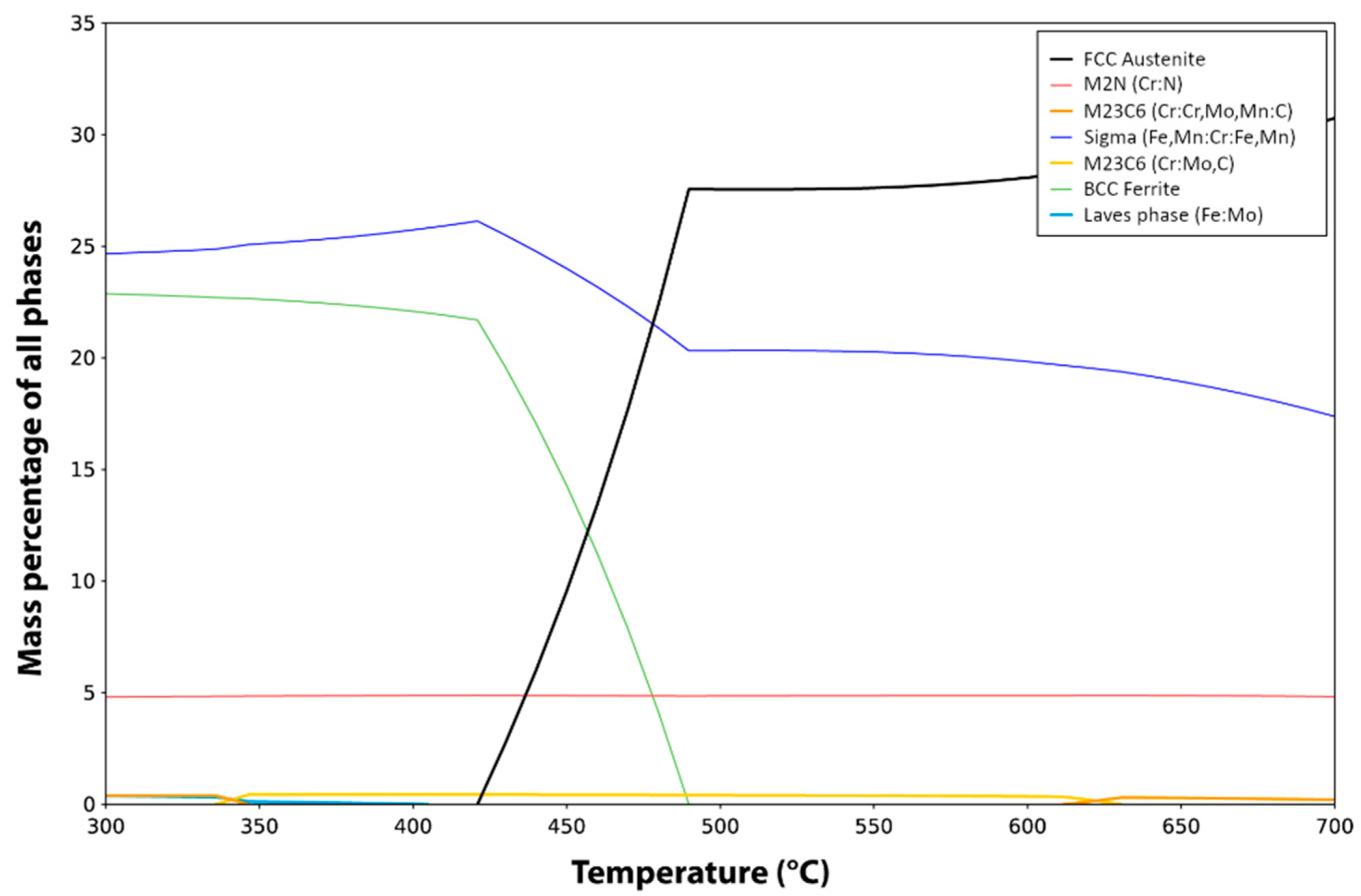
- Chromium nitride has a trigonal symmetry P1 m. According to chemistry and crystallography, these precipitates are identified as (CrxMny)2N, with a much higher quantity Cr, compared to the quantity of Mn. From the X-ray diffraction analysis, it is found that the lattice parameters are a = b = 0.477 nm and c = 0.4466 nm, instead of the theoretical parameters a = b = 0.48 nm and c = 0.4472 nm for the stoichiometric Cr2N. Although they are too small to be correctly chemically analyzed, one can estimate from the equilibrium calculi (Table 2) that these nitrides could be (Cr1.66Mn0.34Mo0.06)N.
- (ii)
- The observed γ’ austenite has an FCC structure with a lattice parameter equal to 0.3592 ± 0.0003 nm deduced from its X-ray diffraction spectrum. While the deformed initial austenite in un-annealed samples contains about 1 wt% nitrogen and has a lattice parameter equal to 0.363 nm, no (or very little) N was found in this austenite. The correlation between lattice parameter and the concentration of N is well documented for austenite (Figure 13 in [25]) and confirms that no (or very little) nitrogen remains in the γ’ austenite. Although the chemical analysis may be only semi-quantitative due to the smallness of the grains, this phase is assumed to contain between 13 wt% and 19 wt% Cr, about 19–21 wt% Mn and a balance of Fe. Further TEM examination showed that, in the γ’ phase, there are large numbers of dislocation arrays, as indicated in Figure 13.Equilibrium calculi confirm that almost no N is expected in this phase. The observed concentration of Mn is also in agreement with the equilibrium data. This concentration of Mn is not far from the initial austenite Mn concentration (22.8 wt%). However, a strong discrepancy is observed for Cr concentration: 7.2 wt% are expected according to the equilibrium calculi, whereas more than 13 wt% Cr were observed (concentration of Cr in the initial austenite is 21.8 wt%).
- (iii)
- The unknown phase is found to have a very complex crystal structure whose determination will be the focus of a separate study. It contains about 20–21 wt% Cr, 25 wt% Mn (slightly increased from the initial γ phase) and the balance of Fe. Almost no N was detected. Its crystal structure was further analyzed by TEM Selected Area Electron (SAED) diffraction, as shown in Figure 14. Diffraction patterns along more than five zone axes have been recorded and confirmed that the unknown phase possesses a BCC superstructure with the lattice parameter a of about 0.9 nm.
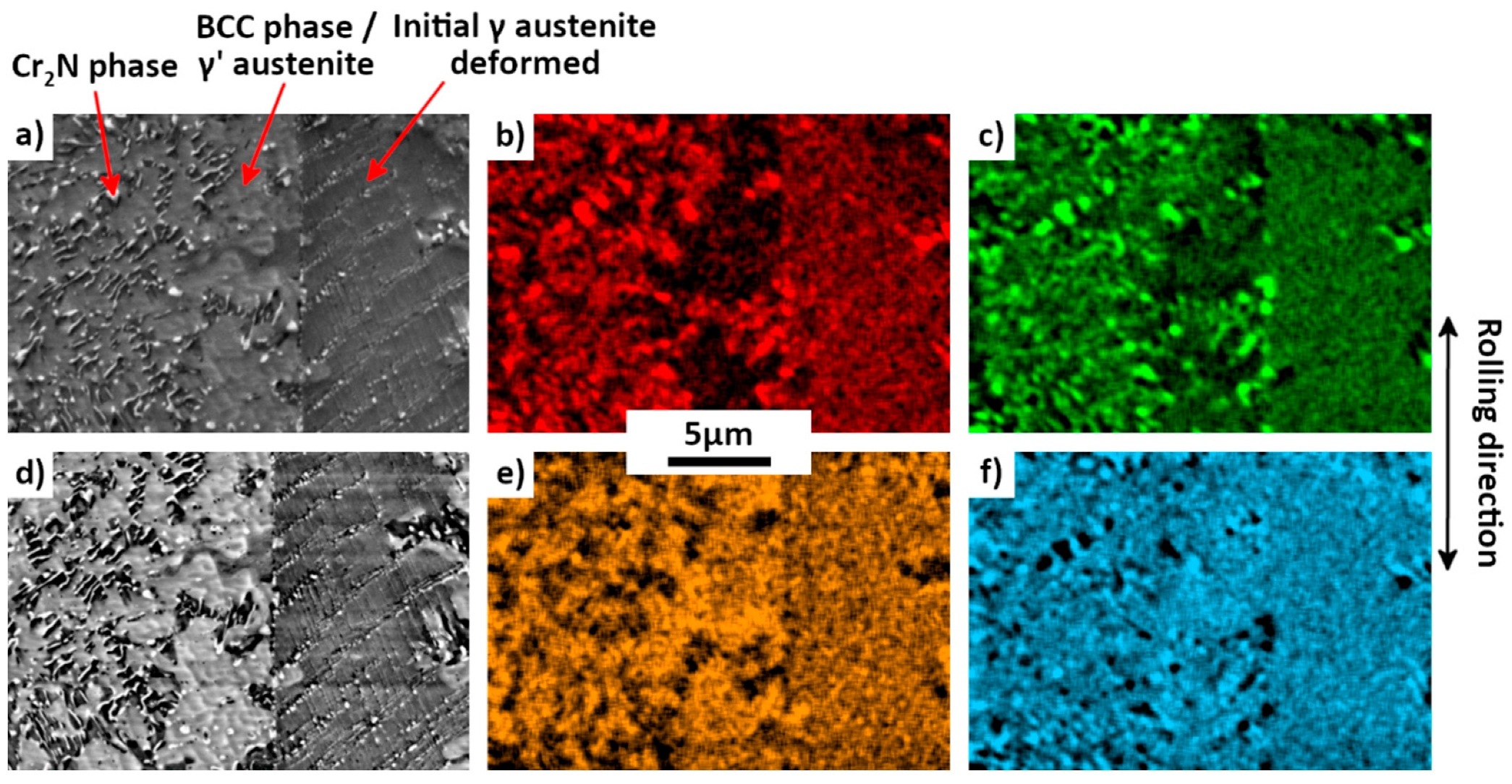
3.5. Microstructure: Evolution of Mediumly-Rolled Sample after Annealing
4. Discussion
5. Conclusions
- (1)
- A secondary hardening mechanism was identified for the Biodur 108© material, when annealed at 575 °C, after cold deformation.
- (2)
- When the material is cold-deformed and annealed, the creation of homogeneously distributed globular Cr2N is observed. For a long annealing time, these precipitates were also created onto deformation bands. They are assumed to be mainly responsible for the secondary hardening.
- (3)
- A new superstructured BCC phase is discovered. Its crystalline structure is complex and will be the focus of a forthcoming paper. This phase is almost free of N and enriched in Mn.
- (4)
- Together with the very small globular Cr2N precipitates and the small grains of the BCC unknown phase, very small grains of an austenite with a very low concentration of N are observed in the rolled and annealed samples.
- (5)
- The nitrogenous perlite observed after long-time annealing at 575 °C of a low-rolled sample, is constituted of three types of lamellae: Cr2N, unknown BCC phase and austenite.
- (6)
- The secondary hardening is then explained by the combination of several mechanisms:
- −
- the creation of small and very small Cr2N precipitates.
- −
- the submicronic (or few microns) size of the new phases.
- −
- the fact that the main part of the newly created microstructure is the superstructured phase.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uggowitzer, P.; Magdowski, R.; Speidel, M.O. Nickel Free High Nitrogen Austenitic Steels. ISIJ Int. 1996, 36, 901–908. [Google Scholar] [CrossRef]
- Li, H.-B.; Jiang, Z.-H.; Zhang, Z.-R.; Xu, B.-Y.; Liu, F.-B. Mechanical Properties of Nickel Free High Nitrogen Austenitic Stainless Steels. J. Iron Steel Res. Int. 2007, 14, 330–334. [Google Scholar] [CrossRef]
- Jung, Y.-S.; Kang, S.; Jeong, K.; Jung, J.-G.; Lee, Y.-K. The effects of N on the microstructures and tensile properties of Fe–15Mn–0.6C–2Cr–xN twinning-induced plasticity steels. Acta Mater. 2013, 61, 6541–6548. [Google Scholar] [CrossRef]
- Balachandran, G.; Bhatia, M.L.; Ballal, N.B.; Rao, P.K. Influence of Thermal and Mechanical Processing on Room Temperature Mechanical Properties of Nickel Free High Nitrogen Austenitic Stainless Steels. ISIJ Int. 2000, 40, 501–510. [Google Scholar] [CrossRef]
- Talha, M.; Behera, C.; Sinha, O. Effect of nitrogen and cold working on structural and mechanical behavior of Ni-free nitrogen containing austenitic stainless steels for biomedical applications. Mater. Sci. Eng. C 2015, 47, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Ohashi, S.; Uematsu, Y. Recent Trends in the Production and Use of High Strength Stainless Steels. ISIJ Int. 1993, 33, 711–720. [Google Scholar] [CrossRef]
- Behjati, P.; Kermanpur, A.; Najafizadeh, A.; Baghbadorani, H.S.; Karjalainen, P.; Jung, J.-G.; Lee, Y.-K. Design of a new Ni-free austenitic stainless steel with unique ultrahigh strength-high ductility synergy. Mater. Des. 2014, 63, 500–507. [Google Scholar] [CrossRef]
- Behjati, P.; Kermanpur, A.; Najafizadeh, A.; Baghbadorani, H.S.; Karjalainen, P.; Jung, J.-G.; Lee, Y.-K. Effect of Nitrogen Content on Grain Refinement and Mechanical Properties of a Reversion-Treated Ni-Free 18Cr-12Mn Austenitic Stainless Steel. Met. Mater. Trans. A 2014, 45, 6317–6328. [Google Scholar] [CrossRef]
- Rasouli, D.; Kermanpur, A.; Ghassemali, E.; Najafizadeh, A. On the Reversion and Recrystallization of Austenite in the Interstitially Alloyed Ni-Free Nano/Ultrafine Grained Austenitic Stainless Steels. Met. Mater. Int. 2019, 25, 846–859. [Google Scholar] [CrossRef]
- Carpenter Technology, Technical Data Sheet. Available online: https://www.carpentertechnology.com/hubfs/7407324/Material%20Saftey%20Data%20Sheets/Biodur%20108.pdf (accessed on 9 September 2022).
- Patnaik, L.; Maity, S.R.; Kumar, S. Status of nickel free stainless steel in biomedical field: A review of last 10 years and what else can be done. Mater. Today Proc. 2020, 26, 638–643. [Google Scholar] [CrossRef]
- Gavriljuk, V.; Petrov, Y.; Shanina, B. Effect of nitrogen on the electron structure and stacking fault energy in austenitic steels. Scr. Mater. 2006, 55, 537–540. [Google Scholar] [CrossRef]
- Lee, T.-H.; Shin, E.; Oh, C.-S.; Ha, H.-Y.; Kim, S.-J. Correlation between stacking fault energy and deformation microstructure in high-interstitial-alloyed austenitic steels. Acta Mater. 2010, 58, 3173–3186. [Google Scholar] [CrossRef]
- Qi-Xun, D.; An-Dong, C.X.-N.W.; Xin-Min, L. Stacking fault energy of cryogenic austenitic steels. Chin. Phys. 2002, 11, 596–600. [Google Scholar] [CrossRef]
- Mosecker, L.; Saeed-Akbari, A. Nitrogen in chromium–manganese stainless steels: A review on the evaluation of stacking fault energy by computational thermodynamics. Sci. Technol. Adv. Mater. 2013, 14, 033001. [Google Scholar] [CrossRef] [PubMed]
- Saller, G.; Spiradek-Hahn, K.; Scheu, C.; Clemens, H. Microstructural evolution of Cr–Mn–N austenitic steels during cold work hardening. Mater. Sci. Eng. A 2006, 427, 246–254. [Google Scholar] [CrossRef]
- Molnár, D.; Lu, S.; Hertzman, S.; Engberg, G.; Vitos, L. Study of the alternative mechanism behind the constant strain hardening rate in high-nitrogen steels. Mater. Charact. 2020, 170, 110726. [Google Scholar] [CrossRef]
- Singh, B.B.; Sivakumar, K.; Bhat, T.B. Effect of cold rolling on mechanical properties and ballistic performance of nitrogen-alloyed austenitic steels. Int. J. Impact Eng. 2009, 36, 611–620. [Google Scholar] [CrossRef]
- Mosecker, L.; Pierce, D.; Schwedt, A.; Beighmohamadi, M.; Mayer, J.; Bleck, W.; Wittig, J. Temperature effect on deformation mechanisms and mechanical properties of a high manganese C+N alloyed austenitic stainless steel. Mater. Sci. Eng. A 2015, 642, 71–83. [Google Scholar] [CrossRef]
- Terazawa, Y.; Ando, T.; Tsuchiyama, T.; Takaki, S. Relationship between Work Hardening Behaviour and Deformation Structure in Ni-free High Nitrogen Austenitic Stainless Steels. Steel Res. Int. 2009, 7, 473–476. [Google Scholar] [CrossRef]
- Moon, J.; Lee, T.-H.; Shin, J.-H.; Lee, J.-W. Hot working behavior of a nitrogen-alloyed Fe–18Mn–18Cr–N austenitic stainless steel. Mater. Sci. Eng. A 2014, 594, 302–308. [Google Scholar] [CrossRef]
- Xu, L.-W.; Li, H.-B.; Jiang, Z.-H.; Cai, M.-H.; Jiao, W.-C.; Feng, H.; Zhang, S.-C.; Lu, P.-C. Hot Deformation Behavior of P550 Steels for Nonmagnetic Drilling Collars. Steel Res. Int. 2020, 91, 2000035. [Google Scholar] [CrossRef]
- Holm, H.; Uggowitzer, P.; Speidel, M. Influence of annealing temperature on the microstructure and mechanical properties of a high nitrogen containing austenitic stainless steel. Scr. Met. 1987, 21, 513–518. [Google Scholar] [CrossRef]
- Feng, S.; XiaoWu, L.; Yang, Q.; ChunMing, L. Effects of Cold Deformation and Aging Process on Precipitation Behavior and Mechanical Properties of Fe-18Cr-18Mn-0.63N High-Nitrogen Austenitic Stainless Steel. Steel Res. Int. 2013, 84, 1034–1039. [Google Scholar] [CrossRef]
- Vanderschaeve, F.; Taillard, R.; Foct, J. Discontinuous precipitation of Cr2N in a high nitrogen, chromium-manganese austenitic stainless steel. J. Mater. Sci. 1995, 30, 6035–6046. [Google Scholar] [CrossRef]
- Rasouli, D.; Kermanpur, A.; Najafizadeh, A. Developing high-strength, ductile Ni-free Fe–Cr–Mn–C–N stainless steels by interstitial-alloying and thermomechanical processing. J. Mater. Res. Technol. 2019, 8, 2846–2853. [Google Scholar] [CrossRef]
- Brodu, E.; Bouzy, E.; Fundenberger, J.-J.; Guyon, J.; Guitton, A.; Zhang, Y. On-axis TKD for orientation mapping of nanocrystalline materials in SEM. Mater. Charact. 2017, 130, 92–96. [Google Scholar] [CrossRef]
- Fundenberger, J.-J.; Morawiec, A.; Bouzy, E.; Lecomte, J.-S. Polycrystal orientation maps from TEM. Ultramicroscopy 2003, 96, 127–137. [Google Scholar] [CrossRef]










| C | Mn | Cr | N | Mo | Si | Fe | |
|---|---|---|---|---|---|---|---|
| Certified Composition wt % | 0.042 | 22.76 | 21.34 | 1.03 | 0.71 | 0.16 | Bal. |
| Measured by SEM-EDX wt(%) | 22.8 ± 0.4 | 21.8 ± 0.4 | 1.1 ± 0.7 | 0.72 ± 0.03 | 0.27 | Bal. |
| Phase | Mass Fraction | Volume Fraction | Mn | Cr | N | Mo | Si | Fe |
|---|---|---|---|---|---|---|---|---|
| austenite γ′ | 52.27% | 50.85% | 20.7 | 7.2 | 0.007 | 0.09 | 0.3 | 71.7 |
| σ phase | 37.84% | 36.70% | 27.9 | 28.4 | 0 | 0.3 | 0.009 | 43.4 |
| Cr2N type | 9.18% | 11.74% | 15.0 | 69.3 | 11.2 | 4.5 | 0 | 0 |
| Phase | Mn | Cr | N | Fe | |
|---|---|---|---|---|---|
| austenite γ | initial composition | 22.76 | 21.34 | 1.03 | 53.96 |
| austenite γ’ | Measured (semi-quantitative) | 19–21 | 13–19 | ≈0 | Bal. |
| Equilibrium calculi | 20.7 | 7.2 | 0.007 | 71.7 | |
| unknown BCC phase | Measured (semi-quantitative) | 25 | 20–21 | ≤0.06 * | Bal. |
| σ phase | Equilibrium calculi | 27.9 | 28.4 | 0 | 43.4 |
| Cr2N type | Equilibrium calculi | 15.0 | 69.3 | 11.2 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siredey-Schwaller, N.; Charbonnier, P.; Zhang, Y.; Guyon, J.; Perroud, O.; Laheurte, P. Secondary Hardening of a High-N Ni-Free Stainless Steel. Materials 2022, 15, 7505. https://doi.org/10.3390/ma15217505
Siredey-Schwaller N, Charbonnier P, Zhang Y, Guyon J, Perroud O, Laheurte P. Secondary Hardening of a High-N Ni-Free Stainless Steel. Materials. 2022; 15(21):7505. https://doi.org/10.3390/ma15217505
Chicago/Turabian StyleSiredey-Schwaller, Nathalie, Pierre Charbonnier, Yudong Zhang, Julien Guyon, Olivier Perroud, and Pascal Laheurte. 2022. "Secondary Hardening of a High-N Ni-Free Stainless Steel" Materials 15, no. 21: 7505. https://doi.org/10.3390/ma15217505
APA StyleSiredey-Schwaller, N., Charbonnier, P., Zhang, Y., Guyon, J., Perroud, O., & Laheurte, P. (2022). Secondary Hardening of a High-N Ni-Free Stainless Steel. Materials, 15(21), 7505. https://doi.org/10.3390/ma15217505







