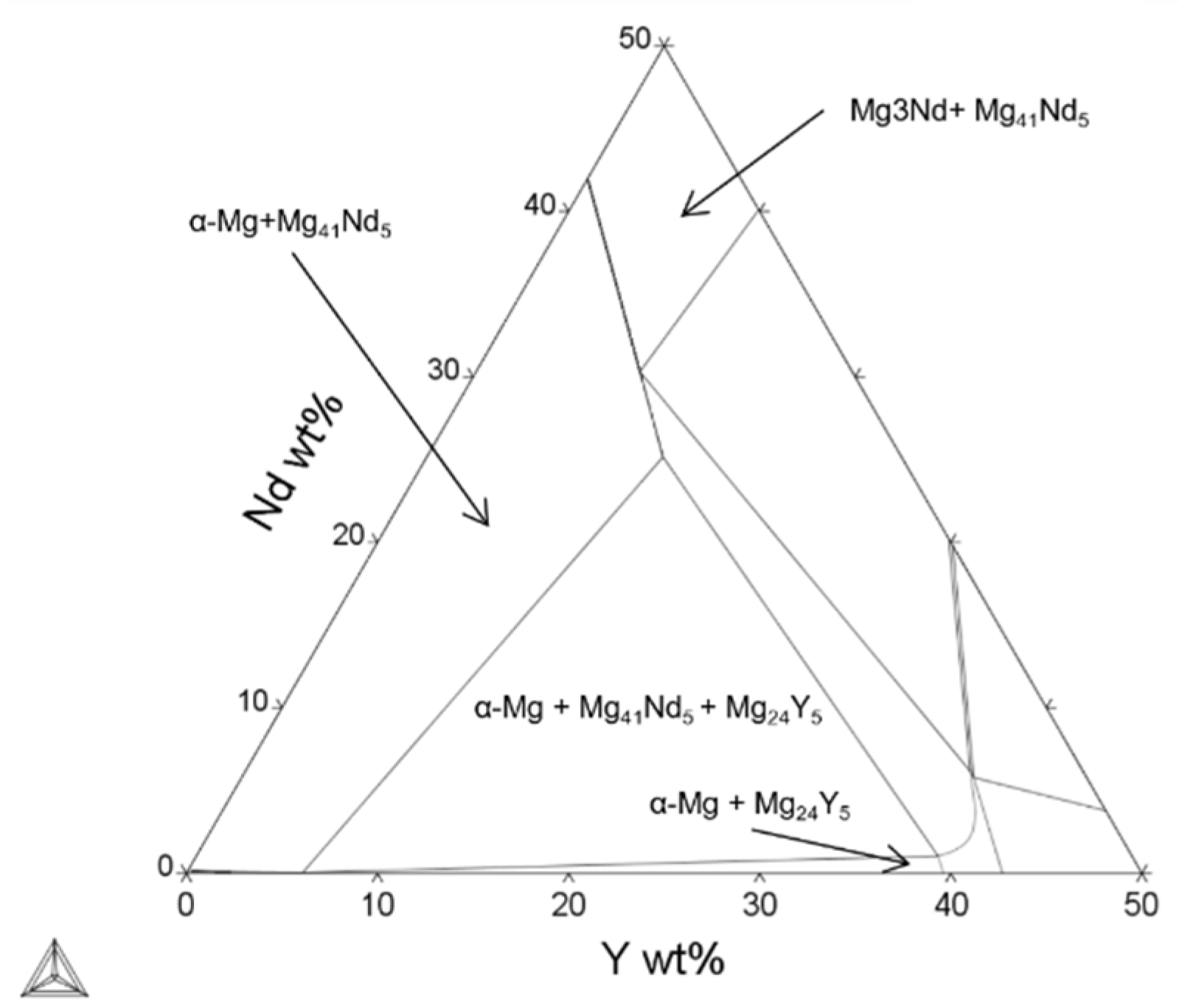
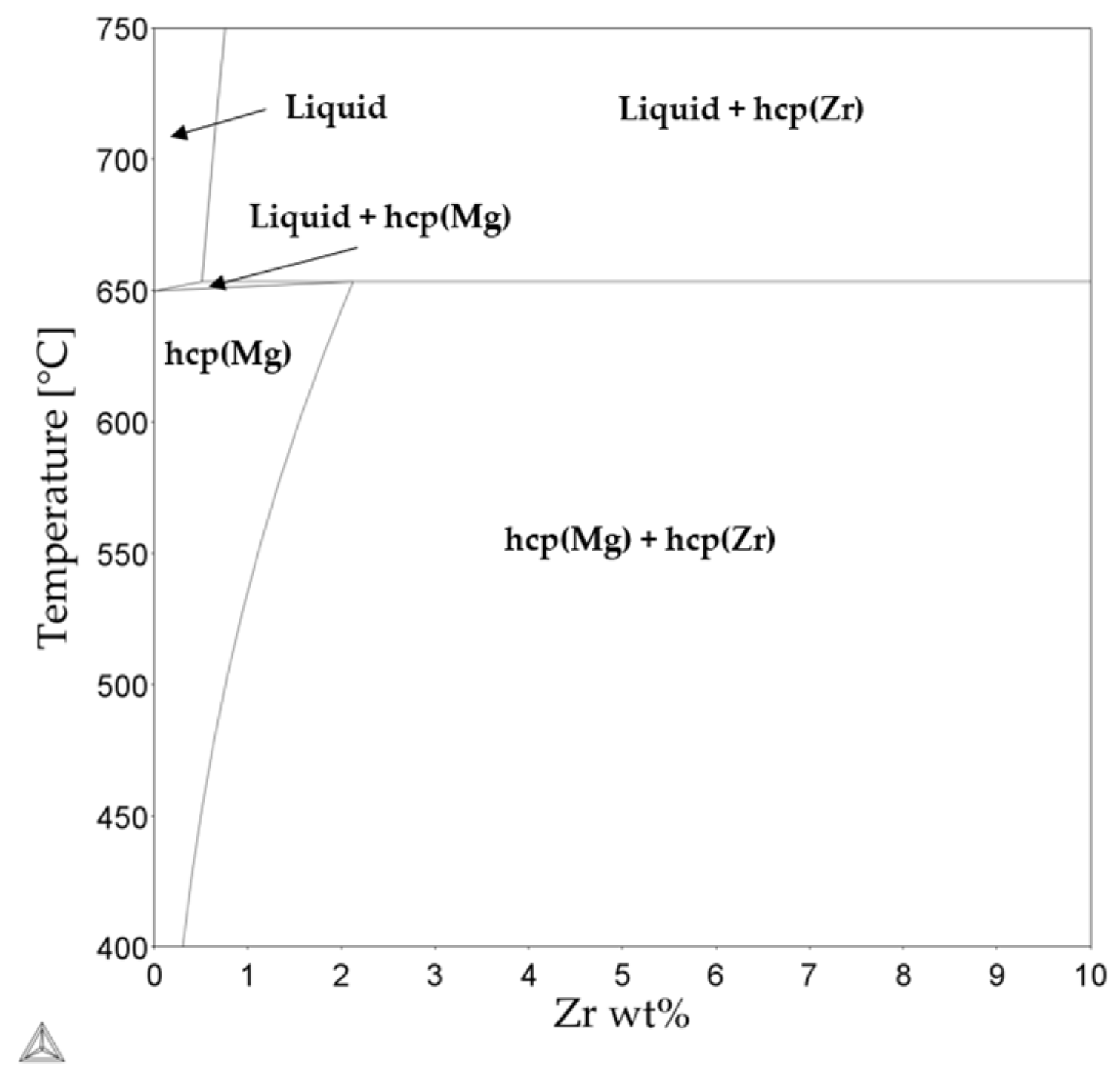
An Enhanced Understanding of the Powder Bed Fusion–Laser Beam Processing of Mg-Y3.9wt%-Nd3wt%-Zr0.5wt% (WE43) Alloy through Thermodynamic Modeling and Experimental Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Gas-Atomized Powder of Mg Alloy WE43: Specification and Characterization
2.2. Additive Manufacturing of Mg Alloy WE43
2.3. Experimental Characterization of Additively-Manufactured Samples
2.4. Computational Modelling of Mg Alloy WE43 Processed by PBF-LB
2.5. Statistical Analysis
3. Results
3.1. Powder Characteristics
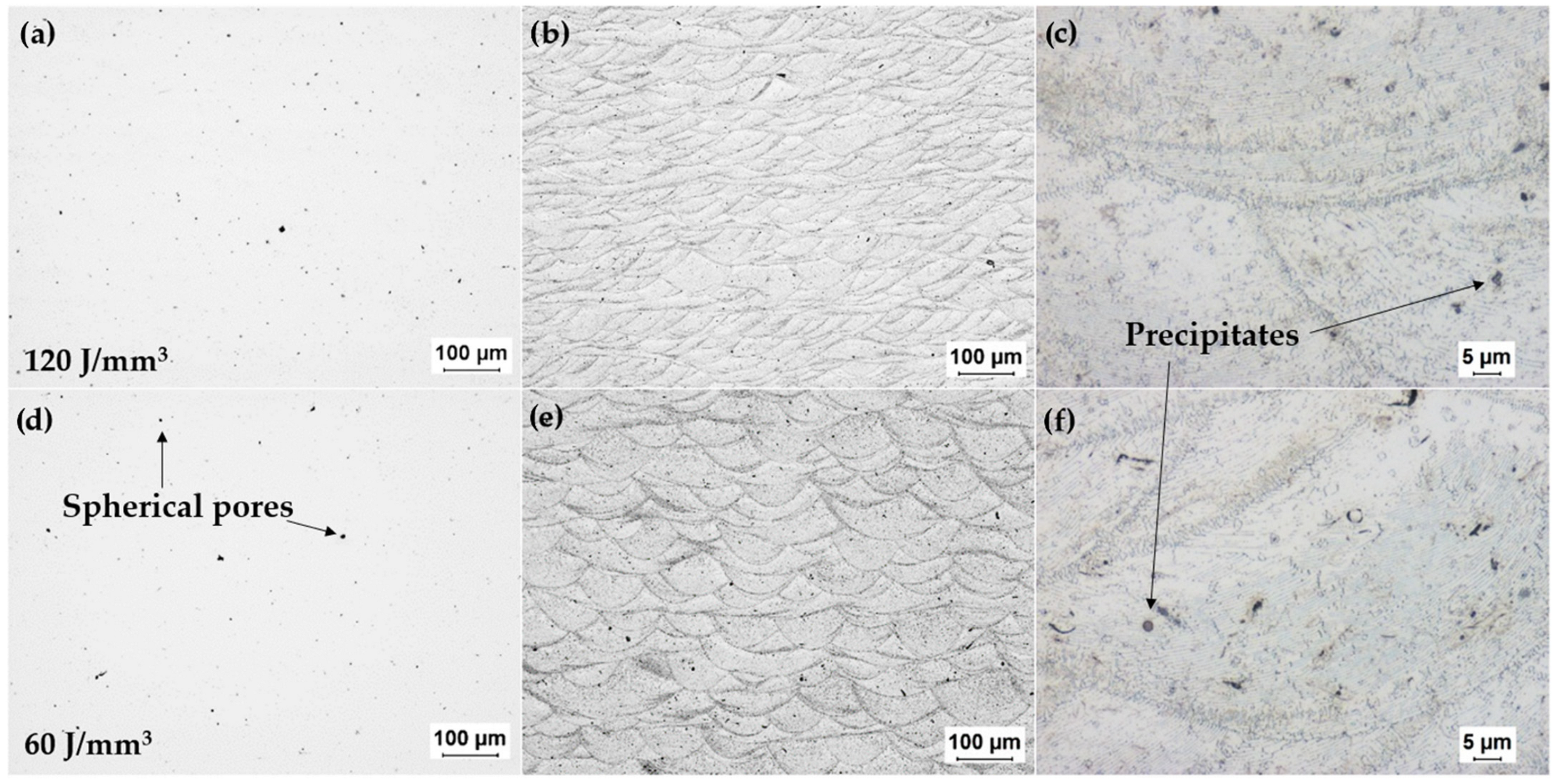
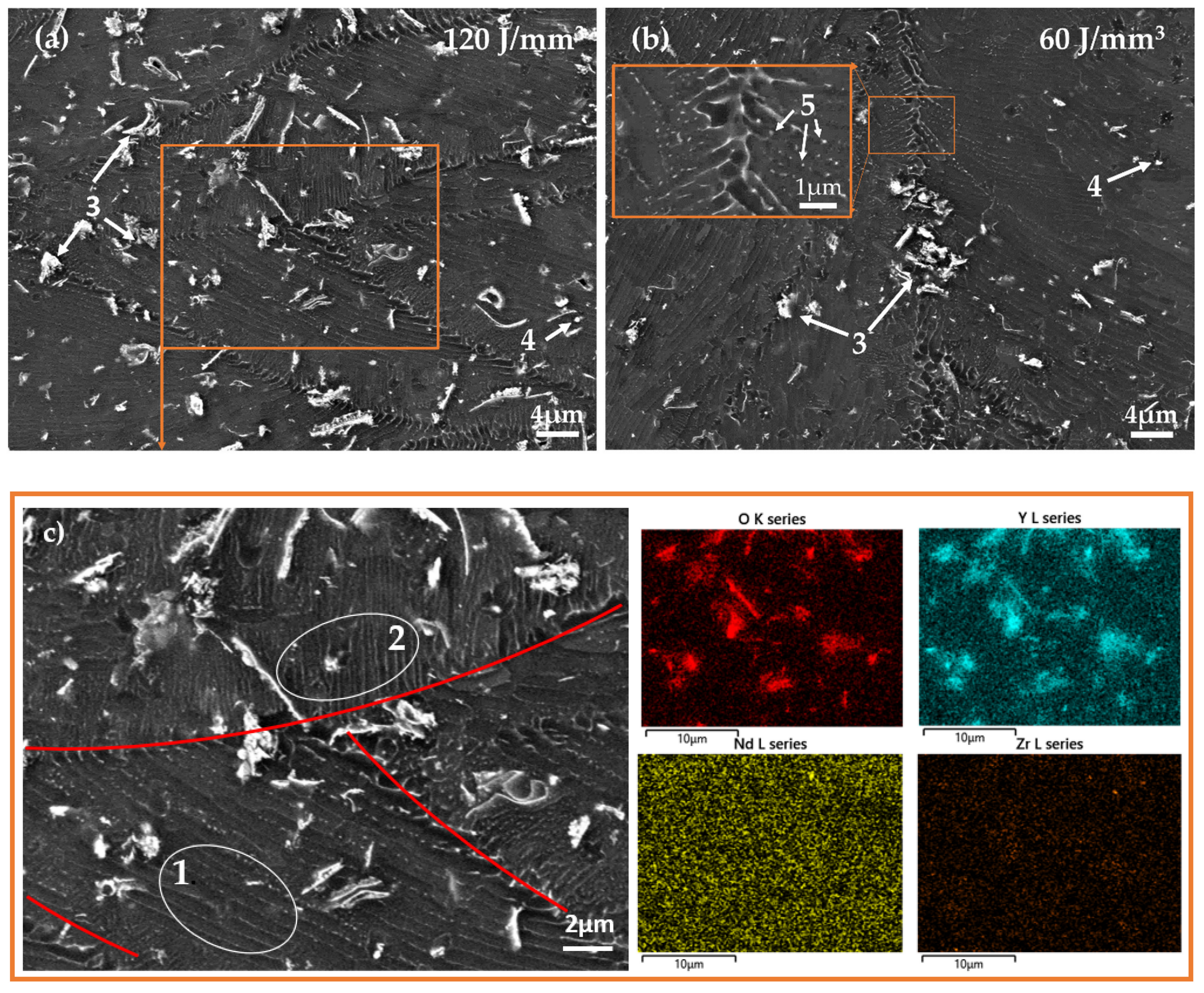
3.2. Experimental Characterization of Additively-Manufactured Samples
3.3. Computational Modelling Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prasad, S.V.S.; Prasad, S.B.; Verma, K.; Mishra, R.K.; Kumar, V.; Singh, S. The role and significance of Magnesium in modern day research-A review. J. Magnes. Alloy. 2021. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Han, H.S.; Loffredo, S.; Jun, I.; Edwards, J.; Kim, Y.C.; Seok, H.K.; Witte, F.; Mantovani, D.; Glyn-Jones, S. Current status and outlook on the clinical translation of biodegradable metals. Mater. Today 2019, 23, 57–71. [Google Scholar] [CrossRef]
- Tsakiris, V.; Tardei, C.; Clicinschi, F.M. Biodegradable Mg alloys for orthopedic implants—A review. J. Magnes. Alloy. 2021. [Google Scholar] [CrossRef]
- Betz, R.R. Limitations of Autograft and Allograft: New Synthetic Solutions. Orthopedics 2002, 25, S561–S570. [Google Scholar] [CrossRef] [PubMed]
- Valtanen, R.S.; Yang, Y.P.; Gurtner, G.C.; Maloney, W.J.; Lowenberg, D.W. Synthetic and Bone tissue engineering graft substitutes: What is the future? Injury 2021, 52, S72–S77. [Google Scholar] [CrossRef] [PubMed]
- Bell, B.G.; Schellevis, F.; Stobberingh, E.; Goossens, H.; Pringle, M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect. Dis. 2014, 14, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, M.; Ram, B.; Sewwandi, H.; Sulfikar; Honda, R.; Chaminda, T. Treatment enhances the prevalence of antibiotic-resistant bacteria and antibiotic resistance genes in the wastewater of Sri Lanka, and India. Environ. Res. 2020, 183, 109179. [Google Scholar] [CrossRef]
- Hofmann, G.O. Biodegradable implants in orthopaedic surgery-A review on the state-of-the-art. Clin. Mater. 1992, 10, 75–80. [Google Scholar] [CrossRef]
- Gusieva, K. Corrosion of magnesium alloys: The role of alloying. Int. Mater. Rev. 2015, 60, 169–194. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Feyerabend, F.; Gavras, S.; Xu, Y.; Willumeit-Römer, R.; Kainer, K.U.; Hort, N. Effects of Intermetallic Microstructure on Degradation of Mg-5Nd Alloy. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2020, 51, 5498–5515. [Google Scholar] [CrossRef]
- Kang, Y.; Huang, Z.; Zhao, H.; Gan, C.; Zhou, N.; Zheng, K.; Zhang, J.; Pan, F.; Huang, J.C.; Wang, S. Comparative study of hot deformation behavior and microstructure evolution of as-cast and extruded we43 magnesium alloy. Metals 2020, 10, 429. [Google Scholar] [CrossRef] [Green Version]
- Refson, K.; Wogelius, R.A.; Fraser, D.G.; Payne, M.C.; Lee, M.H.; Milman, V. Water chemisorption and reconstruction of the MgO surface. Phys. Rev. B 1995, 52, 10823. [Google Scholar] [CrossRef] [Green Version]
- Atrens, A.; Liu, M.; Abidin, N.I.Z.; Song, G.-L. Corrosion of magnesium (Mg) alloys and metallurgical influence. In Corrosion of Magnesium Alloys; Song, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 1, pp. 117–165. ISBN 9781845697082. [Google Scholar]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Liu, M.; Schmutz, P.; Uggowitzer, P.J.; Song, G.; Atrens, A. The influence of yttrium (Y) on the corrosion of Mg-Y binary alloys. Corros. Sci. 2010, 52, 3687–3701. [Google Scholar] [CrossRef]
- Sudholz, A.D.; Gusieva, K.; Chen, X.B.; Muddle, B.C.; Gibson, M.A.; Birbilis, N. Electrochemical behaviour and corrosion of Mg-Y alloys. Corros. Sci. 2011, 53, 2277–2282. [Google Scholar] [CrossRef]
- Leleu, S.; Rives, B.; Bour, J.; Causse, N.; Pébère, N. On the stability of the oxides film formed on a magnesium alloy containing rare-earth elements. Electrochim. Acta 2018, 290, 586–594. [Google Scholar] [CrossRef] [Green Version]
- Südholz, A.D.; Kirkland, N.T.; Buchheit, R.G.; Birbilis, N. Electrochemical properties of intermetallic phases and common impurity elements in magnesium alloys. Electrochem. Solid State Lett. 2011, 14, 2010–2012. [Google Scholar] [CrossRef]
- Syntellix MAGNEZIX®—A MILESTONE in MATERIAL RESEARCH. Available online: https://www.syntellix.de/en/products/technology.html (accessed on 28 June 2019).
- Maier, P.; Griebel, A.; Jahn, M.; Bechly, M.; Menze, R.; Bittner, B.; Schaffer, J. Corrosion Bending Fatigue of RESOLOY®and WE43 Magnesium Alloy Wires. In Magnesium Technology 2019; Springer: Berlin/Heidelberg, Germany, 2019; pp. 175–181. [Google Scholar]
- Gibson, I.; Rosen, D.; Stucker, B. Additive Manufacturing Technologies, 2nd ed.; Springer: New York, NY, USA, 2015; ISBN 978-1-4939-2112-6. [Google Scholar]
- Fernandez-Zelaia, P.; Kirka, M.M.; Dryepondt, S.N.; Gussev, M.N. Crystallographic texture control in electron beam additive manufacturing via conductive manipulation. Mater. Des. 2020, 195, 109010. [Google Scholar] [CrossRef]
- Yang, N.; Song, Y.; Huang, J.; Chen, Y.; Maskery, I. Combinational design of heterogeneous lattices with hybrid region stiffness tuning for additive manufacturing. Mater. Des. 2021, 209, 109955. [Google Scholar] [CrossRef]
- Zhao, D.; Liang, H.; Han, C.; Li, J.; Liu, J.; Zhou, K.; Yang, C.; Wei, Q. 3D printing of a titanium-tantalum Gyroid scaffold with superb elastic admissible strain, bioactivity and in-situ bone regeneration capability. Addit. Manuf. 2021, 47, 102223. [Google Scholar] [CrossRef]
- McGregor, M.; Patel, S.; McLachlin, S.; Vlasea, M. Architectural bone parameters and the relationship to titanium lattice design for powder bed fusion additive manufacturing. Addit. Manuf. 2021, 47, 102273. [Google Scholar] [CrossRef]
- Revilla-León, M.; Sadeghpour, M.; Özcan, M. A Review of the Applications of Additive Manufacturing Technologies Used to Fabricate Metals in Implant Dentistry. J. Prosthodont. 2020, 29, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Lima Corporate Lima Corporate—Our Technology. Available online: https://limacorporate.com/en/patients/technology.html (accessed on 13 December 2021).
- Heinl, P.; Müller, L.; Körner, C.; Singer, R.F.; Müller, F.A. Cellular Ti-6Al-4V structures with interconnected macro porosity for bone implants fabricated by selective electron beam melting. Acta Biomater. 2008, 4, 1536–1544. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.C.; Savalani, M.M.; Man, H.C.; Gibson, I. Layer manufacturing of magnesium and its alloy structures for future applications. Virtual Phys. Prototyp. 2010, 5, 13–19. [Google Scholar] [CrossRef]
- Wei, K.; Gao, M.; Wang, Z.; Zeng, X. Effect of energy input on formability, microstructure and mechanical properties of selective laser melted AZ91D magnesium alloy. Mater. Sci. Eng. A 2014, 611, 212–222. [Google Scholar] [CrossRef]
- Gangireddy, S.; Gwalani, B.; Liu, K.; Faierson, E.J.; Mishra, R.S. Microstructure and mechanical behavior of an additive manufactured (AM) WE43-Mg alloy. Addit. Manuf. 2018, 26, 53–64. [Google Scholar] [CrossRef]
- Hu, D.; Wang, Y.; Zhang, D.; Hao, L.; Jiang, J.; Li, Z.; Chen, Y. Experimental Investigation on Selective Laser Melting of Bulk Net-Shape Pure Magnesium. Mater. Manuf. Process. 2015, 30, 1298–1304. [Google Scholar] [CrossRef]
- Shuai, C.; Liu, L.; Zhao, M.; Feng, P.; Yang, Y.; Guo, W.; Gao, C.; Yuan, F. Microstructure, biodegradation, antibacterial and mechanical properties of ZK60-Cu alloys prepared by selective laser melting technique. J. Mater. Sci. Technol. 2018, 34, 1944–1952. [Google Scholar] [CrossRef]
- Tandon, R.; Wilks, T.; Gieseke, M.; Noelke, C.; Kaierle, S.; Palmer, T. Additive manufacturing of electron® 43 alloy using laser powder bed and directed energy deposition. In Proceedings of the Proceedings Euro PM 2015: International Powder Metallurgy Congress and Exhibition, Reims, France, 4–7 September 2015. [Google Scholar]
- Bär, F.; Berger, L.; Jauer, L.; Kurtuldu, G.; Schäublin, R.; Schleifenbaum, J.H.; Löffler, J.F. Laser additive manufacturing of biodegradable magnesium alloy WE43: A detailed microstructure analysis. Acta Biomater. 2019, 98, 36–49. [Google Scholar] [CrossRef]
- Zumdick, N.A.; Jauer, L.; Kersting, L.C.; Kutz, T.N.; Schleifenbaum, J.H.; Zander, D. Additive manufactured WE43 magnesium: A comparative study of the microstructure and mechanical properties with those of powder extruded and as-cast WE43. Mater. Charact. 2019, 147, 384–397. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.; Pavanram, P.; Leeflang, M.A.; Fockaert, L.I.; Pouran, B.; Tümer, N.; Schröder, K.U.; Mol, J.M.C.; Weinans, H.; et al. Additively manufactured biodegradable porous magnesium. Acta Biomater. 2018, 67, 378–392. [Google Scholar] [CrossRef] [PubMed]
- Esmaily, M.; Zeng, Z.; Mortazavi, A.N.; Gullino, A.; Choudhary, S.; Derra, T.; Benn, F.; D’Elia, F.; Müther, M.; Thomas, S.; et al. A detailed microstructural and corrosion analysis of magnesium alloy WE43 manufactured by selective laser melting. Addit. Manuf. 2020, 35, 1–34. [Google Scholar] [CrossRef]
- Song, S.S. Recent research progress on CALPHAD-based ICME modeling of magnesium alloys. IOP Conf. Ser. Mater. Sci. Eng. 2019, 474, 012024. [Google Scholar] [CrossRef]
- Shi, R.; Luo, A.A. Applications of CALPHAD modeling and databases in advanced lightweight metallic materials. Calphad Comput. Coupling Phase Diagr. Thermochem. 2018, 62, 1–17. [Google Scholar] [CrossRef]
- Geng, S.; Jiang, P.; Shao, X.; Mi, G.; Wu, H.; Ai, Y.; Wang, C.; Han, C.; Chen, R.; Liu, W. Comparison of solidification cracking susceptibility between Al-Mg and Al-Cu alloys during welding: A phase-field study. Scr. Mater. 2018, 150, 120–124. [Google Scholar] [CrossRef]
- Keller, T.; Lindwall, G.; Ghosh, S.; Ma, L.; Lane, B.M.; Zhang, F.; Kattner, U.R.; Lass, E.A.; Heigel, J.C.; Idell, Y.; et al. Application of finite element, phase-field, and CALPHAD-based methods to additive manufacturing of Ni-based superalloys. Acta Mater. 2017, 139, 244–253. [Google Scholar] [CrossRef]
- Karlsson, D.; Chou, C.Y.; Pettersson, N.H.; Helander, T.; Harlin, P.; Sahlberg, M.; Lindwall, G.; Odqvist, J.; Jansson, U. Additive manufacturing of the ferritic stainless steel SS441. Addit. Manuf. 2020, 36, 101580. [Google Scholar] [CrossRef]
- Yusuf, S.M.; Gao, N. Influence of energy density on metallurgy and properties in metal additive manufacturing. Mater. Sci. Technol. 2017, 33, 1269–1289. [Google Scholar] [CrossRef]
- Pakkanen, J. Designing for Additive Manufacturing Product and Process Driven Design for Metals and Polymers; Politecnico di Torino: Torino, Italy, 2018. [Google Scholar]
- Wright, S.I. A Parametric Study of Electron Backscatter Diffraction based Grain Size Measurements. Pract. Metallogr. 2010, 47, 16–33. [Google Scholar] [CrossRef]
- Wright, S.I. Time for a Change—New Perspectives in Grain Size Analysis. Available online: https://edaxblog.com/2014/06/23/time-for-a-change-new-perspectives-in-grain-size-analysis/ (accessed on 8 July 2021).
- Andersson, J.O.; Helander, T.; Höglund, L.; Shi, P.; Sundman, B. Thermo-Calc & DICTRA, computational tools for materials science. Calphad Comput. Coupling Phase Diagr. Thermochem. 2002, 26, 273–312. [Google Scholar] [CrossRef]
- Thermo-Calc Software TCMG5 Mg-Alloys Database.
- Sola, A.; Nouri, A. Microstructural porosity in additive manufacturing: The formation and detection of pores in metal parts fabricated by powder bed fusion. J. Adv. Manuf. Process. 2019, 1, e10021. [Google Scholar] [CrossRef]
- Hyer, H.; Zhou, L.; Benson, G.; McWilliams, B.; Cho, K.; Sohn, Y. Additive manufacturing of dense WE43 Mg alloy by laser powder bed fusion. Addit. Manuf. 2020, 33, 101123. [Google Scholar] [CrossRef]
- Aylward, G.; Findlay, T. SI Chemical Data, 6th ed.; John Wiley & Sons Australia Ltd: Milton, WA, USA, 2007; ISBN 9780470816387. [Google Scholar]
- Kainer, K.U. Magnesium—Alloys and Technologies; Wiley-VCH: Weinheim, Germany, 2003; ISBN 3-527-30570-X. [Google Scholar]
- Ferrari, M.; Lutterotti, L. Method for the simultaneous determination of anisotropic residual stresses and texture by X-ray diffraction. J. Appl. Phys. 1994, 76, 7246–7655. [Google Scholar] [CrossRef]
- Nie, J.F. Precipitation and hardening in magnesium alloys. Metall. Mater. Trans. A 2012, 43, 3891–3939. [Google Scholar] [CrossRef] [Green Version]
- Natarajan, A.R.; Solomon, E.L.S.; Puchala, B.; Marquis, E.A.; Van Der Ven, A. On the early stages of precipitation in dilute Mg-Nd alloys. Acta Mater. 2016, 108, 367–379. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Benn, F.; Derra, T.; Kröger, N.; Zinser, M.; Smeets, R.; Molina-aldareguia, J.M.; Kopp, A.; Llorca, J. Microstructure, mechanical properties, corrosion resistance and cytocompatibility of WE43 Mg alloy scaffolds fabricated by laser powder bed fusion for biomedical applications. Mater. Sci. Eng. C 2021, 119, 111623. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.F.; Muddle, B.C. Characterisation of strengthening precipitate phases in a Mg-Y-Nd alloy. Acta Mater. 2000, 48, 1691–1703. [Google Scholar] [CrossRef]
- Hooper, P.A. Melt pool temperature and cooling rates in laser powder bed fusion. Addit. Manuf. 2018, 22, 548–559. [Google Scholar] [CrossRef]
- Sasha, P.; Viswanathan, S. Grain Refinement of Magnesium by Zirconium: Characterization and Analysis. Trans. Am. Foundry Soc. 2011, 119, 469–480. [Google Scholar]
- Lee, Y.C.; Dahle, A.K.; Stjohn, D.H. The role of solute in grain refinement of magnesium. Metall. Mater. Trans. A 2000, 31, 2895–2906. [Google Scholar] [CrossRef]
- Li, J.; Chen, R.; Ma, Y.; Ke, W. Effect of Zr modification on solidification behavior and mechanical properties of Mg-Y-RE (WE54) alloy. J. Magnes. Alloy. 2013, 1, 346–351. [Google Scholar] [CrossRef] [Green Version]
- Nayeb-Hashemi, A.A.; Clark, J.B. The Mg-Zr (Magnesium-Zirconium) system. Bull. Alloy Phase Diagr. 1985, 6, 246–250. [Google Scholar] [CrossRef]
- Jauer, L.; Meiners, W.; Vervoort, S.; Gayer, C. Selective Laser Melting of Magnesium Alloys. In Proceedings of the World PM 2016 AM-Powder Bed Based Technologies, Hamburg, Germany, 9–13 October 2016. [Google Scholar]

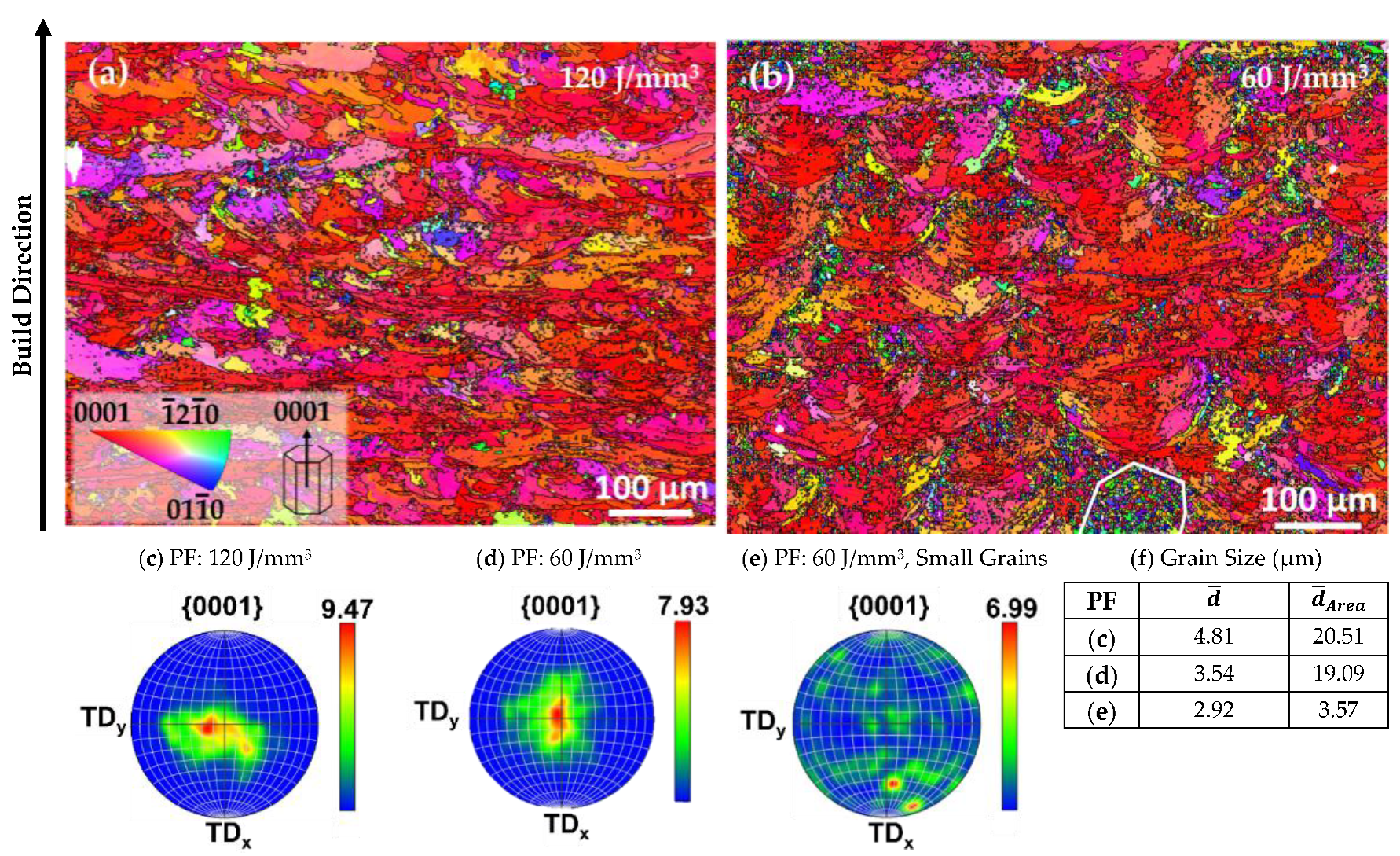
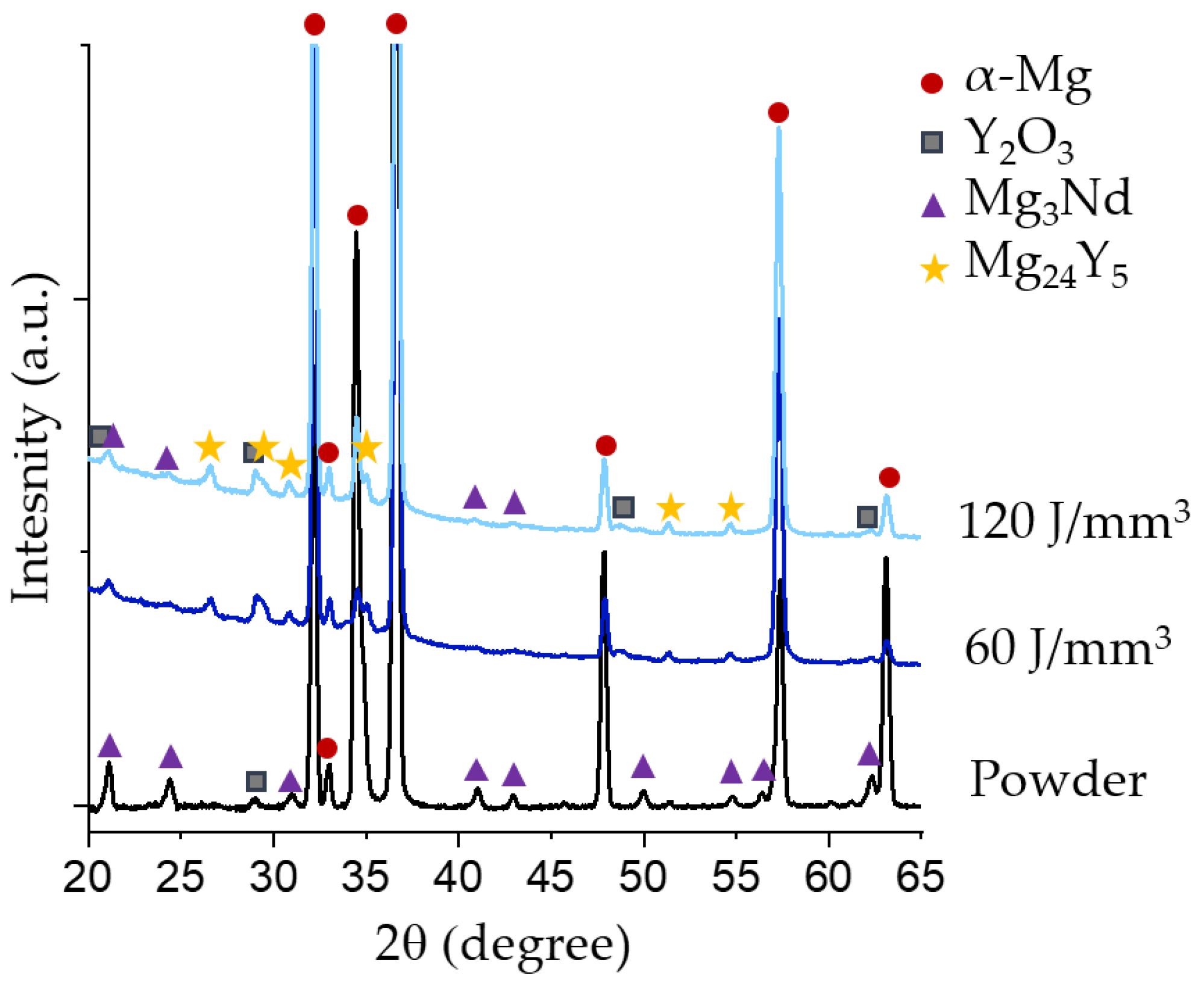
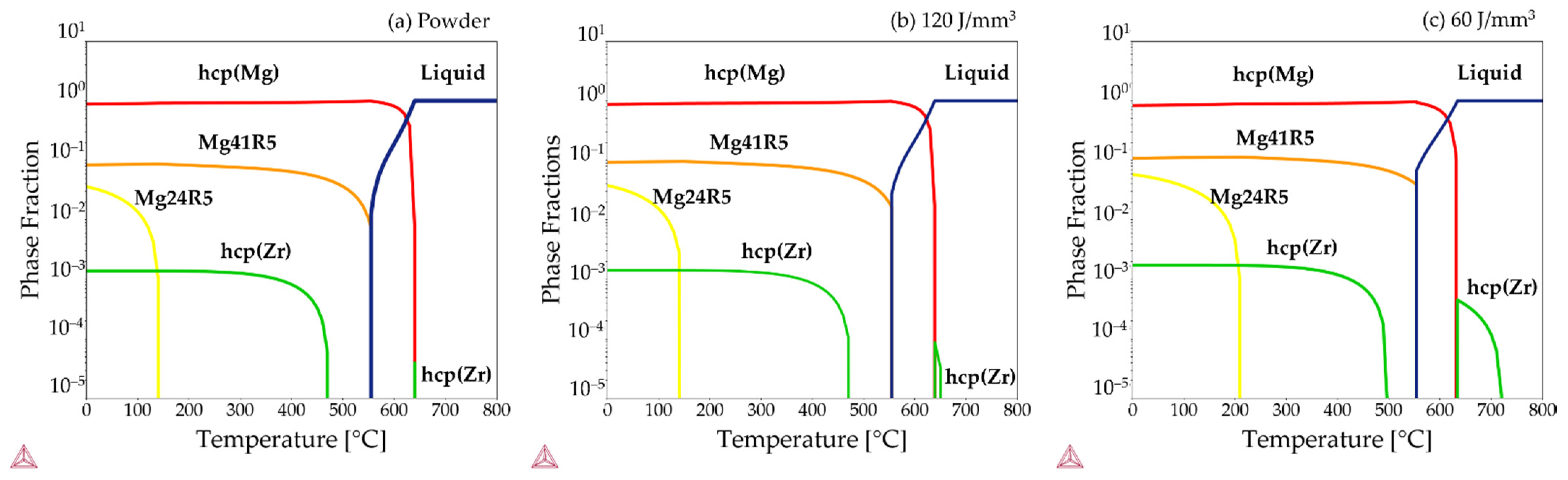
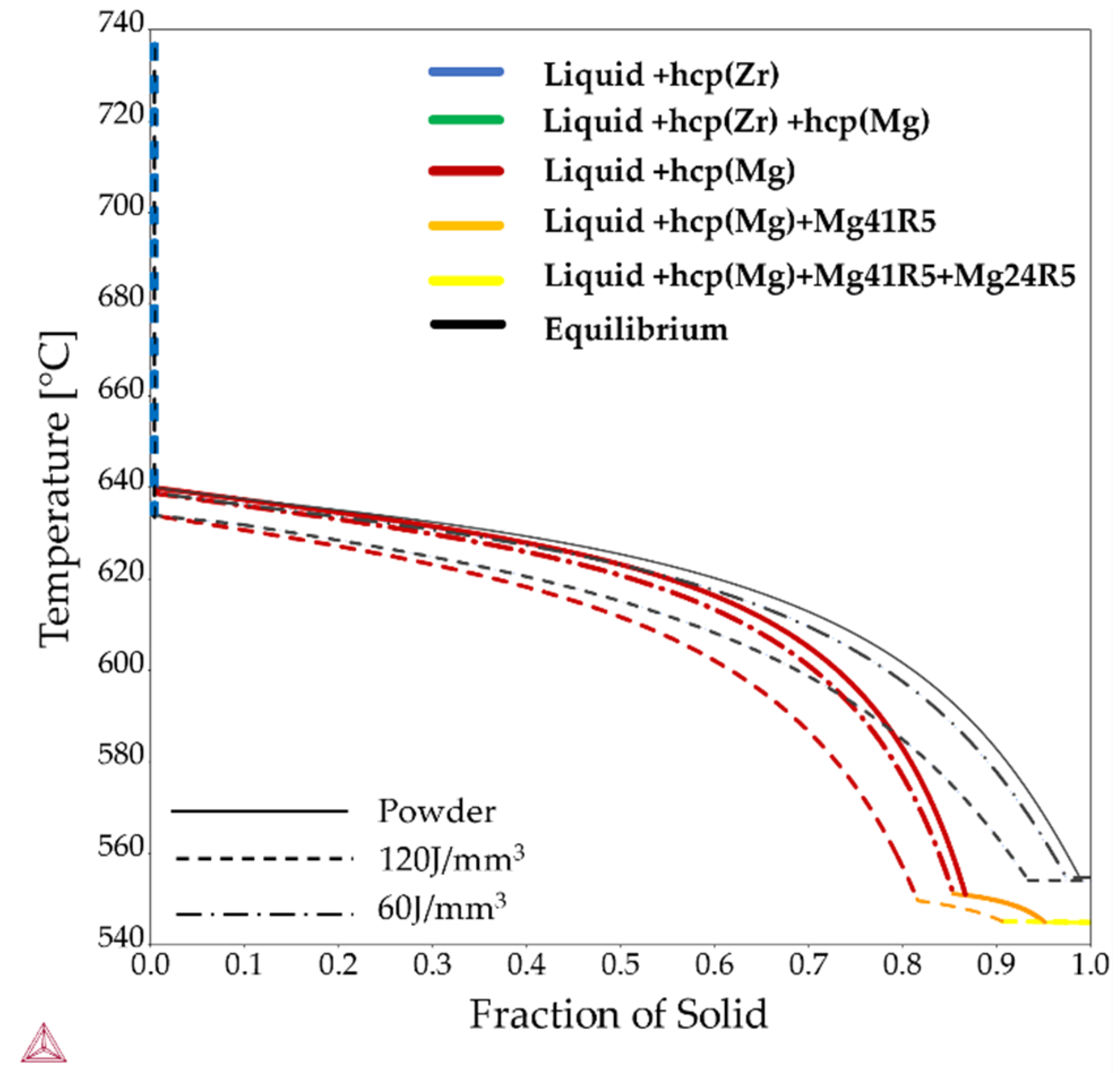
| Element (wt%) | Mg | Y | Nd | Zr |
| balance | 3.9 | 3.0 | 0.49 |
| ED (J/mm3) | P (W) | V (mm/s) | H (mm) | Archimedes Density (%) |
|---|---|---|---|---|
| 120 | 100 | 926 | 0.03 | 99.4 ± 0.05 |
| 60 | 200 | 1111 | 0.1 | 99.8 ± 0.2 |
| Element (wt%) | ||||
|---|---|---|---|---|
| Mg | Y | Nd | Zr | |
| Powder | balance | 3.9 | 3.0 | 0.49 |
| ED = 120 J/mm3 | balance | 5.4 | 3.8 | 0.6 |
| ED = 60 J/mm3 | balance | 4.1 | 3.3 | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nilsson Åhman, H.; Thorsson, L.; Mellin, P.; Lindwall, G.; Persson, C. An Enhanced Understanding of the Powder Bed Fusion–Laser Beam Processing of Mg-Y3.9wt%-Nd3wt%-Zr0.5wt% (WE43) Alloy through Thermodynamic Modeling and Experimental Characterization. Materials 2022, 15, 417. https://doi.org/10.3390/ma15020417
Nilsson Åhman H, Thorsson L, Mellin P, Lindwall G, Persson C. An Enhanced Understanding of the Powder Bed Fusion–Laser Beam Processing of Mg-Y3.9wt%-Nd3wt%-Zr0.5wt% (WE43) Alloy through Thermodynamic Modeling and Experimental Characterization. Materials. 2022; 15(2):417. https://doi.org/10.3390/ma15020417
Chicago/Turabian StyleNilsson Åhman, Hanna, Lena Thorsson, Pelle Mellin, Greta Lindwall, and Cecilia Persson. 2022. "An Enhanced Understanding of the Powder Bed Fusion–Laser Beam Processing of Mg-Y3.9wt%-Nd3wt%-Zr0.5wt% (WE43) Alloy through Thermodynamic Modeling and Experimental Characterization" Materials 15, no. 2: 417. https://doi.org/10.3390/ma15020417
APA StyleNilsson Åhman, H., Thorsson, L., Mellin, P., Lindwall, G., & Persson, C. (2022). An Enhanced Understanding of the Powder Bed Fusion–Laser Beam Processing of Mg-Y3.9wt%-Nd3wt%-Zr0.5wt% (WE43) Alloy through Thermodynamic Modeling and Experimental Characterization. Materials, 15(2), 417. https://doi.org/10.3390/ma15020417