Synthesis and Performances of Shrinkage-Reducing Polycarboxylate Superplasticizer in Cement-Based Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.2.1. Synthesis of Shrinkage-Reducing Monomer
2.2.2. Synthesis of SRP
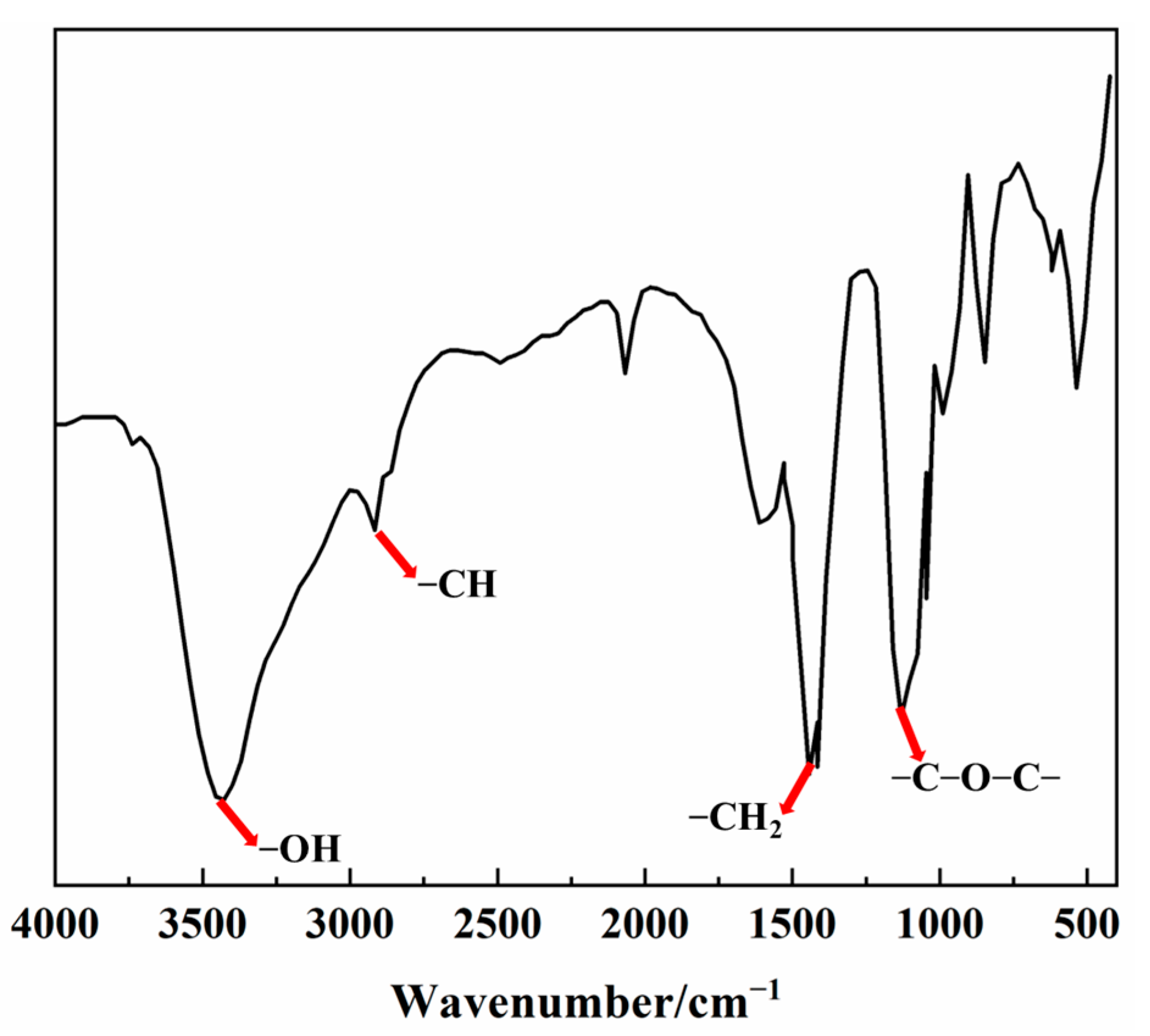
2.3. FTIR Analysis
2.4. GPC Analysis
2.5. Paste Fluidity Test
2.6. Shrinkage Test
2.7. Surface Tension Test
2.8. Evaporation Rate Test
3. Results and Discussion
3.1. Characterization of SRM and SRP
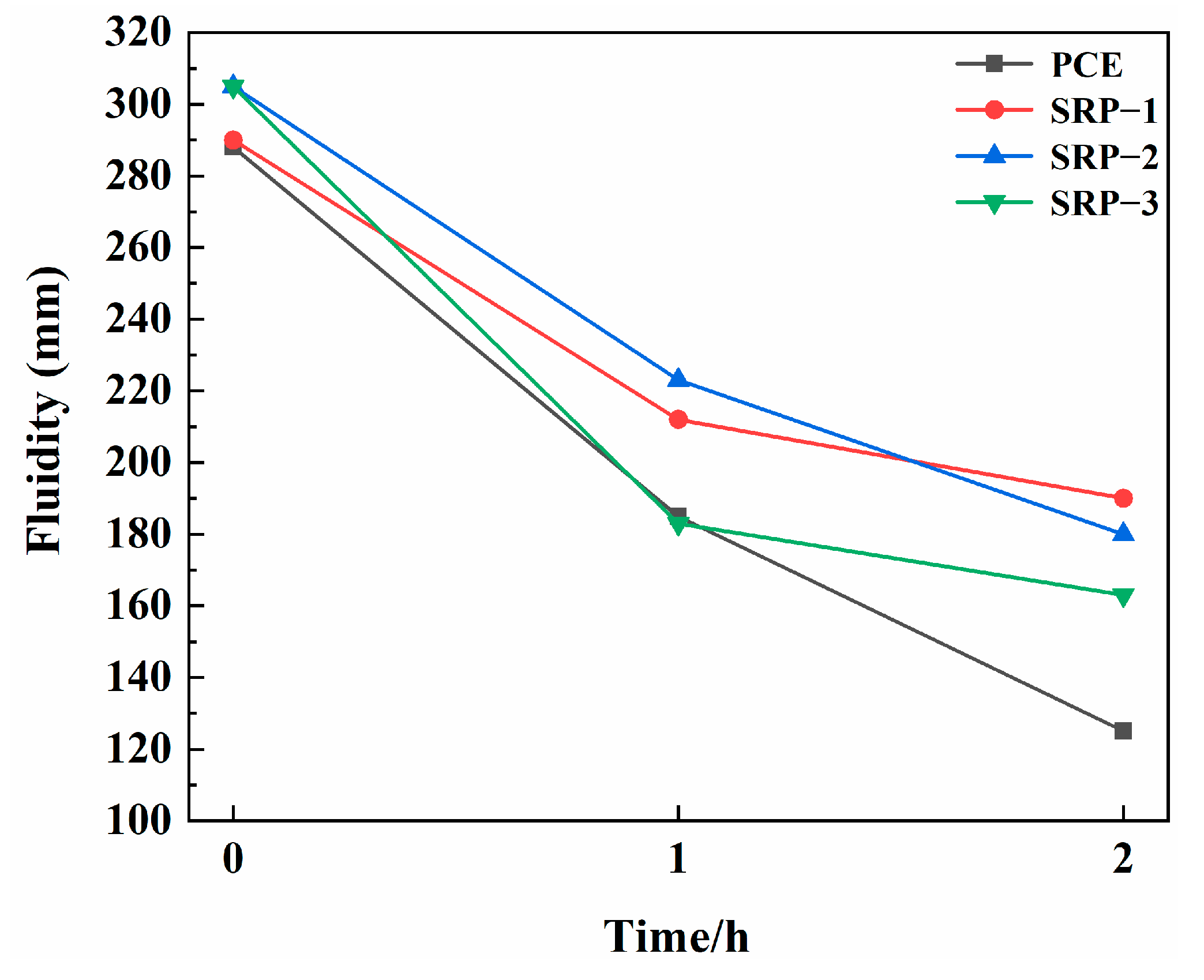
3.2. Fluidity
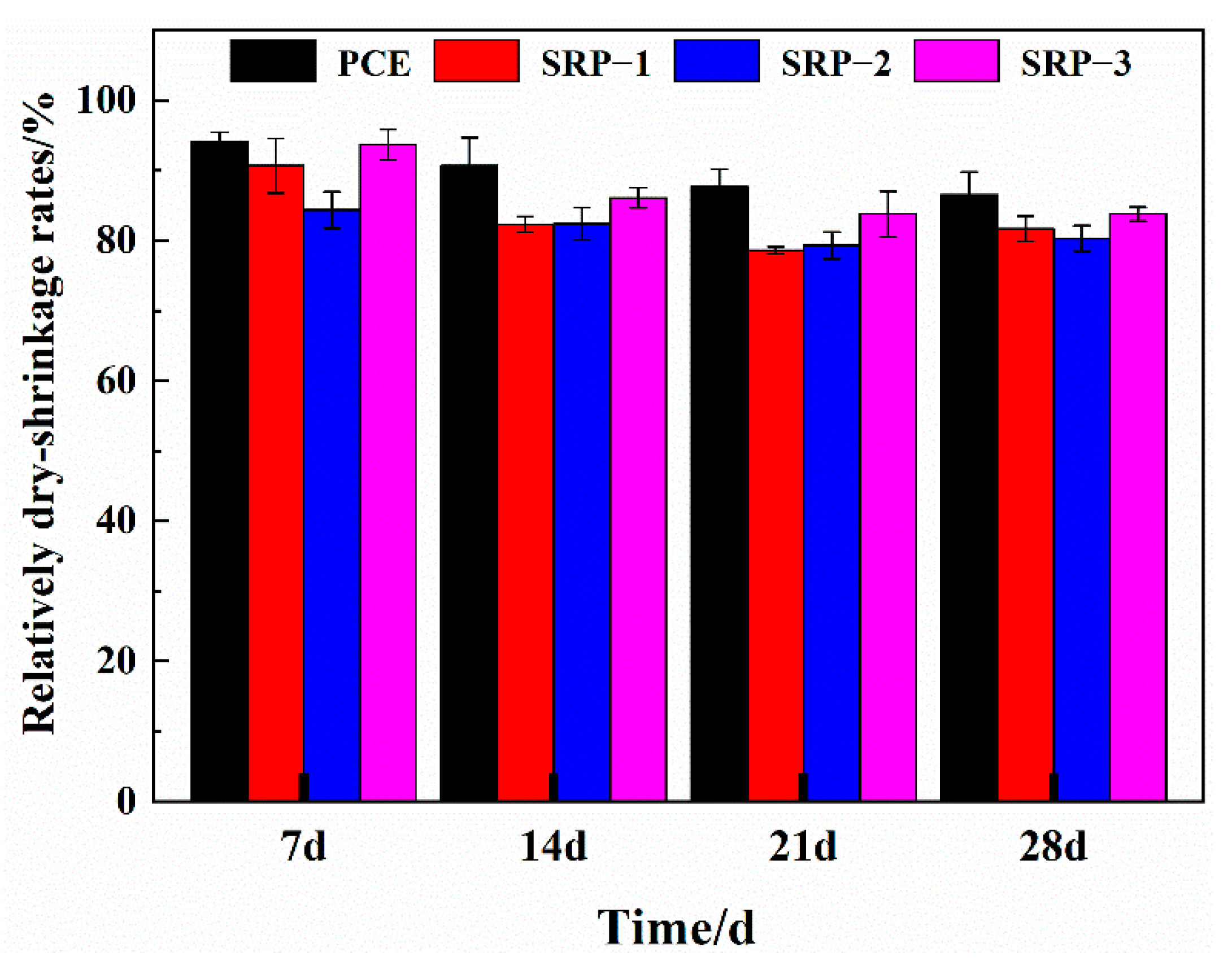
3.3. Drying Shrinkage
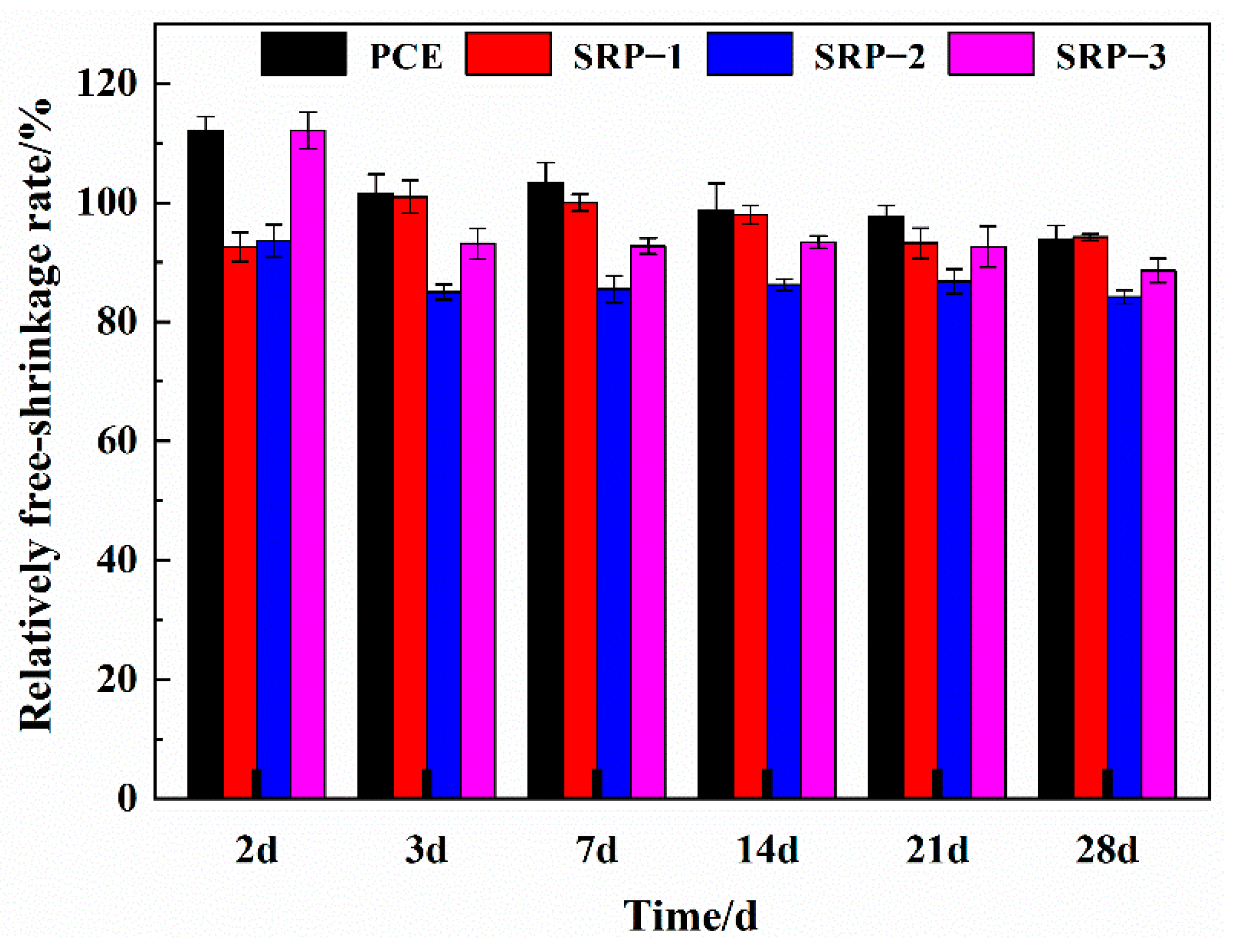
3.4. Free Shrinkage
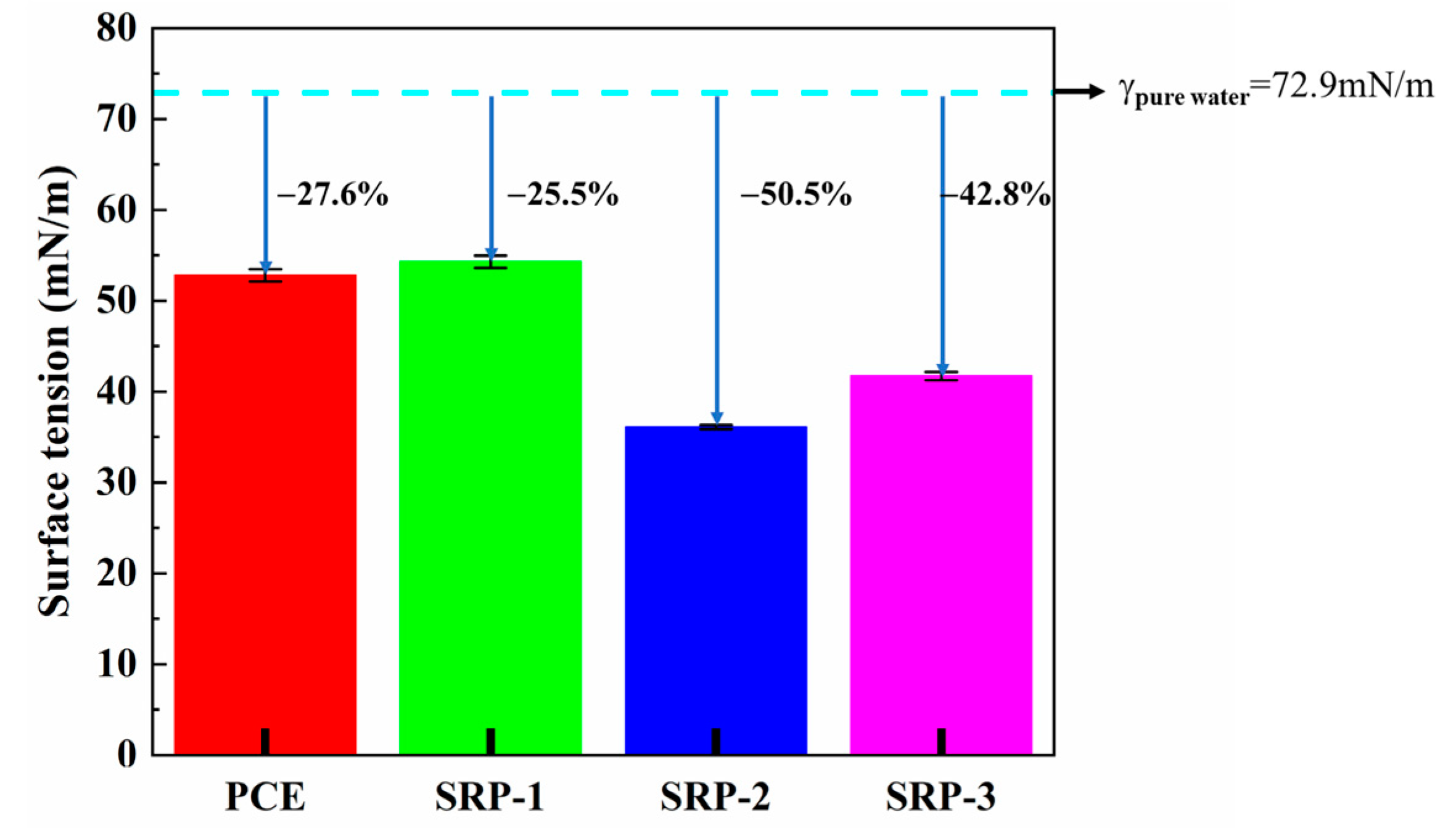
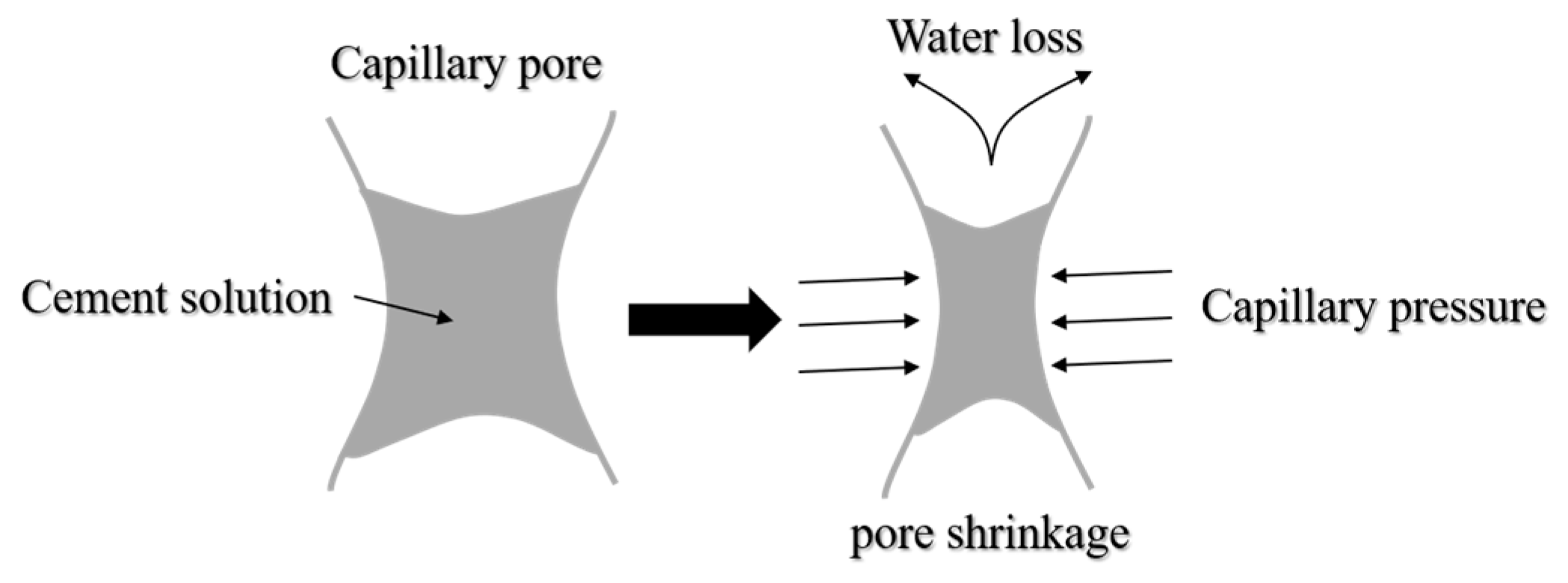
3.5. Surface Tension
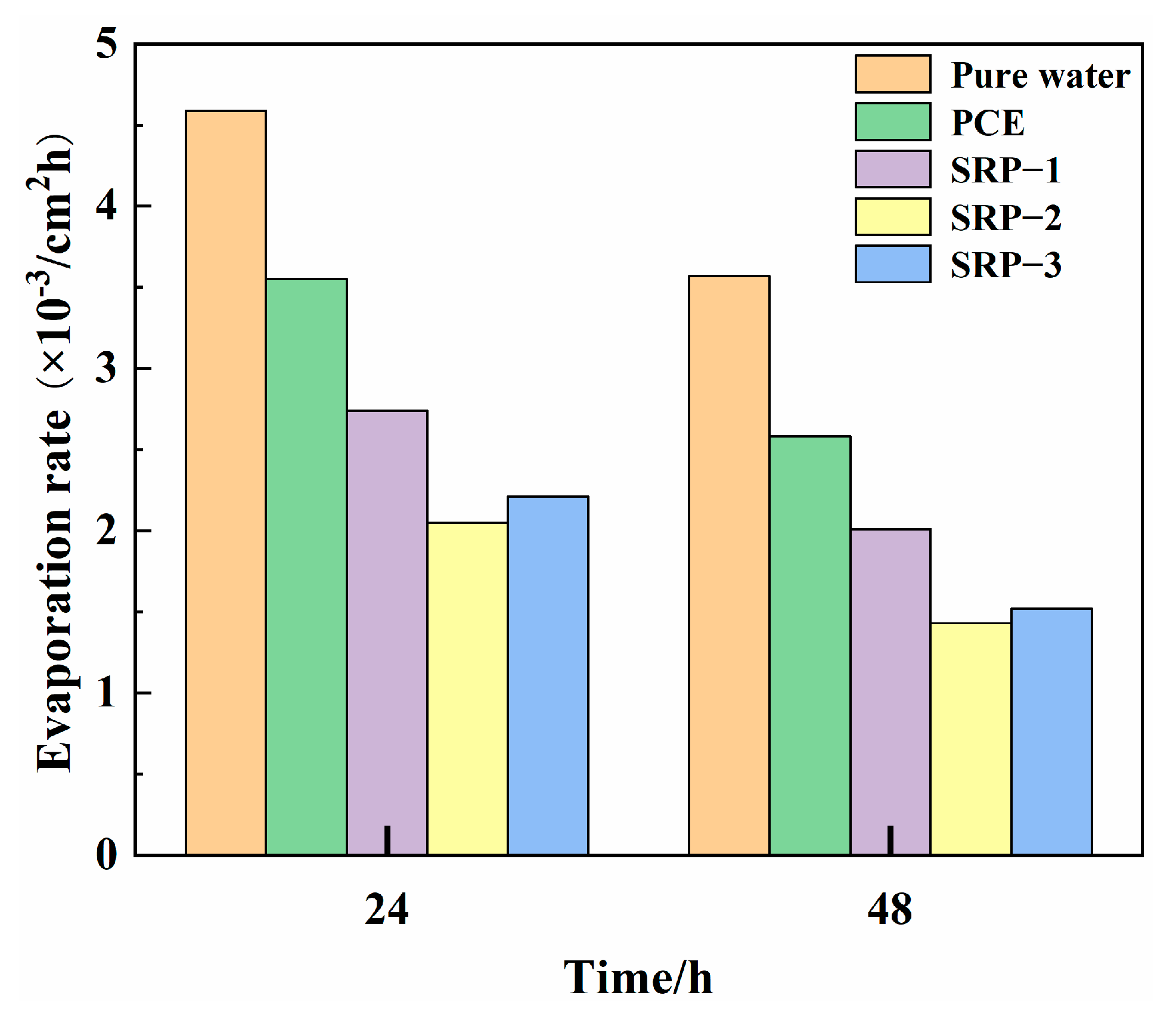
3.6. Evaporation Rate
3.7. Holistic Analysis
4. Conclusions
- (1)
- The molecular structure and molecular weight of the synthesized SRPs were characterized by FTIR and GPC analysis, which confirmed the achievement of the synthesis target and the rationality of the synthesis process;
- (2)
- Compared with conventional PCE, SRP showed the improved fluidity of cement paste, and the fluidity retention ability was increased by 37.2%, indicating that partially replacing AA with the shrinkage-reducing monomer could enhance the dispersing capacity;
- (3)
- Compared with pure water, the surface tension and evaporation rate (within 48 h) of an SRP aqueous solution can be reduced by 50.5% and 57.4%, respectively. SRP effectively reduced the shrinkage of mortar through the combination of two mechanisms, i.e., reducing the surface tension of cement solution in capillary pores and inhibiting the evaporation of water in the solution. This reduction was very significant, especially for drying shrinkage which decreased by over 20%;
- (4)
- The diester structure in SRP molecules showed greater advantages in improving the fluidity of cement paste and reducing the shrinkage of cement-based materials, which was related to the hydrolysis process of ester groups in the molecules and the non-adsorbing small molecule alcohol-based compounds produced by hydrolysis;
- (5)
- The new type of SRP developed in this study can be used as a functional material in cement-based materials and has a potential application in concrete engineering requiring low shrinkage, high workability and long fluidity retention.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, S.; Huang, D.; He, Z. A review of autogenous shrinkage models of concrete. J. Build. Eng. 2021, 44, 103412. [Google Scholar] [CrossRef]
- Akgungor, A.P.; Sevim, O.; Kalkan, I.; Demir, I. Restrained shrinkage cracking of self-consolidating concrete roads. Sci. Eng. Compos. Mater. 2017, 25, 1021–1030. [Google Scholar] [CrossRef]
- Dey, A.; Vastrad, A.V.; Bado, M.F.; Sokolov, A.; Kaklauskas, G. Long-Term Concrete Shrinkage Influence on the Performance of Reinforced Concrete Structures. Materials 2021, 14, 254. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, G.; Tong, Y.; Gu, C. A Numerical Study on Chloride Diffusion in Cracked Concrete. Crystals 2021, 11, 742. [Google Scholar] [CrossRef]
- Li, J.; Wu, Z.; Shi, C.; Yuan, Q.; Zhang, Z. Durability of ultra-high performance concrete—A review. Constr. Build. Mater. 2020, 255, 119296. [Google Scholar] [CrossRef]
- Li, K.; Li, L. Crack-altered durability properties and performance of structural concretes. Cem. Concr. Res. 2019, 124, 105811. [Google Scholar] [CrossRef]
- Czarnecki, L.; Geryo, R.; Kuczyński, K. Concrete Repair Durability. Materials 2020, 13, 4535. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.M.; Wang, Y.L. Concrete Superplasticizer; Chemical Industry Press: Beijing, China, 2011; pp. 1–2. [Google Scholar]
- Cheung, J.; Roberts, L.; Liu, J. Admixtures and sustainability. Cem. Concr. Res. 2017, 114, 79–89. [Google Scholar] [CrossRef]
- Thiyaneswaran, M.P.; Jenova, L.R.; Navaneethan, K.S. Review paper on material properties of high performance concrete. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1055, 012054. [Google Scholar] [CrossRef]
- Sevim, O.; Kalkan, I.; Demir, I.; Akgüngör, A.P. Effects of the sole or combined use of chemical admixtures on properties of self-compacting concrete. Arch. Civ. Mech. Eng. 2021, 21, 150. [Google Scholar] [CrossRef]
- Lai, J.; Zhang, L.; Qian, X.; Shen, C.; Zhang, J. Influence of superplasticizers on early age drying shrinkage of cement paste with the same consistency. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2014, 29, 1201–1207. [Google Scholar] [CrossRef]
- Qian, C.; Zhang, Y.; Huang, H.; Qu, J.; Guo, J. Influences of superplasticizers on the basic and drying creep of concrete. Struct. Concr. 2016, 17, 729–735. [Google Scholar] [CrossRef]
- Ma, B.-G.; Wang, X.-G.; Liang, W.-Q.; Li, X.-G.; He, Z. Study on early-age cracking of cement-based materials with superplasticizers. Constr. Build. Mater. 2007, 21, 2017–2022. [Google Scholar] [CrossRef]
- Qian, X.Q.; Zhan, S.L. Negative Influence of Superplasticisers on Shrinkage and Cracking of Concrete. J. Rail. Sci. Eng. 2004, 48, 19–25. [Google Scholar]
- Liu, L.X.; Yang, C.H.; Wu, F.; Chen, K. Concrete Mixed with Polycarboxylate Superplasticizer. Concrete 2009, 10, 80–82. [Google Scholar]
- Zhan, P.M.; He, Z.H. Application of shrinkage reducing admixture in concrete: A review. Constr. Build. Mater. 2019, 201, 676–690. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, H.; Yu, S.; Tan, C.; Yang, X. Research on influence of admixtures to the plastic shrinkage cracking of mortar. IOP Conf. Ser. Mater. Sci. Eng. 2019, 542, 012002. [Google Scholar] [CrossRef]
- Statkauskas, M.; Grinys, A.; Vaiˇciukynien, D. Investigation of Concrete Shrinkage Reducing Additives. Materials 2022, 15, 3407. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.W.; Ran, Q.P. A Grafted Copolymer Concrete Superplasticizer with Shrinkage-Reducing and Crack Resistance Function and Its Preparation Method. Chinese Patent ZL200610040089.X, 2006. [Google Scholar]
- Sun, Z.P.; Luo, Q. Synthesis and Application Method of a Kind of Shrinkage-Reducing Superplasticizer. Chinese Patent ZL201010102093.0, 2010. [Google Scholar]
- Inoue, K.; Yonezawa, T. Multi-Functional Admixtures for Concrete and Concrete Using the Same. U.S. Patent 20050124737A1, 9 June 2005. [Google Scholar]
- Kinoshita, M.; Saitou, K. Multi-Functional Admixtures for Hydraulic Cement Compositions. U.S. Patent 20050096413A1, 5 May 2009. [Google Scholar]
- Zhang, J.; Ma, Y.; Wang, J.; Gao, N.; Hu, Z.; Liu, J.; Wang, K. A novel shrinkage-reducing polycarboxylate superplasticizer for cement-based materials: Synthesis, performance and mechanisms. Constr. Build. Mater. 2022, 321, 126342. [Google Scholar] [CrossRef]
- Mao, Q.; Ma, J.; Wang, Z.; Lan, M.; Cui, S. Shrinkage reduction of cement-based materials containing polycarboxylate superplasticiser. Mag. Concr. Res. 2019, 73, 217–227. [Google Scholar] [CrossRef]
- Zhang, M.-H.; Sisomphon, K.; Ng, T.S.; Sun, D.J. Effect of superplasticizers on workability retention and initial setting time of cement pastes. Constr. Build. Mater. 2010, 24, 1700–1707. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Z.M. Synthesis, characterization and performance of a polycarboxylate superplasticizer with amide structure. Colloids Surf. A 2014, 448, 119–129. [Google Scholar] [CrossRef]
- Guan, J.; Liu, X.; Lai, G.; Luo, Q.; Qian, S.; Zhan, J.; Wang, Z.; Cui, S. Effect of sulfonation modification of polycarboxylate superplasticizer on tolerance enhancement in sulfate. Constr. Build. Mater. 2021, 273, 122095. [Google Scholar] [CrossRef]
- Erzengin, S.G.; Kaya, K.; Özkorucuklu, S.P.; Özdemir, V.; Yıldırım, G. The properties of cement systems superplasticized with methacrylic ester-based polycarboxylates. Constr. Build. Mater. 2018, 166, 96–109. [Google Scholar] [CrossRef]
- Feng, P.; Zhang, G.; Zhang, W.; Cui, H.; Xin, T. Comparison of ester-based slow-release polycarboxylate superplasticizers with their polycarboxylate counterparts. Colloids Surf. A Physicochem. Eng. Asp. 2022, 633, 127878. [Google Scholar] [CrossRef]
- Zhang, L.; Kong, X.; Xing, F.; Dong, B.; Wang, F. Working mechanism of post-acting polycarboxylate superplasticizers containing acrylate segments. J. Appl. Polym. Sci. 2017, 135, 45753. [Google Scholar] [CrossRef]
- Ilg, M.; Plank, J. Non-adsorbing small molecules as auxiliary dispersants for polycarboxylate superplasticizers. Colloids Surf. A Physicochem. Eng. Asp. 2020, 587, 124307. [Google Scholar] [CrossRef]
- Wadsoe, L.; Karlsson, O.J. Alkaline hydrolysis of polymers with ester groups studied by isothermal calorimetry. Polym. Degrad. Stabil. 2013, 98, 73–78. [Google Scholar] [CrossRef]
- Tran, N.P.; Gunasekara, C.; Law, D.W.; Houshyar, S.; Setunge, S.; Cwirzen, A. A critical review on drying shrinkage mitigation strategies in cement-based materials. J. Build. Eng. 2021, 38, 102210. [Google Scholar] [CrossRef]
- Zhang, T.; Shang, S.; Yin, F.; Aishah, A.; Salmiah, A.; Ooi, T. Adsorptive behavior of surfactants on surface of Portland cement. Cem. Concr. Res. 2001, 31, 1009–1015. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Z.; Yang, Y. Study of the Air-Entraining Behavior Based on the Interactions between Cement Particles and Selected Cationic, Anionic and Nonionic Surfactants. Materials 2020, 13, 3514. [Google Scholar] [CrossRef]
- Qomi, M.A.J.; Brochard, L.; Honorio, T.; Maruyama, I.; Vandamme, M. Advances in atomistic modeling and understanding of drying shrinkage in cementitious materials. Cem. Concr. Res. 2021, 148, 106536. [Google Scholar] [CrossRef]
- Wang, Y.; Li, F.; Fang, W.; Sun, C.; Men, Z. Study of hydrogen bonding interactions in ethylene glycol-water binary solutions by Raman spectroscopy. Spectrochim. Acta Part A 2021, 260, 119916. [Google Scholar] [CrossRef]
- Stetina, T.F.; Clark, A.E.; Li, X. X-ray absorption signatures of hydrogen-bond structure in water–alcohol solutions. Int. J. Quantum Chem. 2018, 119, e25802. [Google Scholar] [CrossRef]
- Hu, J.; Yamahara, H.; Liao, Z.; Yano, Y.; Tabata, H. Characterization of hydrogen bond network of waters around polyethylene glycol by broadband dielectric spectroscopy. Appl. Phys. Lett. 2022, 120, 023702. [Google Scholar] [CrossRef]
- Ma, Y.; Sha, S.; Zhou, B.; Lei, F.; Liu, Y.; Xiao, Y.; Shi, C. Adsorption and dispersion capability of polycarboxylate-based superplasticizers: A review. J. Sustain. Cem. Based 2021, 11, 319–344. [Google Scholar] [CrossRef]
- Ye, Q.; Geert, D.S. Different Effects of NSF and PCE Superplasticizer on Adsorption, Dynamic Yield Stress and Thixotropy of Cement Pastes. Materials 2018, 11, 695. [Google Scholar]
- Yoon, J.; Choi, B.I.; Kim, J.H. Adsorption properties of polycarboxylate ether-based superplasticizer on cement particles and their resultant dispersion. Front. Struct. Civ. Eng. 2022, 16, 506–514. [Google Scholar] [CrossRef]
- Giraudeau, C.; De Lacaillerie, J.-B.D.; Souguir, Z.; Nonat, A.; Flatt, R.J. Surface and Intercalation Chemistry of Polycarboxylate Copolymers in Cementitious Systems. J. Am. Ceram. Soc. 2010, 92, 2471–2488. [Google Scholar] [CrossRef]
- Matsuyama, H.; Young, J.F. Synthesis of calcium silicate hydrate/polymer complexes: Part, I. Anionic and nonionic polymers. J. Mater. Res. 1999, 14, 3379–3388. [Google Scholar] [CrossRef]
- Matsuyama, H.; Young, J.F. Synthesis of calcium silicate hydrate/polymer complexes: Part II. Cationic polymers and complex formation with different polymers. J. Mater. Res. 1999, 14, 3389–3396. [Google Scholar] [CrossRef]
- Lu, Z.; Kong, X.; Zhang, C.; Cai, Y. Effect of highly carboxylated colloidal polymers on cement hydration and interactions with calcium ions. Cem. Concr. Res. 2018, 113, 140–153. [Google Scholar] [CrossRef]
- Tan, H.; Guo, Y.; Ma, B.; Li, X.; Gu, B. Adsorbing behavior of polycarboxylate superplasticizer in the presence of the ester group in side chain. J. Dispers. Sci. Technol. 2017, 38, 743–749. [Google Scholar] [CrossRef]
| Chemical composition (wt %) | |||||||
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Na2Oeq | f-CaO |
| 22.93 | 4.29 | 2.89 | 66.23 | 1.92 | 0.35 | 0.70 | 0.64 |
| Mineral composition (wt %) | |||||||
| C3S | C2S | C3A | C4AF | ||||
| 58.78 | 21.38 | 6.49 | 8.77 | ||||
| Sample | Shrinkage-Reducing Monomer | Composition (Molar Ratio) | ||
|---|---|---|---|---|
| AA | Shrinkage-Reducing Monomer | TPEG | ||
| PCE | - | 3 | 0 | 1 |
| SRP-1 | SRM-1 | 2.5 | 0.5 | 1 |
| SRP-2 | SRM-2 | 2.5 | 0.5 | 1 |
| SRP-3 | SRM-3 | 2.5 | 0.5 | 1 |
| Sample | Cement/g | Water/g | PCE/g | SRP/g |
|---|---|---|---|---|
| PCE | 300 | 87 | 0.6 | - |
| SRP-1 | - | 0.6 | ||
| SRP-2 | - | 0.6 | ||
| SRP-3 | - | 0.6 |
| Sample | SRM-1 | SRM-2 | SRM-3 |
|---|---|---|---|
| Esterification rates/% | 90.95 | 92.04 | 91.80 |
| Sample | PCE | SRP-1 | SRP-2 | SRP-3 |
|---|---|---|---|---|
| Mn | 16,163 | 24,797 | 27,158 | 23,127 |
| Mw | 43,201 | 57,645 | 59,917 | 56,154 |
| Mz | 94,777 | 111,245 | 135,427 | 104,993 |
| PDI | 2.67 | 2.32 | 2.21 | 2.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Liu, X.; Xu, Y.; Lai, G.; Ding, Y.; Chen, Y.; Xia, C.; Wang, Z.; Cui, S. Synthesis and Performances of Shrinkage-Reducing Polycarboxylate Superplasticizer in Cement-Based Materials. Materials 2022, 15, 7002. https://doi.org/10.3390/ma15197002
Li S, Liu X, Xu Y, Lai G, Ding Y, Chen Y, Xia C, Wang Z, Cui S. Synthesis and Performances of Shrinkage-Reducing Polycarboxylate Superplasticizer in Cement-Based Materials. Materials. 2022; 15(19):7002. https://doi.org/10.3390/ma15197002
Chicago/Turabian StyleLi, Shiyu, Xiao Liu, Yurui Xu, Guanghong Lai, Yungchin Ding, Yichen Chen, Chunlei Xia, Ziming Wang, and Suping Cui. 2022. "Synthesis and Performances of Shrinkage-Reducing Polycarboxylate Superplasticizer in Cement-Based Materials" Materials 15, no. 19: 7002. https://doi.org/10.3390/ma15197002
APA StyleLi, S., Liu, X., Xu, Y., Lai, G., Ding, Y., Chen, Y., Xia, C., Wang, Z., & Cui, S. (2022). Synthesis and Performances of Shrinkage-Reducing Polycarboxylate Superplasticizer in Cement-Based Materials. Materials, 15(19), 7002. https://doi.org/10.3390/ma15197002







