Influence of Sulfates on Formation of Ettringite during Early C3A Hydration
Abstract
1. Introduction
2. Materials and Methods
2.1. Minerals
2.2. Methods
2.2.1. Preparation of the Suspensions
2.2.2. Stopping C3A Hydration
2.2.3. DSC Analysis
2.2.4. BET Method Using N2
2.2.5. Scanning Electron Microscopy (SEM)
3. Results and Discussion
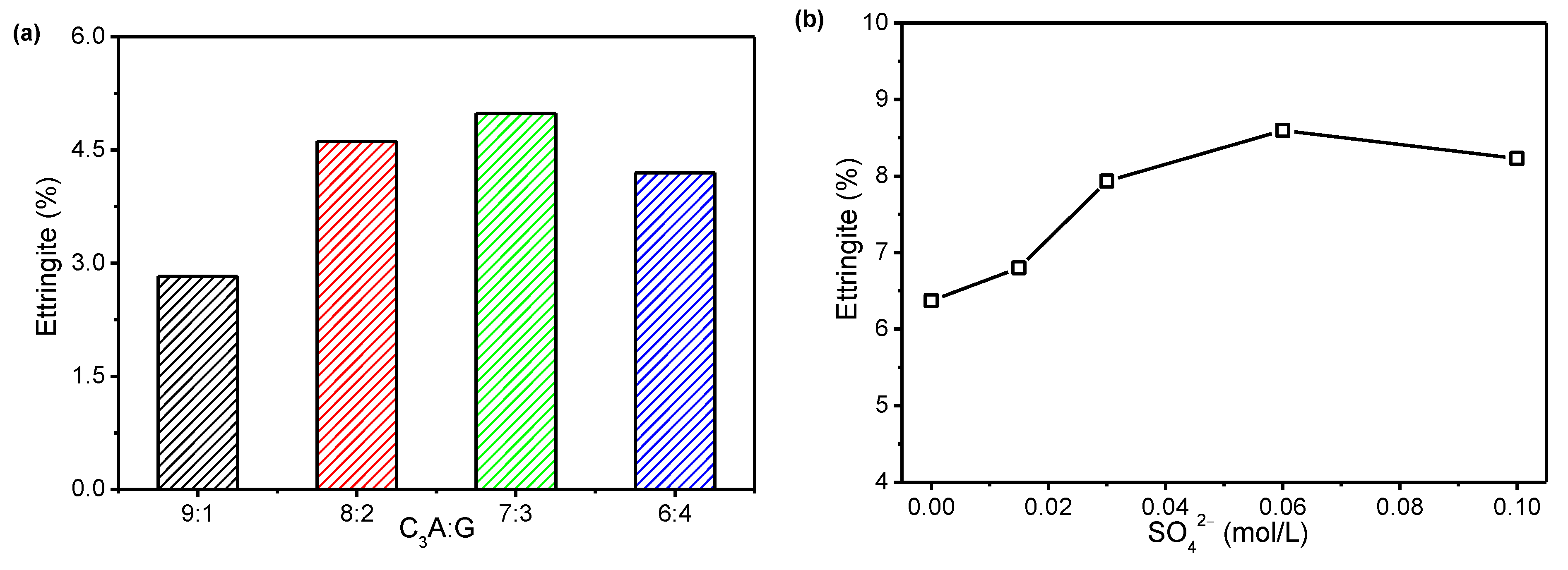
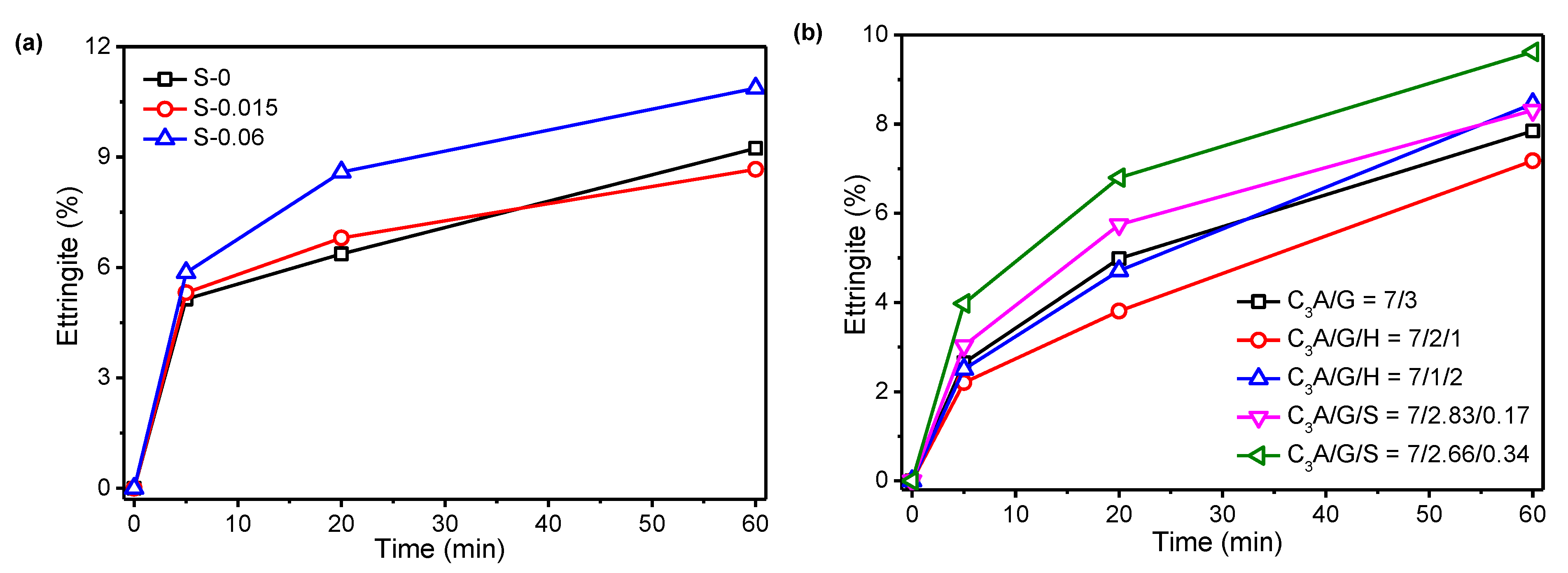
3.1. Effect of Sulfates on the C3A Hydration Kinetics
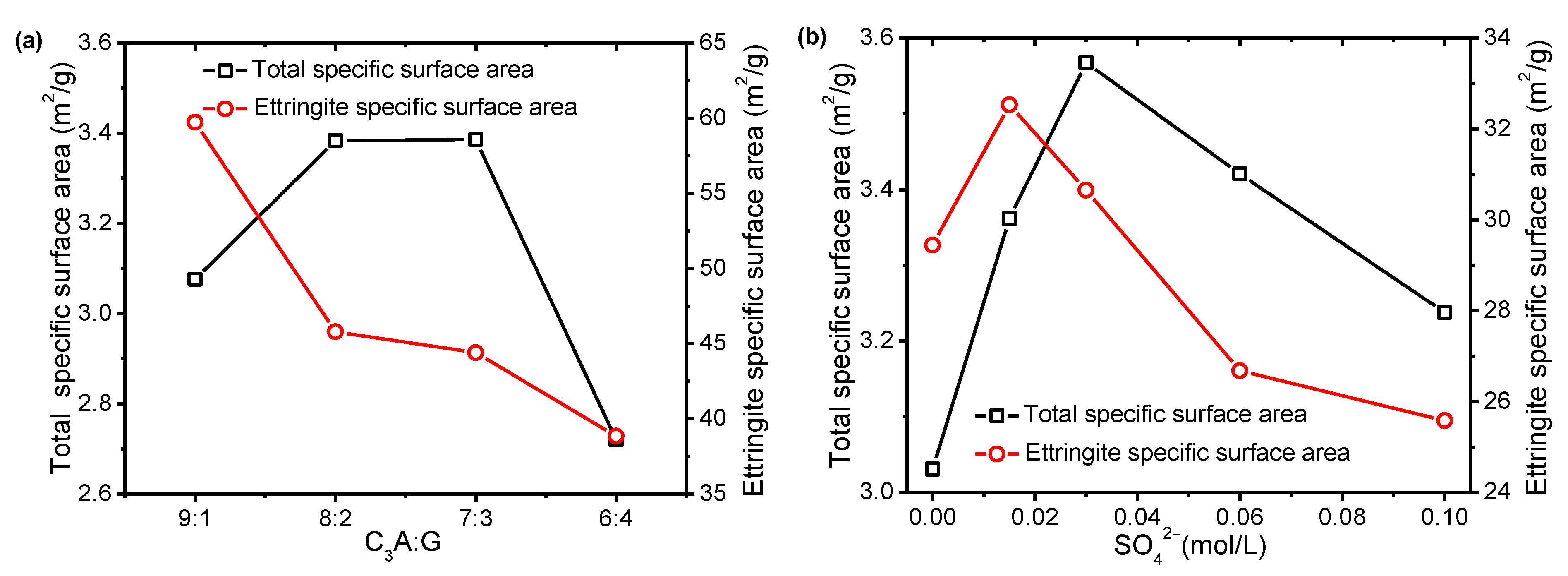
3.2. Effect of Sulfates on SSA along C3A Hydration Reaction
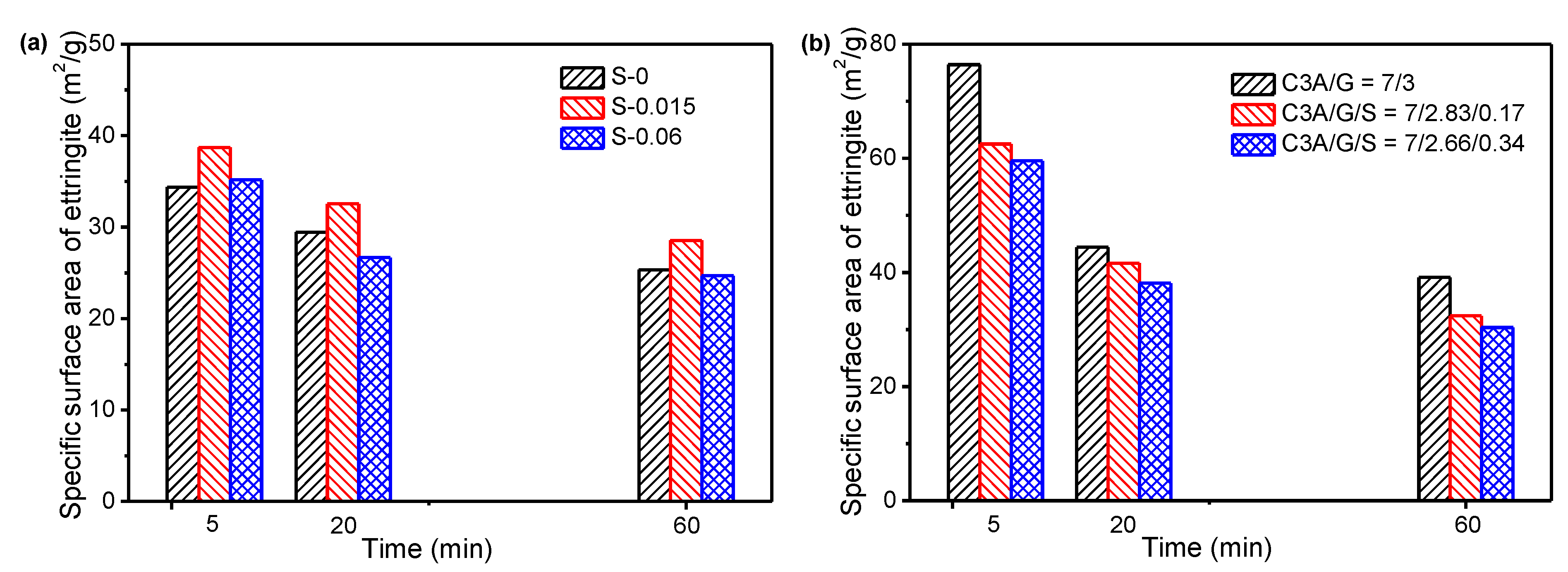
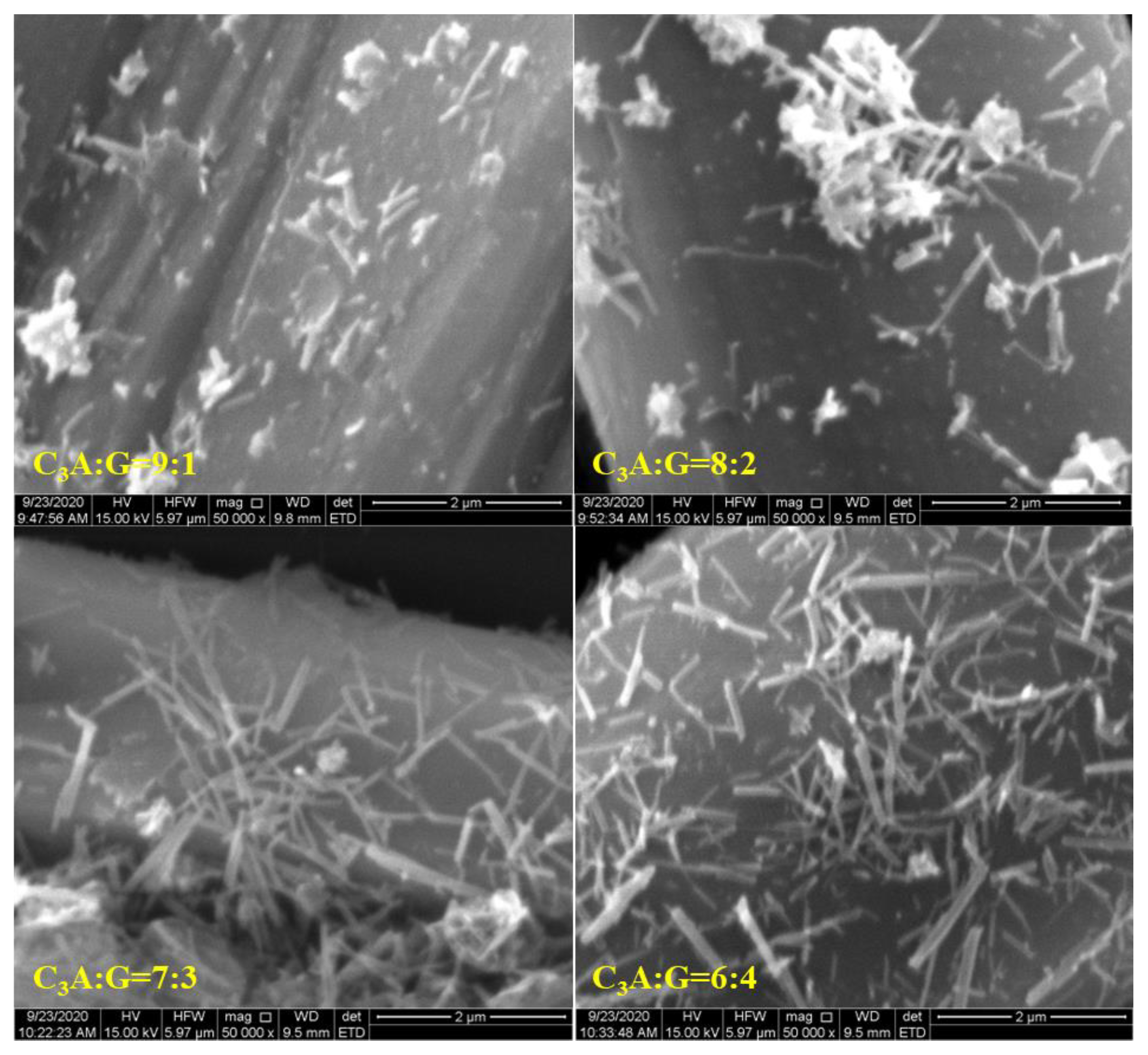
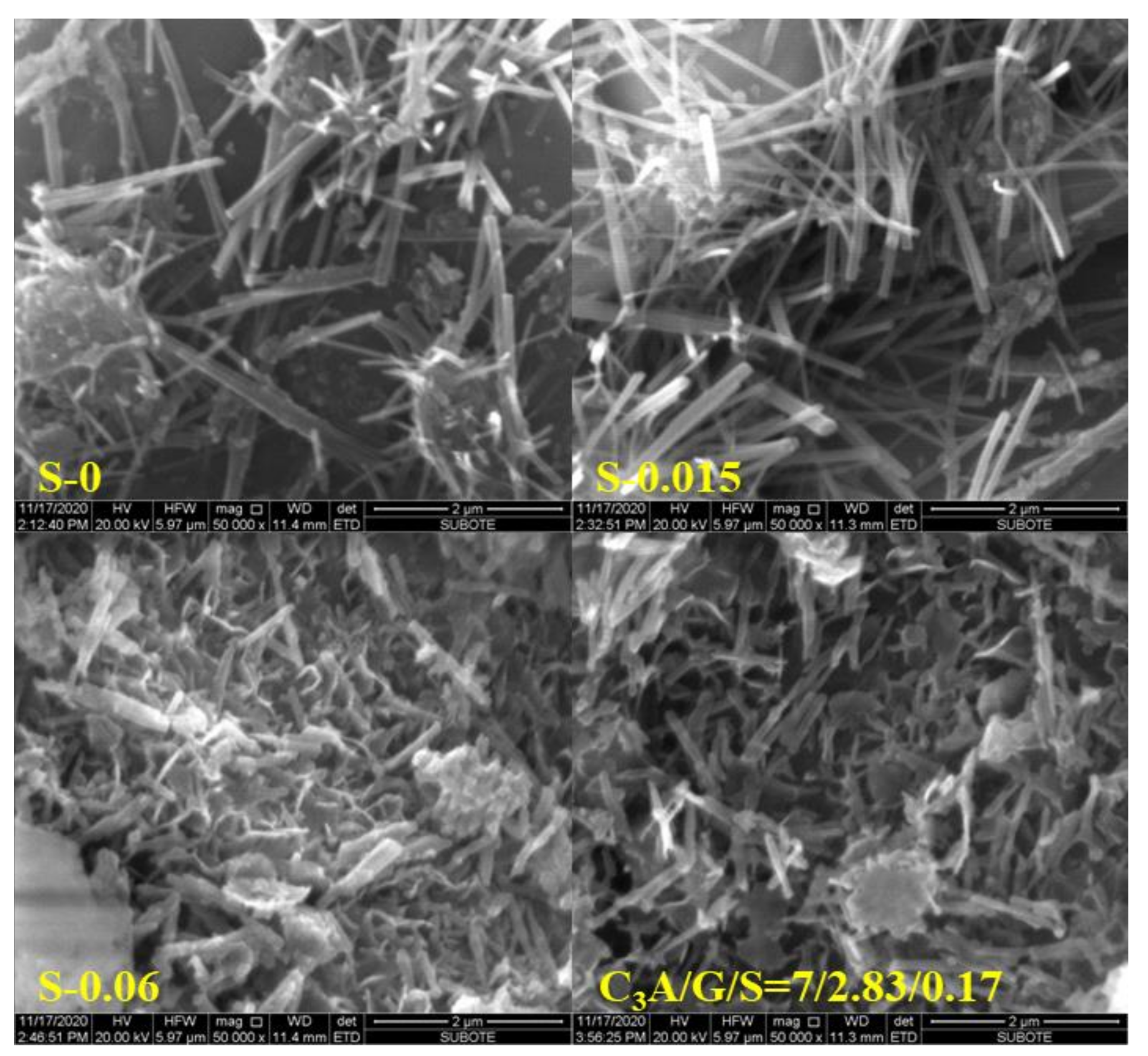
3.3. Effect of Sulfates on SSA and Morphology of Ettringite
4. Conclusions
- A large part of the ettringite precipitated within the first 20 min of the hydration reaction, whereas this reaction became very slow after 20 min. The amount of ettringite precipitated increased first and then decreased with the increasing proportion of gypsum in the C3A-gypsum system and SO42− concentration in the simulated solution. The rate of precipitation of ettringite in first 20 min was greatly promoted by incorporating a small amount of Na2SO4 in a solid in the C3A-gypsum system.
- The specific surface area of the hydrated sample strongly increased within the first 5 min, but obvious differences were found when different sulfates were added after 5 min. The specific surface area of all the C3A-gypsum systems, except those with hemihydrate, slowly increased after 5 min, whereas the specific surface area of the C3A-gypsum system with hemihydrate decreased at 20 min and then increased.
- The specific surface area of ettringite gradually decreased along the C3A hydration reaction. For all the C3A-gypsum systems, except those with hemihydrate, both the slowing down of the precipitation of ettringite and the growth of ettringite resulted in the slow growth of the specific surface area after 5 min. For the C3A-gypsum system with hemihydrate, two processes of ettringite precipitation and gypsum precipitation occurred. The nucleation and growth of ettringite and gypsum resulted in the complex changes in specific surface area, which first increased at the very beginning, then decreased and, finally, increased. In addition, different hydration environments had a very significant effect on the morphology of ettringite. The specific surface area of ettringite in the simulated solution was significantly lower than that in deionized water. The specific surface area of ettringite could be significantly reduced by adding a small amount of Na2SO4 in a solid.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taylor, H.F.W. Cement Chemistry; Academic Press: Cambridge, MA, USA, 1997. [Google Scholar]
- Collepardi, M.; Baldini, G.; Pauri, M.; Corradi, M. Tricalcium aluminate hydration in the presence of lime, gypsum or sodium sulfate. Cem. Concr. Res. 1978, 8, 571–580. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Juilland, P.; Monteiro, P.J.M. Advances in understanding hydration of Portland cement. Cem. Concr. Res. 2015, 78, 38–56. [Google Scholar] [CrossRef]
- Scrivener, K.; Pratt, P. Microstructural studies of the hydration of C3A and C4AF independently and in cement paste. Proc. Br. Ceram. Soc. 1984, 35, 207–219. [Google Scholar]
- Minard, H.; Garrault, S.; Regnaud, L.; Nonat, A. Mechanisms and parameters controlling the tricalcium aluminate reactivity in the presence of gypsum. Cem. Concr. Res. 2007, 37, 1418–1426. [Google Scholar] [CrossRef]
- Zingg, A.; Winnefeld, F.; Holzer, L.; Pakusch, J.; Becker, S.; Gauckler, L. Adsorption of polyelectrolytes and its influence on the rheology, zeta potential, and microstructure of various cement and hydrate phases. J. Colloid Interface Sci. 2008, 323, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Quennoz, A.; Scrivener, K.L. Hydration of C3A-gypsum systems. Cem. Concr. Res. 2012, 42, 1032–1041. [Google Scholar] [CrossRef]
- Kirchheim, A.P.; Rodríguez, E.D.; Myers, R.J.; Gobbo, L.A.; Monteiro, P.J.M.; Molin, D.C.C.D.; de Souza, R.B.; Cincotto, M.A. Effect of gypsum on the early hydration of cubic and Na-doped orthorhombic tricalcium aluminate. Materials 2018, 11, 568. [Google Scholar] [CrossRef]
- Joseph, S.; Skibsted, J.; Cizer, Ö. A quantitative study of the C3A hydration. Cem. Concr. Res. 2019, 115, 145–159. [Google Scholar] [CrossRef]
- Black, L.; Breen, C.; Yarwood, J.; Deng, C.; Phipps, J.; Maitland, G. Hydration of tricalcium aluminate (C3A) in the presence and absence of gypsum—Studied by Raman spectroscopy and X-ray diffraction. J. Mater. Chem. 2006, 16, 1263–1272. [Google Scholar] [CrossRef]
- Meredith, P.; Donald, A.M.; Meller, N.; Hall, C. Tricalcium aluminate hydration: Microstructural observations by in-situ electron microscopy. J. Mater. Sci. 2004, 39, 997–1005. [Google Scholar] [CrossRef]
- Tian, H.; Kong, X.; Su, T.; Wang, D. Comparative study of two PCE superplasticizers with varied charge density in Portland cement and sulfoaluminate cement systems. Cem. Concr. Res. 2019, 115, 43–58. [Google Scholar] [CrossRef]
- Dalas, F.; Pourchet, S.; Rinaldi, D.; Nonat, A.; Sabio, S.; Mosquet, M. Modification of the rate of formation and surface area of ettringite by polycarboxylate ether superplasticizers during early C3A-CaSO4 hydration. Cem. Concr. Res. 2015, 69, 105–113. [Google Scholar] [CrossRef]
- Zhang, Z.; Scherer, G.W.; Bauer, A. Morphology of cementitious material during early hydration. Cem. Concr. Res. 2018, 107, 85–100. [Google Scholar] [CrossRef]
- Fung, W.W.S.; Kwan, A.K.H. Role of water film thickness in rheology of CSF mortar. Cem. Concr. Compos. 2010, 32, 255–264. [Google Scholar] [CrossRef]
- Bensted, J. Effects of the clinker-gypsum grinding temperature upon early hydration of Portland cement. Cem. Concr. Res. 1982, 12, 341–348. [Google Scholar] [CrossRef]
- Sakai, E.; Kang, J.K.; Daimon, M. Influence of superplasticizers on the very early hydration of Ca3Al2O6 in the presence of gypsum, CaSO4 0.5H2O and CaO. Cem. Sci. Concr. Technol. 2002, 56, 36–41. [Google Scholar]
- Geng, G.; Myers, R.J.; Yu, Y.-S.; Shapiro, D.A.; Winarski, R.; Levitz, P.E.; Kilcoyne, D.A.L.; Monteiro, P.J.M. Synchrotron X-ray nanotomographic and spectromicroscopic study of the tricalcium aluminate hydration in the presence of gypsum. Cem. Concr. Res. 2018, 111, 130–137. [Google Scholar] [CrossRef]
- Myers, R.J.; Geng, G.; Rodriguez, E.D.; da Rosa, P.; Kirchheim, A.P.; Monteiro, P.J.M. Solution chemistry of cubic and orthorhombic tricalcium aluminate hydration. Cem. Concr. Res. 2017, 100, 176–185. [Google Scholar] [CrossRef]
- Neto, J.S.A.; de Matos, P.R.; Angeles, G.; Campos, C.E.; Gleize, P.J.; Monteiro, P.J.; Kirchheim, A.P. The role of sodium and sulfate sources on the rheology and hydration of C3A polymorphs. Cem. Concr. Res. 2022, 151, 106639. [Google Scholar] [CrossRef]
- Holly, R.; Peemoeller, H.; Zhang, M.; Reardon, E.; Hansson, C.M. Magnetic resonance in situ study of C3A hydration in the presence of gypsum. J. Am. Ceram. Soc. 2006, 89, 1022–1027. [Google Scholar] [CrossRef]
- Pourchet, S.; Regnaud, L.; Perez, J.P.; Nonat, A. Early C3A hydration in the presence of different kinds of calcium sulfate. Cem. Concr. Res. 2009, 39, 989–996. [Google Scholar] [CrossRef]
- Liu, X.; Feng, P.; Chen, L.; Ye, S. The role of sulfate ions in tricalcium aluminate hydration: New insights. Cem. Concr. Res. 2020, 130, 105973. [Google Scholar] [CrossRef]
- Mantellato, S.; Palacios, M.; Flatt, R.J. Impact of sample preparation on the specific surface area of synthetic ettringite. Cem. Concr. Res. 2016, 86, 20–28. [Google Scholar] [CrossRef]
- Marchon, D.; Juilland, P.; Gallucci, E.; Frunz, L.; Flatt, R.J. Molecular and submolecular scale effects of comb-copolymers on tri-calcium silicate reactivity: Toward molecular design. J. Am. Ceram. Soc. 2017, 100, 817–841. [Google Scholar] [CrossRef]
- Mantellato, S.; Palacios, M.; Flatt, R.J. Reliable specific surface area measurements on anhydrous cements. Cem. Concr. Res. 2015, 67, 286–291. [Google Scholar] [CrossRef]
- Zingg, A.; Holzer, L.; Kaech, A.; Winnefeld, F.; Pakusch, J.; Becker, S.; Gauckler, L. The microstructure of dispersed and non-dispersed fresh cement pastes-new insight by cryo-microscopy. Cem. Concr. Res. 2008, 38, 522–529. [Google Scholar] [CrossRef]
- Hesse, C.; Goetz-Neunhoeffer, F.; Neubauer, J. A new approach in quantitative in-situ XRD of cement pastes: Correlation of heat flow curves with early hydration reactions. Cem. Concr. Res. 2011, 41, 123–128. [Google Scholar] [CrossRef]
- Wang, Y.W.; Kim, Y.-Y.; Christenson, H.K.; Meldrum, F.C. A new precipitation pathway for calcium sulfate dihydrate (gypsum) via amorphous and hemihydrate intermediates. Chem. Commun. 2012, 48, 504–506. [Google Scholar] [CrossRef]
- Ferrari, L.; Kaufmann, J.; Winnefeld, F.; Plank, J. Impact of particle size on interaction forces between ettringite and dispersing comb-polymers in various electrolyte solutions. J. Colloid Interface Sci. 2014, 419, 17–24. [Google Scholar] [CrossRef]


| Code | Na2SO4 (mol/L) | NaNO3 (mol/L) | Na+ (mol/L) | SO42− (mol/L) |
|---|---|---|---|---|
| S-0 | 0 | 0.2 | 0.2 | 0 |
| S-0.015 mol/L | 0.015 | 0.17 | 0.2 | 0.015 |
| S-0.03 mol/L | 0.03 | 0.14 | 0.2 | 0.03 |
| S-0.06 mol/L | 0.06 | 0.08 | 0.2 | 0.06 |
| S-0.1 mol/L | 0.1 | 0 | 0.2 | 0.1 |
| Code | C3A% | G% | PC/% | Mix Water |
|---|---|---|---|---|
| C3A/G = 90/10 | 90 | 10 | 0.1 | Deionized water |
| C3A/G = 80/20 | 80 | 20 | 0.1 | |
| C3A/G = 70/30 | 70 | 30 | 0.1 | |
| C3A/G = 60/40 | 60 | 40 | 0.1 | |
| S-0 | 70 | 30 | 0.1 | S-0 |
| S-0.015 | 70 | 30 | 0.1 | S-0.015 |
| S-0.03 | 70 | 30 | 0.1 | S-0.03 |
| S-0.06 | 70 | 30 | 0.1 | S-0.06 |
| S-0.1 | 70 | 30 | 0.1 | S-0.1 |
| Code | C3A/% | G/% | H/% | S/% | PC/% | Mix Water |
|---|---|---|---|---|---|---|
| S0 | 70 | 30 | 0 | 0 | 0.1 | S0 |
| S0-0.015 | 70 | 30 | 0 | 0 | 0.1 | S0-0.015 |
| S0-0.06 | 70 | 30 | 0 | 0 | 0.1 | S0-0.06 |
| C3A/G = 70/30 | 70 | 30 | 0 | 0 | 0.1 | Deionized water |
| C3A/G/H = 70/20/10 | 70 | 20 | 10 | 0 | 0.1 | |
| C3A/G/H = 70/10/20 | 70 | 10 | 20 | 0 | 0.1 | |
| C3A/G/S = 70/28.3/1.7 | 70 | 28.3 | 0 | 1.7 | 0.1 | |
| C3A/G/S = 70/26.4/3.4 | 70 | 26.4 | 0 | 3.4 | 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Zhang, Q.; Shu, X.; Wang, X.; Ran, Q. Influence of Sulfates on Formation of Ettringite during Early C3A Hydration. Materials 2022, 15, 6934. https://doi.org/10.3390/ma15196934
Yang Y, Zhang Q, Shu X, Wang X, Ran Q. Influence of Sulfates on Formation of Ettringite during Early C3A Hydration. Materials. 2022; 15(19):6934. https://doi.org/10.3390/ma15196934
Chicago/Turabian StyleYang, Yong, Qianqian Zhang, Xin Shu, Xiumei Wang, and Qianping Ran. 2022. "Influence of Sulfates on Formation of Ettringite during Early C3A Hydration" Materials 15, no. 19: 6934. https://doi.org/10.3390/ma15196934
APA StyleYang, Y., Zhang, Q., Shu, X., Wang, X., & Ran, Q. (2022). Influence of Sulfates on Formation of Ettringite during Early C3A Hydration. Materials, 15(19), 6934. https://doi.org/10.3390/ma15196934







