Halochromic Inks Applied on Cardboard for Food Spoilage Monitorization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of pH-Sensitive Aqueous Solutions and Application on Paper Substrates
2.3. Preparation of pH-Sensitive Inks and Application on Cardboard Substrates
2.4. Characterization of pH-Sensitive Aqueous Solutions and Paper Substrates for Color
2.5. Adhesion and Abrasion Properties of Cardboard Substrates
2.6. Evaluation of Cardboard Substrates’ Sensitivity to Gaseous Atmospheres
2.7. Cardboard Detection of Food Spoilage
2.8. Statistical Analysis
3. Results and Discussion
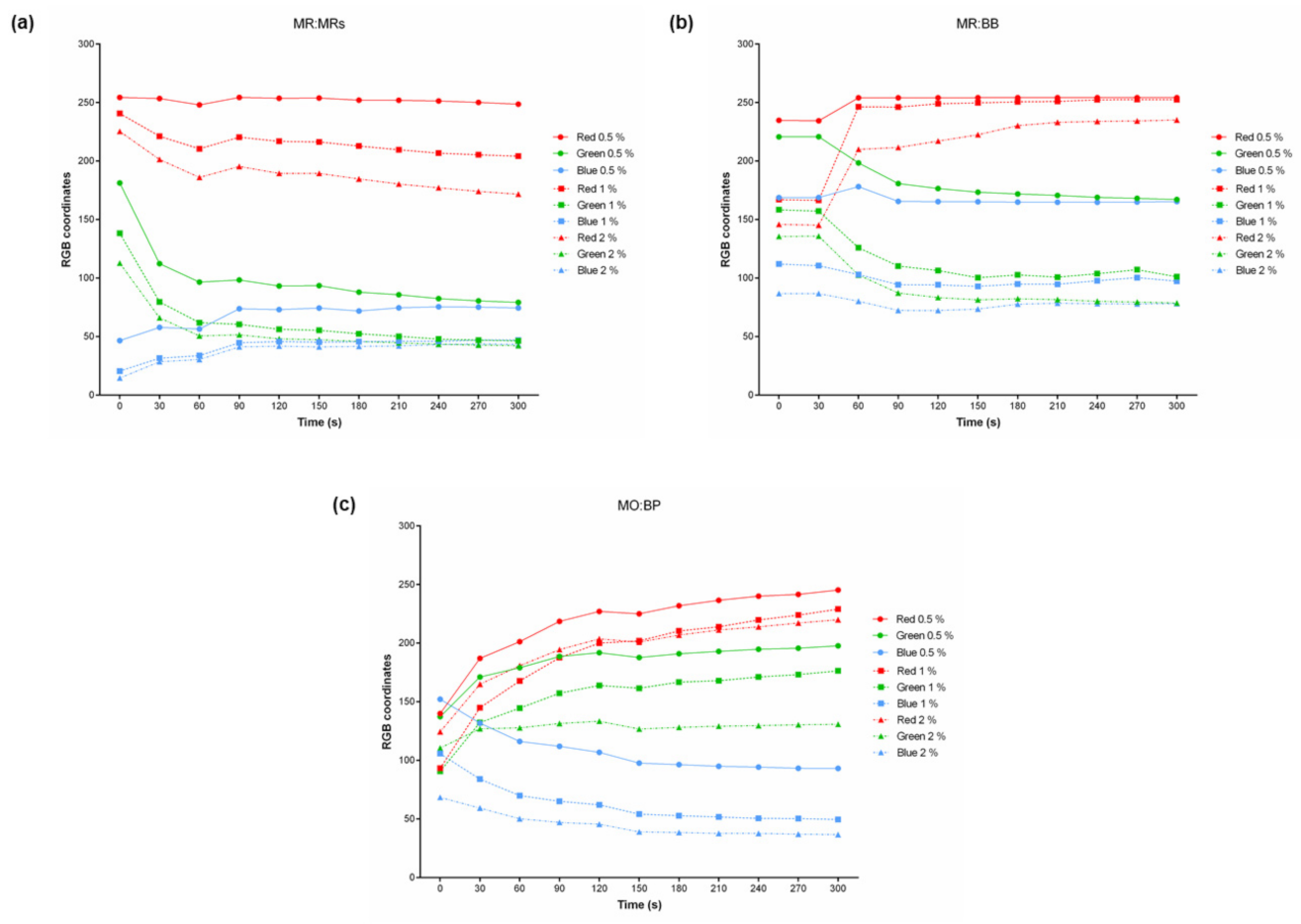
3.1. Measurement of RGB Coordinates of pH-Sensitive Aqueous Solutions
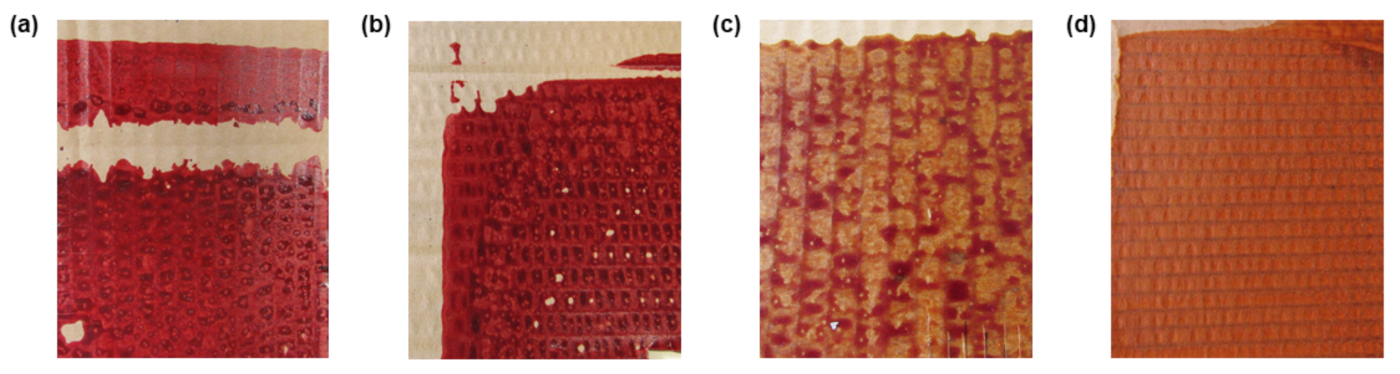
3.2. Measurement of RGB Coordinates of pH-Sensitive Paper
3.3. Influence of Concentration on Color and Time-Response of the Samples
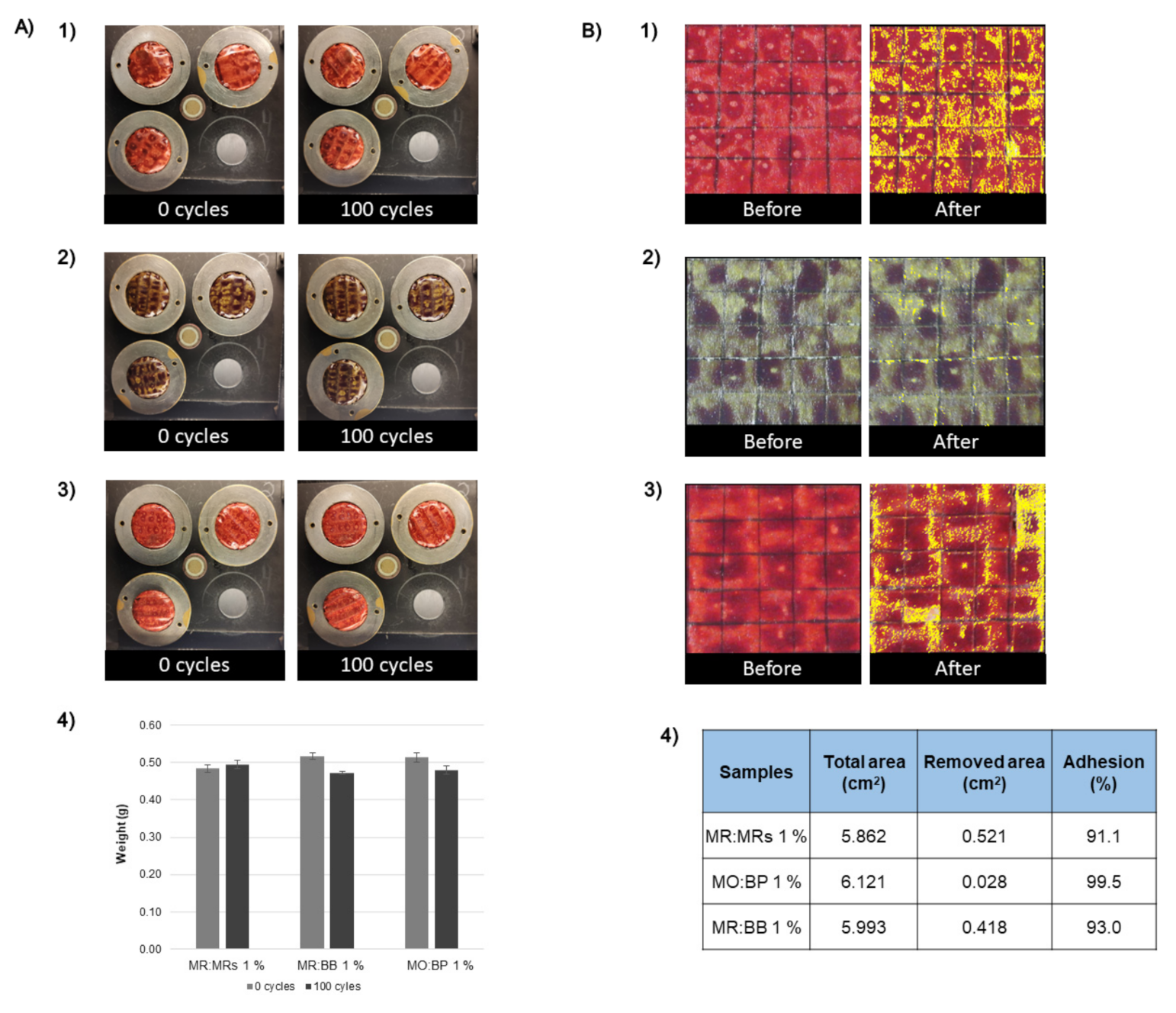
3.4. Adhesive and Abrasive Properties
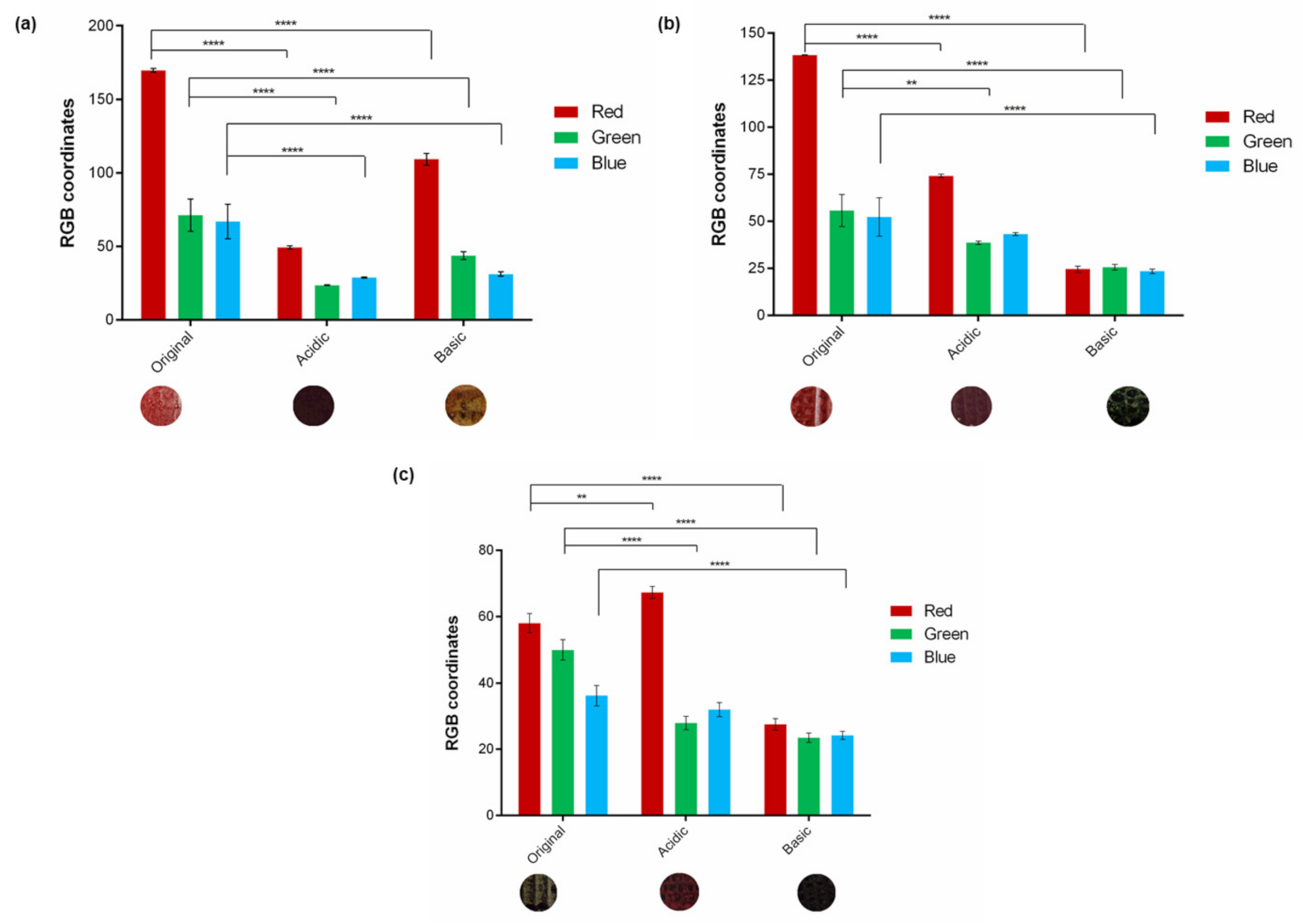
3.5. Measurement of Cardboard Sensitivity to Gaseous Atmospheres
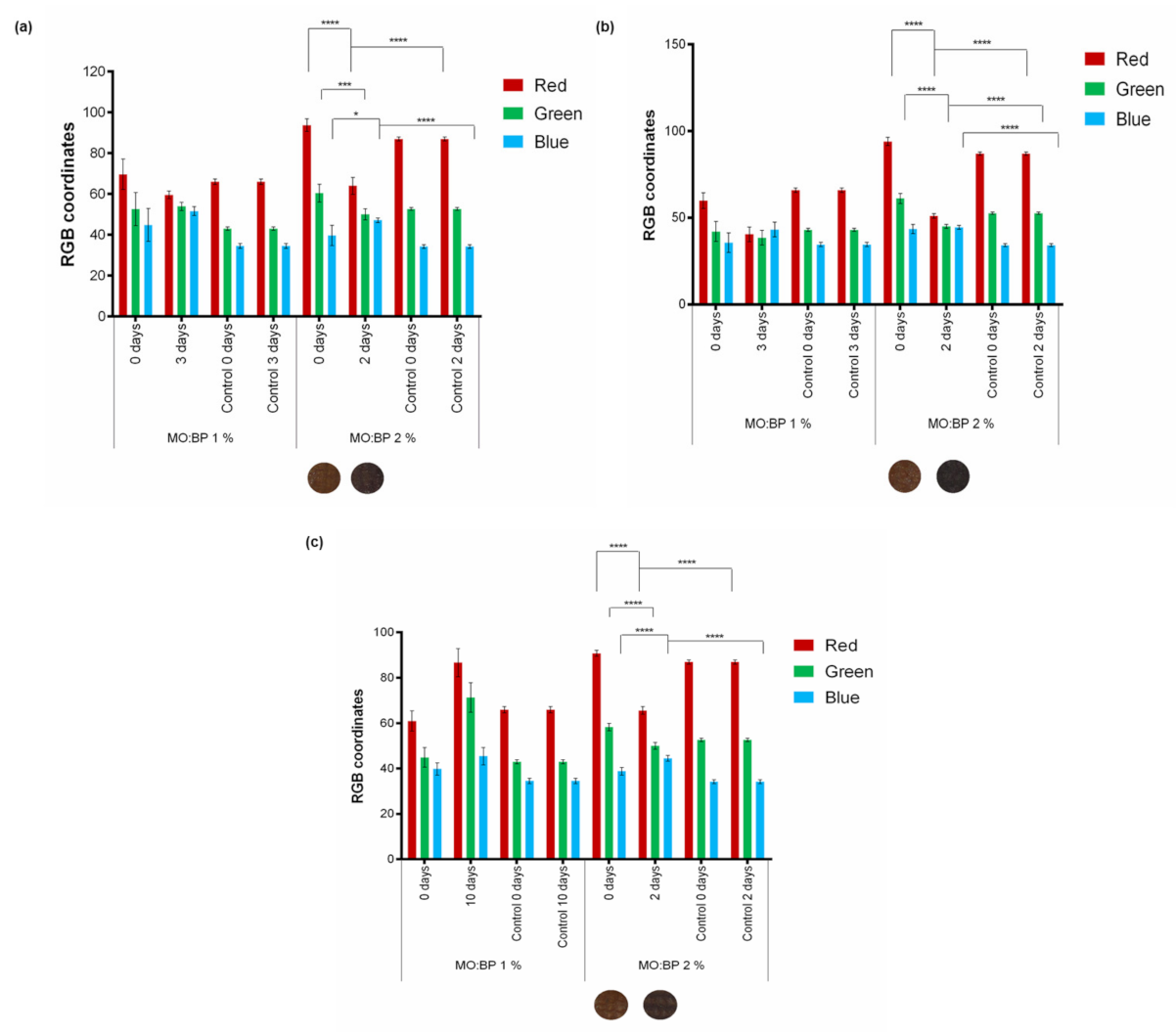
3.6. Cardboard Detection of Food Spoilage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Population Division United Nations, Department of Economic and Social Affairs. World Population Prospects 2019: Highlights (ST/ESA/SER.A/423). 2019. Available online: https://population.un.org/wpp/publications/files/wpp2019_highlights.pdf (accessed on 3 September 2021).
- Skaf, L.; Franzese, P.; Capone, R.; Buonocore, E. Unfolding hidden environmental impacts of food waste: An assessment for fifteen countries of the world. J. Clean. Prod. 2021, 310, 127523. [Google Scholar] [CrossRef]
- United Nations Environment Programme. Food Waste Index Report 2021. 2021. Available online: https://www.unep.org/resources/report/unep-food-waste-index-report-2021 (accessed on 3 September 2021).
- Food and Agriculture Organization of the United Nations. EU Platform on Food Losses and Food Waste. 2016. Available online: http://www.fao.org/family-farming/detail/en/c/428345/ (accessed on 3 September 2021).
- Rodríguez-Félix, F.; Corte-Tarazón, J.A.; Rochín-Wong, S.; Fernández-Quiroz, J.D.; Garzón-García, A.M.; Santos-Sauceda, I.; Plascencia-Martínez, D.F.; Chan-Chan, L.H.; Vásquez-López, C.; Barreras-Urbina, C.G.; et al. Physicochemical, structural, mechanical and antioxidant properties of zein films incorporated with no-ultrafiltered and ultrafiltered betalains extract from the beetroot (Beta vulgaris) bagasse with potential application as active food packaging. J. Food Eng. 2022, 334, 111153. [Google Scholar] [CrossRef]
- Schaefer, D.; Cheung, W.M. Smart Packaging: Opportunities and Challenges. Procedia CIRP 2018, 72, 1022–1027. [Google Scholar] [CrossRef]
- Müller, P.; Schmid, M. Intelligent Packaging in the Food Sector: A Brief Overview. Foods 2019, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Guidelines on submission of a dossier for safety evaluation by the EFSA of active or intelligent substances present in active and intelligent materials and articles intended to come into contact with food. EFSA J. 2009, 7, 4. [Google Scholar]
- Ghaani, M.; Cozzolino, C.A.; Castelli, G.; Farris, S. An overview of the intelligent packaging technologies in the food sector. Trends Food Sci. Technol. 2016, 51, 1–11. [Google Scholar] [CrossRef]
- Ahmed, I.; Lin, H.; Zou, L.; Li, Z.; Brody, A.L.; Qazi, I.M.; Lv, L.; Pavase, T.R.; Khan, M.U.; Khan, S.; et al. An overview of smart packaging technologies for monitoring safety and quality of meat and meat products. Packag. Technol. Sci. 2018, 31, 449–471. [Google Scholar] [CrossRef]
- Kuswandi, B.; Nurfawaidi, A. On-package dual sensors label based on pH indicators for real-time monitoring of beef freshness. Food Control 2017, 82, 91–100. [Google Scholar] [CrossRef]
- Morsy, M.K.; Zór, K.; Kostesha, N.; Alstrøm, T.S.; Heiskanen, A.; El-Tanahi, H.; Sharoba, A.; Papkovsky, D.; Larsen, J.; Khalaf, H.; et al. Development and validation of a colorimetric sensor array for fish spoilage monitoring. Food Control 2016, 60, 346–352. [Google Scholar] [CrossRef]
- Poghossian, A.; Geissler, H.; Schöning, M.J. Rapid methods and sensors for milk quality monitoring and spoilage detection. Biosens. Bioelectron. 2019, 140, 111272. [Google Scholar] [CrossRef]
- Kim, Y.H.; Yang, Y.J.; Kim, J.S.; Choi, D.S.; Park, S.H.; Jin, S.Y.; Park, J.S. Non-destructive monitoring of apple ripeness using an aldehyde sensitive colorimetric sensor. Food Chem. 2018, 267, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Z.; Zhang, M.; Bhandari, B.; Guo, Z. Applicability of a colorimetric indicator label for monitoring freshness of fresh-cut green bell pepper. Postharvest Biol. Technol. 2018, 140, 85–92. [Google Scholar] [CrossRef]
- Rhyne, T.M. Introduction to Color Appearance Models. In Applying Color Theory to Digital Media and Visualization; CRC Press: Boca Raton, FL, USA,, 2017; pp. 1–181. [Google Scholar]
- ASTM D5264—98; Standard Practice for Abrasion Resistance of Printed Materials by the Sutherland Rub Tester. ASTM International: West Conshohocken, PA, USA, 2004; pp. 1–3. [CrossRef]
- ASTM D3359-17; Standard Test Methods for Measuring Adhesion by Tape Test. ASTM International: West Conshohocken, PA, USA, 2012; pp. 1–9. [CrossRef]
- Thomas, O.; Brogat, M. Organic Constituents. In UV-Visible Spectrophotometry of Water and Wastewater; Elsevier: Amsterdam, The Netherlands, 2017; pp. 73–138. [Google Scholar]
- Helmenstine, A. pH Indicator Chart—Colors and Ranges. Science Notes. 2020. Available online: https://sciencenotes.org/ph-indicator-chart-colors-and-ranges/ (accessed on 5 September 2022).
- Shimada, T.; Hasegawa, T. Determination of equilibrium structures of bromothymol blue revealed by using quantum chemistry with an aid of multivariate analysis of electronic absorption spectra. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 185, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Andronic, L.; Duta, A. The influence of TiO2 powder and film on the photodegradation of methyl orange. Mater. Chem. Phys. 2008, 112, 1078–1082. [Google Scholar] [CrossRef]
- Khanjanzadeh, H.; Park, B.-D. Covalent immobilization of bromocresol purple on cellulose nanocrystals for use in pH-responsive indicator films. Carbohydr. Polym. 2021, 273, 118550. [Google Scholar] [CrossRef] [PubMed]
- Tawarah, K.M.; Abu-Shamleh, H.M. A spectrophotometric study of the tautomeric and acid- base equilibria of methyl orange and methyl yellow in aqueous acidic solutions. Dye. Pigment. 1991, 16, 241–251. [Google Scholar] [CrossRef]
- Khan, M.A.; Rahman, M.M.; Gosh, M.K.; Chowdhury, T.A. Mechanical properties study of photocured paperboard surface treated with aliphatic epoxy diacrylate. J. Appl. Polym. Sci. 2003, 87, 1774–1780. [Google Scholar] [CrossRef]
- Claus, P.; Cattenoz, T.; Landaud, S.; Chaillou, S.; Peron, A.-C.; Coeuret, G.; Slimani, S.; Livache, T.; Demarigny, Y.; Picque, D. Discrimination of spoiled beef and salmon stored under different atmospheres by an optoelectronic nose. Comparison with GC-MS measurements. Futur. Foods 2021, 5, 100106. [Google Scholar] [CrossRef]
- Tavares, J.; Martins, A.; Fidalgo, L.; Lima, V.; Amaral, R.; Pinto, C.; Silva, A.; Saraiva, J. Fresh Fish Degradation and Advances in Preservation Using Physical Emerging Technologies. Foods 2021, 10, 780. [Google Scholar] [CrossRef]
- Shao, P.; Liu, L.; Yu, J.; Lin, Y.; Gao, H.; Chen, H.; Sun, P. An overview of intelligent freshness indicator packaging for food quality and safety monitoring. Trends Food Sci. Technol. 2021, 118, 285–296. [Google Scholar] [CrossRef]
- Casaburi, A.; Piombino, P.; Nychas, G.-J.; Villani, F.; Ercolini, D. Bacterial populations and the volatilome associated to meat spoilage. Food Microbiol. 2015, 45, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Mikš-Krajnik, M.; Yoon, Y.-J.; Ukuku, D.O.; Yuk, H.-G. Volatile chemical spoilage indexes of raw Atlantic salmon (Salmo salar ) stored under aerobic condition in relation to microbiological and sensory shelf lives. Food Microbiol. 2016, 53, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Kuswandi, B.; Kinanti, D.P.; Jayus, J.; Abdullah, A.; Heng, L. Simple and Low-cost freshness indicator for strawberries packaging. Acta Manila. Ser. A 2013, 61, 147–159. [Google Scholar]
- Schaude, C.; Meindl, C.; Fröhlich, E.; Attard, J.; Mohr, G.J. Developing a sensor layer for the optical detection of amines during food spoilage. Talanta 2017, 170, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.H.; Naficy, S.; McConchie, R.; Dehghani, F.; Chandrawati, R. Polydiacetylene-based sensors to detect food spoilage at low temperatures. J. Mater. Chem. C 2019, 7, 1919–1926. [Google Scholar] [CrossRef]
- Sutthasupa, S.; Padungkit, C.; Suriyong, S. Colorimetric ammonia (NH3) sensor based on an alginate-methylcellulose blend hydrogel and the potential opportunity for the development of a minced pork spoilage indicator. Food Chem. 2021, 362, 130151. [Google Scholar] [CrossRef] [PubMed]
- Teymouri, Z.; Shekarchizadeh, H. A colorimetric indicator based on copper nanoparticles for volatile sulfur compounds to monitor fish spoilage in intelligent packaging. Food Packag. Shelf Life 2022, 33, 100884. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leite, L.; Boticas, I.; Navarro, M.; Nobre, L.; Bessa, J.; Cunha, F.; Neves, P.; Fangueiro, R. Halochromic Inks Applied on Cardboard for Food Spoilage Monitorization. Materials 2022, 15, 6431. https://doi.org/10.3390/ma15186431
Leite L, Boticas I, Navarro M, Nobre L, Bessa J, Cunha F, Neves P, Fangueiro R. Halochromic Inks Applied on Cardboard for Food Spoilage Monitorization. Materials. 2022; 15(18):6431. https://doi.org/10.3390/ma15186431
Chicago/Turabian StyleLeite, Liliana, Inês Boticas, Miguel Navarro, Luís Nobre, João Bessa, Fernando Cunha, Pedro Neves, and Raúl Fangueiro. 2022. "Halochromic Inks Applied on Cardboard for Food Spoilage Monitorization" Materials 15, no. 18: 6431. https://doi.org/10.3390/ma15186431
APA StyleLeite, L., Boticas, I., Navarro, M., Nobre, L., Bessa, J., Cunha, F., Neves, P., & Fangueiro, R. (2022). Halochromic Inks Applied on Cardboard for Food Spoilage Monitorization. Materials, 15(18), 6431. https://doi.org/10.3390/ma15186431







