1. Introduction
Semiconductor electronics based on planar technologies led to the rapid development of the industry in the second half of the last century [
1]. The breakthrough in modern technologies is mainly associated with the unique properties of nanoobjects [
2]. With all the advantages of nanoobjects, there is a technological complexity involved in their integration into multi-level systems of a larger scale, for example, sensor devices. For this reason, planar structures remain indispensable in many areas of modern technology [
3]. Zinc oxide (ZnO), due to its unique combination of electrophysical, optical, piezoelectric properties, remains one of the most studied film materials [
4].
In biodiagnostics, it has been proposed to implement ZnO-based sensors that use an amplification of the fluorescence and the electrochemical responses from biological objects [
5]. Previously, ZnO films were used in “lab-on-a-chip” technology and biosensors based on acoustic waves [
6]. Furthermore, the development of gas sensors on ZnO films that use various physical effects for sensing is also topical [
7,
8,
9].
Another classical area of application of ZnO film structures is the development of light-emitting devices operating in the near-UV and violet spectral ranges [
10,
11,
12], including optical amplifiers and generators [
13,
14,
15]. In this context, among the advantages of ZnO are their high exciton binding energy (60 meV) and refractive index, their radiation resistance, and their low manufacturing cost [
16,
17]. Moreover, ZnO has become a model material for studying excitonic processes in semiconductors. In addition, due to the high sensitivity of the physical properties of micro/nano-optical resonators and lasers to changes near their surface, laser effects in ZnO can also be used in sensing. In particular, whispering gallery mode (WGM) lasing in ZnO microrods and microwires can be utilized for stress [
18], biological [
19], gas [
20], and temperature [
21] sensing.
Despite the wide range of potential applications, film structures are mainly introduced into individual unique devices and this is mainly due to the great influence of the synthesis conditions on the properties of the films and their low reproducibility. In addition, there is a direct dependence of a film’s properties on its thickness, orientation, and substrate material. All this imposes high requirements on the technology used for the fabrication of ZnO thin films. In this regard, it is very important to develop new directions for the use of ZnO films and methods which are less dependent on the structural-phase composition, synthesis parameters, and material features.
In [
22], the authors proposed the use of the luminescent properties of nanoporous aluminum oxide as markers for biological systems. The idea is to represent the set of oscillations observed in the luminescence spectrum in the form of a barcode in which the bar width corresponds to the oscillation line intensity. However, the fabrication of nanoporous ZnO with high regularity involves some technological difficulties. In [
23], another approach based on using the features of the luminescent properties of ZnO films grown on the rhombohedral plane of sapphire with a gold buffer layer was proposed. In the luminescence spectra of such structures, a set of oscillations was found, which is a consequence of classical film interference. By analogy with porous aluminum oxide, a set of oscillations can be represented as a barcode.
Another example of a possible application of ZnO film structures that does not require high crystallinity and stoichiometry are refractive index sensors. The most sensitive sensors used today are based on fiber optics, waveguides, ring resonators, and plasmonic structures, and often use sensitive spectrometers and laser radiation [
24]. The planar structure of a ‘smooth ZnO film/sapphire substrate’, using a simple LED emitter as a light source and a photodiode for registration, can serve as a prototype of the simplest refractive index sensor. Such a sensor, in particular, can be useful for in situ studies of the photodegradation of optically dense media, such as glucose or sucrose, without the use of labor-intensive spectrophotometric techniques. The resolution of such a sensor depends on the thickness of the ZnO film and the roughness of its surface, which, in turn, is determined by the orientation of the film, the degree and directions of texturing, and the synthesis parameters in general.
ZnO films traditionally exhibit texturing along the [0001], [10
0], and [11
0] directions [
25,
26,
27]. The density of surface free energy calculated in [
28] for the (0001), (10
0), and (11
0) planes was 0.099, 0.209, and 0.123 eV/Å
2, respectively. Due to the fact that they have the lowest value for the (0001) plane, ZnO films are characterized by [0001]-texturing. As a consequence, (0001)-oriented ZnO films are formed even on nonorienting substrates. The greatest structural-geometric similarity of the ZnO and sapphire lattices has been observed for the A (11
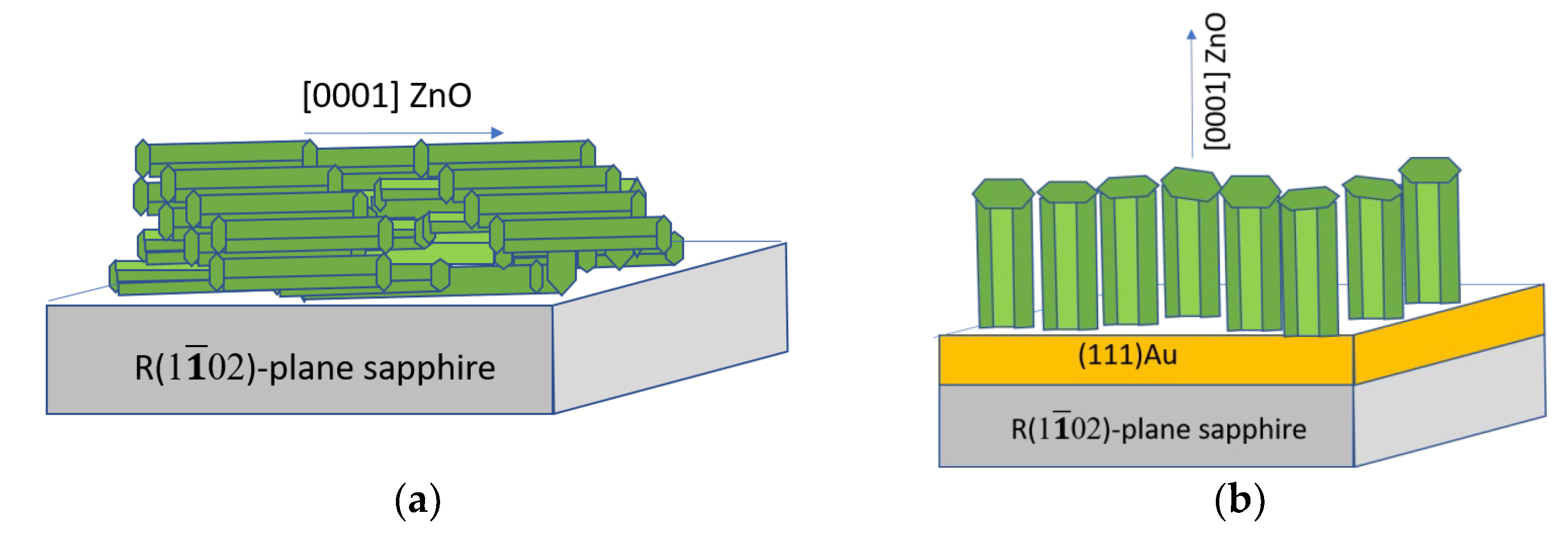
0) sapphire plane, whereas for the C (0001) sapphire plane, the least similarity has been observed. In both cases, [0001]-textured films are formed. A feature of the surface morphology of [0001]-textured films is that growing crystallites form a rough surface. To smooth this surface, a porous buffer sublayer is required, which reduces the density of ZnO seeds and restrains the growth of each nucleus at the expense of neighboring ones. As a result, the crystallites grow at approximately the same rate. Gold can be used for this sublayer. The mechanism of the catalytic synthesis of an ensemble of [0001]-oriented ZnO whisker microcrystals using gold is known [
29,
30]. According to the calculation results from [
28], the free energy densities for the (0001) and (11
0) ZnO planes are close in value, and with an increase in the growth rate, the [11
0] texture quite easily transforms into isolated [0001] ZnO microcrystals. These features make it possible to form bitextured ZnO films with the main [11
0] texture and an ensemble of [0001]-oriented microcrystals.
In accordance with the structural and geometric similarity of the film and substrate lattices, for the synthesis of [11
0]-oriented ZnO films with their basal axis lying in the plane of the substrate, the use of the rhombohedral R (1
02) sapphire plane is suitable [
27,
31]. In this case, the lattice mismatch remains 1.53% along the [0001] direction of ZnO. As we found previously [
32], [10
3] ZnO films are formed on the M (10
0)-plane of sapphire.
This brief analysis demonstrates the possibility of controlling the roughness, texturing, and morphology of ZnO films using the parameters of the synthesis process and the orientation of sapphire substrates.
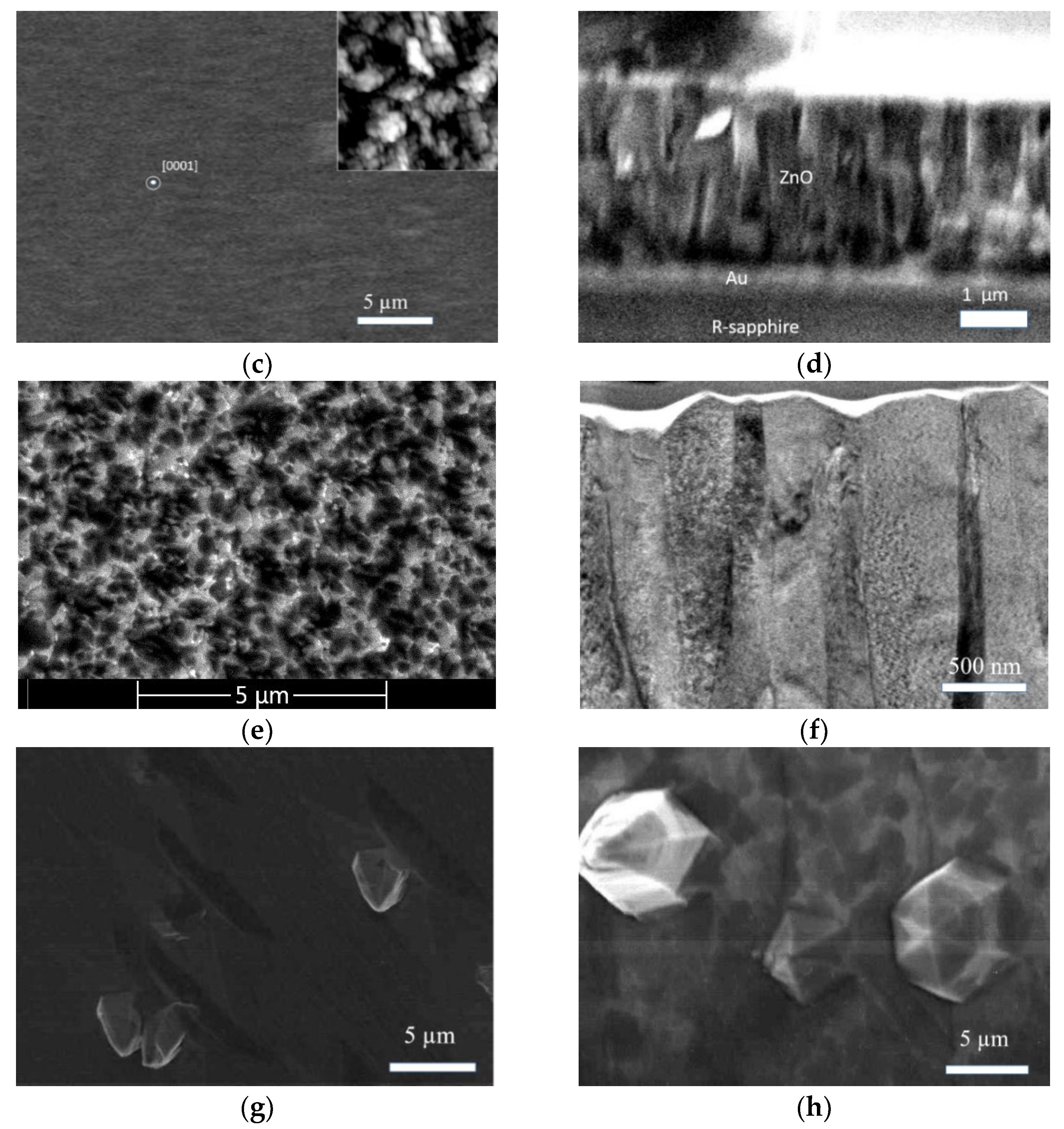
In this work, we studied the effect of texturing, roughness, and morphology features of ZnO films grown on the R (102)-, M (100)-, A (110)-, and C (0001)-planes of sapphire, assessing their optical, interference, and laser characteristics.
2. Materials and Methods
The R (1
02), M (10
0), A (11
0), and C (0001) sapphire planes were used to grow ZnO films. Chemical-mechanical polishing was applied to both sides of the sapphire substrates to obtain a roughness of about 0.2 nm according to the technique described in [
33,
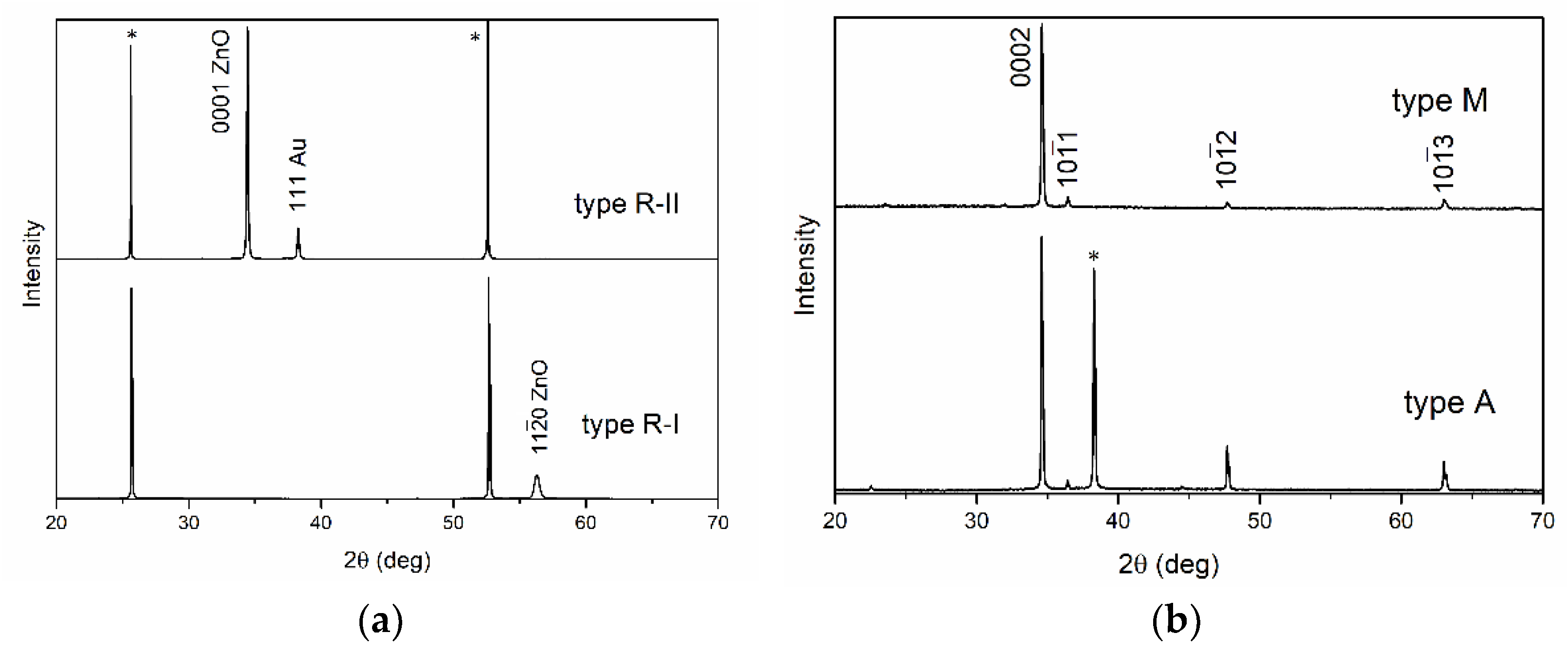
34]. Four samples of ZnO films were obtained on sapphire substrates of each type. Samples are further designated as types R, A, M, and C. Films of type R were considered in two versions: without a buffer layer (type R-I) and with a buffer porous gold layer (type R-II). A porous layer of gold (thickness about 200 nm) was formed via thermal deposition, after which it was heated at a temperature of 730 °C for 30 min. The thickness of a gold layer was chosen in such a way as to provide the possibility of measurements both in reflected and transmitted light.
The films were synthesized via magnetron sputtering (discharge current, 100 mA) at a temperature of 810 °C in an oxygen atmosphere at a pressure of 1.33 Pa. The synthesis time was 25 min. The film growth rate was ~2 nm/s. All types of ZnO films were synthesized under identical conditions. The optimum growth rate of thin films was 2 nm/s. Since the growth rate for (0001) ZnO is the highest and, for comparison, it was desirable to obtain type R-I and R-II films with the same thickness of ~3 µm, the deposition time was set to 35 min for R-I and 25 min for R-II. Since we intended to study the effect of the film thickness on their optical and interference properties, as well as to find out the conditions for the appearance of laser effects, the films of types A and M were deposited for 90 min. The stoichiometric ZnO ceramic target was used in the synthesis process. To increase the chemical stability of the film surface, coating with an aluminum layer about 10 nm thick with further annealing in air at 700 °C was performed.
Structural studies of the films were performed using reflection electron diffraction (horizontal electron diffraction; accelerating voltage is 75 kV) and X-ray diffraction (XRD). XRD patterns were obtained on a PANalytical Empyrean diffractometer in the Bragg–Brentano geometry. Radiation from a copper anode (CuKα2 = 1.54 Å) was used. XRD patterns were analyzed and reflection peaks were identified using the High Score Plus program, using the ICSD database (PDF-2).
Microscopic studies of the samples were carried out via scanning electron microscopy (SEM) and atomic force microscopy (AFM) using Jeol Neoscope 2 (JCM-6000) and Ntegra Prima microscopes, respectively.
The photoluminescence (PL) of the samples was observed under excitation with the 3rd harmonics (355 nm) of a Q-switched Nd:YAG laser. The pulse duration and repetition rate were ~10 ns and 15 Hz. The size of an excitation spot on the samples was ~200 μm. Emissions of the samples were registered with the use of a Peltier-cooled charge-coupled device camera placed behind the exit slit of a monochromator. To obtain a better signal-to-noise ratio, we applied the averaging of spectra using pulse accumulation.
The spectra of directional reflectance (at an angle of 25°) of the samples were measured using a Solar PB2201 UV-Vis spectrophotometer. The transmittance spectra of the samples were measured in the direction normal to their surface using a Solar PB2201 and a Varian Cary 5000 UV-Vis-NIR spectrophotometer.
All measurements were performed at room temperature.
4. Discussion
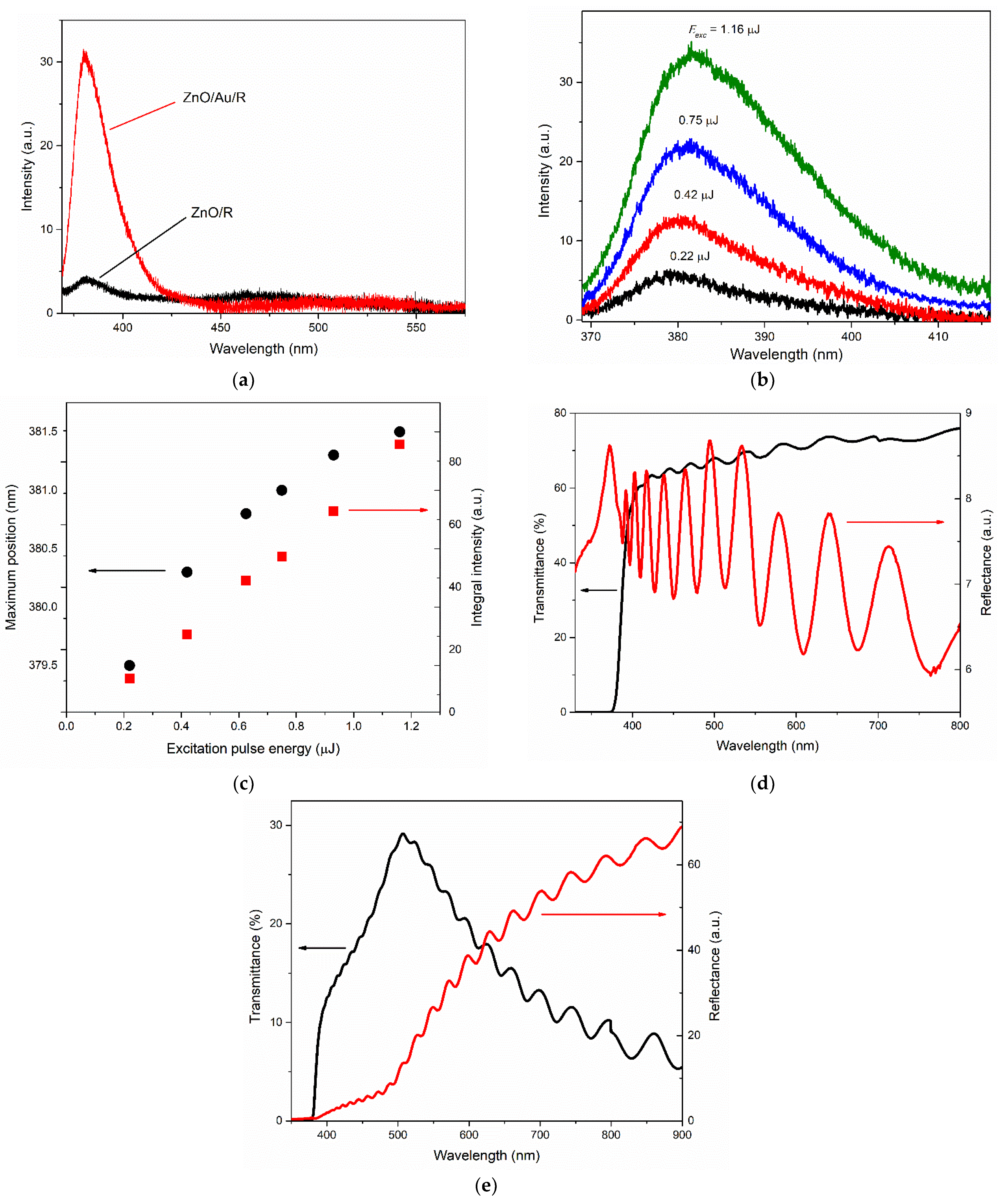
The spectral position of the NBE emission of the studied films in the region of 379–381 nm, the absence of its strong shift, and its linear growth with an increase in the excitation intensity (for all films at relatively low excitation intensities) allow us to expect the participation of exciton mechanisms in the emission, provided that sufficiently low excitation intensities are used, at which the Mott threshold has not yet been exceeded.
In general, all the studied films, except the type-R-I film, demonstrated good UV luminescence intensity for bulk ZnO structures, comparable with other ZnO microcrystalline structures, for example, ceramics [
38] and arrays of isometric microcrystals [
39]. On the other hand, the NBE emission signal from the films was significantly lower as compared to that of ZnO nanocrystal arrays [
40,
41], which are significantly superior to films in terms of their surface-to-volume ratio, as well as ZnO microcrystals with optical modes [
42,
43]. In this case, films of types M and A approached the latter more significantly than the film of type R-II due to the superlinear response to photoexcitation.
At the same time, films of types A and M at low excitation intensities exhibited lower ratios of the NBE and visible emission bands. This ratio (according to the integral intensities) was almost two times lower than for the type-R-II film in the case of the type M film, and four times lower in the case of the type-A film. This presumably can be explained by the violation of stoichiometry in ZnO films. Type A and M films have a lattice parameter d
0001 that is lower than that for the ZnO standard (
Table 1). Meanwhile, it is frequently believed that ZnO lattice parameters change upon variation of the oxygen vacancy (V
o) concentration. For example, in [
44], the (002) peak shift in the XRD of the vacuum- and oxygen-annealed films was attributed to the V
o concentration variation, suggesting that the oxygen vacancies in the ZnO thin films are responsible for the decrease in the lattice parameters. On the other hand, however, a lack of oxygen, or oxygen non-stoichiometry, may result in a larger lattice parameter since a decrease in valency of the cation may increase its ionic radius. Despite the fact that the issue remains debatable, the deviation of the d
0001 parameter from the standard one can be associated with a violation of stoichiometry. In this context, it is noteworthy that for the type-R-II film, for which the ratio of the NBE and visible emission bands was at the maximum, there was an ideal correspondence of the d
0001 parameter with the ZnO standard one (
Table 1). At the same time, in the case of the type-R-I film, for which the ratio of the NBE and visible emission bands was low, the
parameter, on the contrary, was larger than that for the ZnO standard. Thus, it turned out that for the films with parameters corresponding to the ZnO standard, the ratio of the NBE and visible emission bands was maximal.
Despite a good transmittance level, the type-R-I film showed the lowest UV luminescence intensity as compared to other ZnO films studied in this work. Defects located at the boundaries and near them can contribute to the nonradiative recombination of excitons, which generally reduces the yield the NBE emissions. In addition, this film was characterized by an excess of zinc, which could lead to the formation of interstitial Zn
i atoms and, as a consequence, to the appearance of additional centers of nonradiative recombination and an increase in the concentration of free electrons. The latter leads to screening of the electron-hole interaction and, as a result, to an additional decrease in the NBE emission intensity. Moreover, the presence of Zn
i can lead to emissions in the blue region [
45]. This can explain the shorter wavelength maximum position of the visible emission band in the PL spectrum of this film compared to that observed for the type-R-II film, where visible emissions could be attributed mainly to the green luminescence of ZnO, commonly associated with oxygen vacancies. However, it should be noted that the low UV luminescence intensity of the type-R-I film was compensated for by contrast interference because of its low surface roughness.
Due to fundamental structural changes in the texture structure, the use of a porous gold buffer layer during the growth of ZnO on the R-plane of sapphire significantly increased the ZnO NBE emission intensity and the ratio of the UV and visible parts of the emission spectrum (from 1.5 in the case of the R-I-type film to 6 in the case of the type-R-II film). Such a growth method provided a significant smoothing of the surface of a growing c-axis oriented film and, as a result, retained the interference properties of a film, in contrast, for example, to a ZnO film growing on the A-plane of sapphire. It should be noted, however, that in this case the reflectance and transmittance spectra of such a structure were strongly affected by the use of a gold sublayer.
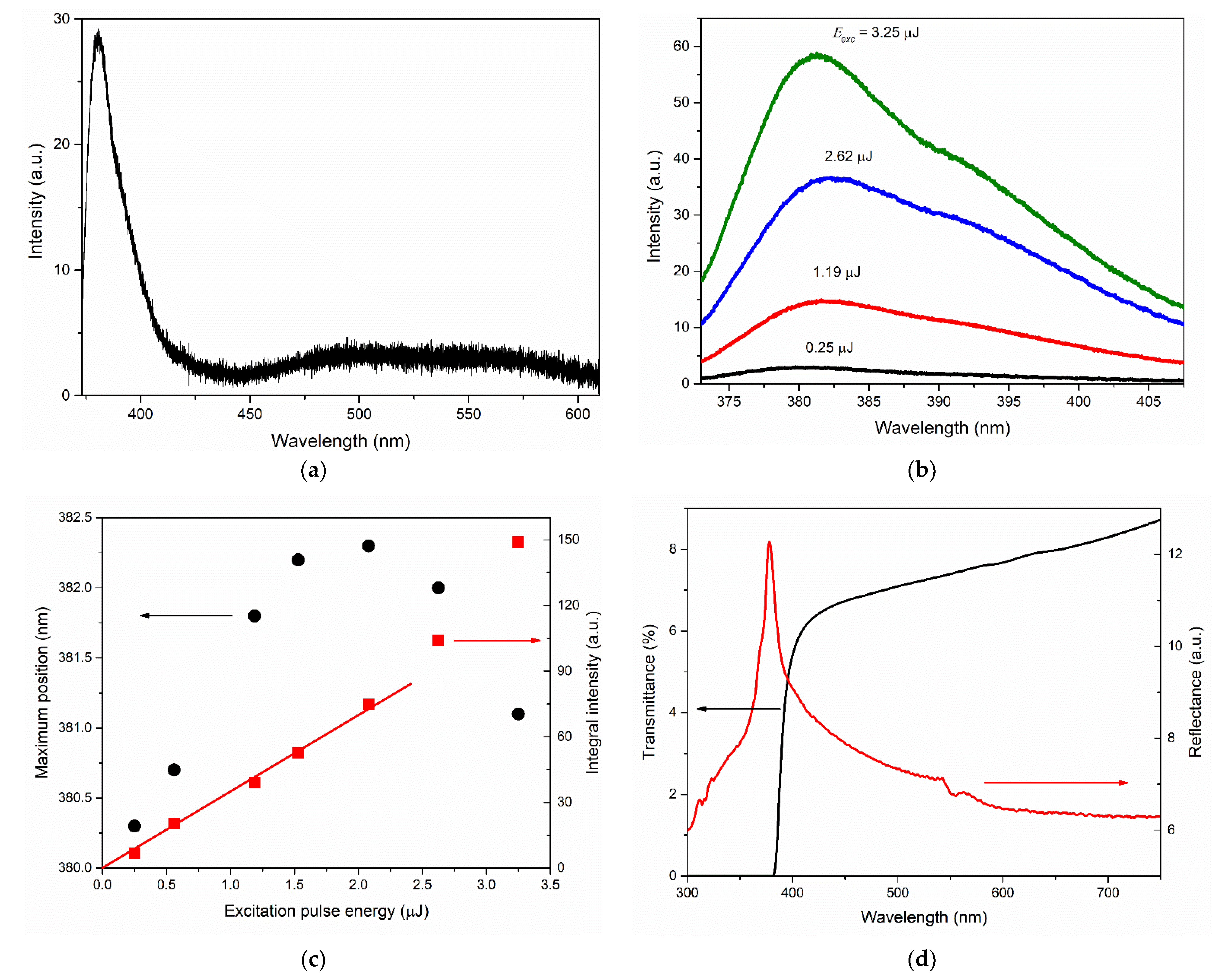
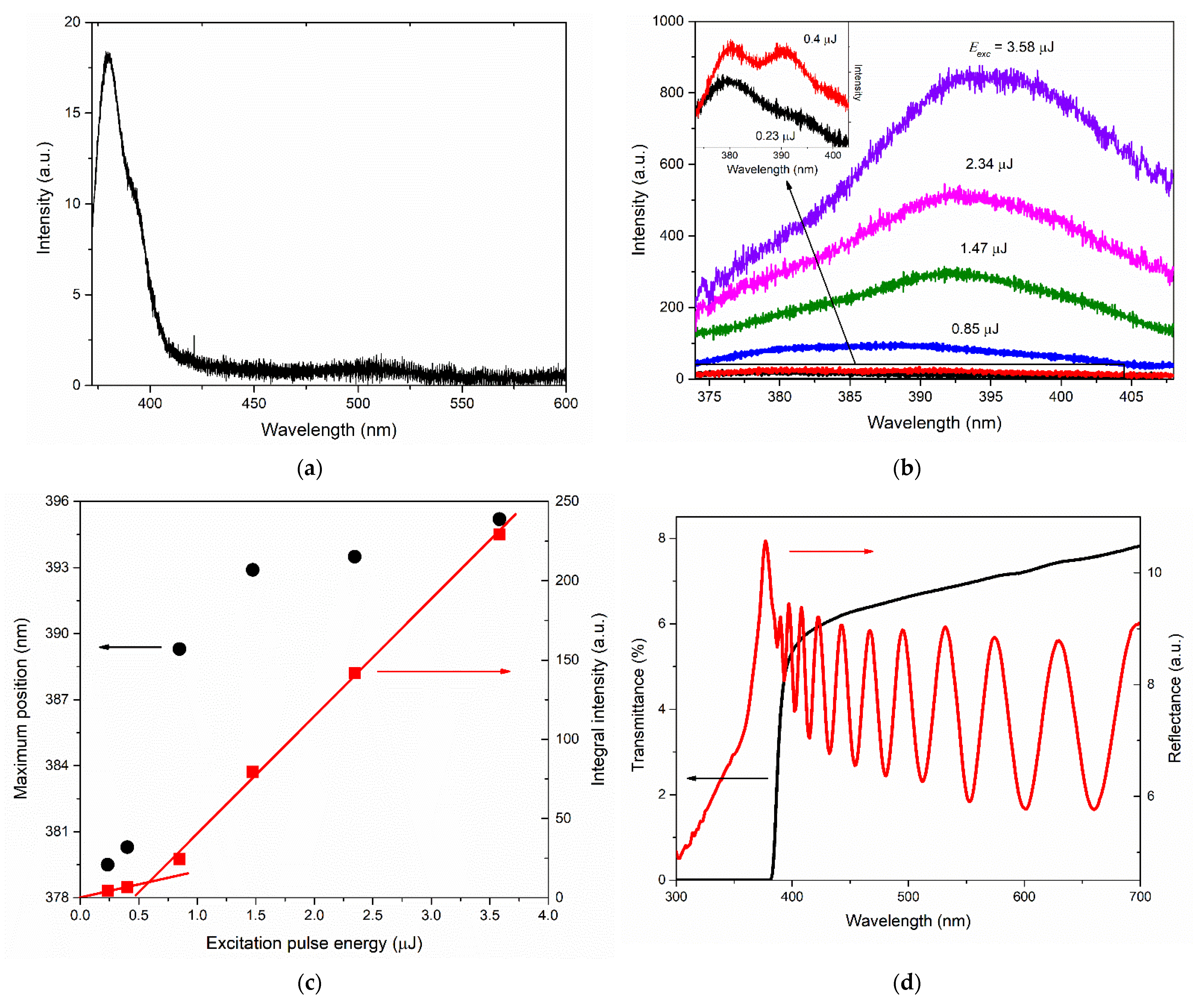
An increase in the growth rate of the NBE emission intensity with an increase in the photoexcitation intensity was observed in the case of films of types A and M. At the same time, the spectral behavior of NBE emissions differed significantly between these films. In particular, the shape of the UV PL spectrum of the type-M film at high excitation intensities distinguished this film strongly from all other samples. The two-band shape of the NBE emission spectrum, its threshold nature, and the rapid redshift of the long-wavelength band with increasing photoexcitation intensity indicated the appearance of stimulated emission (SE) in this film. In our recent work [
46], we observed similar PL spectra, demonstrating the appearance of SE with a similar character and low threshold, in the case of polyhedral ZnO microcrystals supporting WGMs. At the same time, the type-M film differed from the other studied films with
c-axis oriented crystallites due to the presence of large hexagonal [0001] microcrystallites on its surface. Taking into account the absence of SE of this type in other films, as well as the low SE threshold in the type-M film, we assume that SE arises in these hexagonal microcrystallites.
Taking into account the rather high photoexcitation intensities at which superlinearity appeared in the
Iint (
Eexc) dependence in the case of the type-A film, it can be assumed that this occurred in the presence of the electron–hole plasma (EHP). In this case, the blueshift of the NBE emission band observed at the same excitation intensities probably resulted from the dynamic Burstein–Moss effect (the band filling due to the intensive creation of electron-hole pairs via photoexcitation) [
47,
48]. The narrowing of the NBE emission band accompanying these effects indicated the onset of the EHP inversion and the appearance of SE in the film.
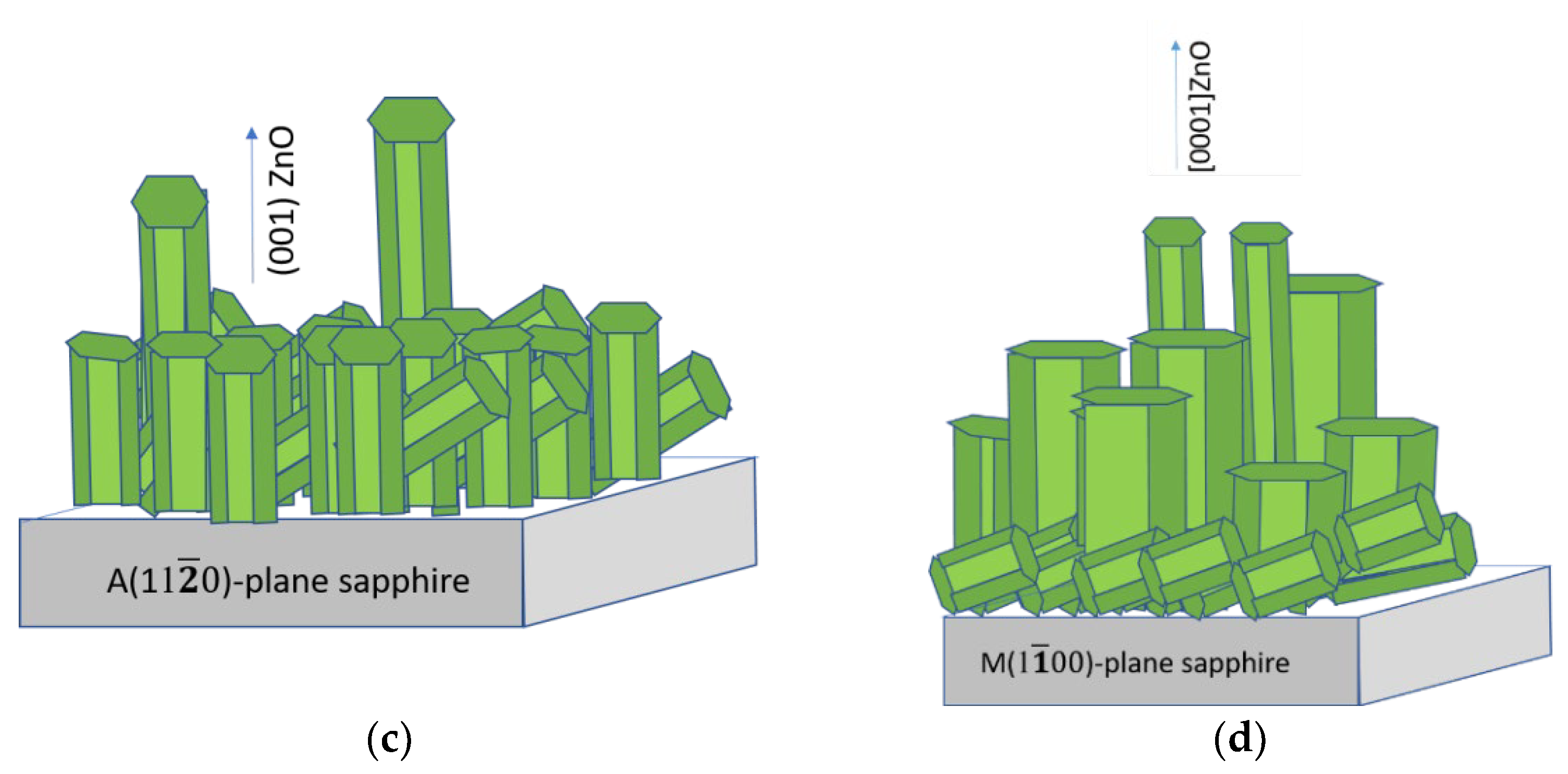
The reasons for such significant differences in the SE spectra of the type-A and type-M films have yet to be clarified, but the following can be preliminarily assumed. The [0001] microcrystals in the type-A film were smaller and had a much lower degree of perfection (prismaticity) compared to similar crystals in the type-M film (see
Figure 2g,h). This probably hindered SE excitation in such microcrystallites, since they could not provide a significant optical path for light amplification, especially at rather low photoexcitation intensities, when exciton recombination mechanisms still existed. In this regard, SE in the case of the type-A film probably took place in a continuous layer of the film or, taking into account its rather large thickness, even more likely, in elongated [10
2] microcrystals on the film’s surface, which distinguished this film from other films studied in this work. The length of the [10
2] microcrystals can provide a single-pass optical gain (see [
49], for example). However, the magnitude of such a gain is not as high as in microcavities. As a result, stimulation occurred only in the EHP regime. EHP formation in such microcrystals was also facilitated by their small (submicron) thickness (transverse size), which caused an increase in the density of electron-hole pairs in the volume of the crystals. This assumption needs to be verified in further studies.
Taking into account the sensitivity of the optical properties of microresonators, especially those with WGMs, to changes near their surface, one can expect sensing properties to be observed in these films as well. In this sense, further studies of ZnO films fabricated on the A- and M-planes of sapphire are relevant in order to elucidate the localization and nature of SE, to control the formation of individual microcrystals on the surface of the films, as well as to develop their possible applications.
The high brightness of NBE emissions of ZnO films grown on the A- and M-planes of sapphire was accompanied by their low transmission in the visible wavelength range and their longer-wavelength transmittance edge compared to the case of the type-R-I film. This was probably due to the greater intensity of light scattering in these films, which is presumably associated with the presence of [0001] microcrystals on their surface. At the same time, the type-M sample, in contrast to the type-A sample, exhibited an interference pattern in the reflection spectrum with a contrast level comparable with the interference in the type-R-I film. We attributed this phenomenon to the smoother surface of the continuous layer (in the regions between individual [0001] microcrystals) in the type-M film compared to the type-A film. Similar characteristics of the reflectance spectra between types-M and type-R-I films (amplitude, interference contrast, etc.) at significantly different transmittance values confirmed the assumptions made.
The high interference contrast observed in the case of type-R-I and type-M films promotes the use of such structures as optical coatings, i.e., antireflection coatings and refractive index sensors. In the latter case, attention should also be paid to the further development of films of the R-II type. The growth of ZnO on a gold nanolayer makes it possible to obtain a
c-axis-oriented film with a significantly lower roughness compared to films grown on the A- and C-planes of sapphire. In addition, we believe that this technology will allow one to obtain interference films of much greater thickness (ten or more micrometers). In view of the much less narrow interference fringes, the efficiency of such films when used as sensitive elements of refractive index sensors increases significantly. In the simplest case of using one film in contact with the analyzed object, for example, a solution, the interference maximum with order
m, is observed at the following wavelength [
50]:
where
d is the thickness of the film with the refractive index
;
is the refractive index of the substance in contact with the film, which depends on the concentration of the analyte substance; and
α is the angle of incidence/observation.
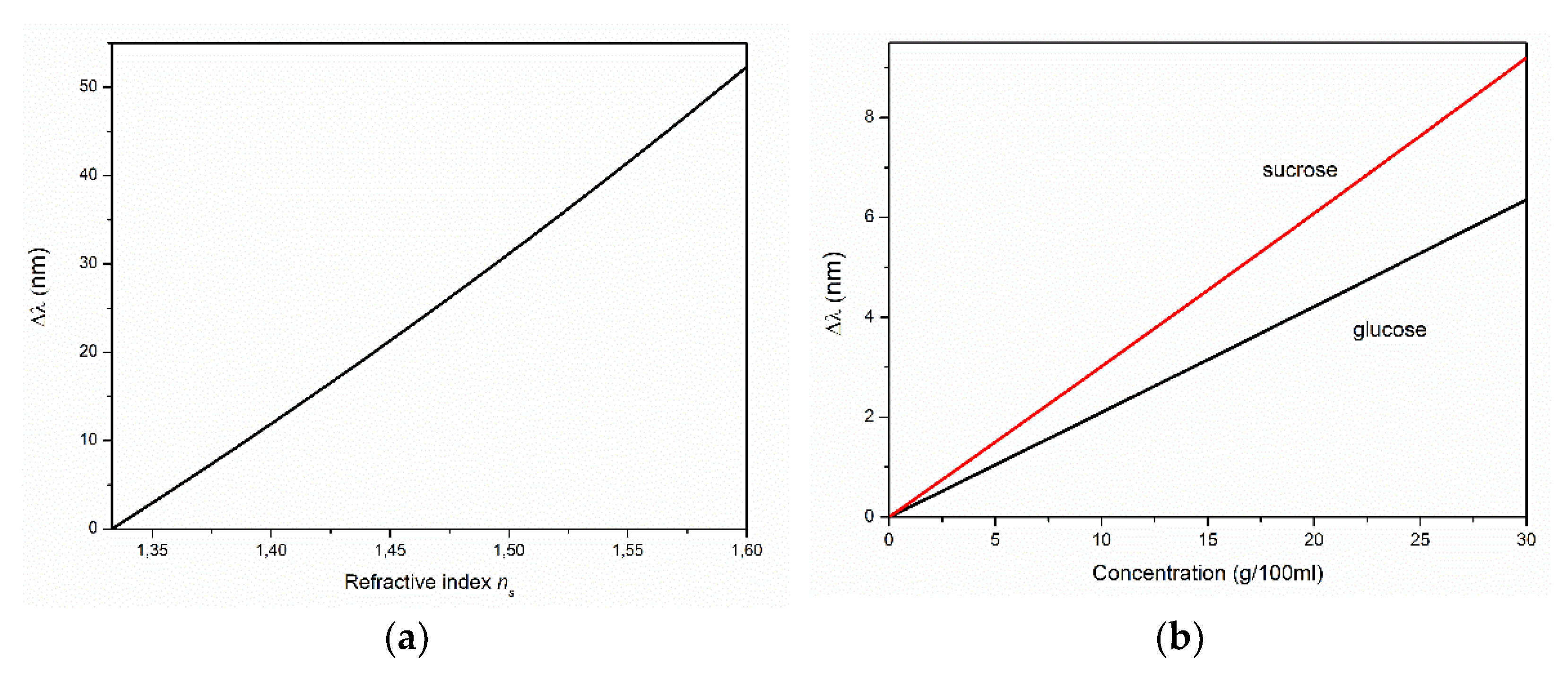
As an example,
Figure 7 shows an estimate of the shift of one of the interference fringes with
m = 13 at
α = 45° with a change in
(
Figure 7a), as well as in the concentrations
of glucose and sucrose in aqueous solutions in contact with the ZnO film with
d = 3 µm (
Figure 7b). For the estimates, we used the linear approximation
from [
51] and the dispersion of
from [
52]. The sensitivity calculated from
Figure 7 was ~190 nm/RIU. With sufficiently narrow interference bands (in the case of thick films), a shift of several nanometers can be easily tracked. The use of a narrow band source and/or two interference films can significantly increase the sensitivity of such a sensor. In this case, for example, films of types R-I, R-II, and/or M can be combined.
With a decrease in the thickness of a continuous gold nanofilm, its transmittance level increases and can reach a maximum of 10–20% at a thickness of 10–30 nm [
53]. In our case, the relatively high transmittance (~30% in the maximum) seemed to be due to the island (porous) nature of the gold layer. From the perspective of using thinner gold layers, this would allow one to effectively use such a structure in reflected light, not only from the side of the ZnO film, but also from the side of the substrate, which is impossible when using opaque substrates, i.e., silicon. This geometry could be useful in the analysis of turbid solutions, which greatly attenuate the light passing through them.
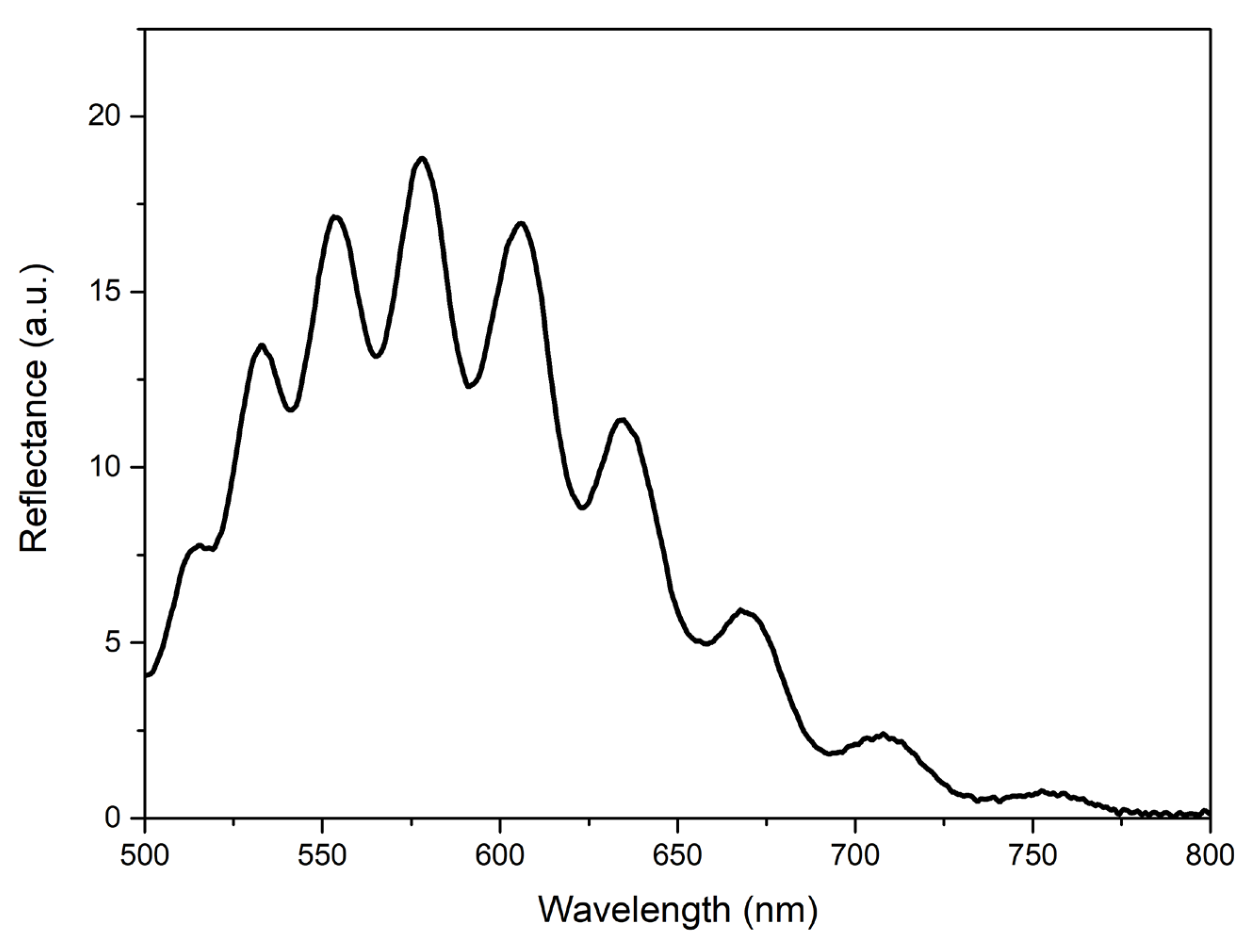
When developing an analyzer device, the developed structure (one or several) can be fixed, for example, in a standard spectrophotometer cuvette or, when working in flow mode, in a transparent tube. Unlike many sensors, such as waveguide-type ones, interference films do not require the use of laser radiation—it is sufficient to use a conventional LED. As an example,
Figure 8 shows the reflectance spectrum obtained from the type-R-II film when illuminated with a conventional commercial LED.
The use of a high-resolution spectrometer is also an option. Moreover, when using a narrow-band LED as a light source, it is possible to use a conventional photodiode without using additional spectral devices. The fabrication technology of such sensors could also be simpler than that required for most sensors based on plasmonic materials, photonic crystals, etc.
Transparent coatings such as Al
2O
3 can be used to stabilize the ZnO film surface.
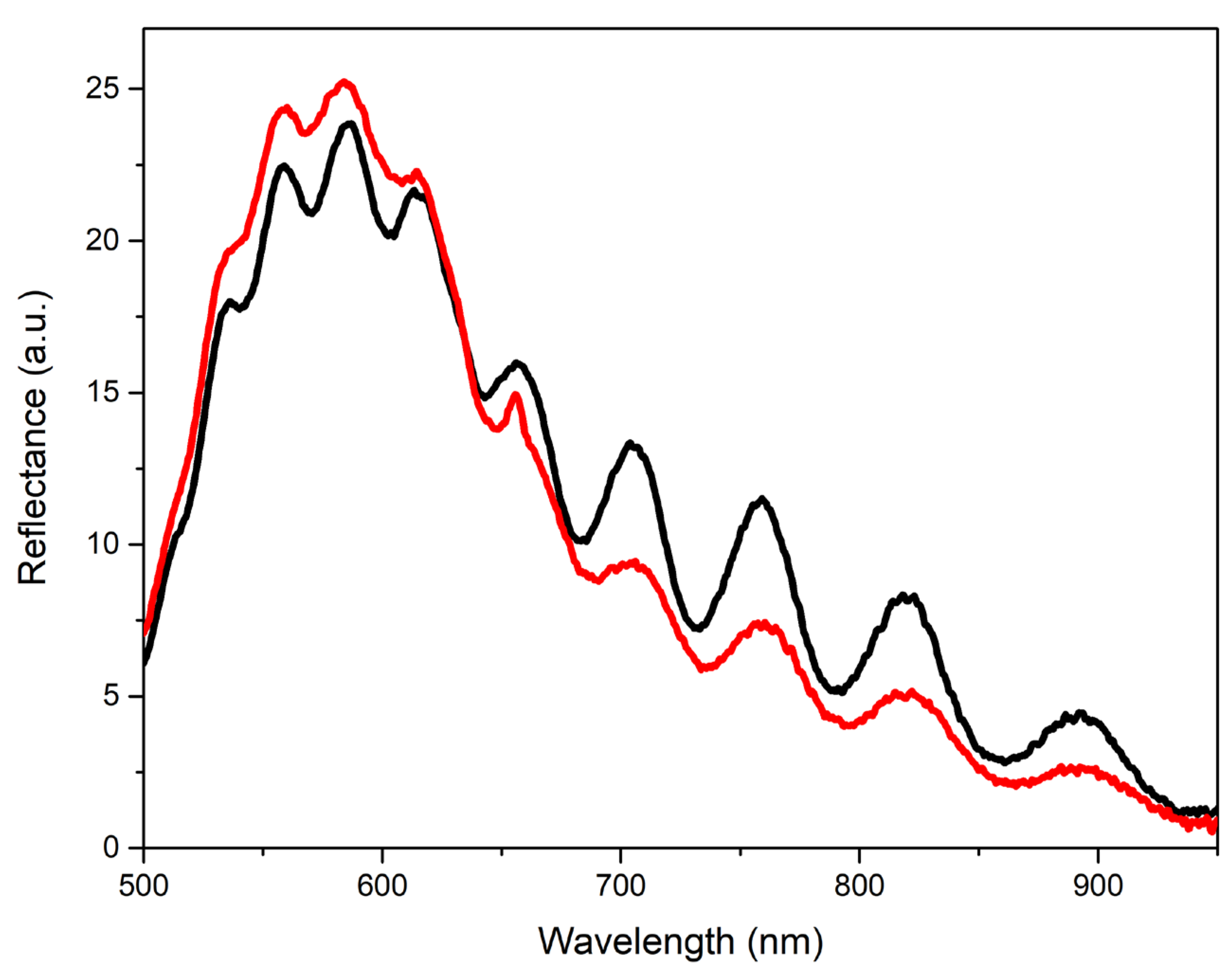
Figure 9 shows the reflection spectra of one of the type-R-II films before and after the deposition of a protective Al
2O
3 layer under illumination by a LED source. In this case, the coating reduced the contrast of the interference fringes. However, work in this direction can also be continued.
5. Conclusions
We studied the synthesis processes, interference, and stimulated emission properties of ZnO film structures grown on R (102)-, M (100)-, A (110)-, and C (0001)-planes of sapphire.
ZnO films grown on the R-plane of sapphire substrate were formed homogeneously, oriented by the (110) plane parallel to the substrate. The Rq value and the height difference of the relief were 12 nm and 47 nm, respectively. In this case, the films had a small crystallite size of about 20 nm. When using a buffer layer of gold, the films were also formed homogeneously, but [0001]-texturing was observed. The RMS surface roughness and the height difference of the relief were 21 nm and 120 nm, respectively. Films grown on A-plane sapphire, as well as those grown on C-plane sapphire, exhibited high roughness values and grew with a (0001) plane parallel to the substrate. Moreover, the surfaces of the films were characterized by the presence of individual [0001] microcrystals up to 3 μm in height and [102] microcrystals, elongated along the surface with longitudinal sizes up to 8 microns. When using an M-plane sapphire as a substrate, ZnO films grew morphologically inhomogeneously. Pronounced large [0001] microcrystals up to 10 µm high and up to 8 µm in diameter were formed in a continuous smooth layer. At the same time, the crystallinity of such films was the highest due to the perfection of the individual microcrystals.
The films grown on the A- and M-planes of sapphire were characterized by the highest brightness of UV radiation. In addition, these films demonstrated stimulated emission of various types. At the same time, in contrast to the ZnO film grown on A-plane sapphire, the film on M-plane sapphire exhibited noticeable interference properties. We assumed that [0001]-microcrystallites formed on the film surface were responsible for the appearance of stimulated emissions, and that this interference was realized in the film layer as a result of the low roughness of its surface in the regions between these microcrystals.
A study of the growth of ZnO films on a porous gold sublayer deposited on the R-plane sapphire substrate revealed differences in the spectral properties of films grown with and without gold. In particular, the use of gold resulted in a significant increase in the intensity of ZnO NBE emission compared to that of the film grown on a bare substrate. Both films exhibited interference properties; however, gold had a noticeable effect on the reflectance and transmittance spectra. In general, due to the significant smoothing of the c-axis-oriented film’s surface, we assumed that the use of gold can facilitate the fabrication of sufficiently thick (tens of micrometers) ZnO films with low surface roughness. The narrow interference fringes of such films will increase their resolution as elements of refractive-index sensors. Estimations of the sensitivity of the observed interference to changes in the refractive index of the surrounding medium—in particular, aqueous solutions of glucose and sucrose—suggested the possibility of using ZnO film structures in in situ studies of the photodegradation of such substances.
The results obtained emphasize the relevance of further studies of such ZnO film structures.