Synthesis and Characterization of Calcium Carbonate Obtained from Green Mussel and Crab Shells as a Biomaterials Candidate
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
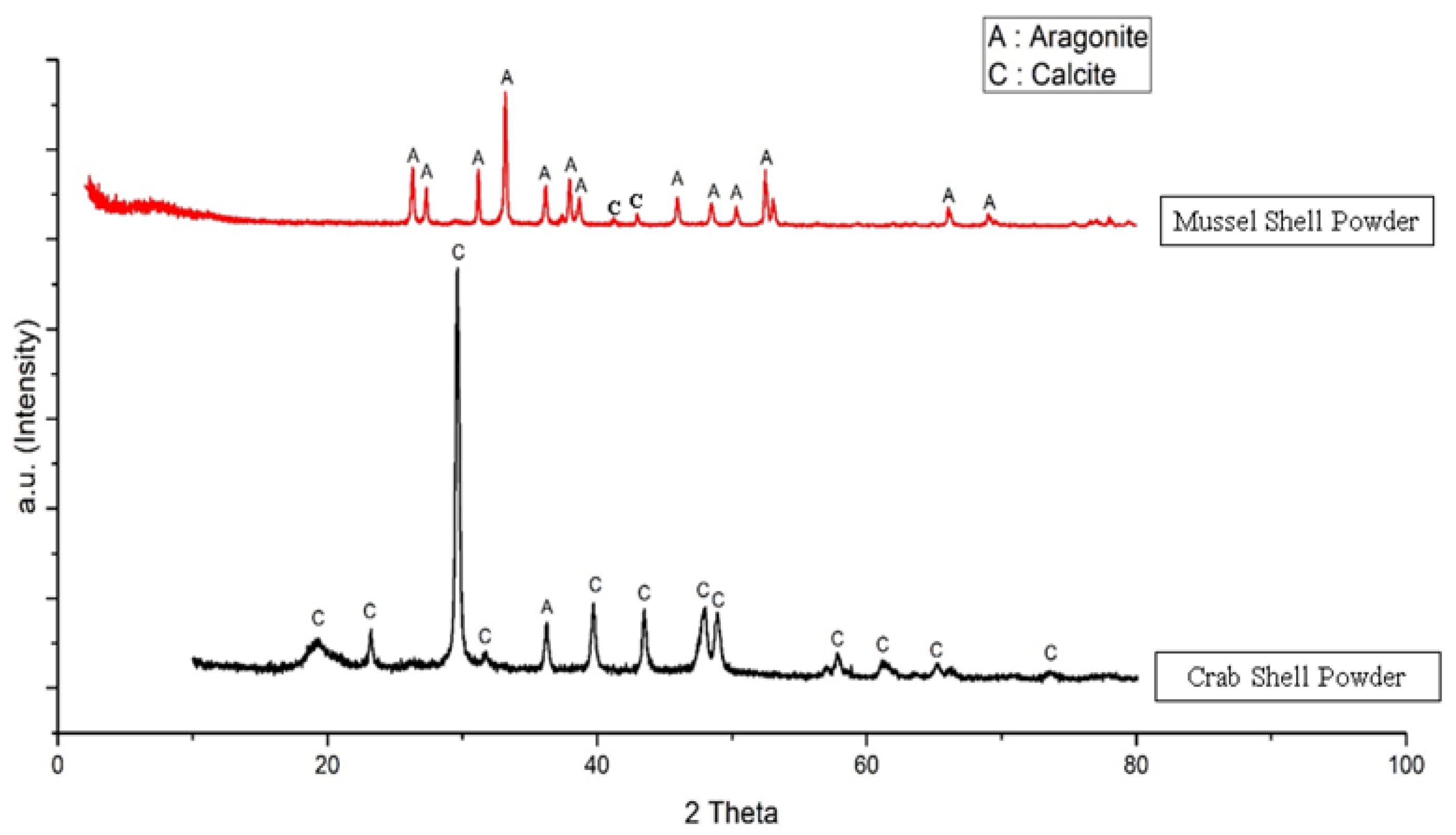
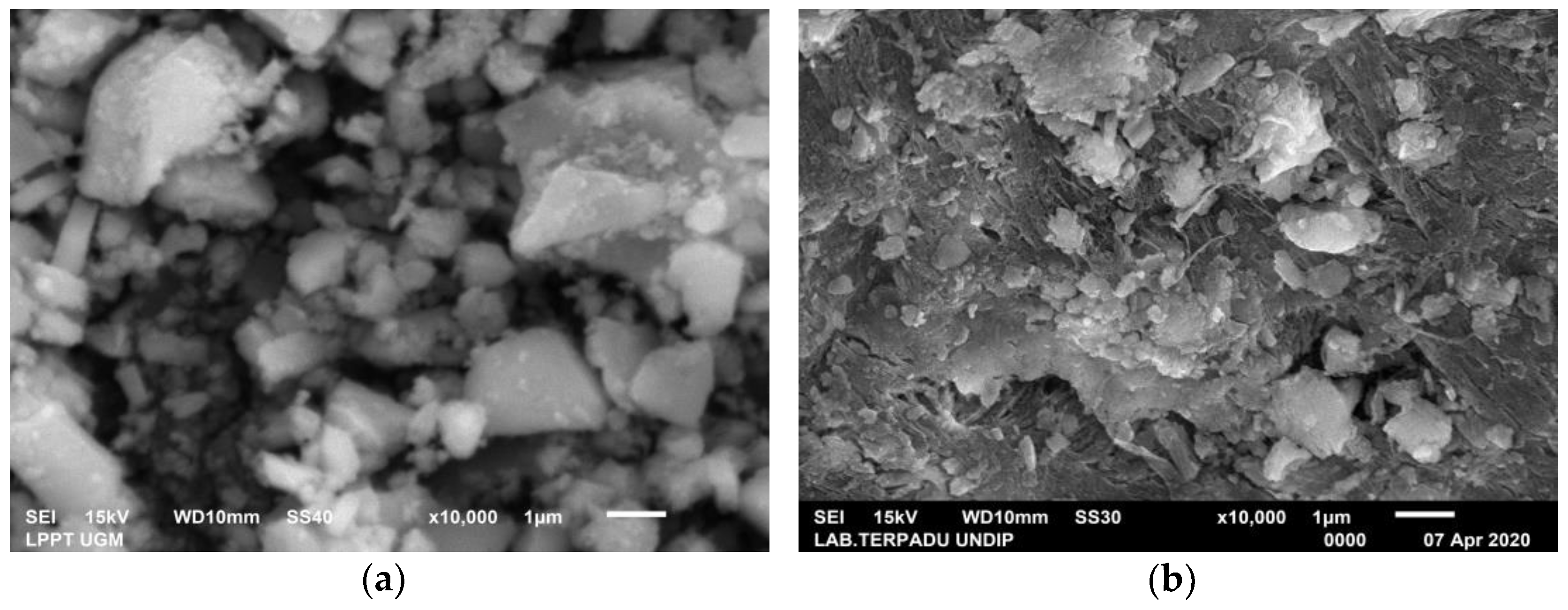
3.1. Characterization of Green Mussel and Crab Shell Powders
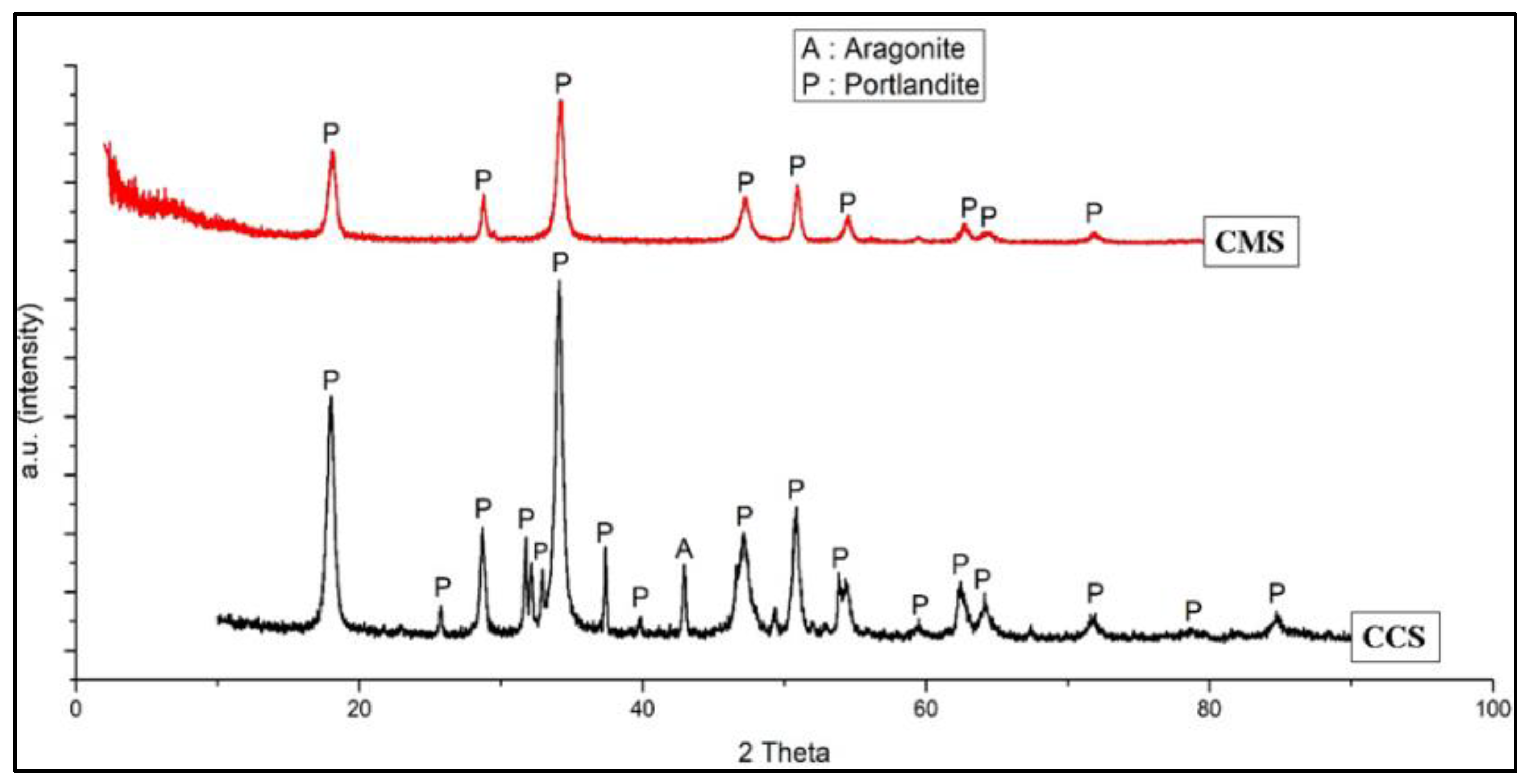
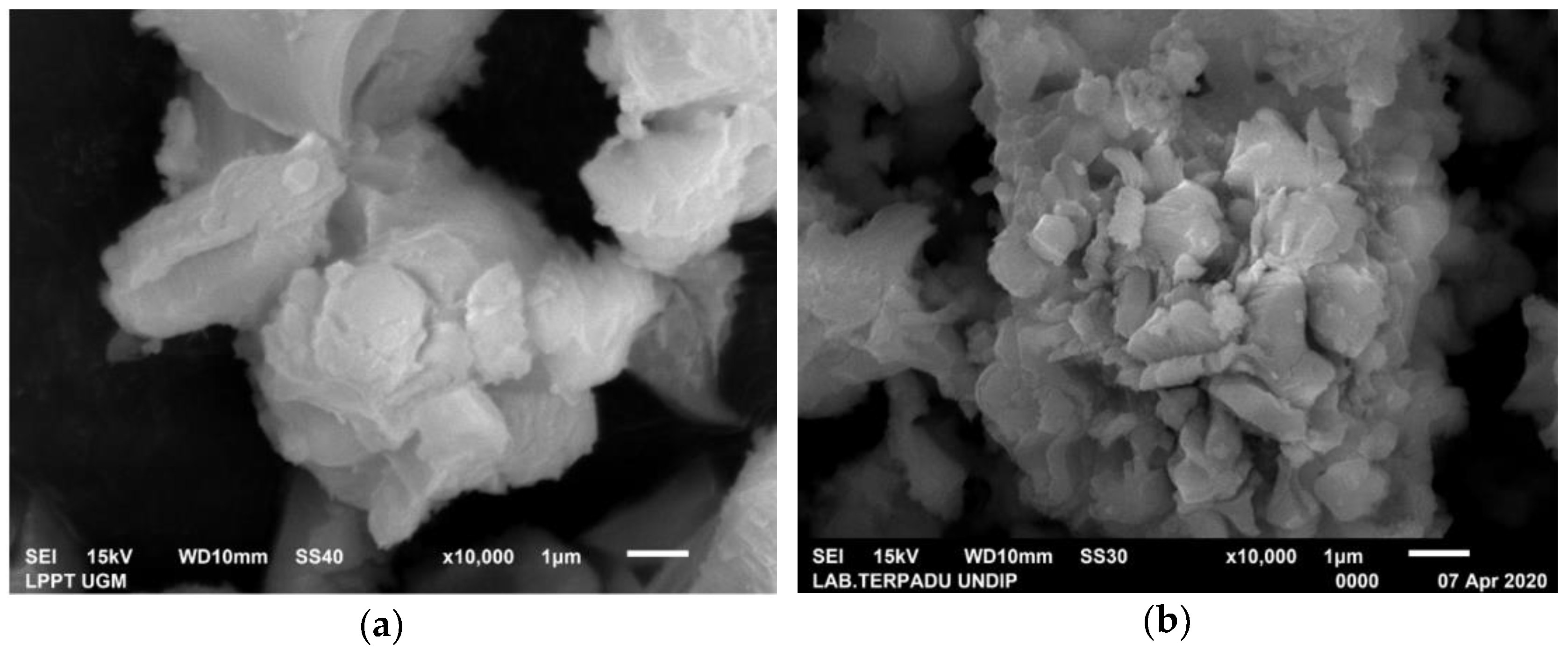
3.2. Characterization of Green Mussel and Crab Shell Powders after Calcination
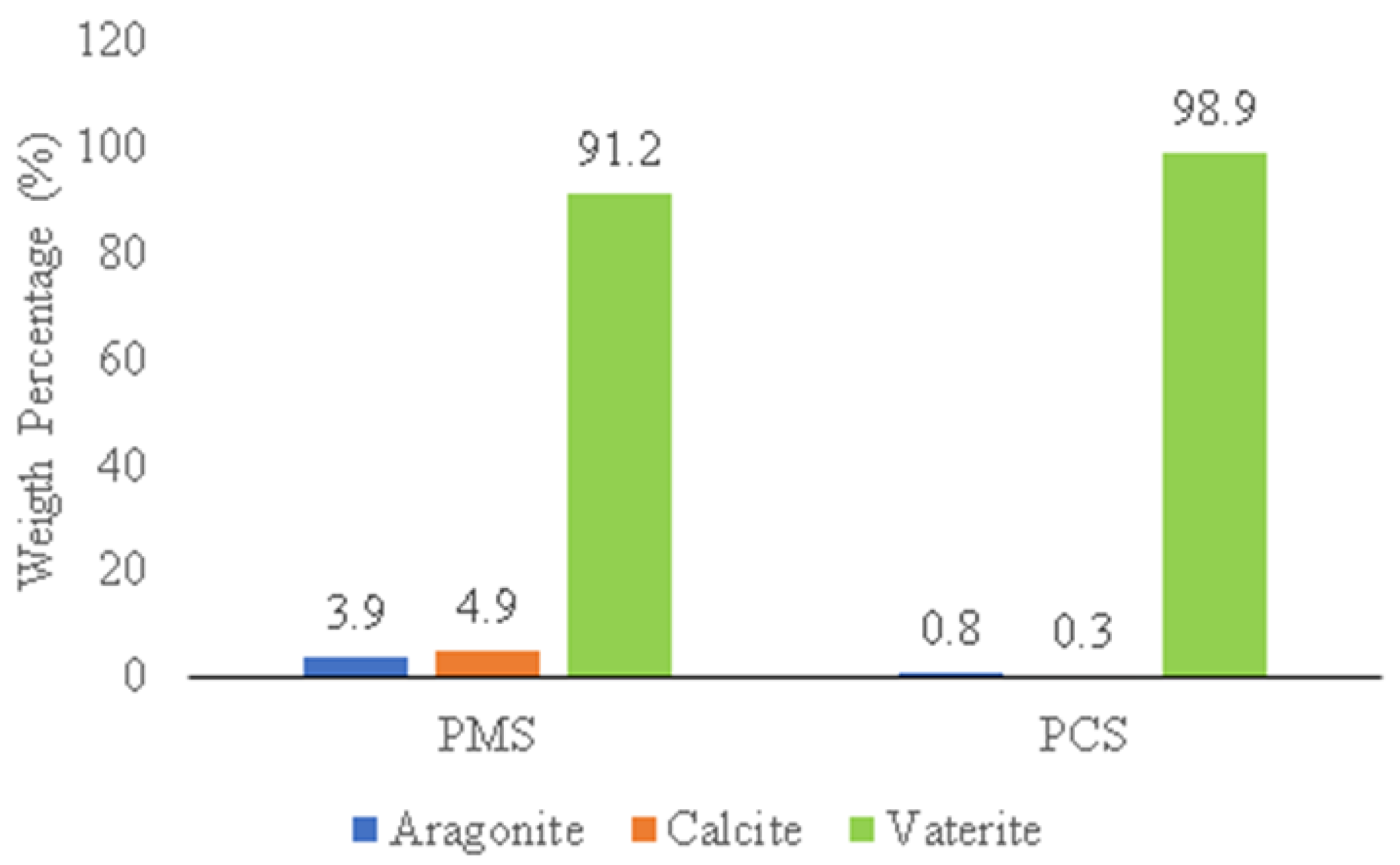
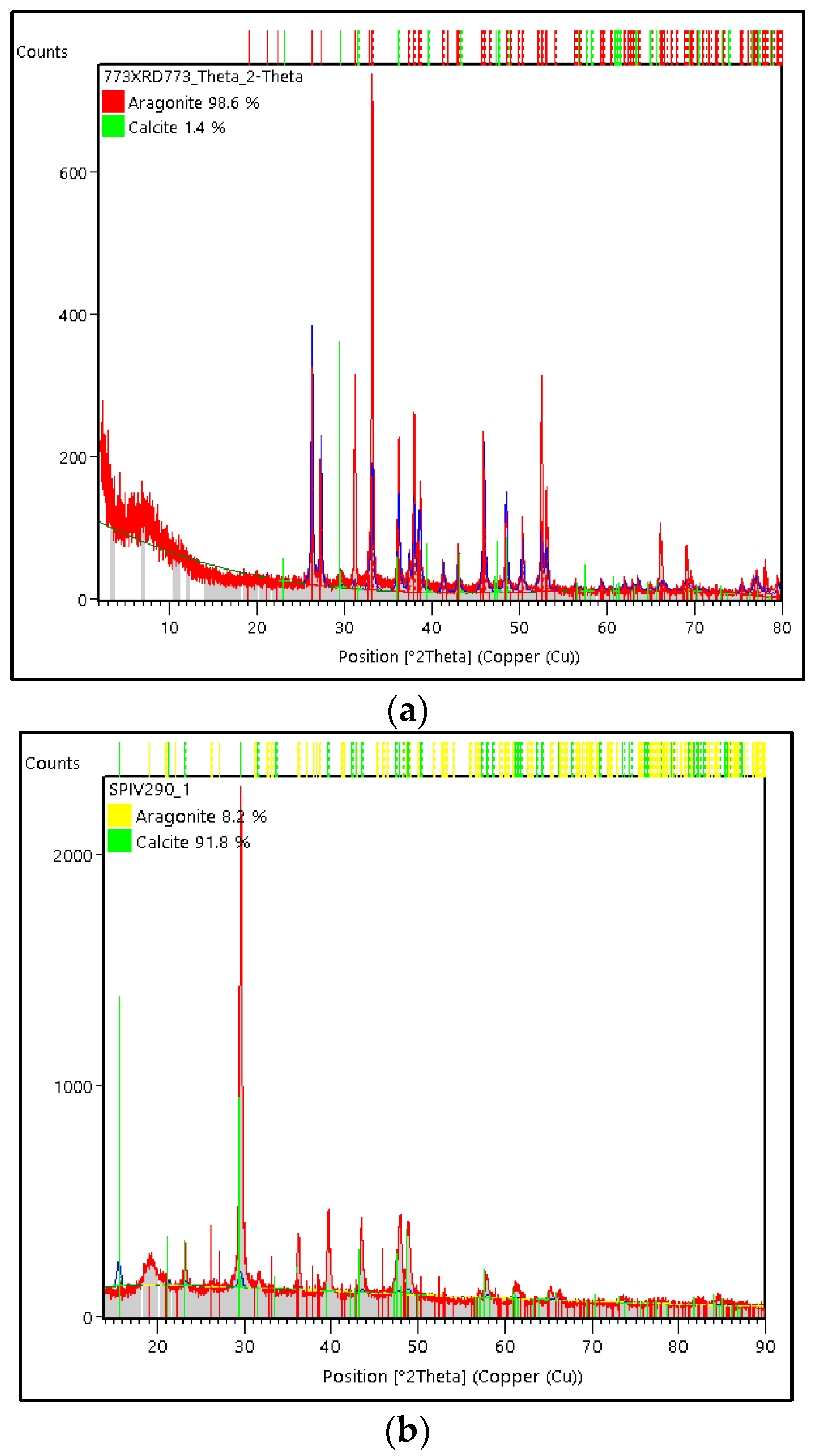
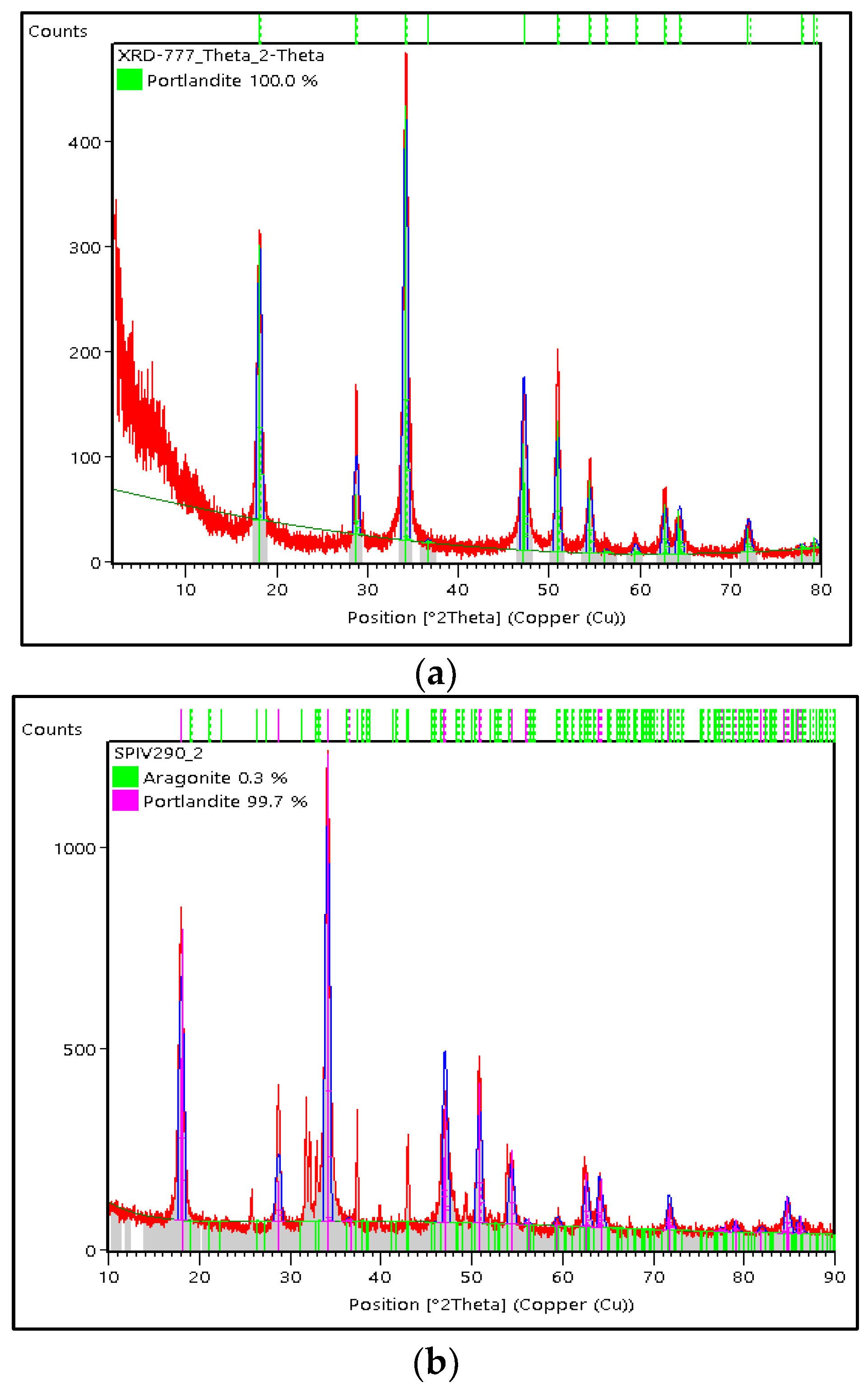
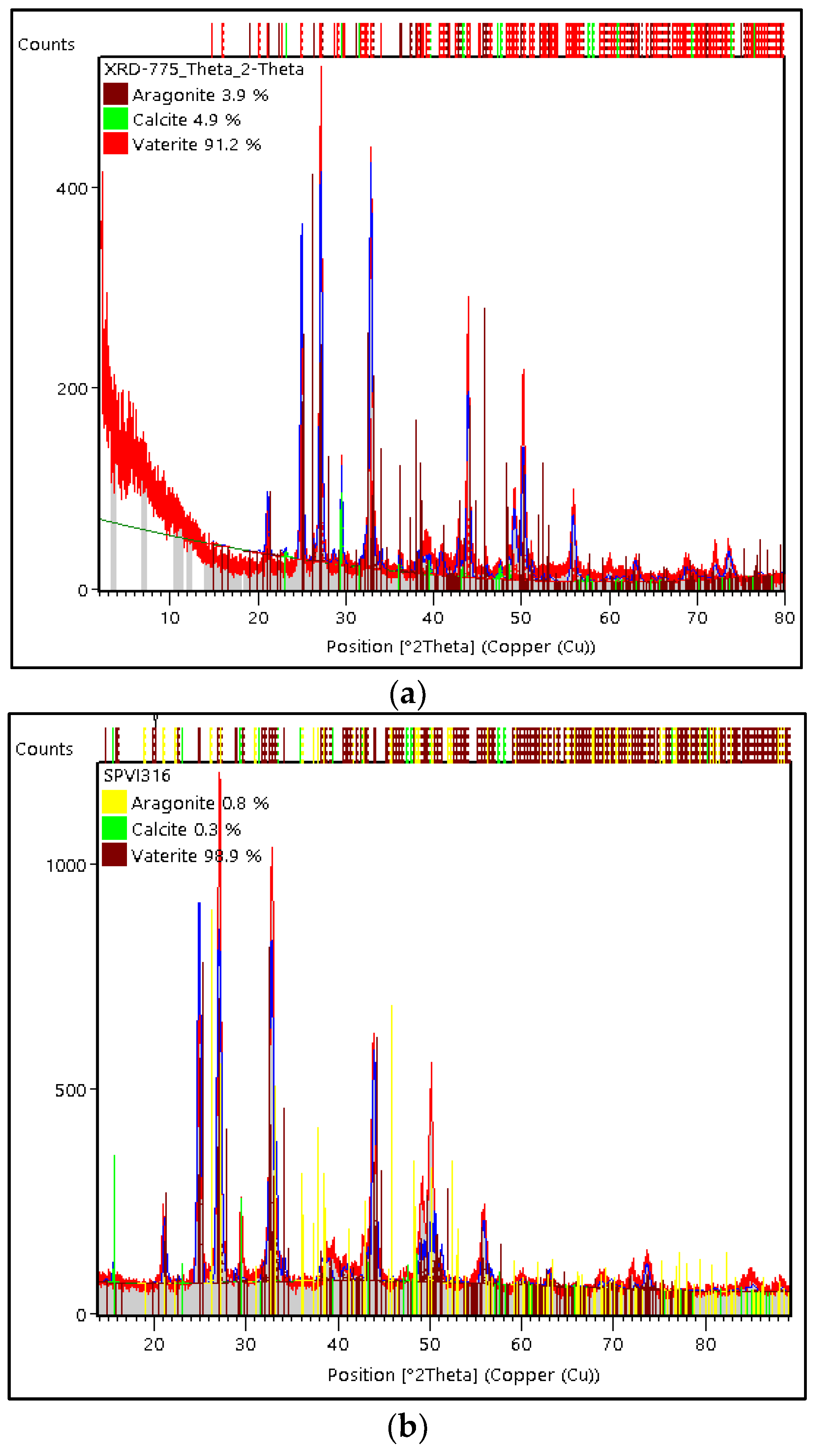
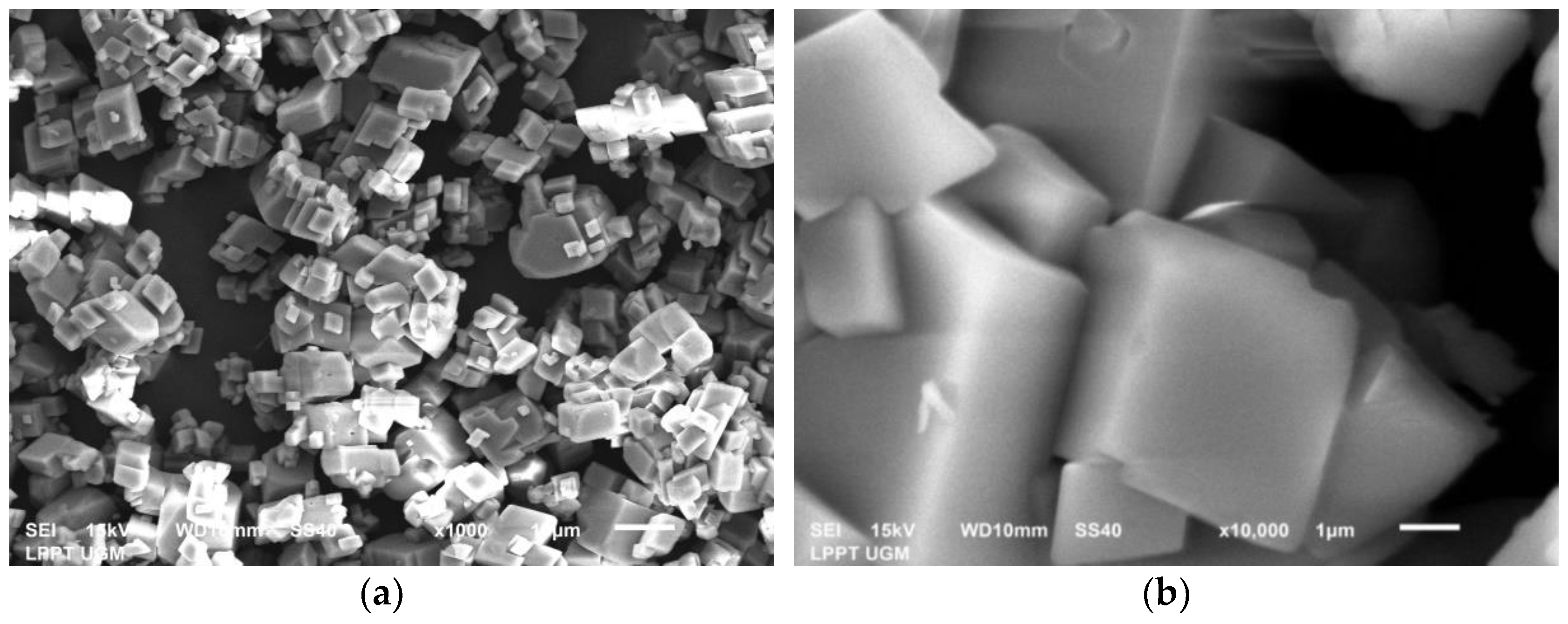
3.3. Characterization of Green Mussel and Crab Shell Powders after Precipitation Process
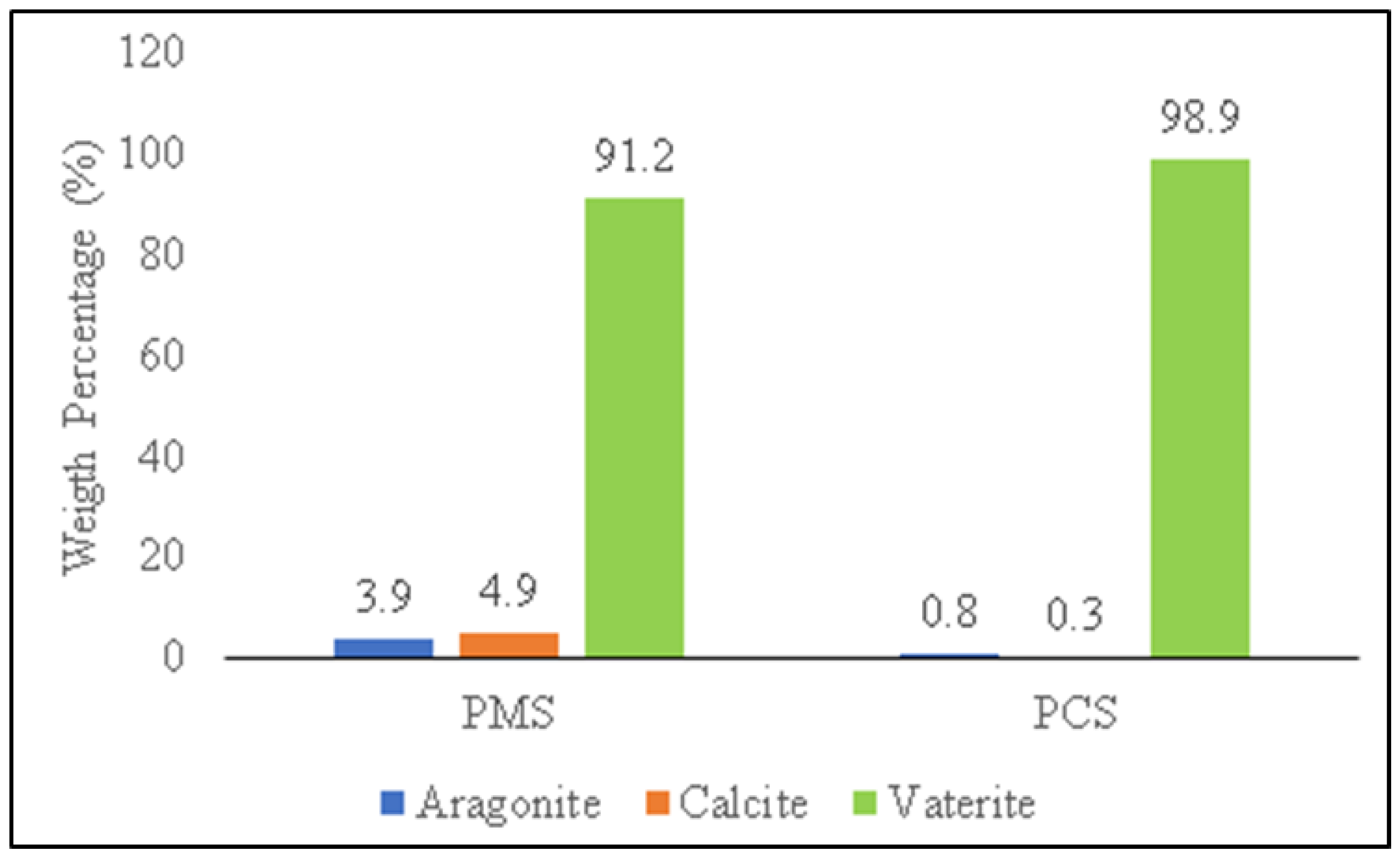
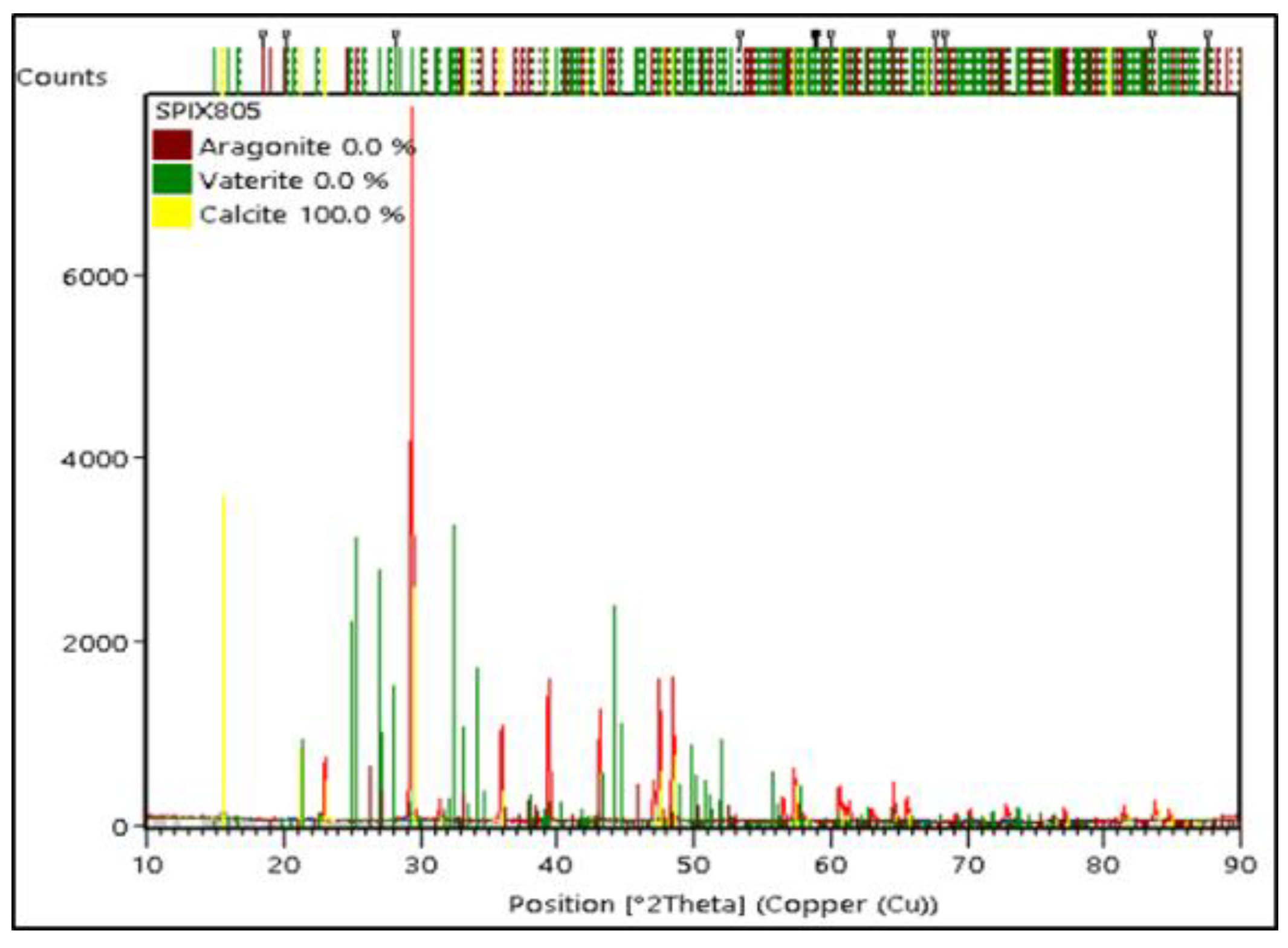
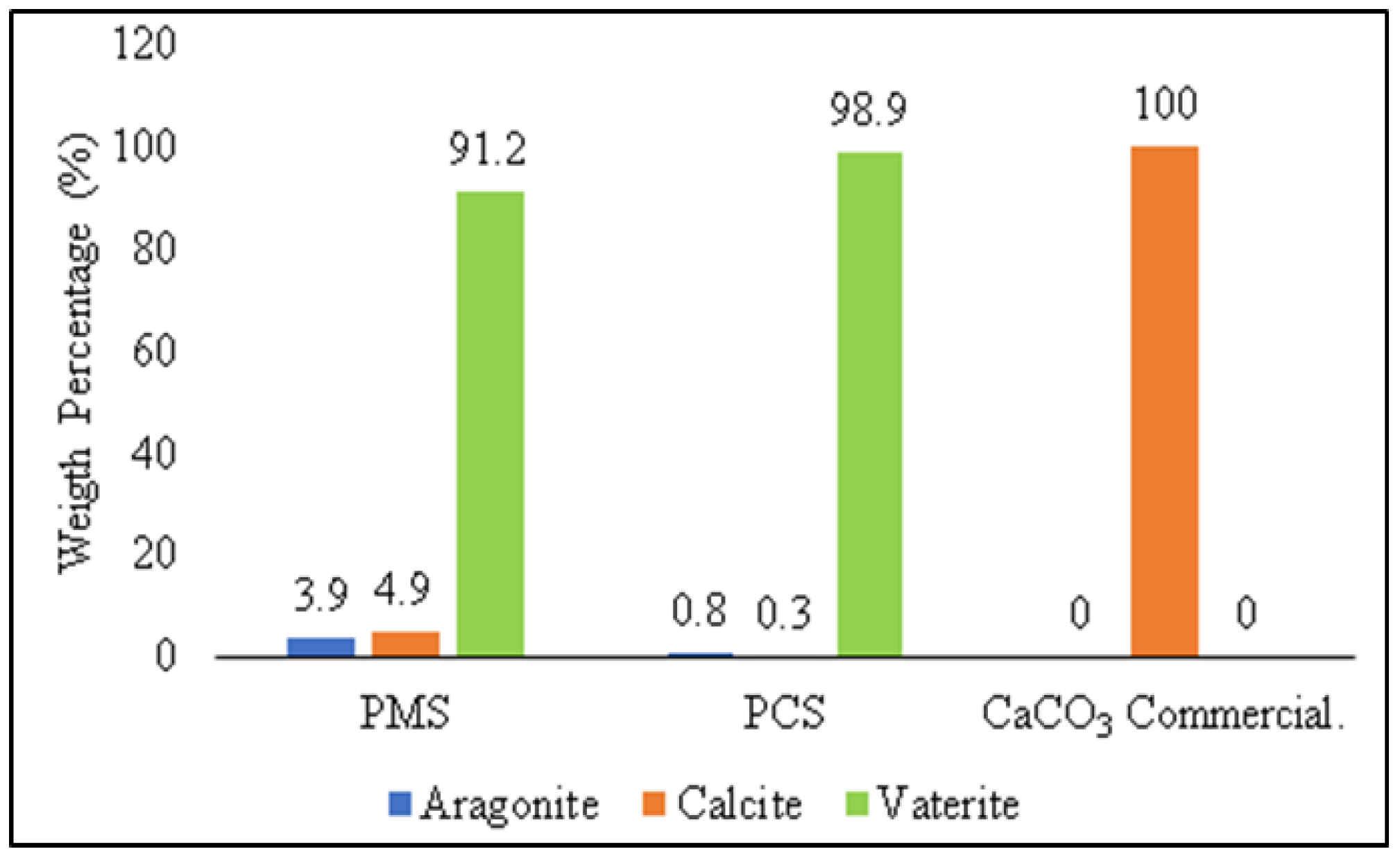
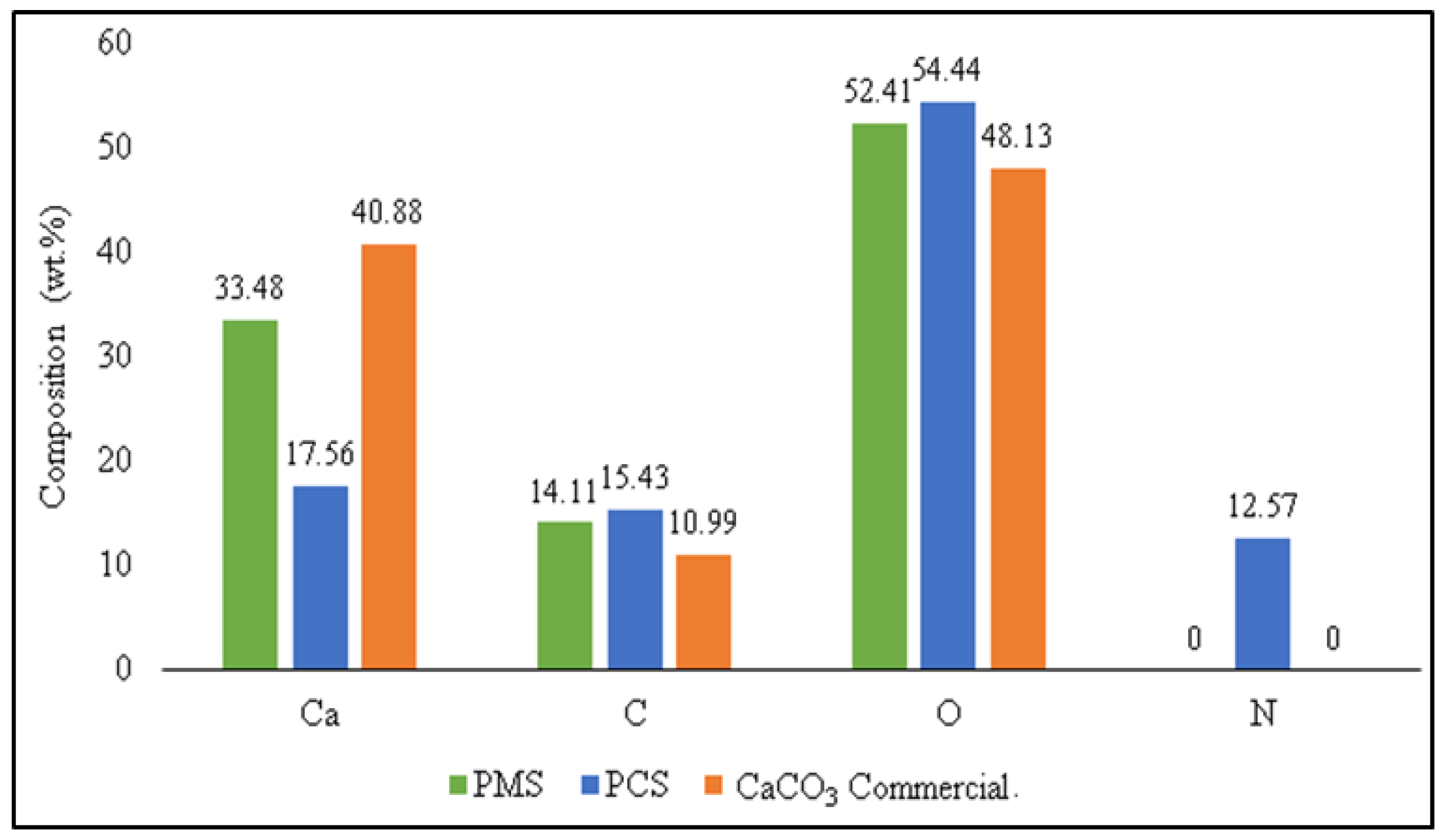
3.4. The Comparison of the Characterization of PMS, PCS, and Commercial CaCO3
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Venugopal, V.; Gopakumar, K. Shellfish: Nutritive Value, Health Benefits, and Consumer Safety. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1219–1242. [Google Scholar] [CrossRef] [PubMed]
- Bps.go.id Fishery. Available online: https://jateng.bps.go.id/subject/56/perikanan.html#subjekViewTab3 (accessed on 2 July 2021).
- Faqir, A. Al Tembus Hingga AS, Ekspor Rajungan Indonesia 2019 Cetak Rp5,3 Triliun|Merdeka.com. Available online: https://www.merdeka.com/uang/tembus-hingga-as-ekspor-rajungan-indonesia-2019-cetak-rp-53-triliun.html (accessed on 2 July 2021).
- Fitriyana, D.F.; Ismail, R.; Santosa, Y.I.; Nugroho, S.; Hakim, A.J.; Syahreza Al Mulqi, M. Hydroxyapatite Synthesis from Clam Shell Using Hydrothermal Method: A Review. In Proceedings of the 2019 International Biomedical Instrumentation and Technology Conference, Special Region of Yogyakarta, Indonesia, 23–24 October 2019; pp. 7–11. [Google Scholar]
- Raya, I.; Mayasari, E.; Yahya, A.; Syahrul, M.; Latunra, A.I. Shynthesis and Characterizations of Calcium Hydroxyapatite Derived from Crabs Shells (Portunus Pelagicus) and Its Potency in Safeguard against to Dental Demineralizations. Int. J. Biomater. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sali, S.S. Natural Calcium Carbonate for Biomedical Applications; Indian Institute Of Technology–Bombay (IITB): Mumbai, India, 2015. [Google Scholar]
- Yang, H.; Yan, N. Transformation of Seafood Wastes into Chemicals and Materials. In Encyclopedia of Sustainability Science and Technology; Springer: New York, NY, USA, 2018; pp. 1–23. [Google Scholar]
- Costa, L.M.M.; de Olyveira, G.M.; Salomão, R. Precipitated Calcium Carbonate Nano-Microparticles: Applications in Drug Delivery. Adv. Tissue Eng. Regen. Med. Open Access 2017, 3, 1–6. [Google Scholar] [CrossRef]
- Bamigboye, G.; Enabulele, D.; Odetoyan, A.O.; Kareem, M.A.; Nworgu, A.; Bassey, D.; Bamigboye, G.O.; Shukla, S.K. Mechanical and Durability Assessment of Concrete Containing Seashells: A Review Mechanical and Durability Assessment of Concrete Containing Seashells: A Review Public Interest Statement. Cogent Eng. 2021, 8, 1883830. [Google Scholar] [CrossRef]
- Fitriyana, D.F.; Nugraha, F.W.; Laroybafih, M.B.; Ismail, R.; Bayuseno, A.P.; Muhamadin, R.C.; Ramadan, M.B.; Qudus, A.R.A.; Siregar, J.P. The Effect of Hydroxyapatite Concentration on the Mechanical Properties and Degradation Rate of Biocomposite for Biomedical Applications. IOP Conf. Ser. Earth Environ. Sci. 2022, 969, 12045. [Google Scholar] [CrossRef]
- Coringa, R.; de Sousa, E.M.; Botelho, J.N.; Diniz, R.S.; de Sá, J.C.; Nogueira da Cruz, M.C.F.; Paschoal, M.A.B.; Gonçalves, L.M. Bone Substitute Made from a Brazilian Oyster Shell Functions as a Fast Stimulator for Bone-Forming Cells in an Animal Model. PLoS ONE 2018, 13, e0198697. [Google Scholar] [CrossRef]
- Oktawati, S.; Mappangara, S.; Chandra, H.; Achmad, H.; Raoda, S.; Ramadhan, J.; Dwipa, G.; Yudin, M. Effectiveness Nacre Pearl Shell (Pinctada Maxima) as Bone Graft for Periodontal Bone Remodeling. Ann. Rom. Soc. Cell Biol. 2021, 25, 8663–8678. [Google Scholar]
- Musa, B.; Raya, I.; Natsir, H. Synthesis and Characterizations of Hydroxyapatite Derived Blood Clam Shells (Anadara Granosa) and Its Potency to Dental Remineralizations. Int. J. Appl. Chem. 2016, 12, 527–538. [Google Scholar]
- Albadr, R.M.; Halfi, S.A.; Ziadan, K.M. The Effectiveness of Oyster Filler on the Physical and Mechanical Properties of Novel Dental Restorative Composite. AIP Conf. Proc. 2020, 2290, 50001. [Google Scholar]
- Fu, W.; Mohd Noor, M.H.; Yusof, L.M.; Ibrahim, T.A.T.; Keong, Y.S.; Jaji, A.Z.; Zakaria, M.Z.A.B. In Vitro Evaluation of a Novel PH Sensitive Drug Delivery System Based Cockle Shell-Derived Aragonite Nanoparticles against Osteosarcoma. J. Exp. Nanosci. 2017, 12, 166–187. [Google Scholar] [CrossRef]
- Saidykhan, L.; Bakar, M.Z.B.A.; Rukayadi, Y.; Kura, A.U.; Latifah, S.Y. Development of Nanoantibiotic Delivery System Using Cockle Shell-Derived Aragonite Nanoparticles for Treatment of Osteomyelitis. Int. J. Nanomed. 2016, 11, 661–673. [Google Scholar] [CrossRef]
- Choi, A.H.; Cazalbou, S.; Ben-Nissan, B. Biomimetics and Marine Materials in Drug Delivery and Tissue Engineering. In Handbook of Bioceramics and Biocomposites; Springer: Berlin/Heidelberg, Germany, 2016; pp. 521–544. ISBN 9783319124605. [Google Scholar]
- Zhao, R.; Yang, R.; Cooper, P.R.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. Molecules 2021, 26, 3007. [Google Scholar] [CrossRef] [PubMed]
- Ismail, R.; Fitriyana, D.F.; Santosa, Y.I.; Nugroho, S.; Hakim, A.J.; Al Mulqi, M.S.; Jamari, J.; Bayuseno, A.P. The Potential Use of Green Mussel (Perna Viridis) Shells for Synthetic Calcium Carbonate Polymorphs in Biomaterials. J. Cryst. Growth 2021, 572, 126282. [Google Scholar] [CrossRef]
- Fitriyana, D.F.; Caesarendra, W.; Nugroho, S.; Haryadi, G.D.; Herawan, M.A.; Rizal, M.; Ismail, R. The Effect of Compressed Air Pressure and Stand-off Distance on the Twin Wire Arc Spray (TWAS) Coating for Pump Impeller from AISI 304 Stainless Steel. Springer Proc. Phys. 2020, 242, 119–130. [Google Scholar] [CrossRef]
- Fitriyana, D.F.; Suhaimi, H.; Sulardjaka; Noferi, R.; Caesarendra, W. Synthesis of Na-P Zeolite from Geothermal Sludge. Springer Proc. Phys. 2020, 242, 51–59. [Google Scholar] [CrossRef]
- Fitriyana, D.F.; Anis, S.; Rachman, A.; Qudus, A.; Aufa, M.; Lakuy, N.; Ismail, R.; Nugroho, S.; Haryadi, G.D.; Bayuseno, A.P. The Effect of Post-Heat Treatment on The Mechanical Properties of FeCrBMnSi Coatings Prepared by Twin Wire Arc Spraying (TWAS) Method on Pump Impeller From 304 Stainless Steel. J. Adv. Res. Fluid Mech. Therm. Sci. 2022, 2, 138–147. [Google Scholar] [CrossRef]
- Huang, Z.Q.; Zhang, G.S.; Tan, Y. Gelatinous Siphon Sheath Templates the Starfruit-Shaped Aragonite Aggregate Growth. J. Nanomater. 2019, 2019, 1–9. [Google Scholar] [CrossRef]
- Ramakrishna, C.; Thenepalli, T.; Ahn, J.W. A Brief Review of Aragonite Precipitated Calcium Carbonate (PCC) Synthesis Methods and Its Applications. Korean Chem. Eng. Res. 2017, 55, 443–455. [Google Scholar] [CrossRef]
- Widyastuti, S.; Intan Ayu Kusuma, P. Synthesis and Characterization of CaCO3 (Calcite) Nano Particles from Cockle Shells (Anadara Granosa Linn) by Precipitation Method. AIP Conf. Proc. 2017, 1855, 030018. [Google Scholar] [CrossRef]
- Oral, Ç.M.; Ercan, B. Influence of PH on Morphology, Size and Polymorph of Room Temperature Synthesized Calcium Carbonate Particles. Powder Technol. 2018, 339, 781–788. [Google Scholar] [CrossRef]
- Loy, C.W.; Matori, K.A.; Lim, W.F.; Schmid, S.; Zainuddin, N.; Wahab, Z.A.; Alassan, Z.N.; Zaid, M.H.M. Effects of Calcination on the Crystallography and Nonbiogenic Aragonite Formation of Ark Clam Shell under Ambient Condition. Adv. Mater. Sci. Eng. 2016, 2016, 2914368. [Google Scholar] [CrossRef]
- Jimoh, O.A.; Ariffin, K.S.; Hussin, H.B.; Temitope, A.E. Synthesis of Precipitated Calcium Carbonate: A Review. Carbonates Evaporites 2018, 33, 331–346. [Google Scholar] [CrossRef]
- Yakymechko, Y.; Sanytsky, M.; Chekanskyi, B. Research of Alteration of Portlandite Crystals Habit as a Factor of Structure Formation Control for Lime-Containing Binders. Eureka Phys. Eng. 2016, 6, 19–28. [Google Scholar] [CrossRef]
- Franus, W.; Panek, R.; Wdowin, M. Sem Investigation of Microstructures in Hydration Products of Portland Cement. In 2nd International Multidisciplinary Microscopy and Microanalysis Congress; Springer: Cham, Switzerland, 2015; Volume 164, pp. 105–112. [Google Scholar]
- Wardhani, S.; Prasetia, F.; Khunur, M.M.; Purwonugroho, D.; Prananto, Y.P. Effect of CO2 Flow Rate and Carbonation Temperature in the Synthesis of Crystalline Precipitated Calcium Carbonate (PCC) from Limestone. Indones. J. Chem. 2018, 18, 573–579. [Google Scholar] [CrossRef]
- Gomez-Villalba, L.S.; López-Arce, P.; Alvarez De Buergo, M.; Fort, R. Atomic Defects and Their Relationship to Aragonite-Calcite Transformation in Portlandite Nanocrystal Carbonation. Cryst. Growth Des. 2012, 12, 4844–4852. [Google Scholar] [CrossRef]
- Ismail, R.; Laroybafih, M.B.; Fitriyana, D.F.; Nugroho, S.; Santoso, Y.I.; Hakim, A.J.; Mulqi, M.S.A.; Priharyoto, A. The Effect of Hydrothermal Holding Time on The Characterization of Hydroxyapatite Synthesized from Green Mussel Shells. J. Adv. Res. Fluid Mech. Therm. Sci. 2021, 80, 84–93. [Google Scholar] [CrossRef]
- Trushina, D.B.; Bukreeva, T.V.; Kovalchuk, M.V.; Antipina, M.N. CaCO3 Vaterite Microparticles for Biomedical and Personal Care Applications. Mater. Sci. Eng. C 2014, 45, 644–658. [Google Scholar] [CrossRef]
- Konopacka-Łyskawa, D.; Kościelska, B.; Łapiński, M. Precipitation of Spherical Vaterite Particles via Carbonation Route in the Bubble Column and the Gas-Lift Reactor. JOM 2019, 71, 1041–1048. [Google Scholar] [CrossRef]
- Prihanto, A.; Muryanto, S.; Ismail, R.; Jamari, J.; Bayuseno, A.P. Practical Insights into the Recycling of Green Mussel Shells (Perna Viridis) for the Production of Precipitated Calcium Carbonate. Environ. Technol. 2022, 1–11. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, Y.; Zhang, X.; Deb, H.; Yao, J. Phase Selection of Calcium Carbonate Crystals under the Induction of Lignin Monomer Model Compounds. CrystEngComm 2020, 22, 2454–2461. [Google Scholar] [CrossRef]
- Kamitakahara, M.; Nagamori, T.; Yokoi, T.; Ioku, K. Carbonate-Containing Hydroxyapatite Synthesized by the Hydrothermal Treatment of Different Calcium Carbonates in a Phosphate-Containing Solution. J. Asian Ceram. Soc. 2015, 3, 287–291. [Google Scholar] [CrossRef]
- Mayorga, I.C.; Astilleros, J.M.; Fernández-Díaz, L. Precipitation of Caco 3 Polymorphs from Aqueous Solutions: The Role of Ph and Sulphate Groups. Minerals 2019, 9, 178. [Google Scholar] [CrossRef]
- Mailafiya, M.M.; Abubakar, K.; Danmaigoro, A.; Chiroma, S.M.; Rahim, E.B.A.; Moklas, M.A.M.; Zakaria, Z.A.B. Cockle Shell-Derived Calcium Carbonate (Aragonite) Nanoparticles: A Dynamite to Nanomedicine. Appl. Sci. 2019, 9, 2897. [Google Scholar] [CrossRef]
- Habte, L.; Khan, M.D.; Shiferaw, N.; Farooq, A.; Lee, M.; Jung, S.; Ahn, J.W. Synthesis, Characterization and Mechanism Study of Green Aragonite Crystals Fromwaste Biomaterials as Calcium Supplement. Sustainability 2020, 12, 5062. [Google Scholar] [CrossRef]
- Konopacka-Łyskawa, D. Synthesis Methods and Favorable Conditions for Spherical Vaterite Precipitation: A Review. Crystals 2019, 9, 223. [Google Scholar] [CrossRef]
- Hamester, M.R.R.; Balzer, P.S.; Becker, D. Characterization of Calcium Carbonate Obtained from Oyster and Mussel Shells and Incorporation in Polypropylene. Mater. Res. 2012, 15, 204–208. [Google Scholar] [CrossRef]
- Awe, O.W.; Minh, D.P.; Lyczko, N.; Nzihou, A.; Zhao, Y. Laboratory-Scale Investigation of the Removal of Hydrogen Sulfide from Biogas and Air Using Industrial Waste-Based Sorbents. J. Environ. Chem. Eng. 2017, 5, 1809–1820. [Google Scholar] [CrossRef]
- Hussein, A.I.; Ab-Ghani, Z.; Che Mat, A.N.; Ab Ghani, N.A.; Husein, A.; Ab Rahman, I. Synthesis and Characterization of Spherical Calcium Carbonate Nanoparticles Derived from Cockle Shells. Appl. Sci. 2020, 10, 7170. [Google Scholar] [CrossRef]


| Element | Composition (wt.%) | |
|---|---|---|
| Crab Shells | Green Mussel Shells | |
| C | 29.44 | 17.56 |
| O | 38.97 | 53.09 |
| Ca | 24.49 | 28.85 |
| Na | 0.38 | 0.50 |
| Mg | 1.4 | - |
| P | 3.33 | - |
| Zr | 1.99 | - |
| Total | 100 | 100 |
| Element | Composition (wt.%) | |||
|---|---|---|---|---|
| Green Mussel Shells | Crab Shells | |||
| Powders | CMS | Powders | CCS | |
| C | 17.56 | 7.13 | 29.44 | 7.38 |
| O | 53.09 | 51.21 | 38.97 | 47.99 |
| Ca | 28.85 | 41.66 | 24.49 | 37.18 |
| Na | 0.5 | - | 0.38 | 0.58 |
| Mg | - | - | 1.4 | 3.89 |
| P | - | - | 3.33 | 2.98 |
| Zr | - | - | 1.99 | - |
| Total | 100 | 100 | 100 | 100 |
| Element | Composition (wt.%) | |||||
|---|---|---|---|---|---|---|
| Green Mussel Shells | Crab Shells | |||||
| Powders | CMS | PMS | Powders | CCS | PCS | |
| C | 17.56 | 7.13 | 14.11 | 29.44 | 7.38 | 15.43 |
| O | 53.09 | 51.21 | 52.41 | 38.97 | 47.99 | 54.44 |
| Ca | 28.85 | 41.66 | 33.48 | 24.49 | 37.18 | 17.56 |
| Na | 0.5 | - | - | 0.38 | 0.58 | - |
| Mg | - | - | - | 1.4 | 3.89 | - |
| P | - | - | - | 3.33 | 2.98 | - |
| Zr | - | - | - | 1.99 | - | - |
| Total | 100 | 100 | 100 | 100 | 100 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismail, R.; Cionita, T.; Shing, W.L.; Fitriyana, D.F.; Siregar, J.P.; Bayuseno, A.P.; Nugraha, F.W.; Muhamadin, R.C.; Junid, R.; Endot, N.A. Synthesis and Characterization of Calcium Carbonate Obtained from Green Mussel and Crab Shells as a Biomaterials Candidate. Materials 2022, 15, 5712. https://doi.org/10.3390/ma15165712
Ismail R, Cionita T, Shing WL, Fitriyana DF, Siregar JP, Bayuseno AP, Nugraha FW, Muhamadin RC, Junid R, Endot NA. Synthesis and Characterization of Calcium Carbonate Obtained from Green Mussel and Crab Shells as a Biomaterials Candidate. Materials. 2022; 15(16):5712. https://doi.org/10.3390/ma15165712
Chicago/Turabian StyleIsmail, Rifky, Tezara Cionita, Wong Ling Shing, Deni Fajar Fitriyana, Januar Parlaungan Siregar, Athanasius Priharyoto Bayuseno, Fariz Wisda Nugraha, Rilo Chandra Muhamadin, Ramli Junid, and Nor Azam Endot. 2022. "Synthesis and Characterization of Calcium Carbonate Obtained from Green Mussel and Crab Shells as a Biomaterials Candidate" Materials 15, no. 16: 5712. https://doi.org/10.3390/ma15165712
APA StyleIsmail, R., Cionita, T., Shing, W. L., Fitriyana, D. F., Siregar, J. P., Bayuseno, A. P., Nugraha, F. W., Muhamadin, R. C., Junid, R., & Endot, N. A. (2022). Synthesis and Characterization of Calcium Carbonate Obtained from Green Mussel and Crab Shells as a Biomaterials Candidate. Materials, 15(16), 5712. https://doi.org/10.3390/ma15165712









