Zeolites Reduce the Transfer of Potentially Toxic Elements from Soil to Leafy Vegetables
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Plant Materials
2.2. Zeolite Collection, Preparation and Characterization
2.3. Soil Collection and Characterization
2.4. Design of the Experiment
2.5. Plant Collection and Analysis
2.6. Quality Assurance
2.7. Calculation of Bioaccumulation and Transfer Factors
2.8. Data Analysis
3. Results and Discussion
3.1. Natural Zeolite
3.2. PTE Concentrations of Soil–Zeolite Mixtures
3.3. PTE Concentration in Plants
3.4. Soil to Plant Transfer
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Medyńska-Juraszek, A.; Bednik, M.; Chohura, P. Assessing the influence of compost and biochar amendments on the mobility and uptake of heavy metals by green leafy vegetables. Int. J. Environ. Res. Public Health 2020, 17, 7861. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kin, K.H. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Palansooriya, K.N.; Shahee, S.M.; Chen, S.C.; Tsang, D.C.W.; Hashimoto, Y.; Hou, D.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef] [PubMed]
- Belviso, C. Zeolite for potential toxic metal uptake from contaminated soil: A brief review. Processes 2020, 8, 820. [Google Scholar] [CrossRef]
- Miclean, M.; Cadar, O.; Levei, E.A.; Roman, R.; Ozunu, A.; Levei, L. Metal (Pb, Cu, Cd, and Zn) transfer along food chain and health risk assessment through raw milk consumption from free-range cows. Int. J. Environ. Res. Public Health 2019, 16, 4064. [Google Scholar] [CrossRef]
- Miclean, M.; Cadar, O.; Levei, L.; Senila, L.; Ozunu, A. Metal contents and potential health risk assessment of crops grown in a former mining district (Romania). J. Environ. Sci. Health Part B 2018, 53, 595–601. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Y.; Tan, S.N.; Yusof, M.L.M.; Ghosh, S.; Chen, Z. Phytoremediation: A promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef]
- Hoaghia, M.A.; Cadar, O.; Moisa, C.; Roman, C.; Kovacs, E. Heavy metals and health risk assessment in vegetables grown in the vicinity of a former non-metallic facility located in Romania. Environ. Sci. Pollut. Res. 2022, 29, 40079–40093. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Z.; Peng, M.; Cheng, X. Contamination levels and the ecological and human health risks of potentially toxic elements (PTEs) in soil of Baoshan area, Southwest China. Appl. Sci. 2022, 12, 1693. [Google Scholar] [CrossRef]
- DalCorso, G.; Fasani, E.; Manara, A.; Visioli, G.; Furini, A. Heavy metal pollutions: State of the art and innovation in phytoremediation. Int. J. Mol. Sci. 2019, 20, 3412. [Google Scholar] [CrossRef] [PubMed]
- Cadar, O.; Dinca, Z.; Senila, M.; Torok, A.I.; Todor, F.; Levei, E.A. Immobilization of potentially toxic elements in contaminated soils using thermally treated natural zeolite. Materials 2021, 14, 3777. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Yi, Y.; Zeng, G. Effects of modified zeolite on the removal and stabilization of heavy metals in contaminated lake sediment using BCR sequential extraction. J. Environ. Manag. 2016, 178, 63–69. [Google Scholar] [CrossRef]
- Eroglu, N.; Emekci, M.; Athanassiou, C. Applications of natural zeolites on agriculture and food production. J. Sci. Food Agric. 2017, 97, 3487–3499. [Google Scholar] [CrossRef]
- Ríos-Reyes, C.A.; Reyes-Mendoza, G.A.; Henao-Martínez, J.A.; Williams, C.; Dyer, A. First report on the geologic occurrence of natural Na–A zeolite and associated minerals in cretaceous mudstones of the Paja formation of Vélez (Santander), Colombia. Crystals 2021, 11, 218. [Google Scholar] [CrossRef]
- Cataldo, E.; Salvi, L.; Paoli, F.; Fucile, M.; Masciandaro, G.; Manzi, D.; Masini, C.M.; Mattii, G.B. Application of zeolites in agriculture and other potential uses: A review. Agronomy 2021, 11, 1547. [Google Scholar] [CrossRef]
- Contin, M.; Miho, L.; Pellegrini, E.; Gjoka, F.; Shkurta, E. Effects of natural zeolites on ryegrass growth and bioavailability of Cd, Ni, Pb, and Zn in an Albanian contaminated soil. J. Soils Sediments 2019, 19, 4052–4062. [Google Scholar] [CrossRef]
- Misaelides, P. Application of natural zeolites in environmental remediation: A short review. Microporous Mesoporous Mater. 2011, 144, 15–18. [Google Scholar] [CrossRef]
- Cadar, O.; Senila, M.; Hoaghia, M.-A.; Scurtu, D.; Miu, I.; Levei, E.A. Effects of thermal treatment on natural clinoptilolite-rich zeolite behavior in simulated biological fluids. Molecules 2020, 25, 2570. [Google Scholar] [CrossRef]
- Kitsopoulos, K.P. Cation-exchange capacity (CEC) of zeolitic volcaniclastic materials: Applicability of the ammonium acetate saturation (AMAS) method. Clays Clay Miner. 1999, 47, 688–696. [Google Scholar] [CrossRef]
- Ghergari, L.; Gal, J. Mineralogy of the pollutant products formed in the Maşca exploration area (Lower Iara valley basin, Cluj County, Romania). Stud. Univ. Babes-Bolyai Geol. 2004, 49, 53–64. [Google Scholar] [CrossRef][Green Version]
- Katoh, M.; Masaki, S.; Sato, T. Single-step extraction to determine soluble lead levels in soil. Int. J. Geomate 2012, 3, 375–380. [Google Scholar] [CrossRef]
- Quevauviller, P.; Rauret, G.; Rubio, R.; López-Sánchez, J.-F.; Ure, A.; Bacon, J.; Muntau, H. Certified reference materials for the quality control of EDTA- and acetic acid-extractable contents of trace elements in sewage sludge amended soils (CRMs 483 and 484). Fresenius J. Anal. Chem. 1997, 357, 611–618. [Google Scholar] [CrossRef]
- Ciesielski, H.; Sterckeman, T. A comparison between three methods for the determination of cation exchange capacity and exchangeable cations in soils. Agronomie 1997, 17, 9–16. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. Off. J. Eur. Union 2006, 364, 5–24.
- Commission Regulation (EU) 2021/1323 of 10 August 2021 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Cadmium in Certain Foodstuffs. Off. J. Eur. Union 2021, 288, 13–18.
- Commission Regulation (EC) 2021/1317 of 9 August 2021 Amending Regulation (EC) No 881/2006 as Regards Maximum Levels of Lead in Certain Foodstuffs. Off. J. Eur. Union 2021, 286, 1–4.
- Cadar, O.; Mocan, T.; Roman, C.; Senila, M. Analytical performance and validation of a reliable method based on graphite furnace atomic absorption spectrometry for the determination of gold nanoparticles in biological tissues. Nanomaterials 2021, 11, 3370. [Google Scholar] [CrossRef]
- Sulaiman, F.R.; Hamzah, H.A. Heavy metals accumulation in suburban roadside plants of a tropical area (Jengka, Malaysia). Ecol. Process. 2018, 7, 28. [Google Scholar] [CrossRef]
- Liu, K.; Lv, J.; Dai, Y.; Zhang, H.; Cao, Y. Cross-species extrapolation of models for predicting lead transfer from soil to wheat grain. PLoS ONE 2016, 11, e0160552. [Google Scholar] [CrossRef] [PubMed]
- Miclean, M.; Levei, E.A.; Senila, M.; Roman, C.; Corods, E. Assessment of Cu, Pb, Zn and Cd availability to vegetable species grown in the vicinity of tailing deposits from Baia Mare area. Rev. Chim-Buchar. 2009, 60, 1–4. [Google Scholar]
- Moirou, A.; Xenidis, A.; Paspaliaris, I. Stabilization Pb, Zn, and Cd contaminated soil by means of natural zeolite. Soil Sediment Contam. Int. J. 2001, 10, 251–267. [Google Scholar] [CrossRef]
- Order No. 756 of 3 November 1997 for the Approval of the Regulation on Environmental Pollution Assessment. Eminent: Ministry of Waters, Forests and Environmental Protection. (Published in: Official Gazette No 303 bis of 6 November 1997). Available online: http://legislatie.just.ro/Public/DetaliiDocumentAfis/151788 (accessed on 15 February 2022). (In Romanian).
- Pachura, P.; Ociepa-Kubicka, A.; Skawron-Grabowska, B. Assessment of the availability of heavy metals to plants based on the translocation index and the bioaccumulation factor. Desalin. Water Treat. 2016, 57, 1469–1477. [Google Scholar] [CrossRef]
- Zhu, Y.-G.; Chen, S.-B.; Yang, J.-C. Effects of soil amendments on lead uptake by two vegetable crops from a lead-contaminated soil from Anhui, China. Environ. Int. 2004, 30, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Sindesi, O.A.; Lewu, M.N.; Ncube, B.; Mulidzi, R.; Lewu, F.B. Mineral composition of potted cabbage (Brassica oleracea var. Capitata L.) grown in zeolite amended sandy soil. Agriculture 2021, 67, 103–112. [Google Scholar] [CrossRef]
- Gül, A.; Erogul, D.; Ongun, A.R. Comparison of the use of zeolite and perlite as substrate for crisp-head lettuce. Sci. Hortic. 2005, 106, 464–471. [Google Scholar] [CrossRef]
- Al Mamun, S.; Chanson, G.; Muliadi; Benyas, E.; Aktar, M.; Lehto, N.; McDowell, R.; Cavanagh, J.; Kellermann, L.; Clucas, L.; et al. Municipal composts reduce the transfer of Cd from soil to vegetables. Environ. Pollut. 2016, 213, 8–15. [Google Scholar] [CrossRef]
- Krippner, J.; Schubert, S. Elevated zinc concentrations did not induce thiols in spinach (Spinacia oleracea) and parsley (Petroselinum crispum). J. Plant Nutr. Soil Sci. 2021, 184, 439–447. [Google Scholar] [CrossRef]

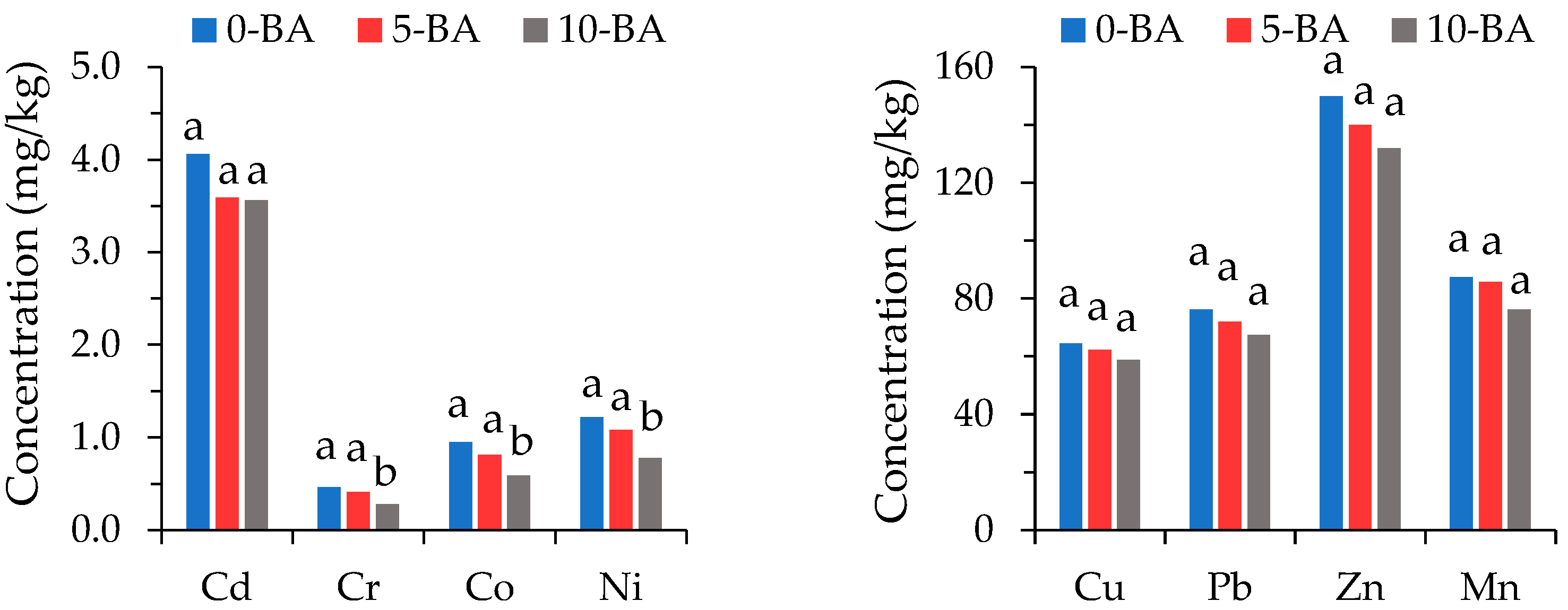
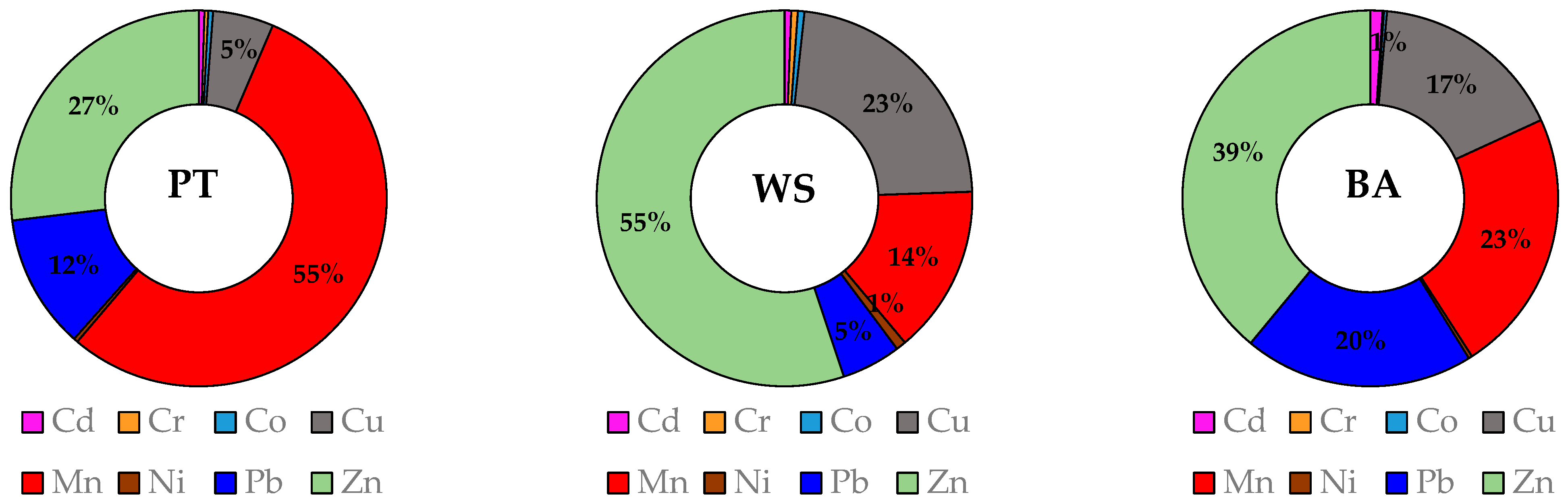


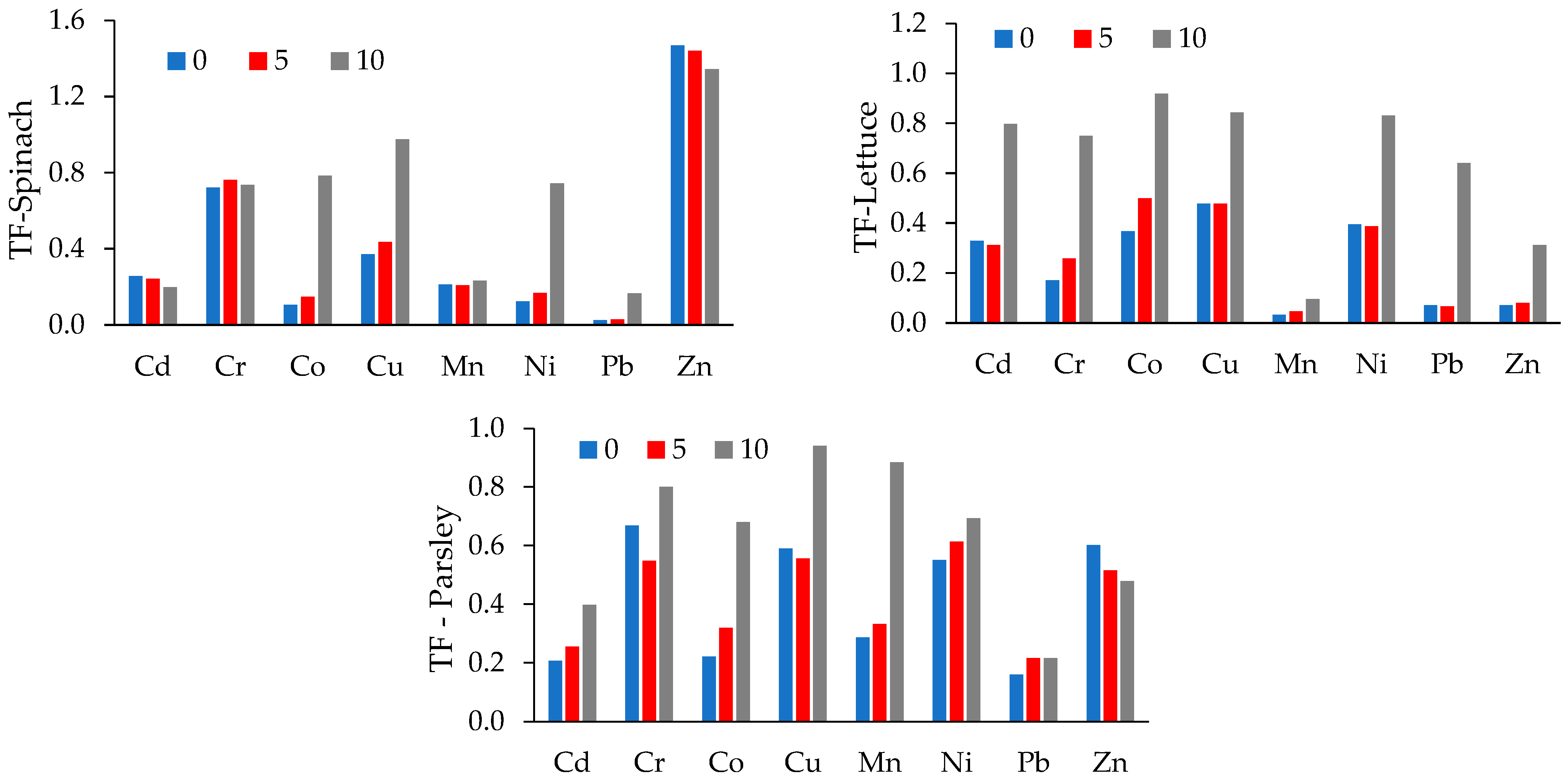
| Dose (%) | Cd | Cr | Co | Cu | Mn | Ni | Pb | Zn | ||
|---|---|---|---|---|---|---|---|---|---|---|
| mg/kg dw | ||||||||||
| Spinach | root | 0 | 6.85 ± 0.70 a | 3.45 ± 0.41 a | 2.84 ± 0.29 a | 68.6 ± 4.77 a | 305 ± 28 a | 10.6 ± 1.6 a | 133 ± 13 a | 375 ± 28 a |
| 5 | 5.94 ± 0.69 a | 2.83 ± 0.30 a | 1.61 ± 0.21 b | 50.6 ± 4.61 b | 241 ± 19 b | 5.91 ± 0.71 b | 96.0 ± 12.6 b | 292 ± 26 b | ||
| 10 | 5.60 ± 0.63 a | 1.92 ± 0.17 b | 0.21 ± 0.02 c | 18.8 ± 1.31 c | 180 ± 15 c | 0.91 ± 0.12 c | 14.0 ± 1.6 c | 208 ± 18 c | ||
| shoot | 0 | 1.75 ± 0.22 a | 2.49 ± 0.16 a | 0.30 ± 0.04 a | 25.5 ± 1.60 a | 64.6 ± 6.6 a | 1.31 ± 0.16 a | 3.46 ± 0.44 a | 550 ± 37 a | |
| 5 | 1.44 ± 0.14 a,b | 2.16 ± 0.19 a | 0.24 ± 0.03 a | 22.1 ± 1.71 a,b | 50.2 ± 4.8 b | 1.00 ± 0.12 a | 2.91 ± 0.30 a,b | 421 ± 30 b | ||
| 10 | 1.11 ± 0.08 b | 1.42 ± 0.15 b | 0.16 ± 0.02 b | 18.3 ± 1.73 b | 42.1 ± 3.9 b | 0.68 ± 0.1 b | 2.32 ± 0.29 b | 280 ± 23 c | ||
| Lettuce | root | 0 | 20.5 ± 2.05 a | 20.4 ± 1.8 a | 17.2 ± 1.1 a | 63.3 ± 4.71 a | 1780 ± 115 a | 28.4 ± 3.17 a | 425 ± 33 a | 1290 ± 76 a |
| 5 | 16.5 ± 1.95 a | 11.6 ± 0.9 b | 10.3 ± 0.7 b | 46.0 ± 3.0 b | 1020 ± 74 b | 22.5 ± 2.8 a | 343 ± 26 b | 800 ± 50 b | ||
| 10 | 5.30 ± 0.44 b | 2.40 ± 0.26 c | 4.00 ± 0.36 c | 20.4 ± 1.64 c | 336 ± 29 c | 7.31 ± 0.86 b | 18.9 ± 2.5 c | 138 ± 13 c | ||
| shoot | 0 | 6.74 ± 0.63 a | 3.48 ± 0.36 a | 6.32 ± 0.47 a | 30.2 ± 1.91 a | 57.2 ± 6.7 a | 11.2 ± 1.2 a | 30.2 ± 4.2 a | 92.6 ± 11.4 a | |
| 5 | 5.14 ± 0.36 b | 3.00 ± 0.27 a | 5.14 ± 0.47 b | 22.0 ± 1.57 b | 48.0 ± 4.1 a | 8.71 ± 0.87 b | 22.6 ± 2.8 a | 64.1 ± 6.4 b | ||
| 10 | 4.21 ± 0.36 b | 1.80 ± 0.20 b | 3.67 ± 0.32 c | 17.2 ± 1.25 c | 32.4 ± 3.2 b | 6.08 ± 0.60 c | 12.1 ± 1.8 b | 43.1 ± 4.3 c | ||
| Parsley | root | 0 | 1.85 ± 0.25 a | 1.42 ± 0.14 a | 0.80 ± 0.10 a | 45.0 ± 3.46 a | 168 ± 14 a | 6.53 ± 0.68 a | 39.8 ± 4.0 a | 164 ± 15 a |
| 5 | 1.22 ± 0.11 b | 0.78 ± 0.08 b | 0.45 ± 0.07 b | 32.4 ± 2.12 b | 109 ± 10 b | 4.72 ± 0.65 b | 23.2 ± 2.9 b | 118 ± 11 b | ||
| 10 | 0.27 ± 0.02 c | 0.34 ± 0.04 c | 0.12 ± 0.01 c | 16.7 ± 1.21 c | 32.0 ± 3.4 c | 2.60 ± 0.41 c | 16.6 ± 1.9 b | 80.3 ± 7.0 c | ||
| shoot | 0 | 0.38 ± 0.04 a | 0.95 ± 0.11 a | 0.18 ± 0.03 a | 26.6 ± 1.80 a | 48.2 ± 4.6 a | 3.60 ± 0.49 a | 6.36 ± 0.69 a | 98.7 ± 9.2 a | |
| 5 | 0.31 ± 0.03 a | 0.43 ± 0.05 b | 0.14 ± 0.02 a,b | 18.0 ± 1.25 b | 36.4 ± 3.8 b | 2.90 ± 0.34 a | 5.00 ± 0.50 b | 61.0 ± 6.1 b | ||
| 10 | 0.11 ± 0.02 b | 0.27 ± 0.03 b | 0.08 ± 0.02 b | 15.7 ± 1.06 b | 28.3 ± 3.8 b | 1.80 ± 0.24 b | 3.59 ± 0.39 c | 38.5 ± 3.8 c | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cadar, O.; Stupar, Z.; Senila, M.; Levei, L.; Moldovan, A.; Becze, A.; Ozunu, A.; Levei, E.A. Zeolites Reduce the Transfer of Potentially Toxic Elements from Soil to Leafy Vegetables. Materials 2022, 15, 5657. https://doi.org/10.3390/ma15165657
Cadar O, Stupar Z, Senila M, Levei L, Moldovan A, Becze A, Ozunu A, Levei EA. Zeolites Reduce the Transfer of Potentially Toxic Elements from Soil to Leafy Vegetables. Materials. 2022; 15(16):5657. https://doi.org/10.3390/ma15165657
Chicago/Turabian StyleCadar, Oana, Zamfira Stupar, Marin Senila, Levente Levei, Ana Moldovan, Anca Becze, Alexandru Ozunu, and Erika Andrea Levei. 2022. "Zeolites Reduce the Transfer of Potentially Toxic Elements from Soil to Leafy Vegetables" Materials 15, no. 16: 5657. https://doi.org/10.3390/ma15165657
APA StyleCadar, O., Stupar, Z., Senila, M., Levei, L., Moldovan, A., Becze, A., Ozunu, A., & Levei, E. A. (2022). Zeolites Reduce the Transfer of Potentially Toxic Elements from Soil to Leafy Vegetables. Materials, 15(16), 5657. https://doi.org/10.3390/ma15165657









