Isothermal Quenching of As-Cast Medium Carbon, High-Silicon AR Steel
Abstract
:1. Introduction
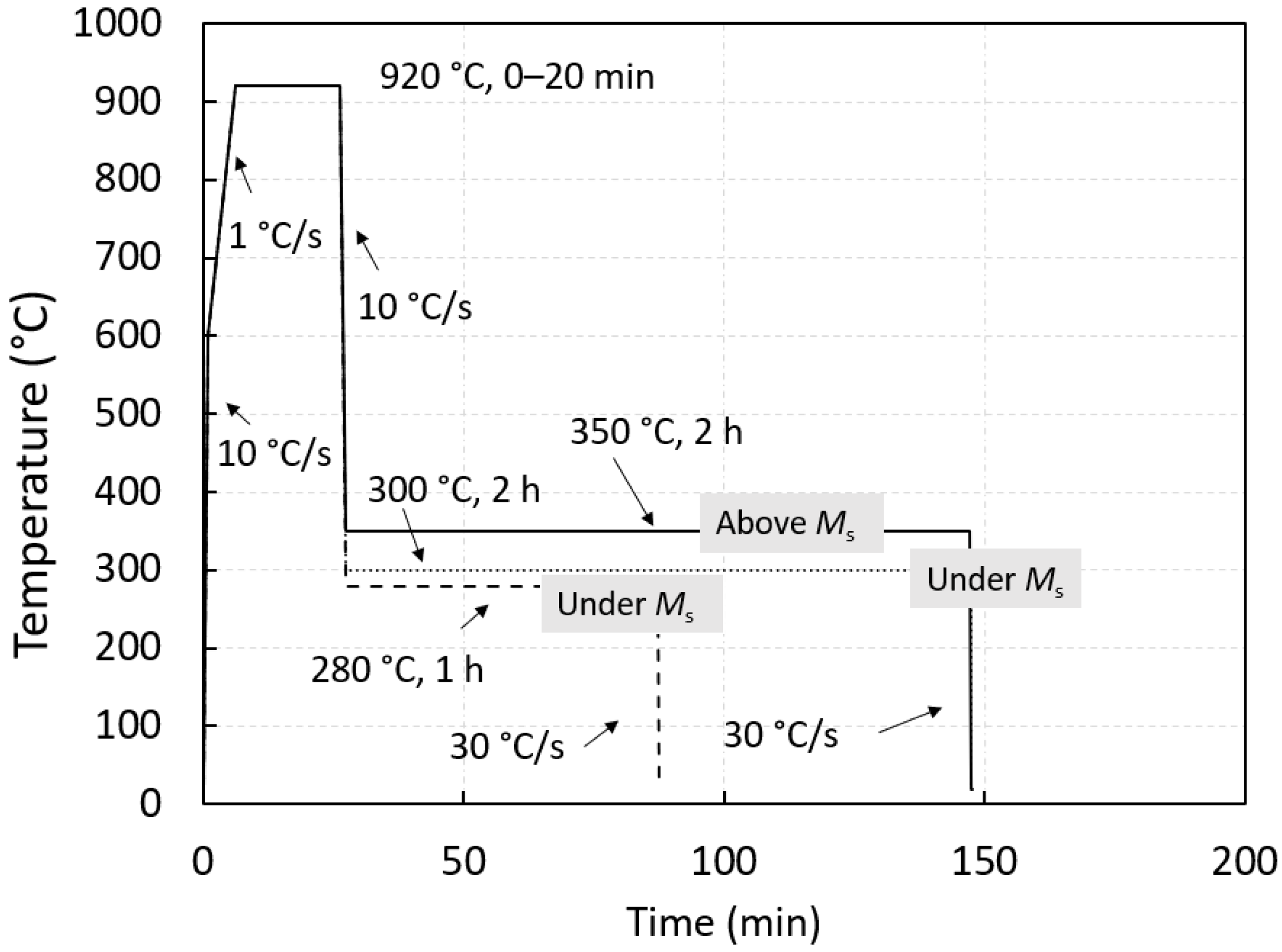
2. Materials and Methods
3. Results and Discussion
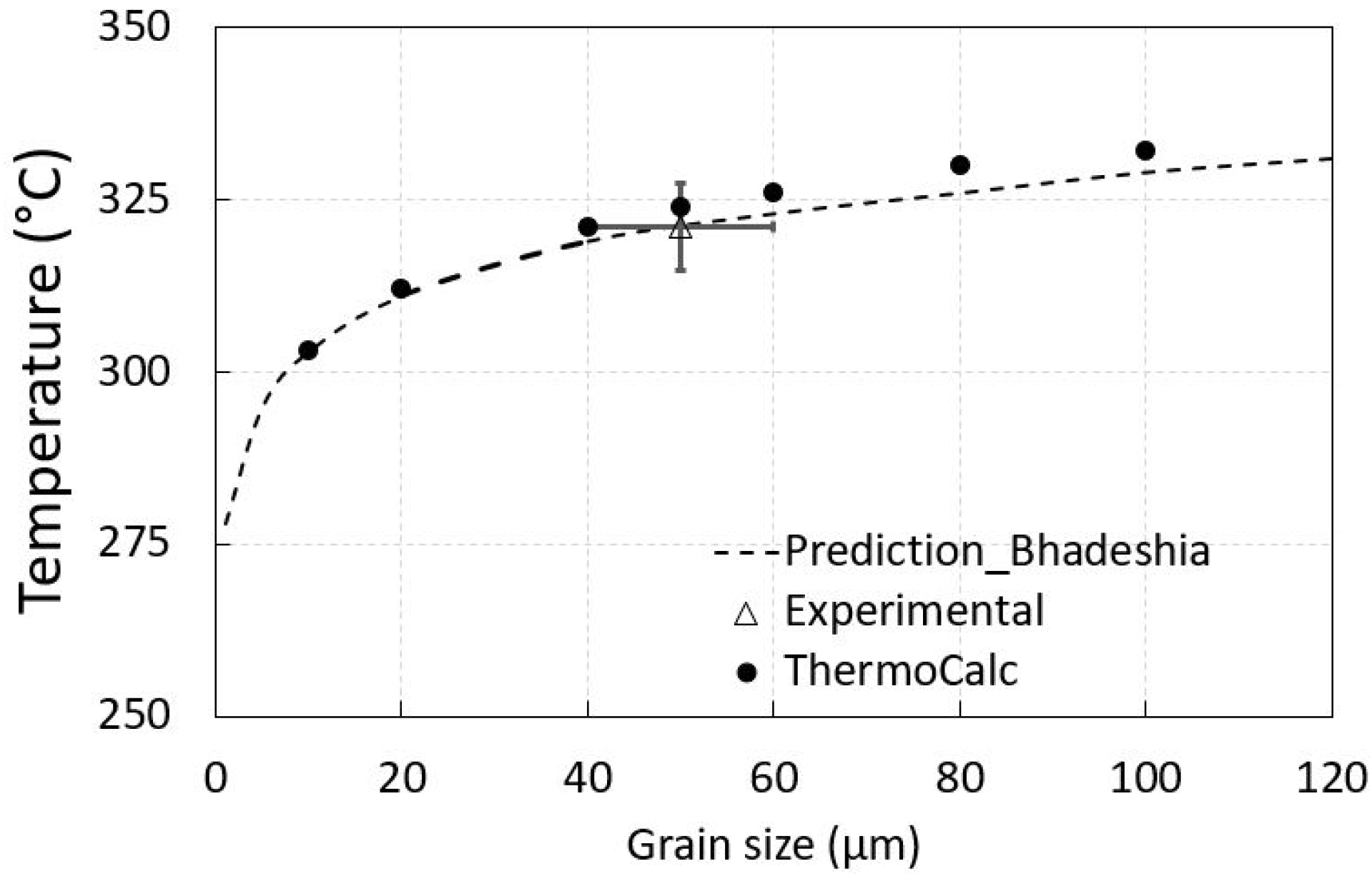
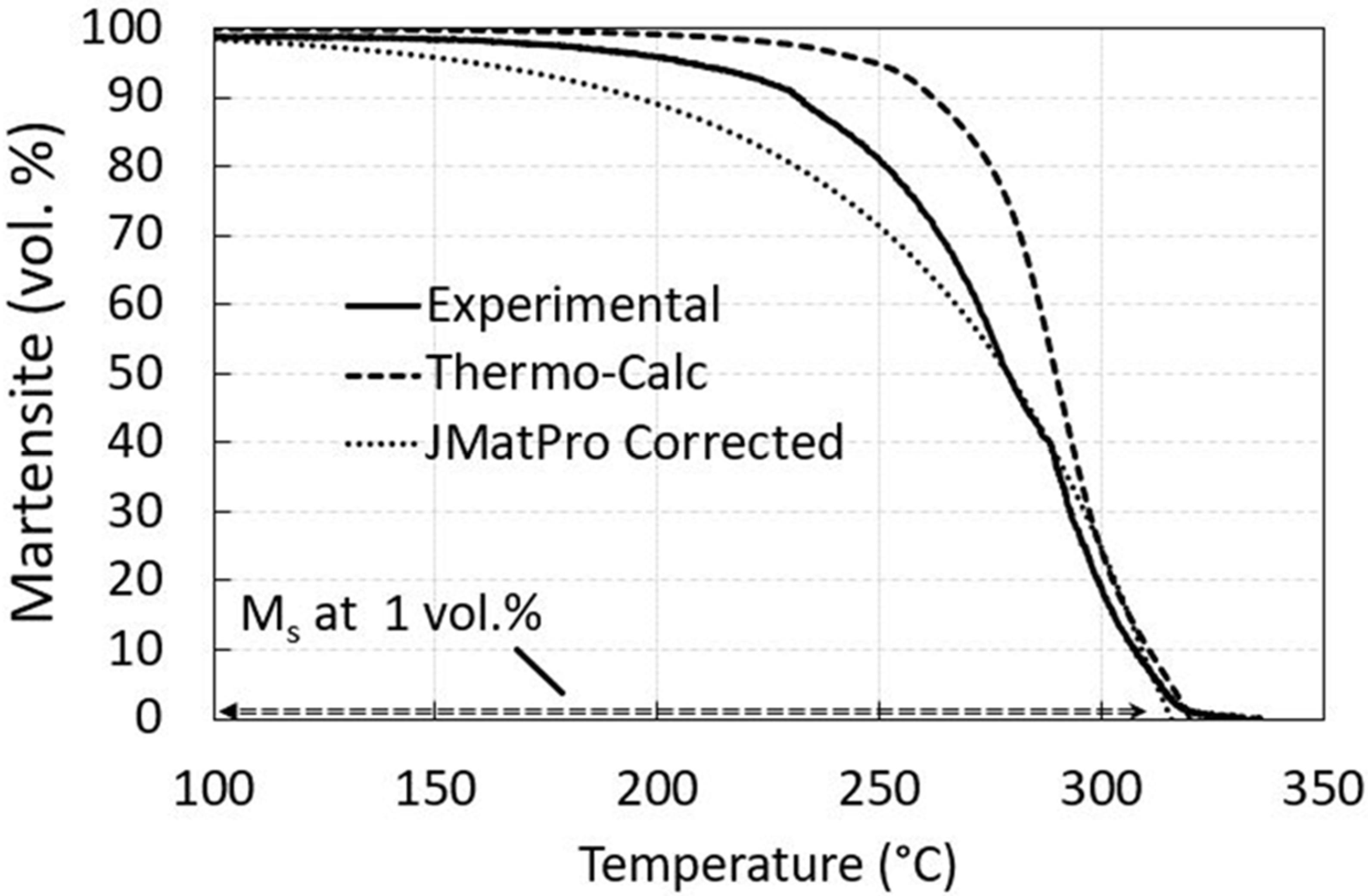
3.1. Thermodynamic Prediction
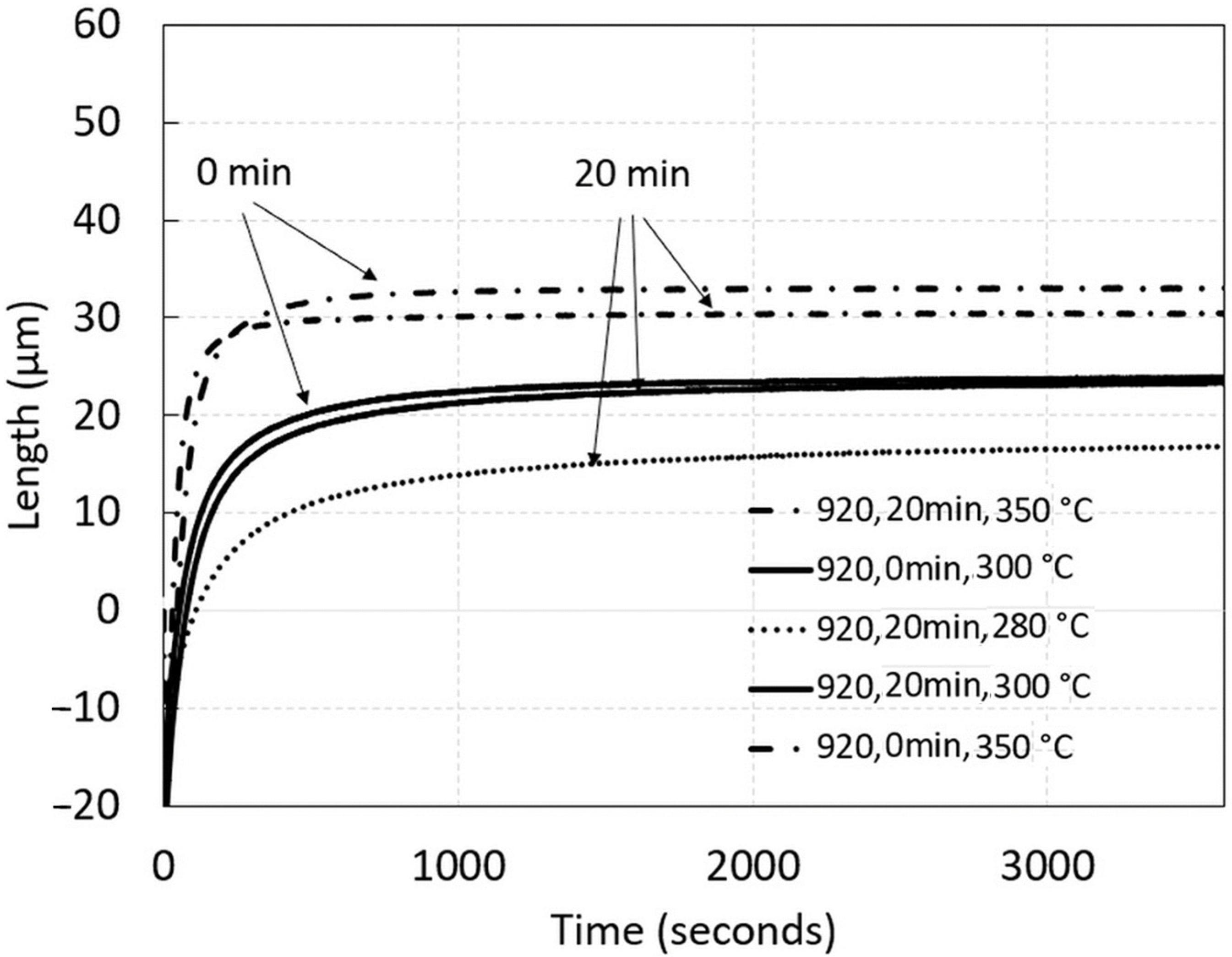
3.2. Dilatometric Measurement
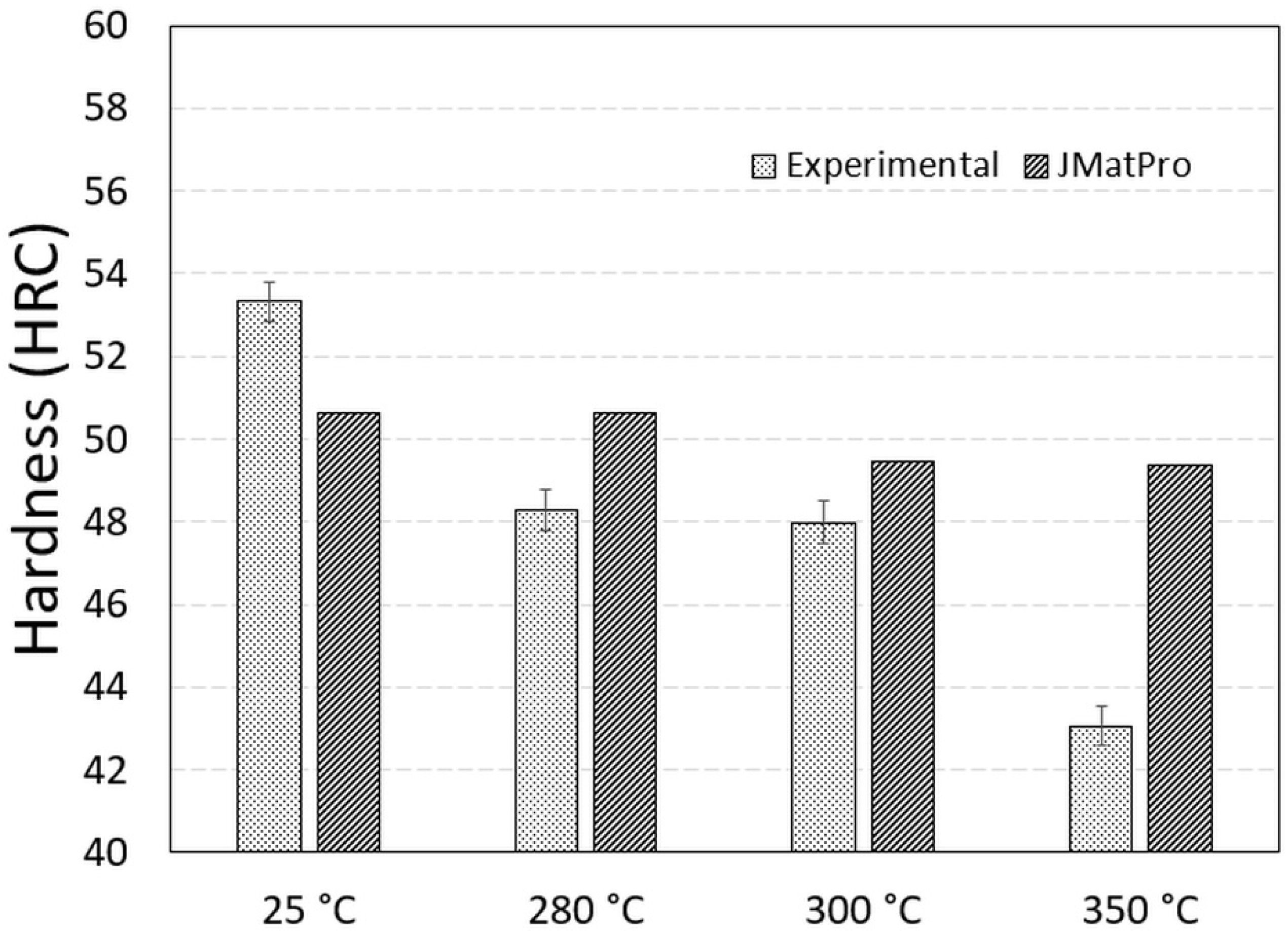
3.3. Hardness Measurement
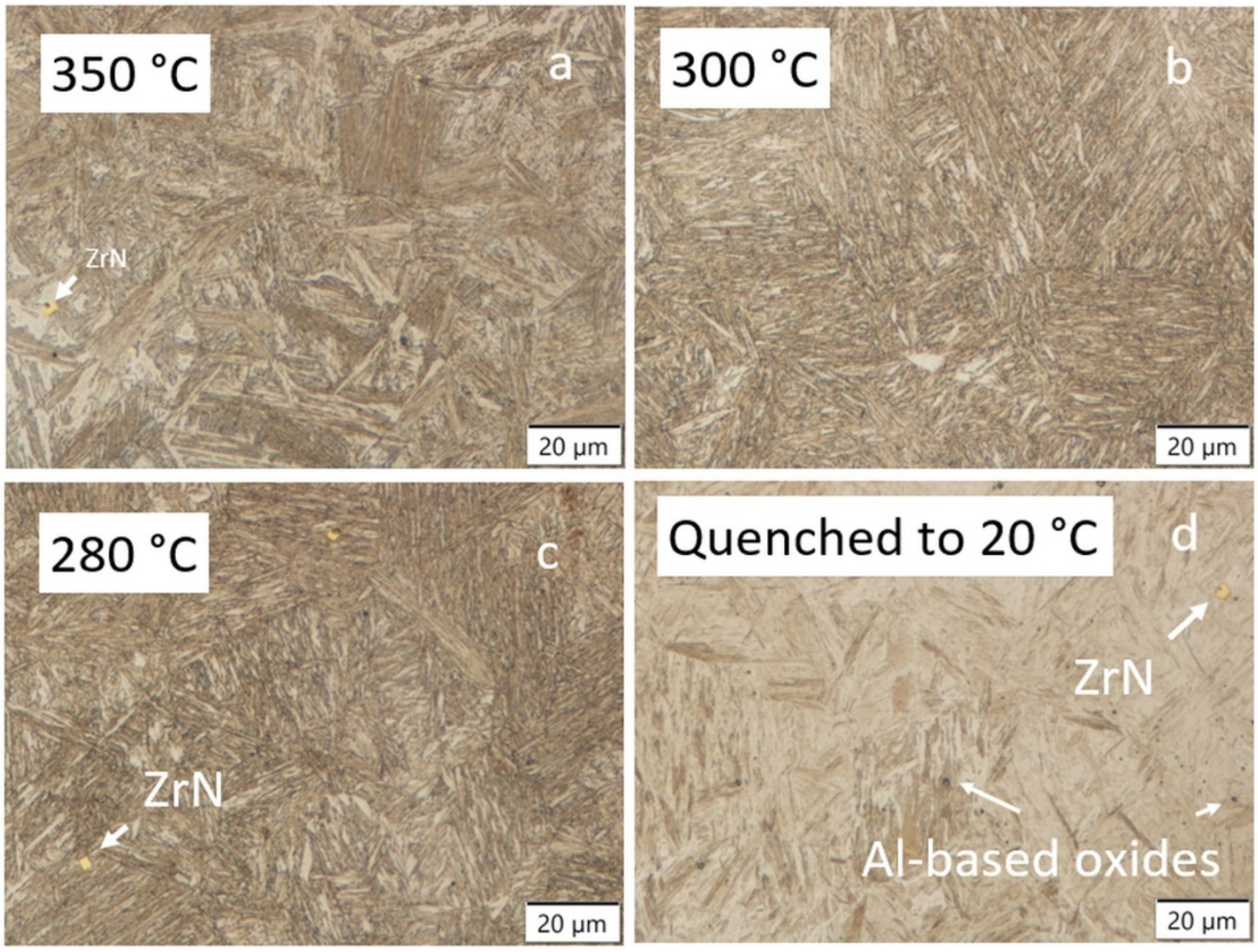
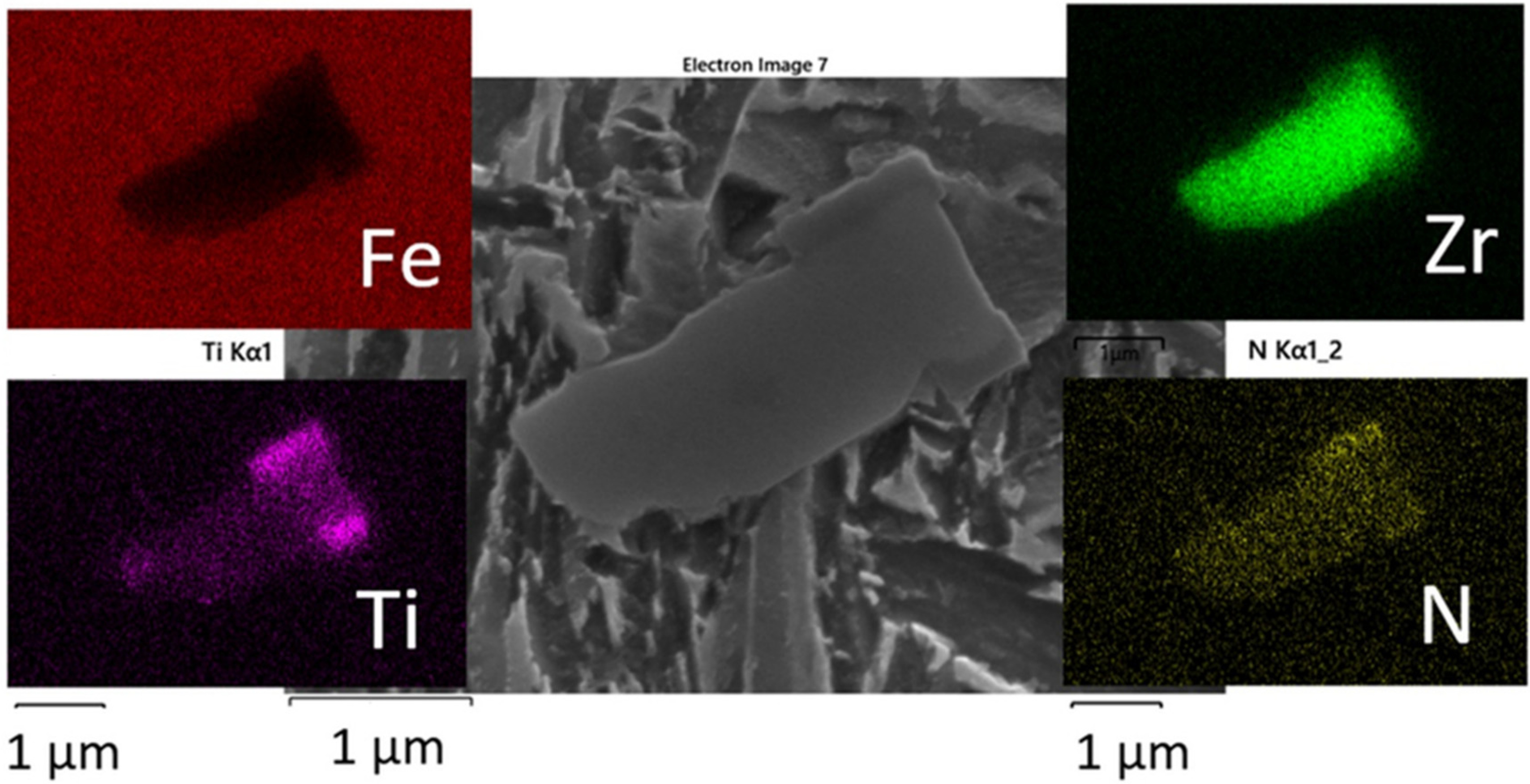
3.4. LOM and SEM-EDS Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Deng, X.T.; Fu, T.L.; Wang, Z.D.; Misra, R.D.K.; Wang, G.D. Epsilon carbide precipitation and wear behaviour of low alloy wear resistant steels. Mater. Sci. Tech. 2016, 32, 320–327. [Google Scholar] [CrossRef]
- Krishna, P.V.; Srikant, R.R.; Iqbal, M.; Sriram, N. Effect of Austempering and Martempering on the Properties of AISI 52100 Steel. Int. Sch. Res. Not. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Mandal, D.; Ghosh, M.; Pal, J.; De, P.K.; Ghosh Chowdhury, S.; Das, S.K.; Das, G.; Ghosh, S. Effect of austempering treatment on microstructure and mechanical properties of high-Si steel. J. Mater. Sci. 2009, 44, 1069–1075. [Google Scholar] [CrossRef]
- Sedlaček, M.; Klančnik, G.; Nagode, A.; Burja, J. Influence of Austempering of As-cast Medium Carbon High-Silicon Steel on Wear Resistance. Materials 2021, 14, 7518. [Google Scholar] [CrossRef] [PubMed]
- Lindström, A. Austempered High Silicon Steel, Investigation of Wear Resistance in a Carbide Free Microstructure. Master’s Thesis, Luleå University of Technology, Luleå, Sweden, 2006. [Google Scholar]
- Hillert, M. The nature of bainite, Review. ISIJ Int. 1995, 35, 1134–1140. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H.; Edmonds, D.V. Bainite in silicon steels: New composition-property approach Part 1. Met. Sci. 1983, 17, 411–419. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H.; Edmonds, D.V. Bainite in silicon steels: New composition-property approach Part 2. Met. Sci. 1983, 17, 420–425. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Bainite in Steels, Transformations, Microstructure and Properties, 2nd ed.; The University Press: Cambridge, UK, 2001. [Google Scholar]
- Davis, J.R. Metals Handbook Desk Edition, 2nd ed.; ASM International: Novelty, OH, USA, 1998. [Google Scholar]
- Heuer, V.; Loeser, K. Dry-Bainitizing—A Clean Approach to Achieve Bainitic Microstructures, in Bainite—From Nano to Macro. In Proceedings of the Symposium on Science and Application of Bainite, Wiesbaden, Germany, 1–2 June 2017. [Google Scholar]
- Samuels, L.E. Light Microscopy of Carbon Steels, 2nd ed.; ASM International: Novelty, OH, USA, 2003. [Google Scholar]
- Garcia-Mateo, C.; Caballero, F.G.; Bhadeshia, H.K.D.H. Acceleration of Low-temperature Bainite. ISIJ Int. 2003, 43, 1821–1825. [Google Scholar] [CrossRef]
- Caballero, F.G.; Santofimia, M.J.; García-Mateo, C.; Chao, J.; García de Andrés, C. Theoretical design and advanced microstructure in super high strength steels. Mater. Design. 2009, 30, 2077–2083. [Google Scholar] [CrossRef]
- Yang, H.-S.; Bhadeshia, H.K.D.H. Designing low carbon, low temperature bainite. Mater. Sci. Technol. 2008, 24, 33–342. [Google Scholar] [CrossRef]
- Smanio, V.; Sourmail, T. Effect of partial martensite transformation on bainite reaction kinetics in different 1%C steels. Solid State Phen. 2011, 172–174, 821–826. [Google Scholar] [CrossRef]
- Matsuzaki, A.; Bhadeshia, H.K.D.H. Effect of austenite grain size and bainite morphology on overall kinetics of bainite transformation in steels. Mater. Sci. Technol. 1999, 15, 518–522. [Google Scholar] [CrossRef]
- Zhao, L.; Qian, L.; Zhou, Q.; Li, D.; Wang, T.; Jia, Z.; Zhang, F.; Meng, J. The combining effects of ausforming and below-Ms or above-Ms austempering on the transformation kinetics, microstructure and mechanical properties of low-carbon bainitic steel. Mater. Des. 2019, 183, 1–14. [Google Scholar] [CrossRef]
- Sharma, S.; Sangal, S.; Mondal, K. Development of new High-Strength Carbide-Free Bainitic Steels. Metall. Mater. Trans. A 2011, 42, 3921–3933. [Google Scholar] [CrossRef]
- Celada-Casero, C.; Sietsma, J.; Santofimia, M.J. The role of the austenite grain size in the martensitic transformation in low carbon steels. Mater. Des. 2019, 167, 1–10. [Google Scholar] [CrossRef]
- Huyan, F.; Hedström, P.; Borgenstam, A. Modelling of the fraction of martensite in low-alloy steels. In Proceedings of the ICOMAT 2014: International Conference on Martensitic Transformations, Bilbao, Spain, 6–11 July 2014. [Google Scholar]
- Huyan, F.; Hedström, P.; Höglund, L.; Borgenstam, A. A Thermodynamic-Based Model to Predict the Fraction of Martensite in Steels. Metall. Mater. Trans. A 2016, 47, 4404–4410. [Google Scholar] [CrossRef]
- Van Bohemen, S.M.C.; Sietsma, J. Kinetics of Martensite formation in plain carbon steels: Critical assessment of possible influence of austenite grain boundaries and autocatalysis. Mater. Sci. Technol. 2014, 30, 1024–1033. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Phase transformations contributing to the properties of modern steels. Bull. Pol. Acad. Sci. Technol. Sci. 2010, 58, 255–265. [Google Scholar] [CrossRef]
- Caballero, F.G.; Bhadeshia, H.K.D.H. Very Strong Bainite. Curr. Opin. Solid State Mater. Sci. 2004, 8, 251–257. [Google Scholar] [CrossRef]
- Garcia-Mateo, C.; Caballero, F.G.; Bhadeshia, H.K.D.H. Development of Hard Bainite. ISIJ Int. 2003, 43, 1238–1243. [Google Scholar] [CrossRef]
- Lee, J.-L.; Bhadeshia, H.K.D.H. A methodology for the prediction of time-temperature-transformation diagrams. Mater. Sci. Eng. A 1993, 171, 223–230. [Google Scholar] [CrossRef]
- Wang, L.; Cai, Q.; Wu, H.; Yu, W. Effects of Si on the stability of retained austenite and temper embrittlement of ultrahigh strength steels. Int. J. Miner. Metall. Mater. 2011, 18, 543. [Google Scholar] [CrossRef]
- He, K.; Baker, T.N. Zr-containing precipitates in a Ti-Nb microalloyed HSLA steel containing 0.016 wt% Zr addition. Mater. Sci. Eng. A 1996, 215, 57–66. [Google Scholar] [CrossRef]
- Baker, T.N. Review or critical assessment, Microalloyed steels. Ironmak. Steelmak. 2016, 43, 264–307. [Google Scholar] [CrossRef]
- Skobir, D.A.; Godec, M.; Balcar, M.; Jenko, M. The influence of the microalloying elements of HSLA steel on the microstructure and mechanical properties. Mater. Technol. 2011, 44, 343–347. [Google Scholar]
- Industrial Furnaces for Heat Treatment. Available online: https://www.unserebroschuere.ch/codere/WebView/ (accessed on 16 February 2022).
- Materials Algorithms Project, Steels: Program Library, MUCG83. Available online: https://www.phase-trans.msm.cam.ac.uk/map/steel/steelprog.html (accessed on 8 December 2021).
- Yang, H.-S.; Bhadeshia, H.K.D.H. Austenite Grain Size and the Martensite-Start Temperature. Scripta Mater. 2009, 62, 493–495. [Google Scholar] [CrossRef]
- ISO 18265:2013; Metallic Materials—Conversion of Hardness Values. ISO: Gerneva, Switzerland, 2003.
- Xia, S.; Zhang, F.; Yang, Z. Microstructure and mechanical properties of 18Mn3Si2CrMo steel subjected to austempering at different temperatures below Ms. Mater. Sci. Eng. A 2018, 724, 103–111. [Google Scholar] [CrossRef]
- Suh, M.S.; Nahm, S.H.; Suh, C.M.; Park, N.K. Impact Toughness of Spring Steel after Bainite and Martensite Transformation. Metals 2022, 12, 304. [Google Scholar] [CrossRef]
- Suh, M.S.; Nahm, S.H.; Suh, C.M.; Pyun, Y.S. VHCF, Tribology Characteristics and UNSM Effects of Bainite and Martensite Spring Steels. Metals 2022, 12, 901. [Google Scholar] [CrossRef]
- Han, X.; Zhang, Z.; Barber, G.C.; Thrush, S.J.; Li, X. Wear resistance of medium carbon steel with different microstructures. Materials 2021, 14, 2015. [Google Scholar] [CrossRef]
- Putatunda, S.K.; Martis, C.; Boileau, J. Influence of austempering temperature on the mechanical properties of a low carbon low alloy steel. Mater. Sci. Eng. A 2011, 528, 5053–5059. [Google Scholar] [CrossRef]
- Baker, T.N. Role of zirconium in microalloyed steels: A review. Mater. Sci. Technol. 2014, 31, 265–294. [Google Scholar] [CrossRef]
- Burja, J.; Koležnik, M.; Batič, B.Š.; Medved, J. Effect of Zr additions on non-metallic inclusions in X11CrNiMo12 steel. Metals 2020, 10, 1183. [Google Scholar] [CrossRef]
- Narita, K. Physical chemistry of the groups IVa(Ti, Zr), Va(V, Nb, Ta) and the rare earth elements in steel. Trans. ISIJ 1975, 15, 145–152. [Google Scholar] [CrossRef]
- Hidalgo, J.; Santofimia, M.J. Effect of Prior Austenite Grain Size Refinement by Thermal Cycling on the Microstructural Features of As-quenched Lath Martensite. Metall. Mater. Trans. A 2016, 47, 5288–5301. [Google Scholar] [CrossRef]



| C | Si | P | S | Mn | Cr + Mo | Cu + Ni | Fe |
|---|---|---|---|---|---|---|---|
| 0.32 | 2.0 | 0.015 | 0.006 | 1.58 | 0.99 | 0.16 | rest |
| Ac1/e1 | Ac3/e3 | Ms | Mf | Bs | |
|---|---|---|---|---|---|
| Experimental | 760 | 830 * | 321 | 180 | - |
| Thermo-Calc | 722 | 820 | 324 ** | 200 *** | - |
| JMatPro | 731 | 826 | 296 | 169 **** | 486 |
| Mucg83 | - | - | 324 | - | 488 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klančnik, G.; Krajnc, L.; Nagode, A.; Burja, J. Isothermal Quenching of As-Cast Medium Carbon, High-Silicon AR Steel. Materials 2022, 15, 5595. https://doi.org/10.3390/ma15165595
Klančnik G, Krajnc L, Nagode A, Burja J. Isothermal Quenching of As-Cast Medium Carbon, High-Silicon AR Steel. Materials. 2022; 15(16):5595. https://doi.org/10.3390/ma15165595
Chicago/Turabian StyleKlančnik, Grega, Luka Krajnc, Aleš Nagode, and Jaka Burja. 2022. "Isothermal Quenching of As-Cast Medium Carbon, High-Silicon AR Steel" Materials 15, no. 16: 5595. https://doi.org/10.3390/ma15165595
APA StyleKlančnik, G., Krajnc, L., Nagode, A., & Burja, J. (2022). Isothermal Quenching of As-Cast Medium Carbon, High-Silicon AR Steel. Materials, 15(16), 5595. https://doi.org/10.3390/ma15165595









