New Ceramic Multi-Unit Dental Abutments with an Antimicrobial Glassy Coating
Abstract
:1. Introduction
2. Materials and Methods
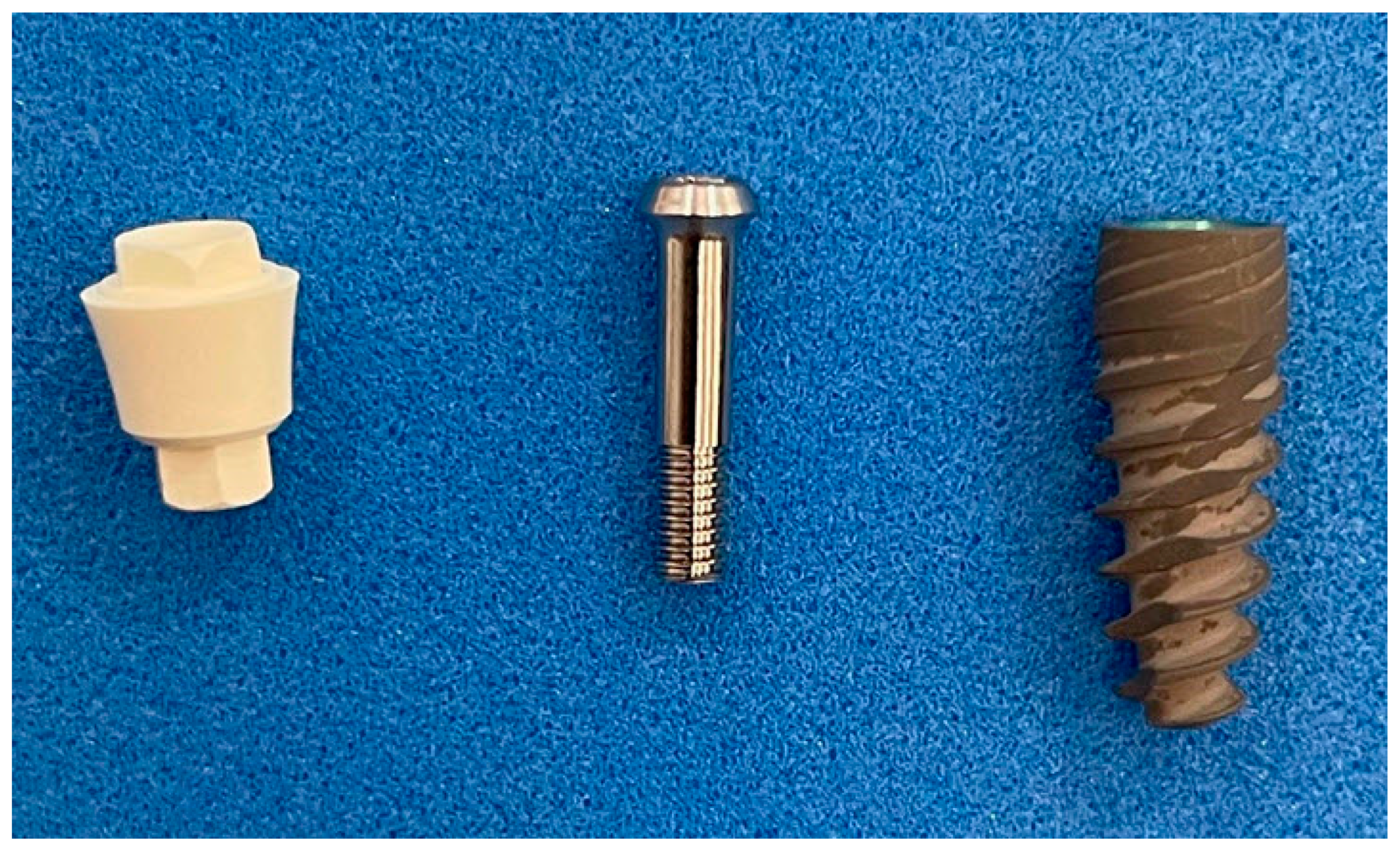
2.1. Dental Implant Components
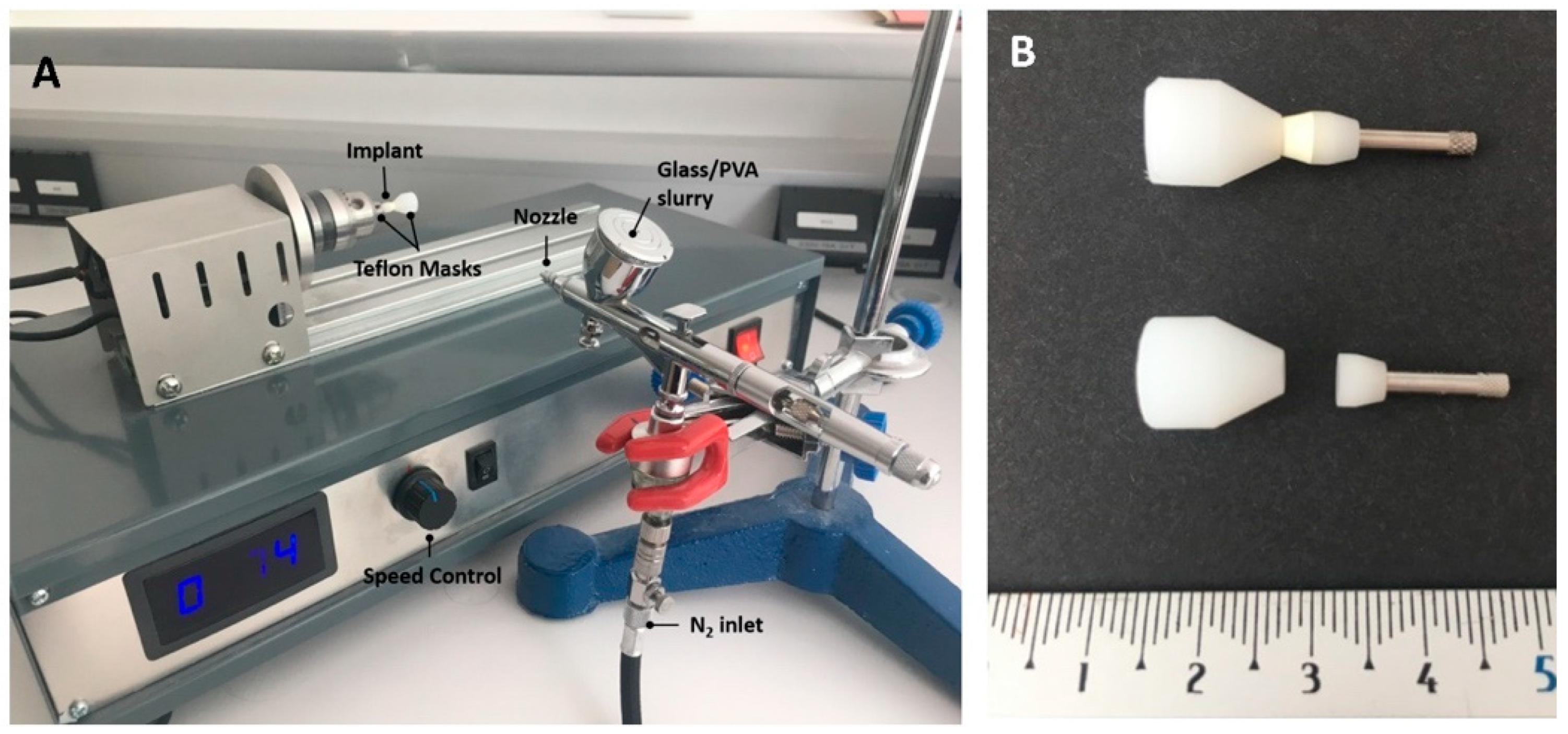
2.2. Preparation of the Coatings
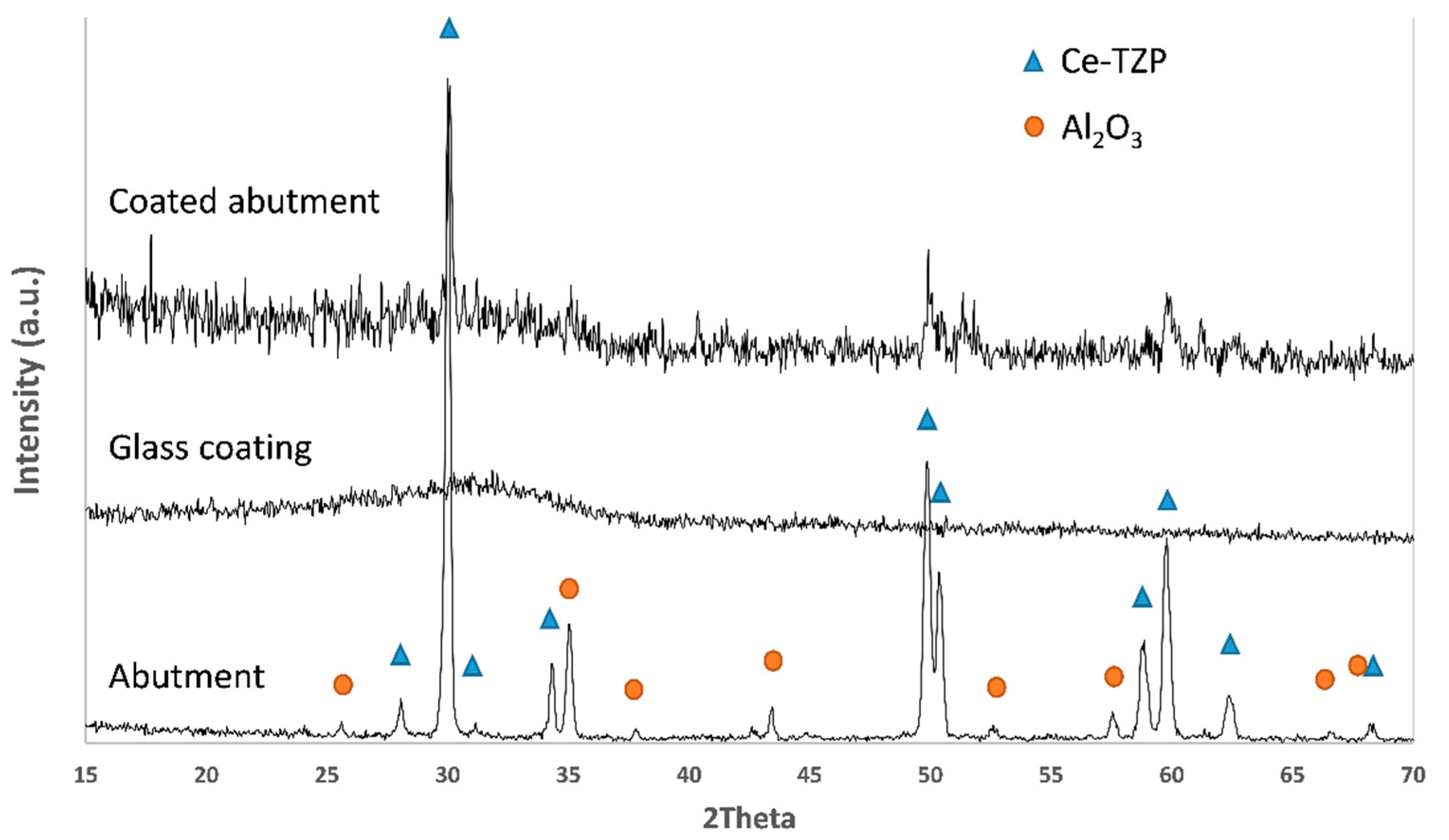
2.3. Characterization of the Coatings
2.4. Cytotoxicity Testing
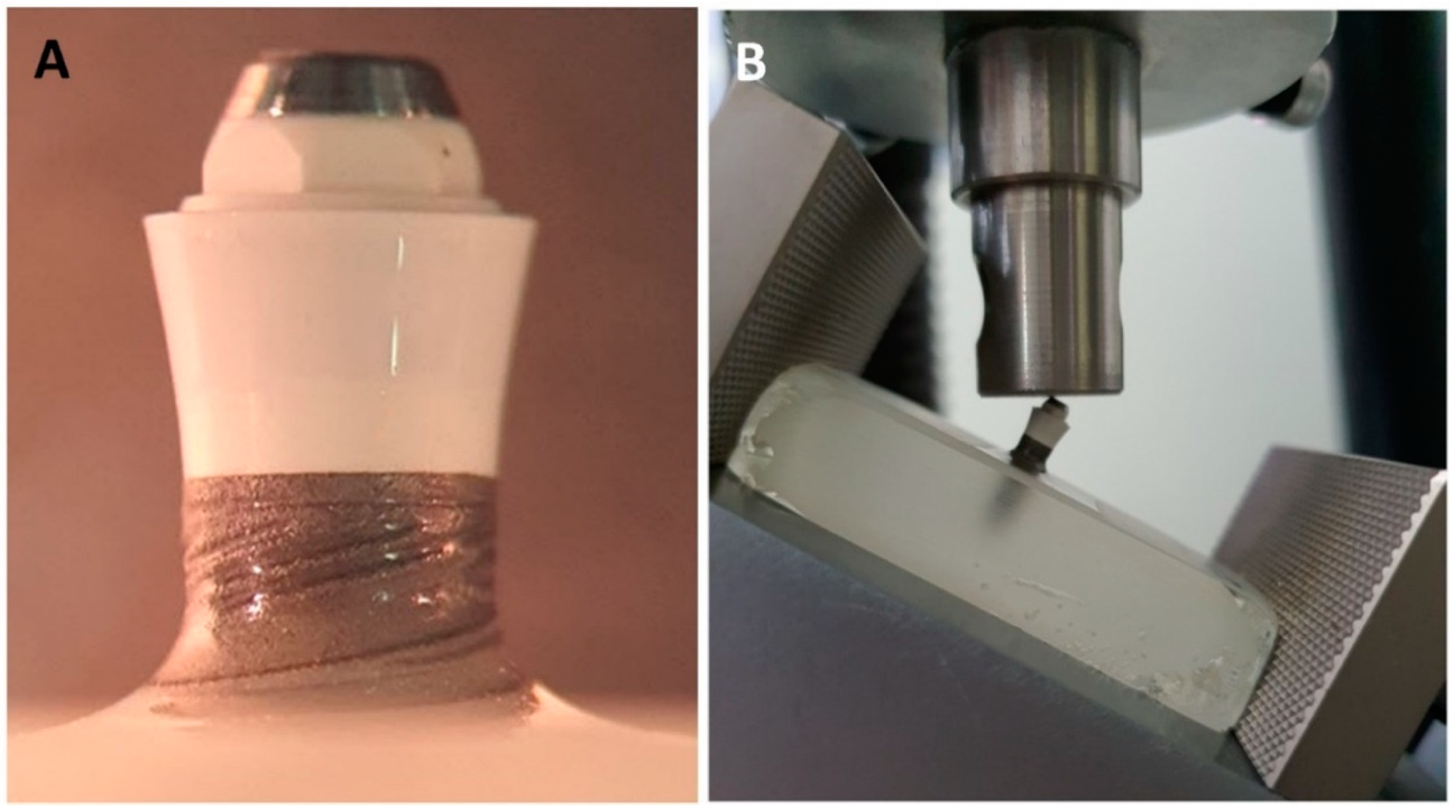
2.5. Mechanical Testing
2.6. Preliminary Clinical Study
3. Results and Discussion
4. Conclusions
- -
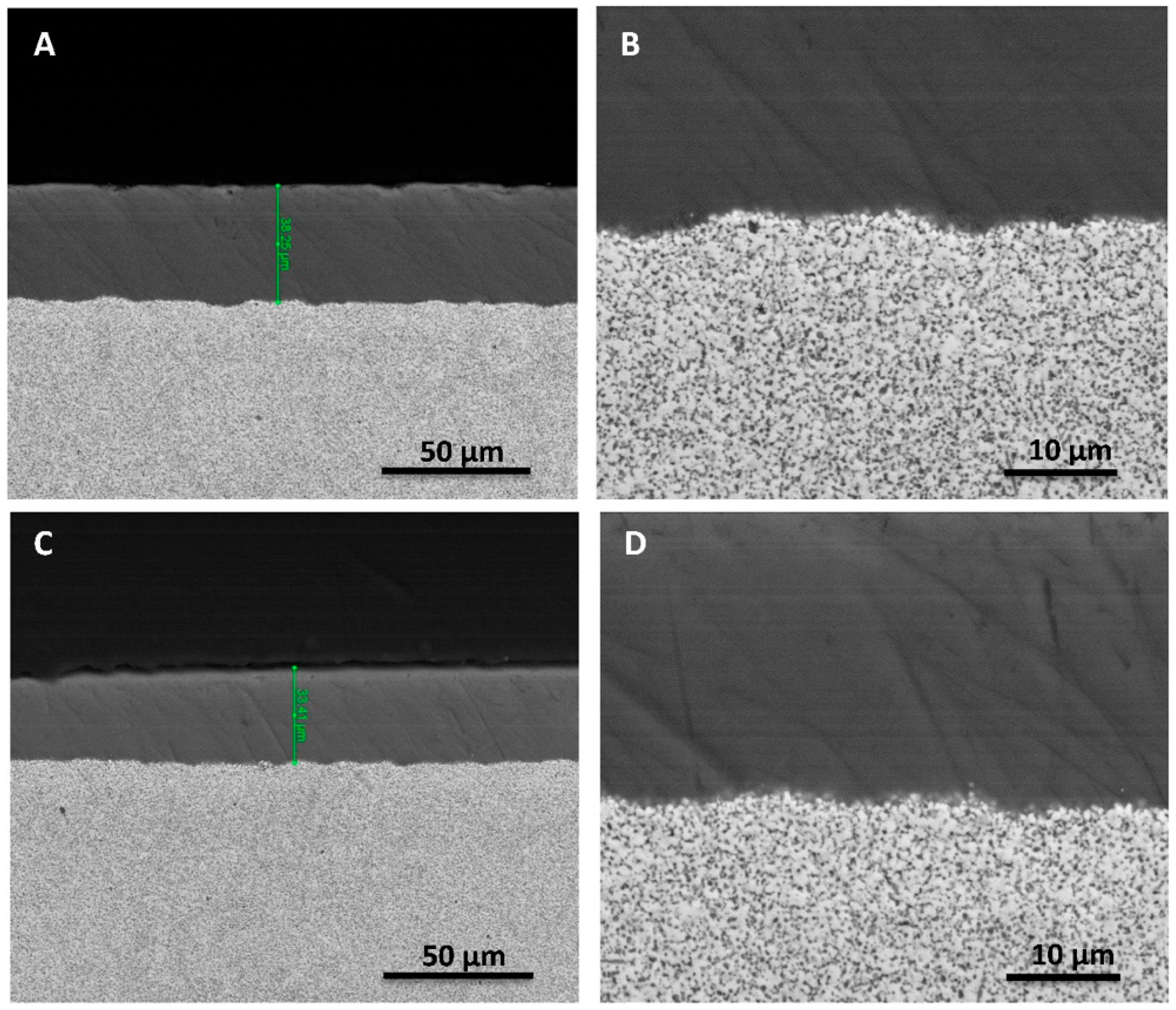
- In the present study, mechanically stable thin antimicrobial soda–lime coatings were successfully deposited on Ce–TZP/Al2O3 abutments (compatible with commercially available Ti implants) by means of suspension spraying and subsequent thermal treatment.
- -
- The antimicrobial coatings survived a load as high as 400 N under cyclic loading conditions over a long period of time (10 × 106 cycles, equivalent to ≈20 years in vivo).
- -
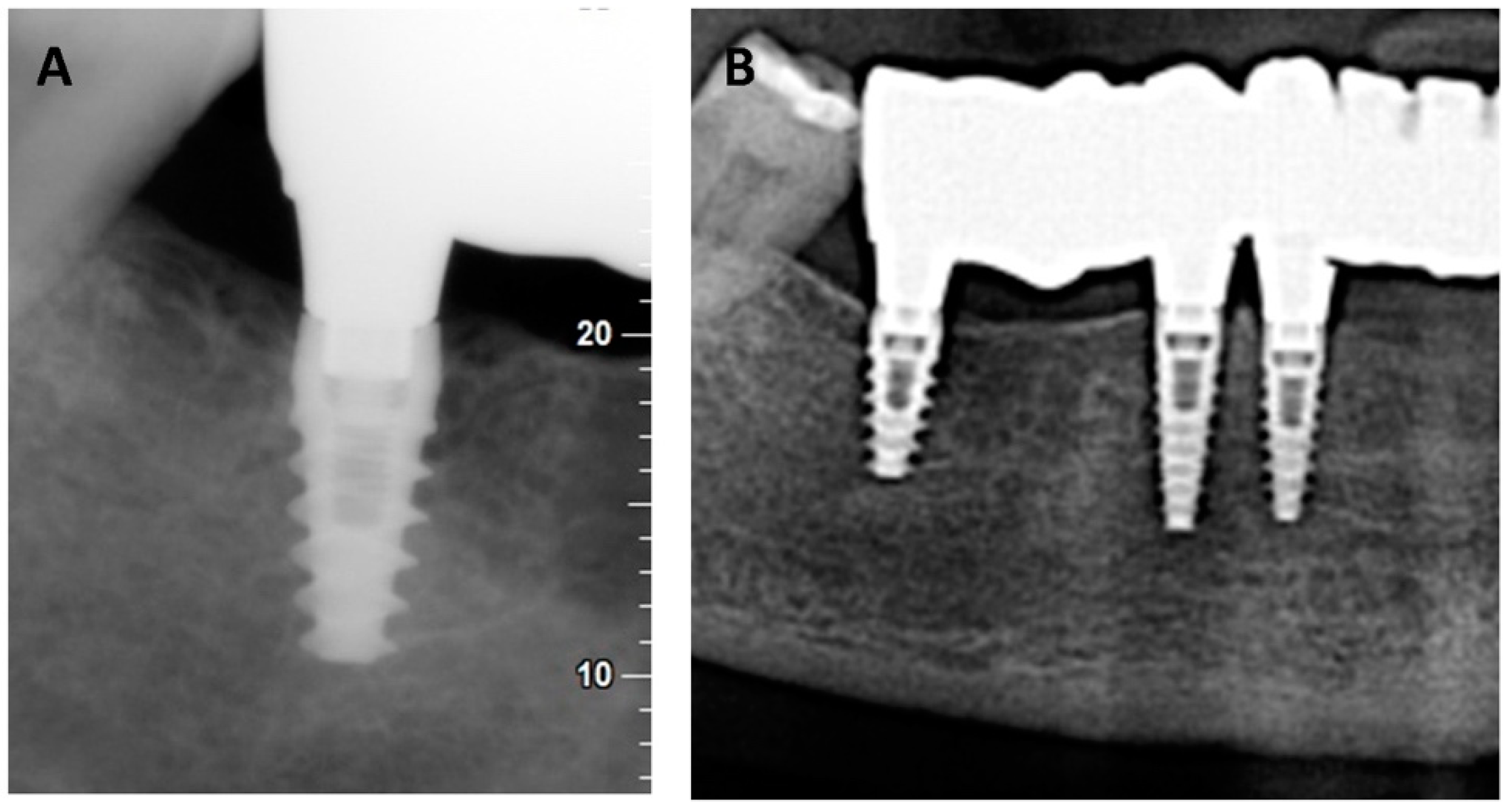
- The coating showed high biocompatibility and mechanical stability one year after implant insertion in a patient with periodontal problems.
- -
- Therefore, these new, biocompatible and antimicrobial glassy coatings placed on ceramic composite dental implant components could be promising candidates to reduce the risk of peri-implant infections in the anterior dentition.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pjetursson, B.; Asgeirsson, A.; Zwahlen, M.; Sailer, I. Improvements in implant dentistry over the last decade: Comparison of survival and complication rates in older and newer publications. Int. J. Oral Maxillofac. Implant. 2014, 29, 308–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papaspyridakos, P.; Chen, C.J.; Chuang, S.K.; Weber, H.P.; Galluci, G.O. A systematic review of biologic and technical complications with fixed implant rehabilitations for edentutlous patients. Int. J. Oral Maxillofac. Implant. 2012, 27, 102–110. [Google Scholar]
- Cionca, N.; Hashim, D.; Mombelli, A. Zirconia dental implants: Where are we now, and where are we heading? Periodontology 2000 2017, 73, 241–258. [Google Scholar] [CrossRef] [PubMed]
- López-Píriz, R.; Cabal, B.; Goyos-Ball, L.; Fernández, A.; Bartolomé, J.F.; Moya, J.S.; Torrecillas, R. Current state-of-the-art and future perspectives of the three main modern implant-dentistry concerns: Aesthetic requirements, mechanical properties, and peri-implantitis prevention, J. Biomed. Mater. Res. Part A 2019, 107, 1466–1475. [Google Scholar] [CrossRef] [PubMed]
- Sailer, I.; Zembic, A.R.; Jung, E.; Hämmerle, C.H.F.; Mattiola, A. Single-Tooth Implant Reconstructions: Esthetics Factors Influencing the Decision between Titanium and Zirconia Abutments in Anterior Regions. Eur. J. Esthet. Dent. 2007, 2, 296–310. [Google Scholar]
- Li, R.W.K.; Chow, T.W.; Matinlinna, J.P. Ceramic dental biomaterials and CAD/CAM technology: State of the art. JADA 2014, 58, 208–216. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Píriz, R.; Fernández, A.; Goyos-Ball, L.; Rivera, S.; Díaz, L.A.; Fernández-Domínguez, M.; Prado, C.; Moya, J.S.; Torrecillas, R. Performance of a New Al2O3/Ce–TZP Ceramic Nanocomposite Dental Implant: A Pilot Study in Dogs. Materials 2017, 10, 614. [Google Scholar] [CrossRef] [Green Version]
- Benzaid, R.; Chevalier, J.; Saâdaoui, M.; Fantozzi, G.; Nawa, M.; Diaz, L.A.; Torrecillas, R. Fracture toughness, strength and slow crack growth in a ceria stabilized zirconia–alumina nanocomposite for medical applications. Biomaterials 2008, 29, 3636–3641. [Google Scholar] [CrossRef]
- Ban, S.; Sato, H.; Suehiro, Y.; Nakanishi, H.; Nawa, M. Biaxial Flexure Strength and Low Temperature Degradation of Ce-TZP/Al2O3 Nanocomposite and Y-TZP as Dental Restoratives, Biomed Mater Res Part B: Appl. Biomater. 2008, 87B, 492–498. [Google Scholar] [CrossRef]
- Tanaka, K.; Tamura, J.; Kawanabe, K.; Nawa, M.; Uchida, M.; Kokubo, T.; Nakamura, T. Phase stability after aging and its influence on Pin-on-disk wear properties of Ce-TZP/Al2O3 nanocomposite and conventional Y-TZP. J. Biomed. Mater. Res. 2003, 67A, 200–207. [Google Scholar] [CrossRef]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.-L. Peri-implantitis. J. Periodontol. 2018, 89, S267–S290. [Google Scholar] [CrossRef] [PubMed]
- Tallarico, M.; Canullo, L.; Caneva, M.; Özcan, M. Microbial colonization at the implant-abutment interface and its possible influence on periimplantitis: A systematic review and metaanalysis. J. Prosthodont. Res. 2017, 61, 233–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinebrunner, L.; Wolfart, S.; Böbmann, K.; Kern, M. In vitro evaluation of bacterial leakage along the implant–abutment interface of different implant systems. Int. J. Oral Maxillofac Implants 2005, 20, 875–881. [Google Scholar]
- Souza, J.G.S.; Costa Oliveira, B.E.; Bertolini, M.; Veloso Lima, C.; Retamal-Valdes, B.; de Faveri, M.; Feres, M.; Barão, V.A.R. Titanium particles and ions favor dysbiosis in oral biofilms. J. Periodont Res. 2020, 55, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Kotsakis, G.A.; Olmedo, D.G. Periimplantitis is not periodontitis: Scientific discoveries shed light on microbiome-biomaterial interactions that may determine disease phenotype. Periodontol 2000 2021, 86, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Papi, P.; Letizia, C.; Pilloni, A.; Petramala, L.; Saraceno, V.; Rosella, D.; Pompa, G. Peri-implant diseases and metabolic syndrome components: A systematic review. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 866–875. [Google Scholar] [CrossRef]
- Ustaoğlu, G.; Erdal, E. Relationship between risk markers for cardiovascular disease and peri-implant diseases. Int. J. Implant. Dent. 2020, 6, 73. [Google Scholar] [CrossRef]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019, 5, 934–936. [Google Scholar] [CrossRef] [Green Version]
- Marouf, N.; Cai, W.; Said, K.N.; Daas, H.; Diab, H.; Chinta, V.R.; Hssain, A.A.; Nicolau, B.; Sanz, M.; Tamimi, F. Association between periodontitis and severity of COVID-19 infection: A case–control study. J Clin Periodontol. 2021, 48, 483–491. [Google Scholar] [CrossRef]
- Durkin, M.J.; Hsueh, K.; Yh, S.; Feng, Q.; Jafarzadeh, R.S.; Munshi, K.D.; Lockhart, P.B.; Thornhill, M.H.; Henderson, M.H.; Fraser, V.J. An evaluation of dental antibiotic prescribing practices in the United States. JADA 2017, 148, 878–886.e871. [Google Scholar] [CrossRef] [Green Version]
- Carinci, F.; Lauritano, D.; Bignozzi, C.A.; Pazzi, D.; Candotto, V.; de Oliveira, P.S.; Scarano, A. A new strategy against peri-implantitis: Antibacterial internal coating. Int. J. Mol. Sci. 2019, 20, 3897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghensi, P.; Bettio, E.; Maniglio, D.; Bonomi, E.; Piccoli, F.; Gross, S.; Caciagli, P.; Segata, N.; Nollo, G.; Tessarolo, F. Dental implants with anti-biofilm properties: A pilot study for developing a new sericin-based coating. Materials 2019, 12, 2429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almohandes, A.; Abrahamsson, I.; Dahlén, G.; Berglundh, T. Effect of biofilm formation on implant abutments with an antibacterial coating. A pre-clinical in vivo study. Clin. Oral Impl. Res. 2021, 32, 756–766. [Google Scholar] [CrossRef] [PubMed]
- de Avila, E.D.; van Oirschot, B.A.; van den Beucken, J.J.J.P. Biomaterial-based possibilities for managing peri-implantitis. J. Periodont. Res. 2020, 55, 165–173. [Google Scholar] [CrossRef]
- Cabal, B.; Cafini, F.; Esteban-Tejeda, L.; Alou, L.; Bartolome, J.F.; Sevillano, D.; López-Piriz, R.; Torrecillas, R.; Moya, J.S. Inhibitory Effect on In Vitro Streptococcus oralis Biofilm of a Soda-Lime Glass Containing Silver Nanoparticles Coating on Titanium Alloy. PLoS ONE 2012, 7, e42393. [Google Scholar] [CrossRef] [Green Version]
- López-Píriz, R.; Solá-Linares, E.; Granizo, J.J.; Díaz-Güemes, I.; Enciso, S.; Bartolomé, J.F.; Cabal, B.; Esteban-Tejeda, L.; Torrecillas, R.; Moya, J.S. Radiologic Evaluation of Bone Loss at Implants with Biocide Coated Titanium Abutments: A Study in the Dog. PLoS ONE 2012, 7, e52861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, A.; Guitián, F.; López-Píriz, R.; Bartolomé, J.F.; Cabal, B.; Esteban-Tejeda, L.; Torrecillas, R.; Moya, J.S. Bone loss at implant with titanium abutments coated by soda lime glass containing silver nanoparticles: A histological study in the dog. PLoS ONE 2014, 9, e86926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Píriz, R.; Solá-Linares, E.; Rodriguez-Portugal, M.; Malpica, B.; Díaz-Güemes, I.; Enciso, S.; Esteban-Tejeda, L.; Cabal, B.; Granizo, J.J.; Moya, J.S. Evaluation in a Dog Model of Three Antimicrobial Glassy Coatings: Prevention of Bone Loss around Implants and Microbial Assessments. PLoS ONE 2015, 10, e0140374. [Google Scholar] [CrossRef]
- Sobolev, A.; Valkov, A.; Kossenko, A.; Wolicki, I.; Zinigrad, M.; Borodianskiy, K. Bioactive Coating on Ti Alloy with High Osseointegration and Antibacterial Ag Nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 39534–39544. [Google Scholar] [CrossRef]
- Ahamed, M.; Karns, M.; Goodson, M. DNA damage response to different surface chemistry of silver nanoparticles in mammalian cells. Toxicol Appl Pharmacol. 2008, 233, 404–410. [Google Scholar] [CrossRef]
- Esteban-Tejeda, L.; Díaz, L.A.; Cabal, B.; Prado, C.; López-Piriz, R.; Torrecillas, R.; Moya, J.S. Biocide glass-ceramic coating on titanium alloy and zirconium oxide for dental applications. Mater. Lett. 2013, 111, 59–62. [Google Scholar] [CrossRef]
- Moya, J.S.; Esteban-Tejeda, L.; Pecharromán, C.; Mello-Castanho, S.R.H.; Da Silva, A.C.; Malpartida, F. Glass powders with a high content of calcium oxide: A step towards a "green" universal biocide. Adv Eng Mater. 2011, 13, B256–B260. [Google Scholar] [CrossRef]
- Rius-Rocabert, S.; Arranz-Herrero, J.; Fernández-Valdés, A.; Marciello, M.; Moreno, S.; Llinares-Pinel, F.; Presa, J.; Hernández-Alcoceba, R.; López-Píriz, R.; Torrecillas, R.; et al. Broad virus inactivation using inorganic micro/nano-particulate materials. Mat. Today Bio 2022, 13, 100191. [Google Scholar] [CrossRef] [PubMed]
- López-Esteban, S.; Bartolomé, J.F.; Díaz, L.A.; Esteban-Tejeda, L.; Prado, C.; López-Piriz, R.; Torrecillas, R.; Moya, J.S. Mechanical performance of a biocompatible biocide soda-lime glass-ceramic. J Mech Behav Biomed Mater. 2014, 34, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Tejeda. L.; Zheng, K.; Prado, C.; Cabal, B.; Torrecillas, R.; Boccaccini, A.R.; Moya, J.S. Bone tissue scaffolds based on antimicrobial SiO2-Na2O-Al2O3-CaOB2O3 glass, J. Non. Cryst. Solids 2016, 432, 73–80. [CrossRef]
- Cabal, B.; Alou, L.; Cafini, F.; Couceiro, R.; Sevillano, D.; Esteban-Tejeda, L.; Guitián, F.; Torrecillas, R.; Moya, J.S. A New Biocompatible and Antibacterial Phosphate Free Glass-Ceramic for Medical Applications. Sci. Rep. 2014, 4, 5440. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Detsch, R.; Esteban-Tejeda, L.; Grünewald, A.; Moya, J.S.; Boccaccini, A.R. Influence of dissolution products of a novel Ca-enriched silicate bioactive glass-ceramic on VEGF release from bone marrow stromal cells. Biomed. Glas 2017, 3, 104–110. [Google Scholar] [CrossRef]
- Moya, J.S.; Martínez, A.; López-Píriz, R.; Guitián, F.; Díaz, L.A.; Esteban-Tejeda, L.; Cabal, B.; Sket, F.; Fernández-García, E.; Tomsia, A.P.; et al. Histological response of soda-lime glass-ceramic bactericidal rods implanted in the jaws of beagle dogs. Sci. Rep. 2016, 6, 31478. [Google Scholar] [CrossRef]
- ISO Norm 10993 (2021); Biological Evaluation of Medical Devices-Part 5, Tests for In Vitro Cytotoxicity and Part 12: Sample Preparation and Reference Materials. ISO: Geneva, Switzerland, 2021.
- ISO Norm 14801 (2016); Dynamic Loading Test for Endosseous Dental Implants. ISO: Geneva, Switzerland, 2016.
- Odin, G.; Savoldelli, C.; Bouchard, P.-O.; Tillier, Y. Determination of Young's modulus of mandibular bone using inverse analysis. Med. Eng. Phys. 2010, 32, 630–637. [Google Scholar] [CrossRef] [Green Version]
- Alonso-Pérez, R.; Bartolomé, J.F.; Ferreiroa, A.; Salido, M.P.; Pradíes, G. Evaluation of the Mechanical Behavior and Marginal Accuracy of Stock and Laser-Sintered Implant Abutments. Int. J. Prosthodont. 2017, 30, 136–138. [Google Scholar] [CrossRef] [Green Version]
- Alonso-Pérez, R.; Bartolomé, J.F.; Ferreiroa, A.; Salido, M.P.; Pradíes, G. Original vs. non-original abutments for screw-retained single implant crowns: An in vitro evaluation of internal fit, mechanical behaviour and screw loosening. Clin. Oral Implants Res. 2018, 29, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Pérez León, P.; Bartolomé, J.F.; Lombardía, C.; Pradíes, G. Mechanical fatigue behaviour of different lengths screw-retained restorations connected to two designs prosthetic connection level. J. Oral Rehabil. 2019, 46, 747–755. [Google Scholar] [CrossRef]
- Alonso-Pérez, R.; Bartolomé, J.F.; Fraile, C.; Pradíes, G. Original versus nonoriginal cast-to gold abutment-implant connection: Analysis of the internal fit and long-term fatigue performance, J. Prosthet. Dent. 2021, 126, e1–e94. [Google Scholar] [CrossRef]
- Giner, S.; Bartolomé, J.F.; Gomez-Cogolludo, P.; Castellote, C.; Pradíes, G. Fatigue fracture resistance of titanium and chairside CAD-CAM zirconia implant abutments supporting zirconia crowns: An in vitro comparative and finite element analysis study. J. Prosthet. Dent. 2021, 125, 503.e1–503.e9. [Google Scholar] [CrossRef] [PubMed]
- Giner, S.; Bartolomé, J.F.; Gomez-Cogolludo, P.; Castellote, C.; Pradíes, G. Mechanical Performance of Chairside Ceramic CAD/CAM Restorations and Zirconia Abutments with Different Internal Implant Connections: In Vitro Study and Finite Element Analysis. Materials 2021, 14, 5009. [Google Scholar] [CrossRef]
- Morneburg, T.R.; Pröschel, P.A. Measurement of masticatory forces and implant loads: A methodologic clinical study. Int. J. Prosthodont. 2002, 15, 20–27. [Google Scholar] [PubMed]
- Morneburg, T.R.; Pröschel, P.A. In vivo forces on implants influenced by occlusal scheme and food consistency. Int. J. Prosthodont. 2003, 16, 481–486. [Google Scholar]
- Studart, A.R.; Filser, F.; Kocher, P.; Lüthy, H.; Gauckler, L.J. Cyclic fatigue in water of veneer–framework composites for all-ceramic dental bridges. Dent. Mater. 2007, 23, 177–185. [Google Scholar] [CrossRef]
- Esteban-Tejeda, L.; Malpartida, F.; Díaz, L.A.; Torrecillas, R.; Rojo, F.; Moya, J.S. Glass-(nAg, nCu) Biocide Coatings on Ceramic Oxide Substrates. PLoS ONE 2012, 7, e33135. [Google Scholar] [CrossRef] [Green Version]
- Moya, J.S.; Cabal, B.; Sanz, J.; Da Silva, A.C.; Mello-Castanho, S.; Torrecillas, R.; Rojo, F. Mechanism of calcium lixiviation in soda-lime glasses with a strong biocide activity. Mater. Lett. 2012, 70, 113–115. [Google Scholar] [CrossRef]
- Gehrke, P.; Johannson, D.; Fischer, C.; Stawarczyk, B.; Beuer, F. In Vitro Fatigue and Fracture Resistance of One- and Two-Piece CAD/CAM Zirconia Implant Abutments. Int. J. Oral Maxillofac. Implant. 2015, 30, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.; Aguilar, L.; Gatti, A.; Abduo, J.; Lee, P.V.S.; Ackland, D. Load response of the natural tooth and dental implant: A comparative biomechanics study. J. Adv. Prosthodont. 2019, 11, 169–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Píriz, R.; Goyos-Ball, L.; Cabal, B.; Martínez, S.; Moya, J.S.; Bartolomé, J.F.; Torrecillas, R. New Ceramic Multi-Unit Dental Abutments with an Antimicrobial Glassy Coating. Materials 2022, 15, 5422. https://doi.org/10.3390/ma15155422
López-Píriz R, Goyos-Ball L, Cabal B, Martínez S, Moya JS, Bartolomé JF, Torrecillas R. New Ceramic Multi-Unit Dental Abutments with an Antimicrobial Glassy Coating. Materials. 2022; 15(15):5422. https://doi.org/10.3390/ma15155422
Chicago/Turabian StyleLópez-Píriz, Roberto, Lidia Goyos-Ball, Belén Cabal, Susana Martínez, José S. Moya, José F. Bartolomé, and Ramón Torrecillas. 2022. "New Ceramic Multi-Unit Dental Abutments with an Antimicrobial Glassy Coating" Materials 15, no. 15: 5422. https://doi.org/10.3390/ma15155422
APA StyleLópez-Píriz, R., Goyos-Ball, L., Cabal, B., Martínez, S., Moya, J. S., Bartolomé, J. F., & Torrecillas, R. (2022). New Ceramic Multi-Unit Dental Abutments with an Antimicrobial Glassy Coating. Materials, 15(15), 5422. https://doi.org/10.3390/ma15155422








