Recovery and Separation of Dysprosium from Waste Neodymium Magnets through Cyphos IL 104 Extraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Apparatus
2.3. Pretreatment and Complete Dissolving
2.4. Ionic Liquid Extraction
3. Results and Discussion
3.1. Elemental Analysis
3.2. Extraction
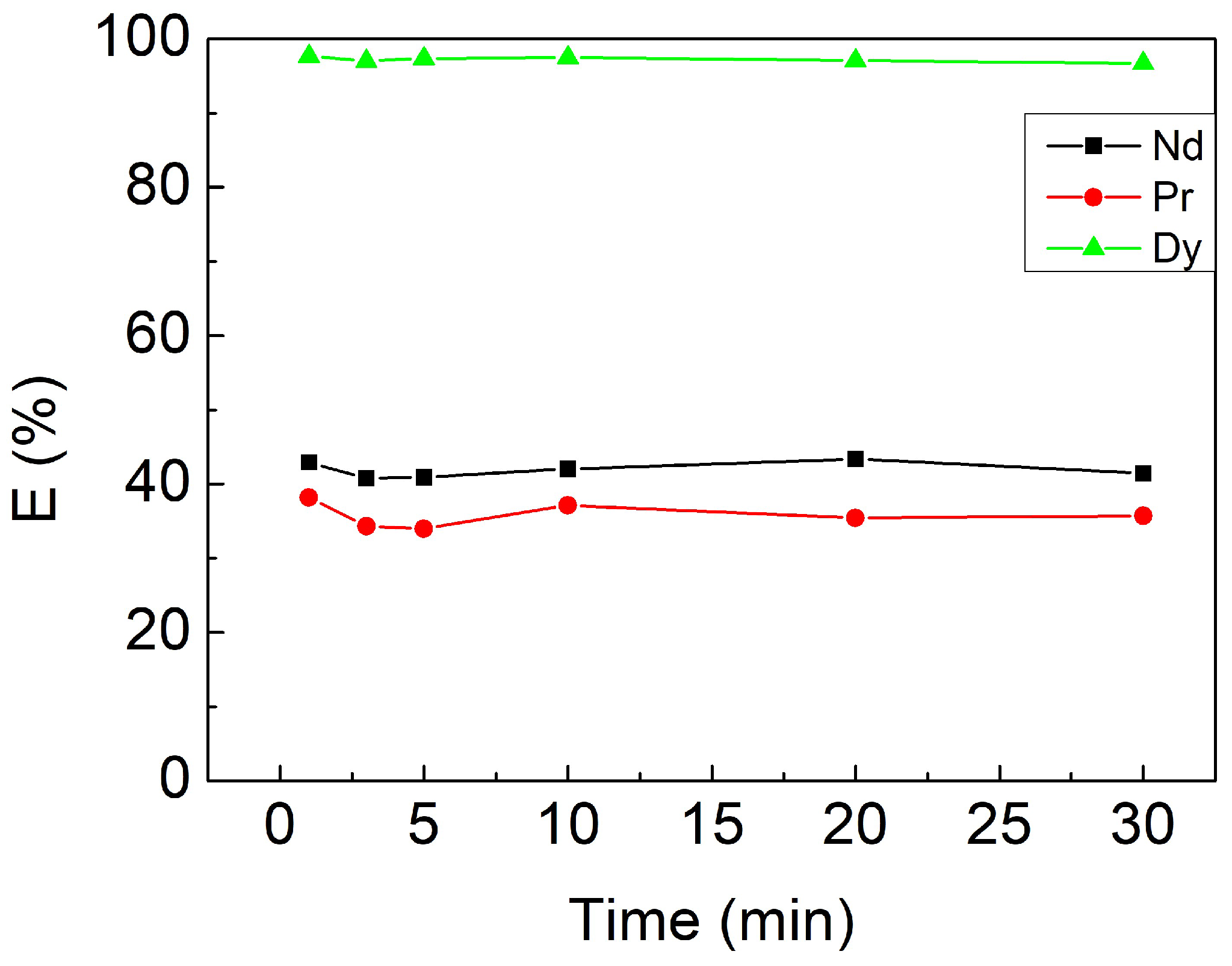
3.2.1. Effect of Contacting Time
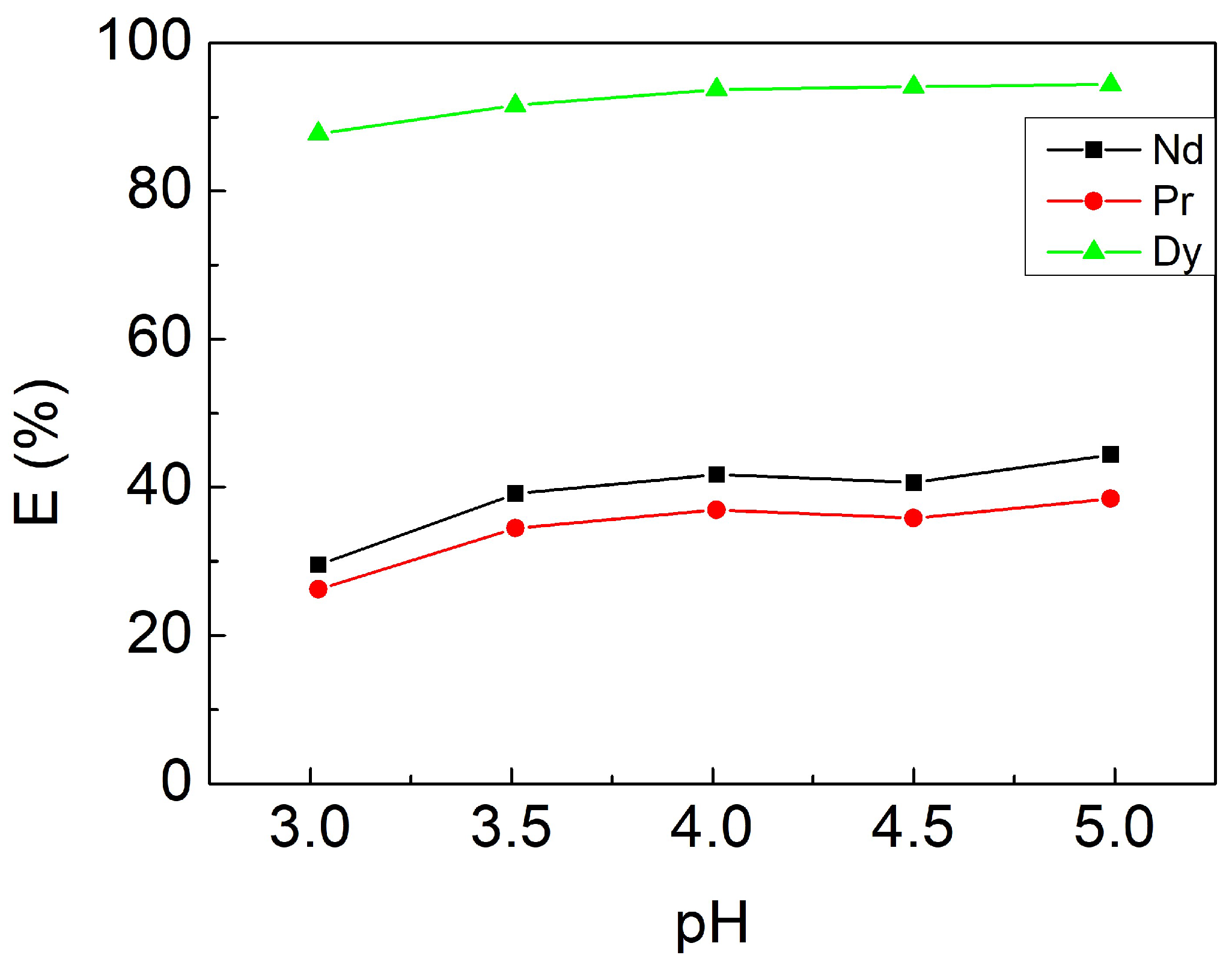
3.2.2. Effect of Initial pH Value
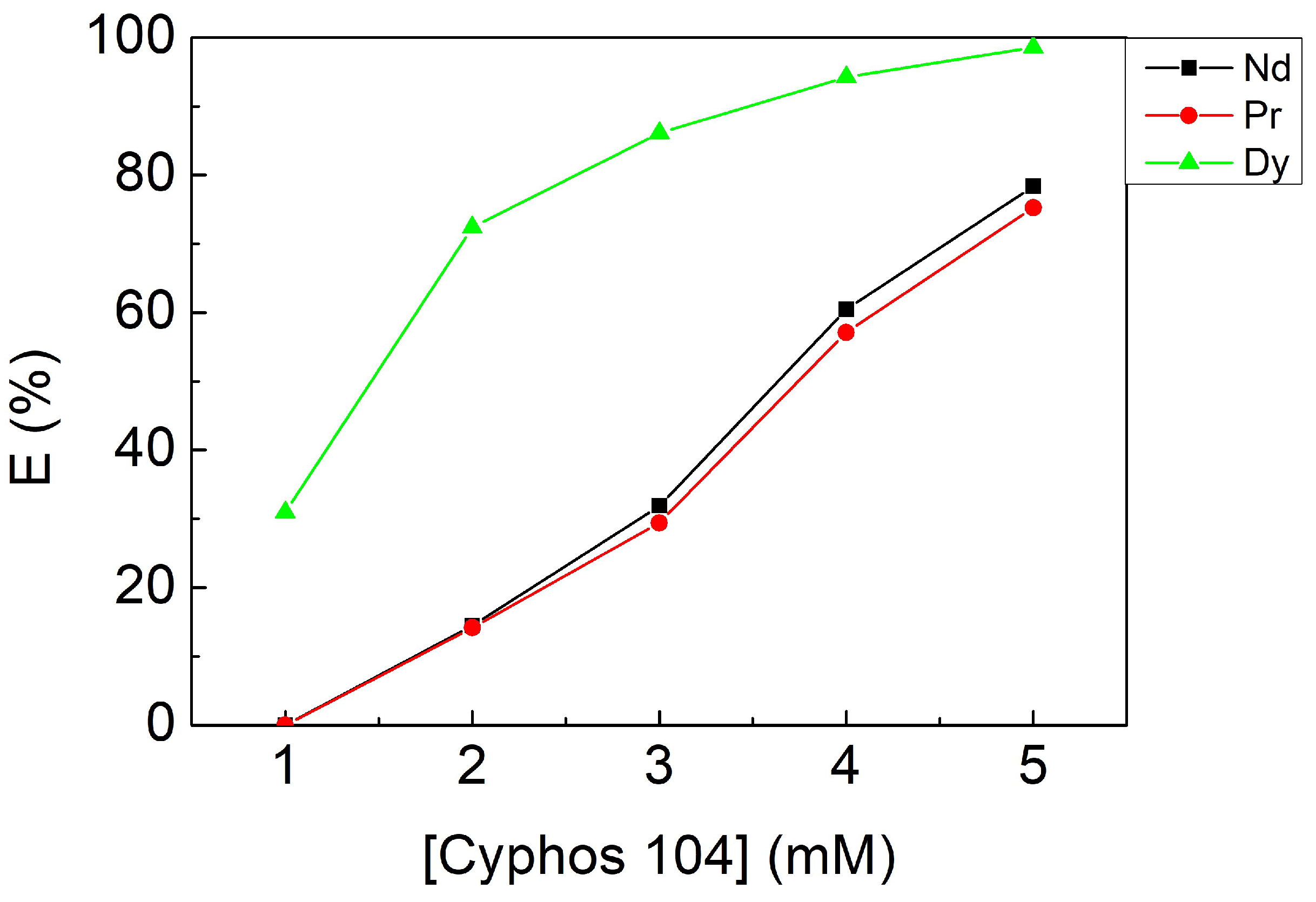
3.2.3. Effect of Cyphos IL 104 Concentration
3.2.4. Effect of O/A Ratio
3.3. Stripping
3.3.1. Stripping Agent
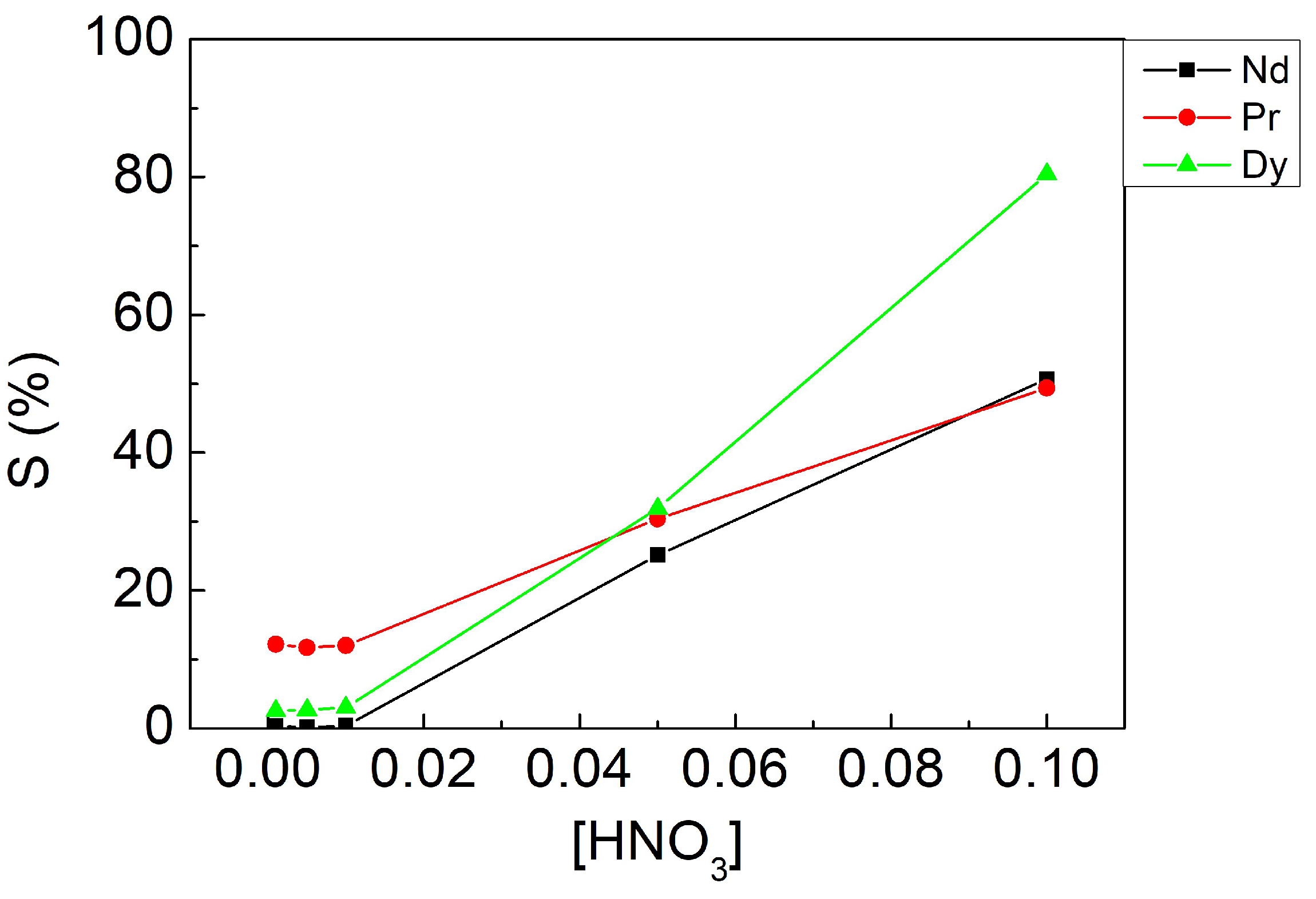
3.3.2. Effect of Stripping Agent Concentration
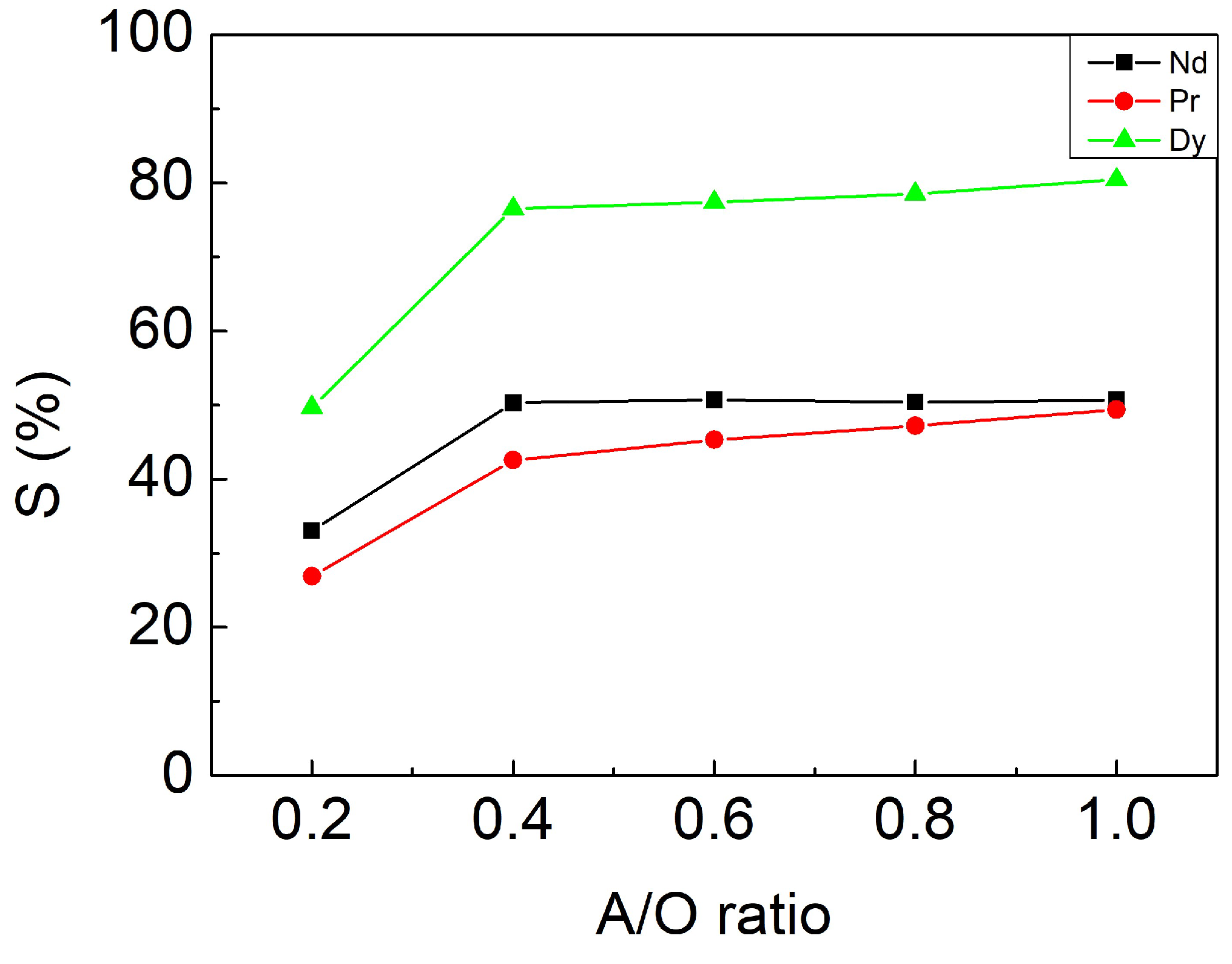
3.3.3. Effect of A/O Ratio
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Jha, M.K.; Kumari, A.; Panda, R.; Kumar, J.R.; Yoo, K.; Lee, J.Y. Review on hydrometallurgical recovery of rare earth metals. Hydrometallurgy 2016, 165, 2–26. [Google Scholar] [CrossRef]
- Jordens, A.; Cheng, Y.P.; Waters, K.E. A review of the beneficiation of rare earth element bearing minerals. Miner. Eng. 2013, 41, 97–114. [Google Scholar] [CrossRef]
- Kumari, A.; Panda, R.; Jha, M.K.; Kumar, J.R.; Lee, J.Y. Process development to recover rare earth metals from monazite mineral: A review. Miner. Eng. 2015, 79, 102–115. [Google Scholar] [CrossRef]
- US Geological Survey. Mineral Commodity Summaries 2021; US Geological Survey: Reston, VA, USA, 2021.
- Zhang, Y.; Gu, F.; Su, Z.; Liu, S.; Anderson, C.; Jiang, T. Hydrometallurgical recovery of rare earth elements from NdFeB permanent magnet scrap: A review. Metals 2020, 10, 841. [Google Scholar] [CrossRef]
- Gambogi, J.; Cordier, D.J. Minerals Yearbook: Rare Earths; US Geological Survey: Reston, VA, USA, 2016. [Google Scholar]
- Baba, K.; Hiroshige, Y.; Nemoto, T. Rare-earth magnet recycling. Hitachi Rev. 2013, 62, 452–455. [Google Scholar]
- Yang, Y.; Walton, A.; Sheridan, R.; Güth, K.; Gauß, R.; Gutfleisch, O.; Buchert, M.; Steenari, B.-M.; Van Gerven, T.; Jones, P.T.; et al. REE Recovery from End-of-Life NdFeB Permanent Magnet Scrap: A Critical Review. J. Sustain. Metall. 2017, 3, 122–149. [Google Scholar] [CrossRef]
- Kaya, M. Electronic Waste and Printed Circuit Board Recycling Technologies; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Nijman, S. UN Report: Time to Seize Opportunity, Tackle Challenge of e-Waste. 2019. Available online: https://www.unep.org/news-and-stories/press-release/un-report-time-seize-opportunity-tackle-challenge-e-waste (accessed on 7 July 2022).
- Petridis, N.E.; Petridis, K.; Stiakakis, E. Global e-waste trade network analysis. Resour. Conserv. Recycl. 2020, 158, 104742. [Google Scholar] [CrossRef]
- Reuter, M.; Hudson, C.; Van Schaik, A.; Heiskanen, K.; Meskers, C.; Hagelüken, C. Metal Recycling: Opportunities, Limits, Infrastructure; A report of the working group on the global metal flows to the international resource panel; United Nations Environment Programme: Nairobi, Kenya, 2013. [Google Scholar]
- Gergoric, M.; Ekberg, C.; Foreman, M.; Steenari, B.-M.; Retegan, T. Characterization and Leaching of Neodymium Magnet Waste and Solvent Extraction of the Rare-Earth Elements Using TODGA. J. Sustain. Metall. 2017, 3, 638–645. [Google Scholar] [CrossRef] [Green Version]
- Gergoric, M.; Ekberg, C.; Steenari, B.-M.; Retegan, T. Separation of Heavy Rare-Earth Elements from Light Rare-Earth Elements Via Solvent Extraction from a Neodymium Magnet Leachate and the Effects of Diluents. J. Sustain. Metall. 2017, 3, 601–610. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, M.; Forsberg, K.; Kloo, L.; Martinez De La Cruz, J.; Rasmuson, Å. Separation of ND(III), DY(III) and Y(III) by solvent extraction using D2EHPA and EHEHPA. Hydrometallurgy 2015, 156, 215–224. [Google Scholar] [CrossRef]
- Pavón, S.; Fortuny, A.; Coll, M.; Sastre, A. Neodymium recovery from NdFeB magnet wastes using Primene 81R· Cyanex 572 IL by solvent extraction. J. Environ. Manag. 2018, 222, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Li, J.; Zhang, W.; Gong, A.; Yuan, X.; Liu, Y. Extraction and Back-Extraction Behaviors of La (III), Ce (III), Pr (III), and Nd (III) Single Rare Earth and Mixed Rare Earth by TODGA. Sensors 2021, 21, 8316. [Google Scholar] [CrossRef] [PubMed]
- Belfqueh, S.; Seron, A.; Chapron, S.; Arrachart, G.; Menad, N. Evaluating organic acids as alternative leaching reagents for rare earth elements recovery from NdFeB magnets. J. Rare Earths, 2022, in press. [CrossRef]
- AliAkbari, R.; Marfavi, Y.; Kowsari, E.; Ramakrishna, S. Recent Studies on Ionic Liquids in Metal Recovery from E-Waste and Secondary Sources by Liquid-Liquid Extraction and Electrodeposition: A Review. Mater. Circ. Econ. 2020, 2, 10. [Google Scholar] [CrossRef]
- Chen, J. Application of Ionic Liquids on Rare Earth Green Separation and Utilization; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Ardakani, E.K.; Kowsari, E.; Ehsani, A. Imidazolium-derived polymeric ionic liquid as a green inhibitor for corrosion inhibition of mild steel in 1.0 M HCl: Experimental and computational study. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124195. [Google Scholar] [CrossRef]
- Dermani, A.K.; Kowsari, E.; Ramezanzadeh, B.; Amini, R. Utilizing imidazole based ionic liquid as an environmentally friendly process for enhancement of the epoxy coating/graphene oxide composite corrosion resistance. J. Ind. Eng. Chem. 2019, 79, 353–363. [Google Scholar] [CrossRef]
- Kowsari, E. Ionic Liquids for Green Energy Applications; Nova Science Publishers: Hauppauge, NY, USA, 2016. [Google Scholar]
- Holbrey, J.D.; Visser, A.E.; Spear, S.K.; Reichert, W.M.; Swatloski, R.P.; Broker, G.A.; Rogers, R.D. Mercury (II) partitioning from aqueous solutions with a new, hydrophobic ethylene-glycol functionalized bis-imidazolium ionic liquid. Green Chem. 2003, 5, 129–135. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, Q.; Shen, X. Complexation and extraction investigation of rubidium ion by calixcrown-C2mimNTf2 system. Sep. Purif. Technol. 2019, 227, 115704. [Google Scholar] [CrossRef]
- Wei, G.-T.; Yang, Z.; Chen, C.-J. Room temperature ionic liquid as a novel medium for liquid/liquid extraction of metal ions. Anal. Chim. Acta 2003, 488, 183–192. [Google Scholar] [CrossRef]
- Zuo, Y.; Liu, Y.; Chen, J.; Li, D.Q. The separation of cerium (IV) from nitric acid solutions containing thorium (IV) and lanthanides (III) using pure [C8mim] PF6 as extracting phase. Ind. Eng. Chem. Res. 2008, 47, 2349–2355. [Google Scholar] [CrossRef]
- Lee, C.H.; Chen, W.S. Extraction of cesium from aqueous solution through t-bambp/c2 mimntf2 and recovery of cesium from waste desalination brine. Desalination Water Treat. 2021, 235, 193–199. [Google Scholar] [CrossRef]
- Xu, C.; Yuan, L.; Shen, X.; Zhai, M. Efficient removal of caesium ions from aqueous solution using a calix crown ether in ionic liquids: Mechanism and radiation effect. Dalton Trans. 2010, 39, 3897–3902. [Google Scholar] [CrossRef] [PubMed]
- Hidayah, N.N.; Abidin, S.Z. Extraction of light, medium and heavy rare-earth elements using synergist extractants developed from ionic liquid and conventional extractants. C. R. Chim. 2019, 22, 728–744. [Google Scholar] [CrossRef]
- Riaño, S.; Sobekova Foltova, S.; Binnemans, K. Separation of neodymium and dysprosium by solvent extraction using ionic liquids combined with neutral extractants: Batch and mixer-settler experiments. RSC Adv. 2020, 10, 307–316. [Google Scholar] [CrossRef] [Green Version]
- Kumari, A.; Sinha, M.; Sahu, S.K.; Pandey, B. Solvent Extraction and Separation of Trivalent Lanthanides Using Cyphos IL 104, a Novel Phosphonium Ionic Liquid as Extractant. Solvent Extr. Ion Exch. 2016, 34, 469–484. [Google Scholar] [CrossRef]
- Cieszynska, A.; Wiśniewski, M. Extractive recovery of palladium (II) from hydrochloric acid solutions with Cyphos® IL 104. Hydrometallurgy 2012, 113, 79–85. [Google Scholar] [CrossRef]
- Dhiman, S.; Gupta, B. Cyphos IL 104 assisted extraction of indium and recycling of indium, tin and zinc from discarded LCD screen. Sep. Purif. Technol. 2020, 237, 116407. [Google Scholar] [CrossRef]
- Nayak, S.; Devi, N. Studies on extraction of gallium (III) from chloride solution using Cyphos IL 104 and its removal from photodiodes and red mud. Hydrometallurgy 2017, 171, 191–197. [Google Scholar] [CrossRef]
- Nayak, S.; Devi, N. Studies on the solvent extraction of indium (III) from aqueous chloride medium using Cyphos IL 104. Mater. Today Proc. 2020, 30, 258–261. [Google Scholar] [CrossRef]
- Pospiech, B. Studies on extraction and permeation of cadmium (II) using Cyphos IL 104 as selective extractant and ion carrier. Hydrometallurgy 2015, 154, 88–94. [Google Scholar] [CrossRef]
- Rybka, P.; Regel-Rosocka, M. Nickel (II) and cobalt (II) extraction from chloride solutions with quaternary phosphonium salts. Sep. Sci. Technol. 2012, 47, 1296–1302. [Google Scholar] [CrossRef]
- Kumari, A.; Sinha, M.K.; Sahu, S.K.; Pandey, B.D. Investigation of a novel ionic liquid, Cyphos IL 104 for the solvent extraction of mineral acids. Hydrometallurgy 2016, 165, 159–165. [Google Scholar] [CrossRef]


| Reference | Extractant | Diluent | αDy/Nd | αDy/Pr | αPr/Dy | αPr/Gd | αGd/Nd |
|---|---|---|---|---|---|---|---|
| [15] | D2EHPA | hexane | 6.8 ± 0.6 | 7.6 ± 0.8 | |||
| [14] | TODGA | Solvent 70 | 39.0 ± 1.1 | 51.7 ± 2.1 | |||
| [17] | Cyanex 572 | kerosene | 237 | ||||
| [17] | P81R-Cy572 | kerosene | 86 | ||||
| [31] | HTTA | toluene | 764 | 1.7 | |||
| [31] | A336 | [C2mim][NTf2] | 82 | 142 | |||
| [32] | [C101][SCN] | Cyanex 923 | 3.43 ± 0.24 | ||||
| [33] | [Cyphos 104] | kerosene | 20 | ||||
| [33] | Cyanex 272 | kerosene | 4 |
| Element | Fe | Nd | Pr | Dy | Co | B | Ni | Cu |
|---|---|---|---|---|---|---|---|---|
| Wt(%) | 64.62% | 20.73% | 5.08% | 3.55% | 3.25% | 1.25% | 0.65% | 0.87% |
| Element | Fe | Nd | Pr | Dy | Co | B | Ni | Cu |
|---|---|---|---|---|---|---|---|---|
| Wt(%) | 0.62% | 59.51% | 14.47% | 9.77% | 8.96% | 3.27% | 2.01% | 1.35% |
| S | Nd | Pr | Dy |
|---|---|---|---|
| HCl | 41.87% | 40.19% | 65.28% |
| HNO3 | 50.37% | 45.63% | 81.25% |
| H2SO4 | 48.02% | 43.64% | 76.42% |
| H2C2O4 | 35.01% | 35.78% | 61.21% |
| NH4OH | 5.29% | 11.05% | 1.74% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.-S.; Jian, G.-C.; Lee, C.-H. Recovery and Separation of Dysprosium from Waste Neodymium Magnets through Cyphos IL 104 Extraction. Materials 2022, 15, 5281. https://doi.org/10.3390/ma15155281
Chen W-S, Jian G-C, Lee C-H. Recovery and Separation of Dysprosium from Waste Neodymium Magnets through Cyphos IL 104 Extraction. Materials. 2022; 15(15):5281. https://doi.org/10.3390/ma15155281
Chicago/Turabian StyleChen, Wei-Sheng, Guo-Cai Jian, and Cheng-Han Lee. 2022. "Recovery and Separation of Dysprosium from Waste Neodymium Magnets through Cyphos IL 104 Extraction" Materials 15, no. 15: 5281. https://doi.org/10.3390/ma15155281
APA StyleChen, W.-S., Jian, G.-C., & Lee, C.-H. (2022). Recovery and Separation of Dysprosium from Waste Neodymium Magnets through Cyphos IL 104 Extraction. Materials, 15(15), 5281. https://doi.org/10.3390/ma15155281








