Aqueous-Based Synthesis of Photocatalytic Copper Sulfide Using Sulfur Waste as Sulfurizing Agent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Copper Sulfide
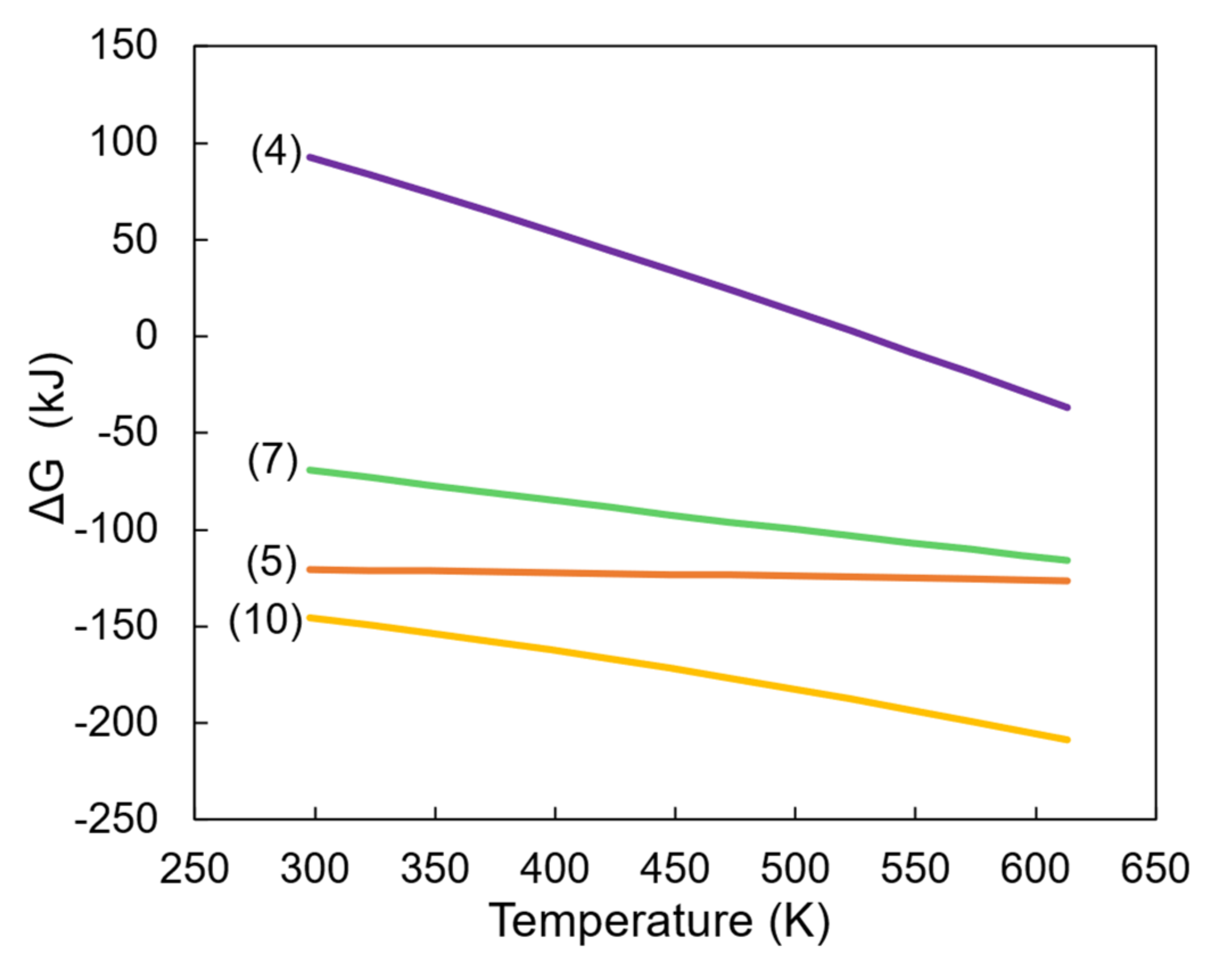
2.3. Thermodynamic Calculations
2.4. Photocatalytic Activity Experiment
2.5. Characterization Methods
3. Results
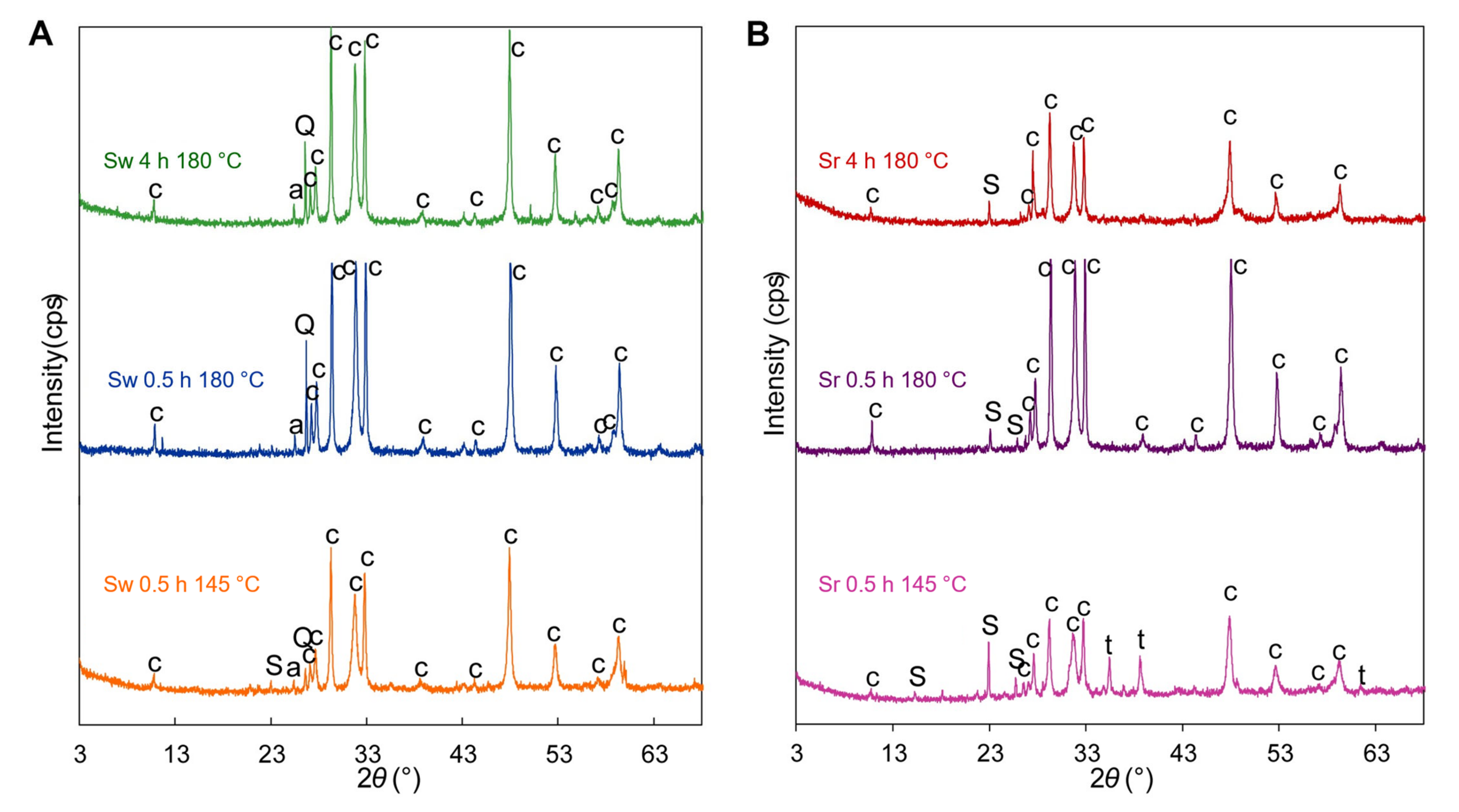
3.1. Products of Hydrothermal Synthesis
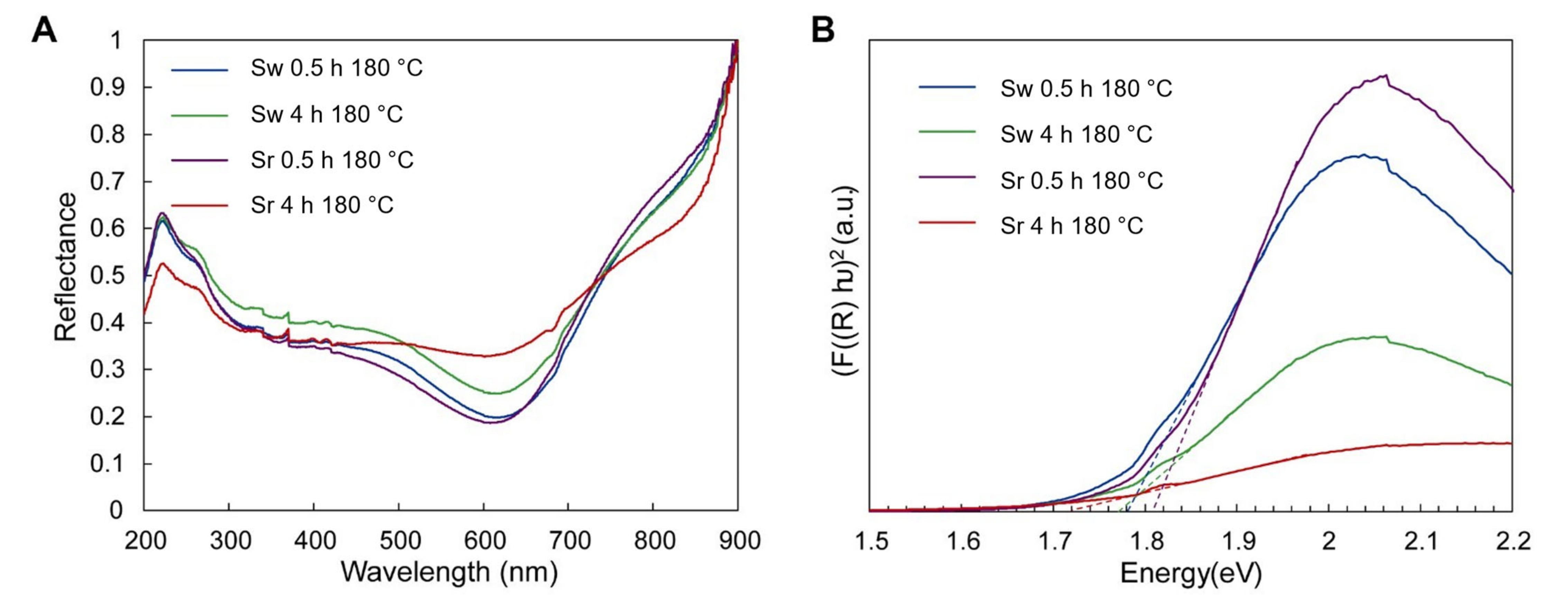
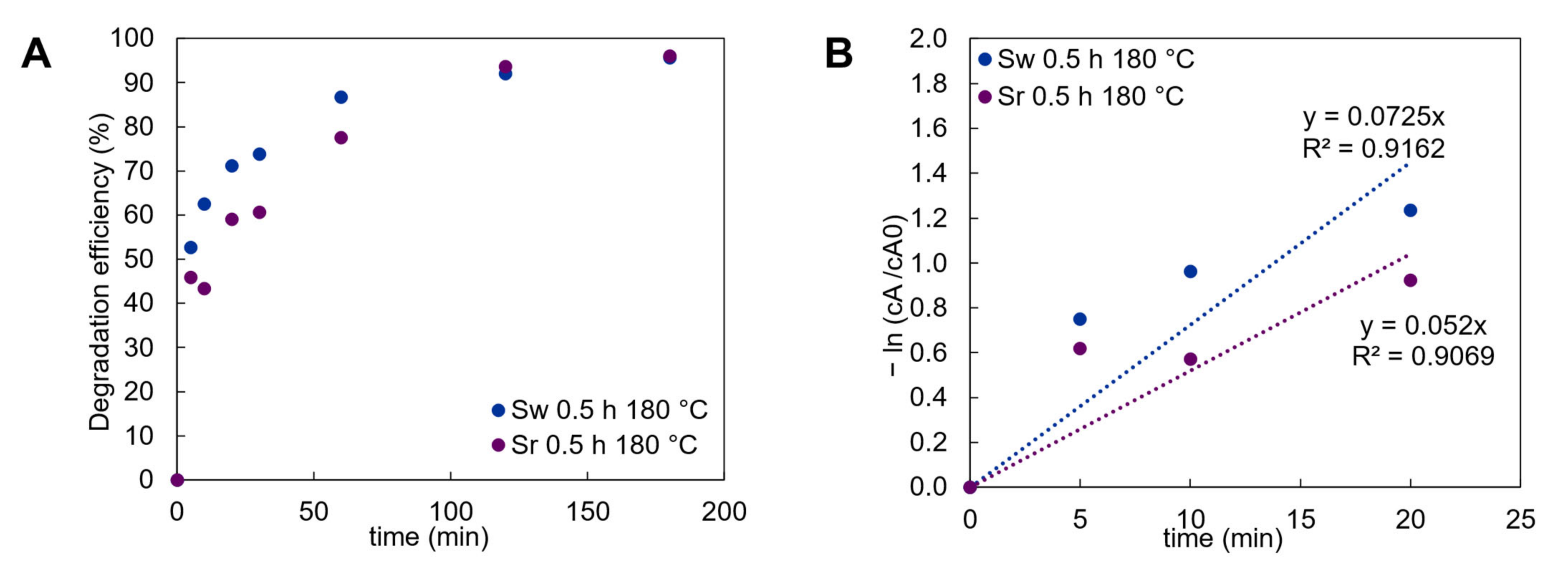
3.2. Photocatalytic Effect
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Roy, P.; Srivastava, S.K. Nanostructured Copper Sulfides: Synthesis, Properties and Applications. CrystEngComm 2015, 17, 7801–7815. [Google Scholar] [CrossRef]
- Barqi, J.; Masoudpanah, S.M.; Bafghi, M.S. Facile Synthesis of Plate-like Copper Sulfide Powder as an Electrode Material for High-Performance Supercapacitors. J. Mater. Sci. Mater. Electron. 2020, 31, 17614–17623. [Google Scholar] [CrossRef]
- Majumdar, D. Recent Progress in Copper Sulfide Based Nanomaterials for High Energy Supercapacitor Applications. J. Electroanal. Chem. 2021, 880, 114825. [Google Scholar] [CrossRef]
- Xiong, F.; Yuan, K.; Aftab, W.; Jiang, H.; Shi, J.; Liang, Z.; Gao, S.; Zhong, R.; Wang, H.; Zou, R. Copper Sulfide Nanodisk-Doped Solid–Solid Phase Change Materials for Full Spectrum Solar-Thermal Energy Harvesting and Storage. ACS Appl. Mater. Interfaces 2021, 13, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Kalimuldina, G.; Nurpeissova, A.; Adylkhanova, A.; Adair, D.; Taniguchi, I.; Bakenov, Z. Morphology and Dimension Variations of Copper Sulfide for High-Performance Electrode in Rechargeable Batteries: A Review. ACS Appl. Energy Mater. 2020, 3, 11480–11499. [Google Scholar] [CrossRef]
- Coughlan, C.; Ibáñez, M.; Dobrozhan, O.; Singh, A.; Cabot, A.; Ryan, K.M. Compound Copper Chalcogenide Nanocrystals. Chem. Rev. 2017, 117, 5865–6109. [Google Scholar] [CrossRef] [PubMed]
- Tirado, J.; Roldán-Carmona, C.; Muñoz-Guerrero, F.A.; Bonilla-Arboleda, G.; Ralaiarisoa, M.; Grancini, G.; Queloz, V.I.E.; Koch, N.; Nazeeruddin, M.K.; Jaramillo, F. Copper Sulfide Nanoparticles as Hole-Transporting-Material in a Fully-Inorganic Blocking Layers n-i-p Perovskite Solar Cells: Application and Working Insights. Appl. Surf. Sci. 2019, 478, 607–614. [Google Scholar] [CrossRef]
- Masar, M.; Urbanek, M.; Urbanek, P.; Machovska, Z.; Maslik, J.; Yadav, R.S.; Skoda, D.; Machovsky, M.; Kuritka, I. Synthesis, Characterization and Examination of Photocatalytic Performance of Hexagonal Covellite CuS Nanoplates. Mater. Chem. Phys. 2019, 237, 121823. [Google Scholar] [CrossRef]
- Wu, L.; Gao, J.; Qin, Z.; Sun, Y.; Tian, R.; Zhang, Q.; Gao, Y. Deactivated-Desulfurizer-Derived Hollow Copper Sulfide as Anode Materials for Advanced Sodium Ion Batteries. J. Power Sources 2020, 479, 228518. [Google Scholar] [CrossRef]
- Goel, S.; Chen, F.; Cai, W. Synthesis and Biomedical Applications of Copper Sulfide Nanoparticles: From Sensors to Theranostics. Small 2014, 10, 631–645. [Google Scholar] [CrossRef]
- Ingle, A.P.; Duran, N.; Rai, M. Bioactivity, Mechanism of Action, and Cytotoxicity of Copper-Based Nanoparticles: A Review. Appl. Microbiol. Biotechnol. 2014, 98, 1001–1009. [Google Scholar] [CrossRef]
- Shalabayev, Z.; Baláž, M.; Daneu, N.; Dutková, E.; Bujňáková, Z.; Kaňuchová, M.; Danková, Z.; Balážová, Ĺ.; Urakaev, F.; Tkáčiková, Ĺ.; et al. Sulfur-Mediated Mechanochemical Synthesis of Spherical and Needle-Like Copper Sulfide Nanocrystals with Antibacterial Activity. ACS Sustain. Chem. Eng. 2019, 7, 12897–12909. [Google Scholar] [CrossRef]
- Shamraiz, U.; Badshah, A.; Hussain, R.A.; Nadeem, M.A.; Saba, S. Surfactant Free Fabrication of Copper Sulphide (CuS–Cu2S) Nanoparticles from Single Source Precursor for Photocatalytic Applications. J. Saudi Chem. Soc. 2017, 21, 390–398. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Jiang, Q.; Deng, R.; Xie, X.; Meng, J. Controllable Preparation, Formation Mechanism and Photocatalytic Performance of Copper Base Sulfide Nanoparticles. Mater. Chem. Phys. 2020, 254, 123504. [Google Scholar] [CrossRef]
- Ge, Z.-H.; Zhao, L.-D.; Wu, D.; Liu, X.; Zhang, B.-P.; Li, J.-F.; He, J. Low-Cost, Abundant Binary Sulfides as Promising Thermoelectric Materials. Mater. Today 2016, 19, 227–239. [Google Scholar] [CrossRef]
- Singh, N.; Taunk, M. Structural, Optical, and Electrical Studies of Sonochemically Synthesized CuS Nanoparticles. Semiconductors 2020, 54, 1016–1022. [Google Scholar] [CrossRef]
- An, C.; Ni, Y.; Wang, Z.; Li, X.; Liu, X. Facile Fabrication of CuS Microflower as a Highly Durable Sodium-Ion Battery Anode. Inorg. Chem. Front. 2018, 5, 1045–1052. [Google Scholar] [CrossRef]
- Kim, H.; Sadan, M.K.; Kim, C.; Choe, S.-H.; Cho, K.-K.; Kim, K.-W.; Ahn, J.-H.; Ahn, H.-J. Simple and Scalable Synthesis of CuS as an Ultrafast and Long-Cycling Anode for Sodium Ion Batteries. J. Mater. Chem. A 2019, 7, 16239–16248. [Google Scholar] [CrossRef]
- Kalimuldina, G.; Taniguchi, I. Sulfur-Rich CuS1+x Cathode for Lithium Batteries. Mater. Lett. 2021, 282, 128705. [Google Scholar] [CrossRef]
- Isac, L.; Duta, A.; Kriza, A.; Manolache, S.; Nanu, M. Copper Sulfides Obtained by Spray Pyrolysis—Possible Absorbers in Solid-State Solar Cells. Thin Solid Films 2007, 515, 5755–5758. [Google Scholar] [CrossRef]
- Mousavi-Kamazani, M.; Zarghami, Z.; Salavati-Niasari, M. Facile and Novel Chemical Synthesis, Characterization, and Formation Mechanism of Copper Sulfide (Cu2S, Cu2S/CuS, CuS) Nanostructures for Increasing the Efficiency of Solar Cells. J. Phys. Chem. C 2016, 120, 2096–2108. [Google Scholar] [CrossRef]
- Kozhevnikova, N.S.; Maskaeva, L.N.; Markov, V.P.; Lipina, O.A.; Chufarov, A.U.; Kuznetsov, M. V One-Pot Green Synthesis of Copper Sulfide (I) Thin Films with p-Type Conductivity. Mater. Chem. Phys. 2020, 242, 122447. [Google Scholar] [CrossRef]
- Sagade, A.A.; Sharma, R. Copper Sulphide (CuxS) as an Ammonia Gas Sensor Working at Room Temperature. Sens. Actuators B Chem. 2008, 133, 135–143. [Google Scholar] [CrossRef]
- Zhu, T.; Xia, B.; Zhou, L.; Wen, D.; Lou, X. Arrays of Ultrafine CuS Nanoneedles Supported on a CNT Backbone for Application in Supercapacitors. J. Mater. Chem. 2012, 22, 7851. [Google Scholar] [CrossRef]
- Hong, J.; Kim, B.-S.; Yang, S.; Jang, A.-R.; Lee, Y.-W.; Pak, S.; Lee, S.; Cho, Y.; Kang, D.; Shin, H.S.; et al. Chalcogenide Solution-Mediated Activation Protocol for Scalable and Ultrafast Synthesis of Single-Crystalline 1-D Copper Sulfide for Supercapacitors. J. Mater. Chem. A 2019, 7, 2529–2535. [Google Scholar] [CrossRef]
- Liu, J.; Xue, D. Rapid and Scalable Route to CuS Biosensors: A Microwave-Assisted Cu-Complex Transformation into CuS Nanotubes for Ultrasensitive Nonenzymatic Glucose Sensor. J. Mater. Chem. 2011, 21, 223–228. [Google Scholar] [CrossRef]
- Wang, D.; Li, Q.; Xing, Z.; Yang, X. Copper Sulfide Nanoplates as Nanosensors for Fast, Sensitive and Selective Detection of DNA. Talanta 2018, 178, 905–909. [Google Scholar] [CrossRef]
- Ajibade, P.A.; Oluwalana, A.E. Enhanced Photocatalytic Degradation of Ternary Dyes by Copper Sulfide Nanoparticles. Nanomaterials 2021, 11, 2000. [Google Scholar] [CrossRef]
- Zhou, S.; Gong, L.; Zhao, X.; Liang, Q.; Zhang, W.; Wang, L.; Yu, K.; Zhou, B. Synthesis and Photocatalytic Performance of Copper Sulfide by a Simple Solvothermal Method. Chem. Phys. Lett. 2020, 759, 138034. [Google Scholar] [CrossRef]
- Khan, M.D.; Malik, M.A.; Akhtar, J.; Mlowe, S.; Revaprasadu, N. Phase Pure Deposition of Flower-like Thin Films by Aerosol Assisted Chemical Vapor Deposition and Solvent Mediated Structural Transformation in Copper Sulfide Nanostructures. Thin Solid Films 2017, 638, 338–344. [Google Scholar] [CrossRef]
- Castillo, A.C.; Lázaro, R.C.A.; Ojeda, E.M.L.; De La Mota Gonzalez, M.A.; López, M.A.Q.; Moreno, M.M.; Diaz, V.R.G.; Castellanos, J.F.G. Characterization of amorphous cus thin films obtained from fast time and low temperature of deposition. Chalcogenide Lett. 2016, 13, 217. [Google Scholar]
- Yadav, S.; Shrivas, K.; Bajpai, P.K. Role of Precursors in Controlling the Size, Shape and Morphology in the Synthesis of Copper Sulfide Nanoparticles and Their Application for Fluorescence Detection. J. Alloys Compd. 2019, 772, 579–592. [Google Scholar] [CrossRef]
- Botha, N.L.; Ajibade, P.A. Effect of Temperature on Crystallite Sizes of Copper Sulfide Nanocrystals Prepared from Copper(II) Dithiocarbamate Single Source Precursor. Mater. Sci. Semicond. Process. 2016, 43, 149–154. [Google Scholar] [CrossRef]
- Kadam, S.L.; Bulakhe, R.N.; Kadam, R.A.; Yewale, M.A. Electrochemical Synthesis of CuS Thin Film for Supercapacitor Application. Macromol. Symp. 2020, 392, 1900209. [Google Scholar] [CrossRef]
- Achimovičová, M.; Dutková, E.; Tóthová, E.; Bujňáková, Z.; Briančin, J.; Kitazono, S. Structural and Optical Properties of Nanostructured Copper Sulfide Semiconductor Synthesized in an Industrial Mill. Front. Chem. Sci. Eng. 2019, 13, 164–170. [Google Scholar] [CrossRef]
- Baláž, M.; Tešinský, M.; Marquardt, J.; Škrobian, M.; Daneu, N.; Rajňák, M.; Baláž, P. Synthesis of Copper Nanoparticles from Refractory Sulfides Using a Semi-Industrial Mechanochemical Approach. Adv. Powder Technol. 2020, 31, 782–791. [Google Scholar] [CrossRef]
- Romero-Jaime, A.K.; Vargas-Hernández, D.; Acosta-Enríquez, M.C.; Tánori-Córdova, J.C.; Valenzuela-Badilla, J.; Castillo, S.J. Novel Route for Simplified and Efficient Synthesis of Spiky-like Copper Sulfide Nanoballs by Soft Chemistry Method and Their Basic Physicochemical Characterizations. Mater. Sci. Semicond. Process. 2020, 107, 104830. [Google Scholar] [CrossRef]
- Song, Z.; Lei, H.; Li, B.; Wang, H.; Wen, J.; Li, S.; Fang, G. Enhanced Field Emission from in Situ Synthesized 2D Copper Sulfide Nanoflakes at Low Temperature by Using a Novel Controllable Solvothermal Preferred Edge Growth Route. Phys. Chem. Chem. Phys. 2015, 17, 11790–11795. [Google Scholar] [CrossRef]
- Saranya, M.; Santhosh, C.; Ramachandran, R.; Kollu, P.; Saravanan, P.; Vinoba, M.; Jeong, S.K.; Grace, A.N. Hydrothermal Growth of CuS Nanostructures and Its Photocatalytic Properties. Powder Technol. 2014, 252, 25–32. [Google Scholar] [CrossRef]
- Shahi, S.; Saeednia, S.; Iranmanesh, P.; Hatefi Ardakani, M. Influence of Synthesis Parameters on the Optical and Photocatalytic Properties of Solvo/Hydrothermal CuS and ZnS Nanoparticles. Luminescence 2021, 36, 180–191. [Google Scholar] [CrossRef]
- Huang, W.; Cheng, Y.; Fei, X.; Xu, W.; Chang, S.; Song, S.; Huang, C. Designing of CuS Growing on Bi2WO6 Nanosheet Heterostructures Based on a Photoelectrochemical Aptasensor for Detecting Ofloxacin. Microchim. Acta 2020, 187, 583. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Bahadur, A.; Anwer, S.; Ali, S.; Saeed, A.; Muhammad Irfan, R.; Li, H.; Javed, M.; Raheel, M.; Shoaib, M. Shape and Phase-Controlled Synthesis of Specially Designed 2D Morphologies of l-Cysteine Surface Capped Covellite (CuS) and Chalcocite (Cu2S) with Excellent Photocatalytic Properties in the Visible Spectrum. Appl. Surf. Sci. 2020, 526, 146691. [Google Scholar] [CrossRef]
- Auyoong, Y.L.; Yap, P.L.; Huang, X.; Abd Hamid, S.B. Optimization of Reaction Parameters in Hydrothermal Synthesis: A Strategy towards the Formation of CuS Hexagonal Plates. Chem. Cent. J. 2013, 7, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulla, R.; Jones, D.R.; Dunnill, C.W. Economical and Facile Route to Produce Gram-Scale and Phase-Selective Copper Sulfides for Thermoelectric Applications. ACS Sustain. Chem. Eng. 2020, 8, 14234–14242. [Google Scholar] [CrossRef]
- Sun, S.; Li, P.; Liang, S.; Yang, Z. Diversified Copper Sulfide (Cu 2−x S) Micro-/Nanostructures: A Comprehensive Review on Synthesis, Modifications and Applications. Nanoscale 2017, 9, 11357–11404. [Google Scholar] [CrossRef] [PubMed]
- Olatunde, O.C.; Onwudiwe, D.C. Stoichiometric Phases and Mechanism of Crystal Phase Selectivity of Copper-Based Ternary Sulphides. Mater. Sci. Semicond. Process. 2021, 125, 105627. [Google Scholar] [CrossRef]
- Mikhlin, Y.; Nasluzov, V.; Ivaneeva, A.; Vorobyev, S.; Likhatski, M.; Romanchenko, A.; Krylov, A.; Zharkov, S.; Meira, D.M. Formation, Evolution and Characteristics of Copper Sulfide Nanoparticles in the Reactions of Aqueous Cupric and Sulfide Ions. Mater. Chem. Phys. 2020, 255, 123600. [Google Scholar] [CrossRef]
- Li, Z.; Chen, M.; Huang, P.; Zhang, Q.; Song, S. Effect of grinding with sulfur on surface properties and floatability of three nonferrous metal oxides. Trans. Nonferrous. Met. Soc. China 2017, 27, 2474–2480. [Google Scholar] [CrossRef]
- Kundu, J.; Pradhan, D. Controlled Synthesis and Catalytic Activity of Copper Sulfide Nanostructured Assemblies with Different Morphologies. ACS Appl. Mater. Interfaces 2014, 6, 1823–1834. [Google Scholar] [CrossRef]
- Dambrauskas, T.; Baltakys, K.; Eisinas, A. Formation and Thermal Stability of Calcium Silicate Hydrate Substituted with Al3+ Ions in the Mixtures with CaO/SiO2 = 1.5. J. Therm. Anal. Calorim. 2018, 131, 501–512. [Google Scholar] [CrossRef]
- Gineika, A.; Baltakys, K.; Dambrauskas, T. The Application of Silica Gel Waste for the Two-Step Synthesis of Wollastonite in Temperature Range of 200–950 °C. J. Therm. Anal. Calorim. 2019, 138, 2263–2273. [Google Scholar] [CrossRef]
- Jiang, R.; Jia, C.-S.; Wang, Y.-Q.; Peng, X.-L.; Zhang, L.-H. Prediction of Gibbs Free Energy for the Gases Cl2, Br2, and HCl. Chem. Phys. Lett. 2019, 726, 83–86. [Google Scholar] [CrossRef]
- Lide, D.R. Standard Thermodynamic Properties of Chemical Substances. In CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2000; p. 2661. [Google Scholar]
- Goldberg, R.N.; Parker, V.B. Thermodynamics of solution of so2(g) in water and of aqueous sulfur dioxide solutions. J. Res. Natl. Bur. Stand. 1985, 90, 341–358. [Google Scholar] [CrossRef] [PubMed]
- Technology, R.I. Thermodynamic Data for Copper; CM Digitaltryck AB Bromma: Stockholm, Sweden, 2000. [Google Scholar]
- Sarapajevaite, G.; Baltakys, K. Thermal stability and decomposition of synthetic covellite samples. J. Therm. Anal. Calorim. 2022, 1–13. [Google Scholar] [CrossRef]
- Escobedo-Morales, A.; Ruiz-López, I.I.; del Ruiz-Peralta, M.; Tepech-Carrillo, L.; Sánchez-Cantú, M.; Moreno-Orea, J.E. Automated Method for the Determination of the Band Gap Energy of Pure and Mixed Powder Samples Using Diffuse Reflectance Spectroscopy. Heliyon 2019, 5, e01505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laperdrix, E.; Sahibed-dine, A.; Costentin, G.; Bensitel, M.; Lavalley, J.-C. Evidence of the Reverse Claus Reaction on Metal Oxides: Influence of Their Acid--Base Properties. Appl. Catal. B Environ. 2000, 27, 137–142. [Google Scholar] [CrossRef]
- Hong, T.; Wei, Y.; Li, L.; Mumford, K.A.; Stevens, G.W. An Investigation into the Precipitation of Copper Sulfide from Acidic Sulfate Solutions. Hydrometallurgy 2020, 192, 105288. [Google Scholar] [CrossRef]
- Sadovnikov, S.I. Thermal Stability and Recrystallization of Semiconductor Nanostructured Sulfides and Sulfide Solid Solutions. J. Alloys Compd. 2019, 788, 586–599. [Google Scholar] [CrossRef]
- Morselli, D.; Campagnolo, L.; Prato, M.; Papadopoulou, E.L.; Scarpellini, A.; Athanassiou, A.; Fragouli, D. Ceria/Gold Nanoparticles in Situ Synthesized on Polymeric Membranes with Enhanced Photocatalytic and Radical Scavenging Activity. ACS Appl. Nano Mater. 2018, 1, 5601–5611. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Zaen, R.; Oktiani, R. Correlation between Crystallite Size and Photocatalytic Performance of Micrometer-Sized Monoclinic WO3 Particles. Arab. J. Chem. 2020, 13, 1283–1296. [Google Scholar] [CrossRef]


| Sulfurizing Agent | Duration (h) | Temperature (°C) | Abbreviation |
|---|---|---|---|
| Sulfur waste | 0.5 | 145 | Sw 0.5 h 145 °C |
| 180 | Sw 0.5 h 180 °C | ||
| 4 | Sw 4 h 180 °C | ||
| Sulfur reference | 0.5 | 145 | Sr 0.5 h 145 °C |
| 180 | Sr 0.5 h 180 °C | ||
| 4 | Sr 4 h 180 °C |
| Component | |||
|---|---|---|---|
| H+ (aq) | 0 | 0 | 0 |
| H2O (l) | –285.8 | 70.0 | 75.3 |
| S (l) | 1.4 | 35.2 | 23.6 |
| SO2 (aq) | –323.8 | 159.5 | 195.0 |
| H2S (g) | –20.6 | 205.8 | 34.2 |
| HS− (aq) | –17.6 | 62.8 | NG |
| Cu2+ (aq) | 64.8 | –99.6 | NG |
| CuO (s) | –157.3 | 42.6 | 42.3 |
| CuS (s) | –79.5 | 120.9 | 76.3 |
| Sample | Waste | Reference |
|---|---|---|
| Crystallite Size ± Standard Dev. (nm) | ||
| 0.5 h 145 °C | 32.8 ± 9.5 | 27.2 ± 10.5 |
| 0.5 h 180 °C | 38.7 ± 9.4 | 34.8 ± 10.6 |
| 4 h 180 °C | 39.5 ± 9.3 | 36.6 ± 9.7 |
| Sample | Atomic Ratio Cu:S |
|---|---|
| Sw 0.5 h 145 °C | 1.01:1 |
| Sw 0.5 h 180 °C | 1.06:1 |
| Sw 4 h 180 °C | 0.87:1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarapajevaite, G.; Morselli, D.; Baltakys, K. Aqueous-Based Synthesis of Photocatalytic Copper Sulfide Using Sulfur Waste as Sulfurizing Agent. Materials 2022, 15, 5253. https://doi.org/10.3390/ma15155253
Sarapajevaite G, Morselli D, Baltakys K. Aqueous-Based Synthesis of Photocatalytic Copper Sulfide Using Sulfur Waste as Sulfurizing Agent. Materials. 2022; 15(15):5253. https://doi.org/10.3390/ma15155253
Chicago/Turabian StyleSarapajevaite, Gabriele, Davide Morselli, and Kestutis Baltakys. 2022. "Aqueous-Based Synthesis of Photocatalytic Copper Sulfide Using Sulfur Waste as Sulfurizing Agent" Materials 15, no. 15: 5253. https://doi.org/10.3390/ma15155253
APA StyleSarapajevaite, G., Morselli, D., & Baltakys, K. (2022). Aqueous-Based Synthesis of Photocatalytic Copper Sulfide Using Sulfur Waste as Sulfurizing Agent. Materials, 15(15), 5253. https://doi.org/10.3390/ma15155253







