Effect of Pre-Anodized Film on Micro-Arc Oxidation Process of 6063 Aluminum Alloy
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials
2.2. Surface Treatment Based on Anodizing Techniques
2.3. Coating Characterizations
2.4. Specific Energy Consumption
2.5. Corrosion Performance
3. Results
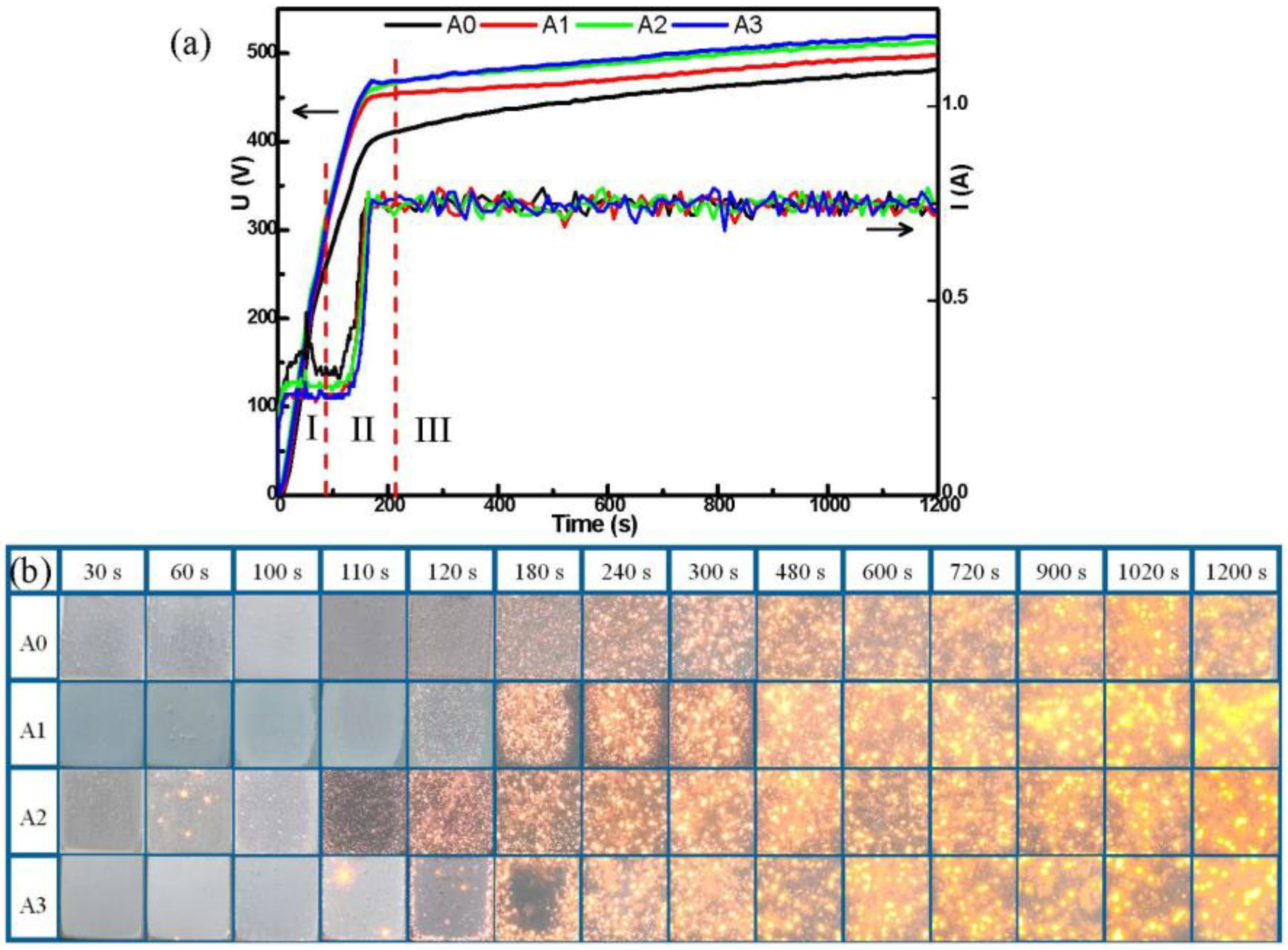
3.1. Voltage Behavior and Discharges Evolution
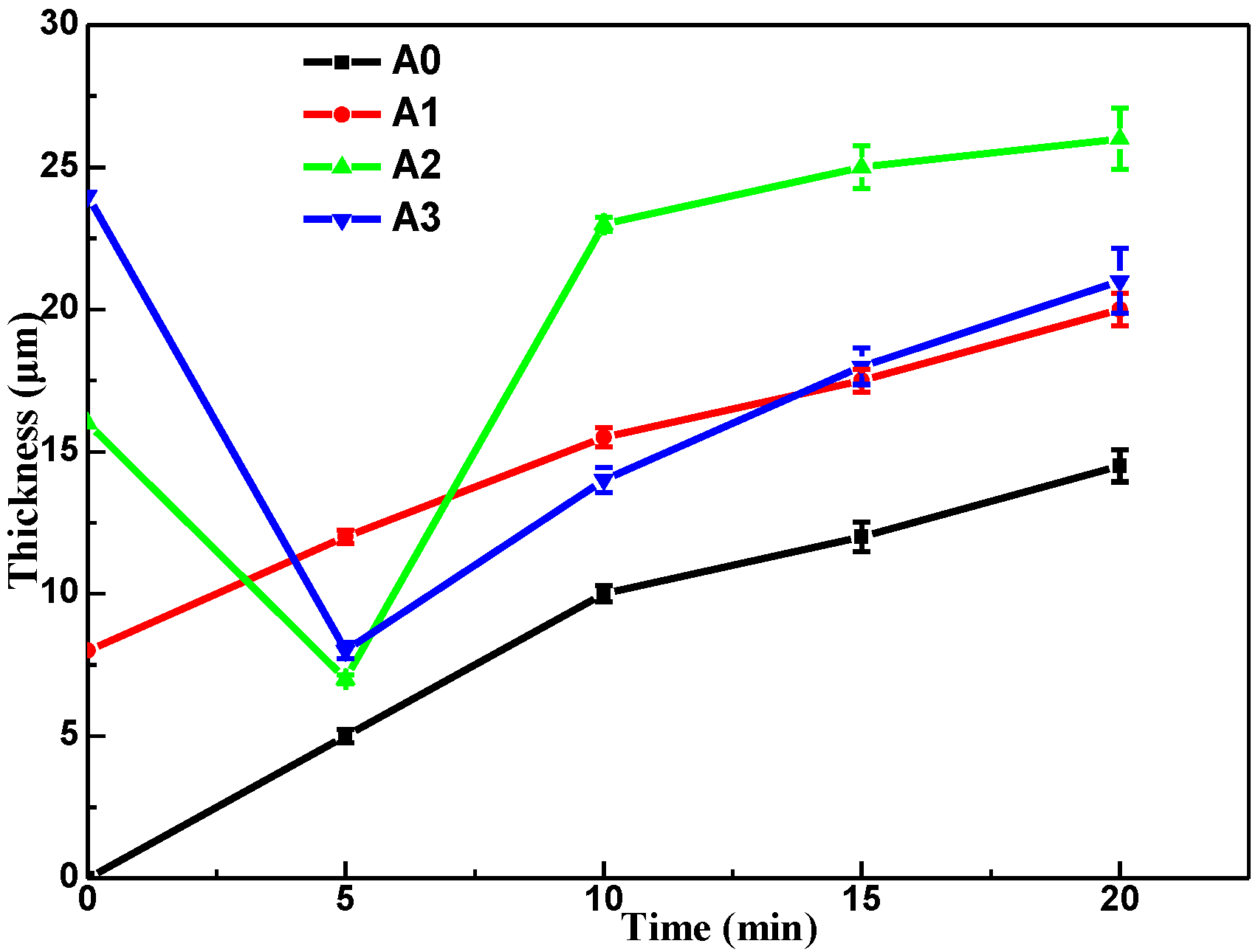
3.2. Coating Thickness and Efficiency Analysis
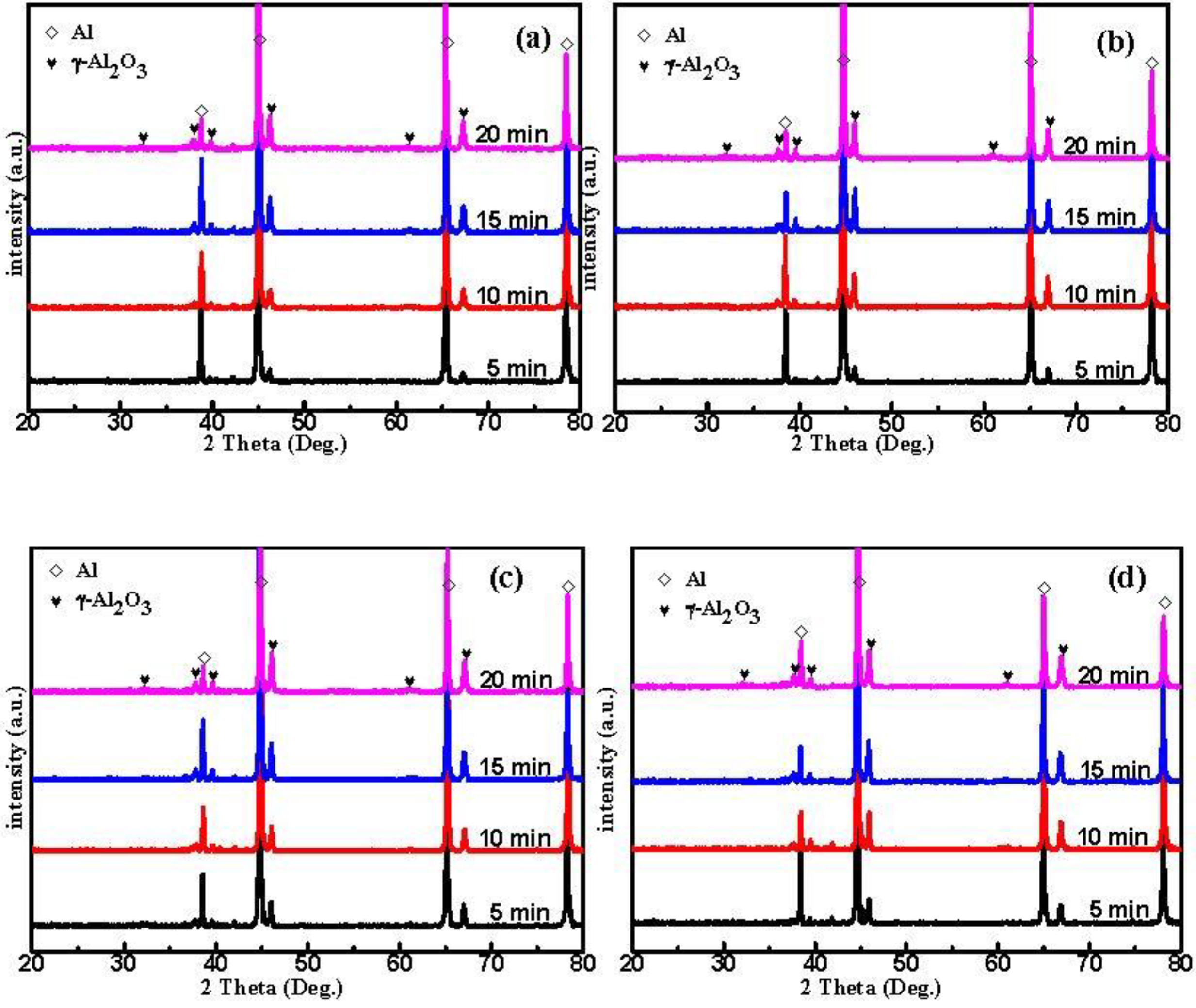
3.3. Phase Composition
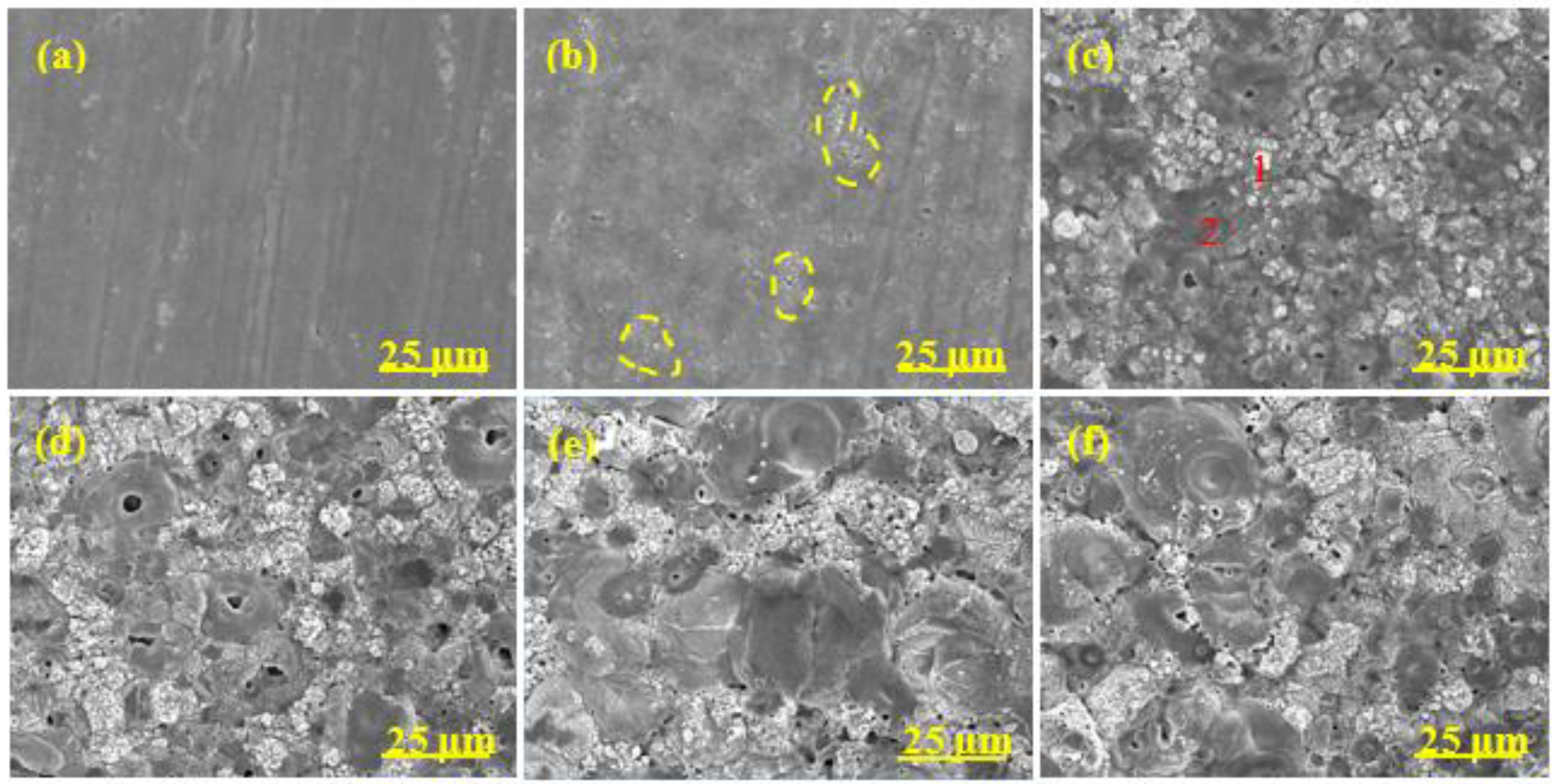
3.4. Surface Morphology
3.5. Polished Cross-Section Morphology
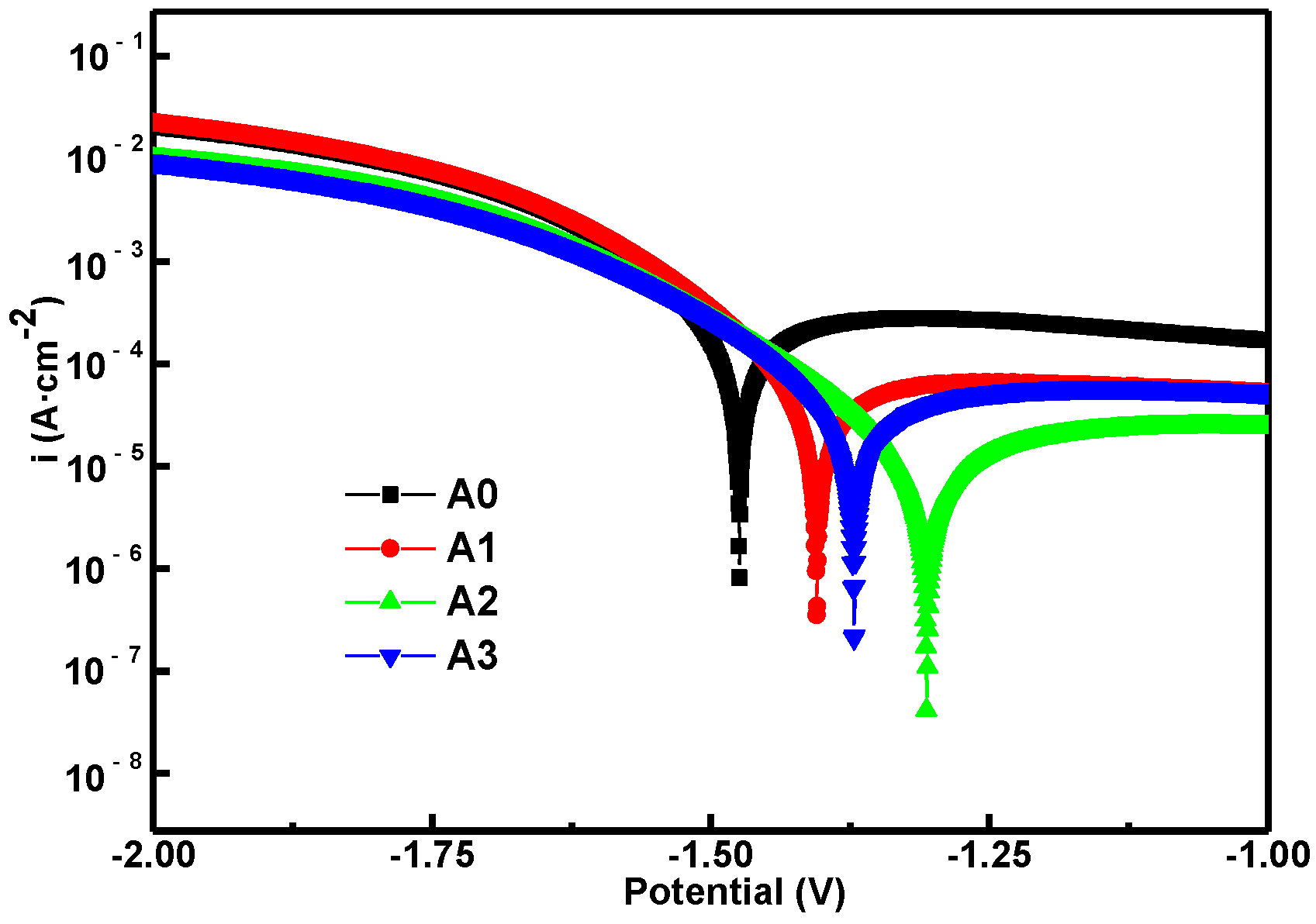
3.6. Corrosion Evaluation
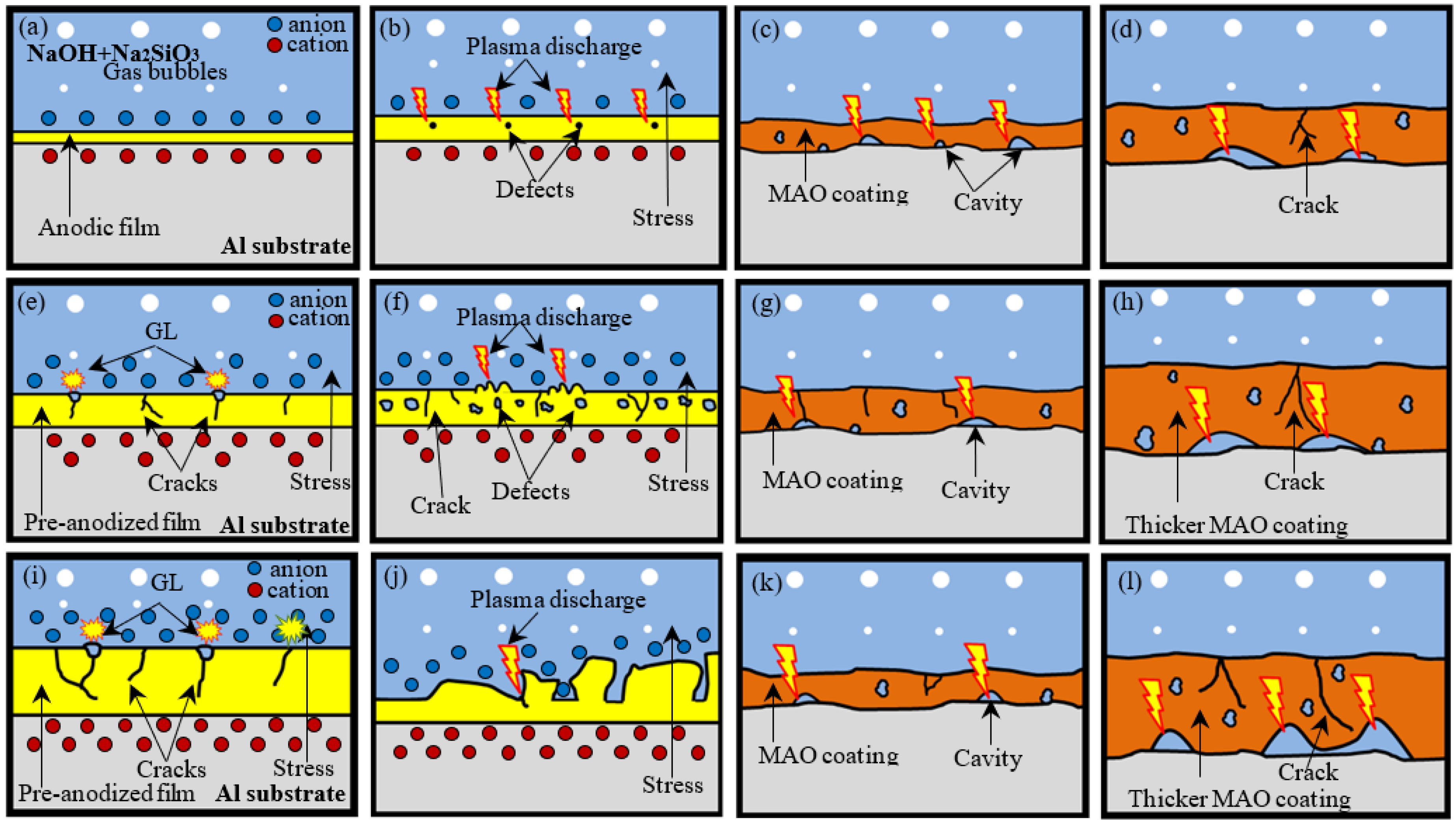
3.7. Growth Model for MAO Coatings
4. Conclusions
- The pre-anodization can change the spark of the early reaction and shorten the early reaction process of the MAO reaction, reducing energy consumption. The thickness of pre-anodized at 16 μm has the best energy-saving effect (2.78 kW·h·m−2μm−1) after oxidation for 10 min, saving 47%;
- All the pre-anodization appeared to be a breakdown by GL when the working voltage approached the breakdown voltage. The thicker pre-anodized film (beyond 8 μm) will be locally lost from the aluminum surface and appear to fragment thinning, due to the high-temperature sparking;
- The γ-Al2O3 phase content after pre-anodized is significantly increased in MAO coatings, associated with an increase in the reaction energy by pre-anodized that enhanced the local transient pressure as well as the temperature to promote the formation of γ-Al2O3 phases;
- After pre-anodized, the corrosion resistance of MAO coating was significantly improved. Better corrosion resistance was obtained of the pre-anodized compared to the without pre-anodized after 10 min oxidation, the corrosion rate was reduced by an order of magnitude;
- On the basis of the pre-anodized film, it is believed that the corrosion resistance of the MAO coating can be further improved by changing the electrical parameters or the composition of the electrolyte.
Author Contributions
Funding
Conflicts of Interest
References
- Matykina, E.; Arrabal, R.; Mohamed, A.; Skeldon, P.; Thompson, G.E. Plasma electrolytic oxidation of pre-anodized aluminium. Corros. Sci. 2009, 51, 2897–2905. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A.; Dowey, S.J. Plasma electrolysis for surface engineering. Surf. Coat. Technol. 1999, 122, 73–93. [Google Scholar] [CrossRef]
- Wang, D.D.; Liu, X.T.; Wang, Y.; Zhang, Q.; Li, D.L.; Liu, X.R.; Su, H.; Zhang, Y.H.; Yu, S.X.; Shen, D.J. Role of the electrolyte composition in establishing plasma discharges and coating growth process during a micro-arc oxidation. Surf. Coat. Technol. 2020, 402, 126349. [Google Scholar] [CrossRef]
- Li, Z.Y.; Cai, Z.B.; Cui, Y.; Liu, J.H.; Zhu, M.H. Effect of oxidation time on the impact wear of micro-arc oxidation coating on aluminum alloy. Wear 2019, 426, 285–295. [Google Scholar] [CrossRef]
- Ji, S.P.; Weng, Y.C.; Wu, Z.Z.; Ma, Z.Y.; Tian, X.B.; Fu, R.K.Y.; Lin, H.; Wu, G.S.; Chu, P.K.; Pan, F. Excellent corrosion resistance of P and Fe modified micro-arc oxidation coating on Al alloy. J. Alloys Compd. 2017, 710, 452–459. [Google Scholar] [CrossRef]
- Echeverry-Rendon, M.; Allain, J.P.; Robledo, S.M.; Echeverria, F.; Harmsen, M.C. Coatings for biodegradable magnesium-based supports for therapy of vascular disease: A general view. Mater. Sci. Eng. C 2019, 102, 150–163. [Google Scholar] [CrossRef]
- Santos-Coquillat, A.; Tenorio, R.G.; Mohedano, M.; Martinez-Campos, E.; Arrabal, R.; Matykina, E. Tailoring of antibacterial and osteogenic properties of Ti6Al4V by plasma electrolytic oxidation. Appl. Surf. Sci. 2018, 454, 157–172. [Google Scholar] [CrossRef]
- Sridhar, S.; Arunnellaiappan, T.; Rameshbabu, N.; Mika, S.; Viswanathan, A. Solar photocatalytic activity of nitrogen doped TiO2 coating by micro-arc oxidation. Surf. Eng. 2017, 33, 779–786. [Google Scholar] [CrossRef]
- Leijtens, T.; Eperon, G.E.; Barker, A.J.; Grancini, G.; Zhang, W.; Ball, J.M.; Kandada, A.R.S.; Snaith, H.J.; Petrozza, A. Carrier trapping and recombination: The role of defect physics in enhancing the open circuit voltage of metal halide perovskite solar cells. Energy Environ. Sci. 2016, 9, 3472–3481. [Google Scholar] [CrossRef] [Green Version]
- Phuong, N.V.; Fazal, B.R.; Moon, S. Cerium-and phosphate-based sealing treatments of PEO coated AZ31. Mg alloy. Surf. Coat. Technol. 2017, 309, 86–95. [Google Scholar] [CrossRef]
- Liu, C.Y.; Tsai, D.S.; Wang, J.M.; Tsai, J.T.J.; Chou, C.C. Particle Size Influences on the Coating Microstructure through Green Chromia Inclusion in Plasma Electrolytic Oxidation. ACS Appl. Mater. Interfaces 2017, 9, 21864–21871. [Google Scholar] [CrossRef]
- Lu, X.P.; Mohedano, M.; Blawert, C.; Matykina, E.; Arrabal, R.; Kainer, K.U.; Zheludkevich, M.L. Plasma electrolytic oxidation coatings with particle additions—A review. Surf. Coat. Technol. 2016, 307, 1165–1182. [Google Scholar] [CrossRef]
- Aliofkhazraei, M.; Macdonald, D.D.; Matykina, E.; Parfenov, E.V.; Egorkin, V.S.; Curran, J.A.; Troughton, S.C.; Sinebryukhov, S.L.; Gnedenkov, S.V.; Lampke, T.; et al. Review of plasma electrolytic oxidation of titanium substrates: Mechanism, properties, applications and limitations. Appl. Surf. Sci. Adv. 2021, 5, 100121. [Google Scholar] [CrossRef]
- Matykina, E.; Arrabal, R.; Skeldon, P.; Thompson, G.E. Optimisation of the plasma electrolytic oxidation process efficiency on aluminium. Surf. Interface Anal. 2010, 42, 221–226. [Google Scholar] [CrossRef]
- Snizhko, L.O.; Yerokhin, A.L.; Gurevina, N.L.; Patalakha, V.A.; Matthews, A. Excessive oxygen evolution during plasma electrolytic oxidation of aluminium. Thin Solid Films 2007, 516, 460–464. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Shatrov, A.; Samsonov, V.; Shashkov, P.; Pilkington, A.; Leyland, A.; Matthews, A. Oxide ceramic coatings on aluminium alloys produced by a pulsed bipolar plasma electrolytic oxidation process. Surf. Coat. Technol. 2005, 199, 150–157. [Google Scholar] [CrossRef]
- Troughton, S.C.; Nomine, A.; Nomine, A.V.; Henrion, G.; Clyne, T.W. Synchronised electrical monitoring and high speed video of bubble growth associated with individual discharges during plasma electrolytic oxidation. Appl. Surf. Sci. 2015, 359, 405–411. [Google Scholar] [CrossRef] [Green Version]
- Gebarowski, W.; Pietrzyk, S. Growth Characteristics of the Oxide Layer on Aluminium in the Process of Plasma Electrolytic Oxidation. Arch. Metall. Mater. 2014, 59, 407–411. [Google Scholar] [CrossRef]
- Pillai, A.M.; Rajendra, A.; Sharma, A.K. Influence of process parameters on growth behaviour and properties of coatings obtained by plasma electrolytic oxidation (PEO) on AA 6061. J. Appl. Electrochem. 2018, 48, 543–557. [Google Scholar] [CrossRef]
- Ly, X.N.; Yang, S. Influence of current mode on microstructure and corrosion behavior of micro-arc oxidation (MAO) biodegradable Mg-Zn-Ca alloy in Hank’s solution. Surf. Coat. Technol. 2019, 358, 331–339. [Google Scholar] [CrossRef]
- del Olmo, R.; Mohedano, M.; Mingo, B.; Arrabal, R.; Matykina, E. LDH Post-Treatment of Flash PEO Coatings. Coatings 2019, 9, 354. [Google Scholar] [CrossRef] [Green Version]
- O’Hara, M.; Troughton, S.C.; Francis, R.; Clyne, T.W. The incorporation of particles suspended in the electrolyte into plasma electrolytic oxidation coatings on Ti and Al substrates. Surf. Coat. Technol. 2020, 385, 125354. [Google Scholar] [CrossRef]
- Matykina, E.; Arrabal, R.; Skeldon, P.; Thompson, G.E.; Belenguer, P. AC PEO of aluminium with porous alumina precursor films. Surf. Coat. Technol. 2010, 205, 1668–1678. [Google Scholar] [CrossRef]
- Mohedano, M.; Matykina, E.; Arrabal, R.; Mingo, B.; Zheludkevich, M.L. PEO of rheocast A356 Al alloy: Energy efficiency and corrosion properties. Surf. Interface Anal. 2016, 48, 953–959. [Google Scholar] [CrossRef]
- Matykina, E.; Arrabal, R.; Mohedano, M.; Mingo, B.; Gonzalez, J.; Pardo, A.; Merino, M.C. Recent advances in energy efficient PEO processing of aluminium alloys. Trans. Nonferrous Met. Soc. China 2017, 27, 1439–1454. [Google Scholar] [CrossRef]
- Mohedano, M.; Mingo, B.; Mora-Sanchez, H.; Matykina, E.; Arrabal, R. Effects of pre-anodizing and phosphates on energy consumption and corrosion performance of PEO coatings on AA6082. Surf. Coat. Technol. 2021, 409, 126892. [Google Scholar] [CrossRef]
- Ling, K.; Mo, Q.F.; Lv, X.Y.; Qin, G.M.; Yang, W.W.; Li, L.W.; Li, W.Z. Growth characteristics and corrosion resistance of micro-arc oxidation coating on Al-Mg composite plate. Vacuum 2022, 195, 110640. [Google Scholar] [CrossRef]
- Ikonopisov, S. Theory of Electrical Breakdown during Formation of Barrier Anodic Films. Electrochim. Acta 1977, 22, 1077–1082. [Google Scholar] [CrossRef]
- Vijh, A.K. Sparking voltages and side reactions during anodization of valve metals in terms of electron tunnelling. Corros. Sci. 1971, 11, 411–417. [Google Scholar] [CrossRef]
- Wu, Y.K.; Yang, Z.; Wang, R.Q.; Wu, G.R.; Chen, D.; Wang, D.D.; Liu, X.T.; Li, D.L.; Guo, C.H.; Yu, S.X.; et al. An investigation of microstructure evolution for plasma electrolytic oxidation (PEO) coated Al in an alkaline silicate. Surf. Coat. Technol. 2018, 351, 136–152. [Google Scholar] [CrossRef]
- Stojadinovic, S.; Vasilic, R.; Petkovic, M.; Nedic, Z.; Kasalica, B.; Belca, I.; Zekovic, L. Luminescence properties of oxide films formed by anodization of aluminum in 12-tungstophosphoric acid. Electrochim. Acta 2010, 55, 3857–3863. [Google Scholar] [CrossRef]
- Stojadinovic, S.; Vasilic, R.; Peric, M. Investigation of plasma electrolytic oxidation on valve metals by means of molecular spectroscopy—A review. Rsc. Adv. 2014, 4, 25759–25789. [Google Scholar] [CrossRef]
- Stojadinovic, S.; Vasilic, R. Characterization of Porous Anodic Aluminum Oxide Films by Luminescence Methods—A Review. Curr. Nanosci. 2015, 11, 547–559. [Google Scholar] [CrossRef]
- Stojadinovic, S.; Zekovic, L.; Belca, I.; Kasalica, B. Galvanoluminescence of oxide films formed by anodization of aluminum in phosphoric acid. Electrochem. Commun. 2004, 6, 427–431. [Google Scholar] [CrossRef]
- Stojadinovic, S.; Peric, M.; Radic-Peric, J.; Vasilic, R.; Petkovic, M.; Zekovic, L. Luminescence of the B-1 Sigma(+)-X-1 Sigma(+), band system of MgO during plasma electrolytic oxidation of magnesium alloy. Surf. Coat. Technol. 2012, 206, 2905–2913. [Google Scholar] [CrossRef]
- Kasalica, B.; Petkovic, M.; Belca, I.; Stojadinovic, S.; Zekovic, L. Electronic transitions during plasma electrolytic oxidation of aluminum. Surf. Coat. Technol. 2009, 203, 3000–3004. [Google Scholar] [CrossRef]
- Zhao, Z.Q.; Pan, Q.L.; Yan, J.K.; Ye, J.; Liu, Y.R. Direct current micro-arc oxidation coatings on Al-Zn-Mg-Mn-Zr extruded alloy with tunable structures and properties templated by discharge stages. Vacuum 2018, 150, 155–165. [Google Scholar] [CrossRef]
- Gu, W.C.; Lv, G.H.; Chen, H.; Chen, G.L.; Feng, W.R.; Zhang, G.L.; Yang, S.Z. Investigation of morphology and composition of plasma electrolytic oxidation coatings in systems of Na2SiO3-NaOH and (NaPO3)(6)-NaOH. J. Mater. Process. Technol. 2007, 182, 28–33. [Google Scholar]
- Arrabal, R.; Matykina, E.; Skeldon, P.; Thompson, G.E. Incorporation of zirconia particles into coatings formed on magnesium by plasma electrolytic oxidation. J. Mater. Sci. 2008, 43, 1532–1538. [Google Scholar] [CrossRef]
- Gao, Y.H.; Yerokhin, A.; Parfenov, E.; Matthews, A. Application of Voltage Pulse Transient Analysis during Plasma Electrolytic Oxidation for Assessment of Characteristics and Corrosion Behaviour of Ca- and P-containing Coatings on Magnesium. Electrochim. Acta 2014, 149, 218–230. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Duan, X.Y.; Zheng, W.Q.; Zhao, Y.W. Long time corrosion test of AZ31B Mg alloy via micro-arc oxidation (MAO) technology. Mater. Res. Express 2019, 6, 126416. [Google Scholar] [CrossRef]
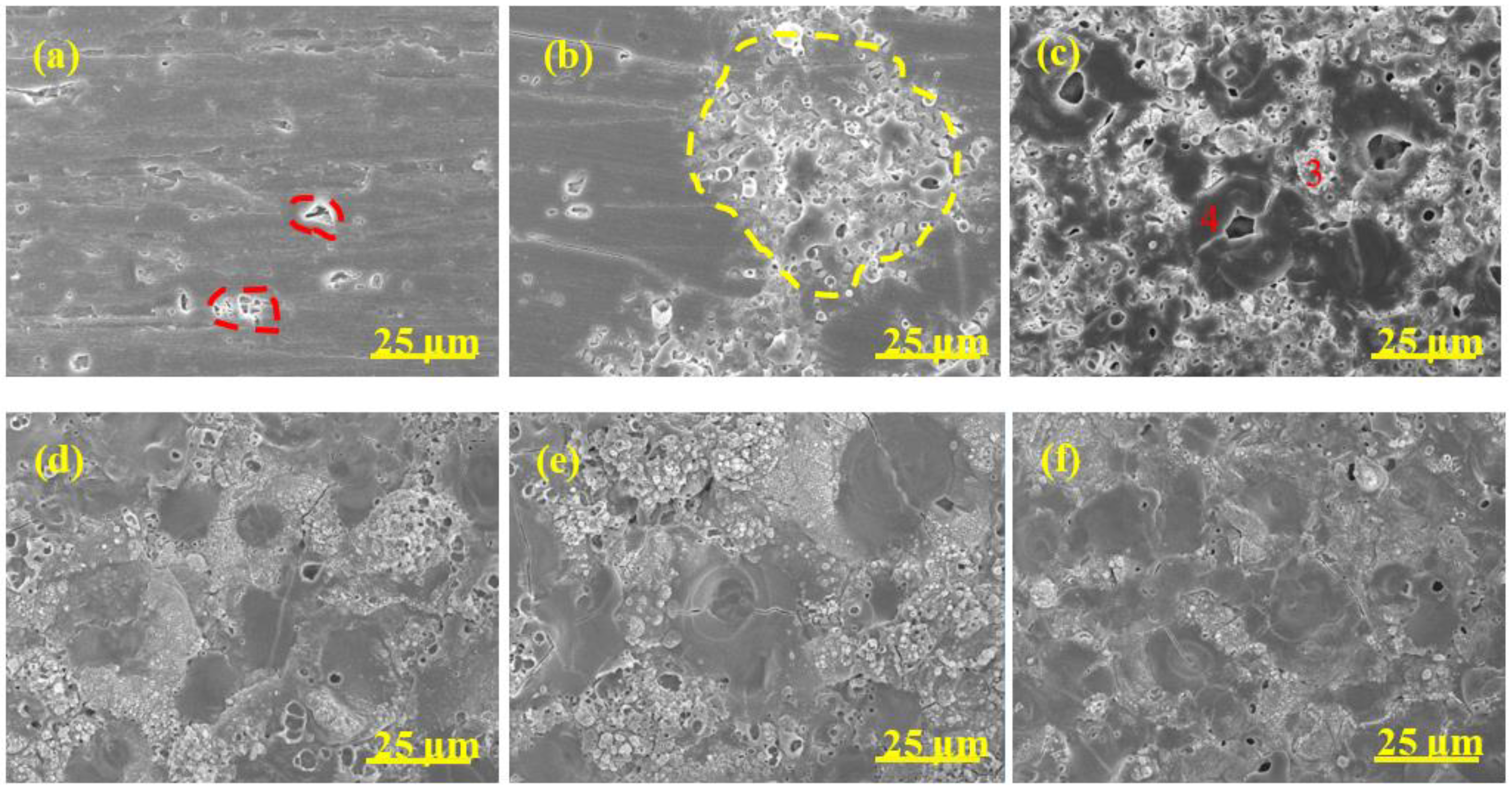
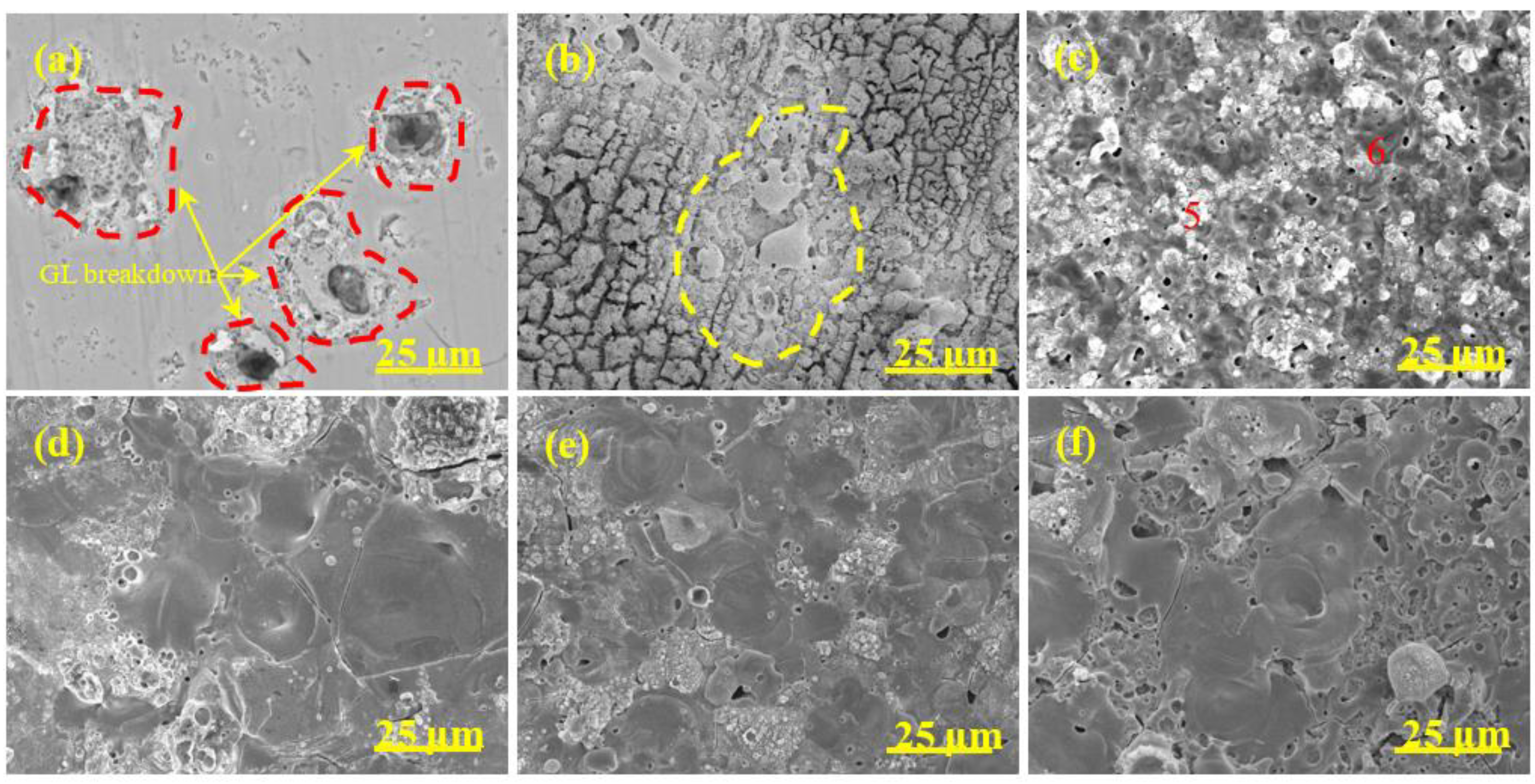

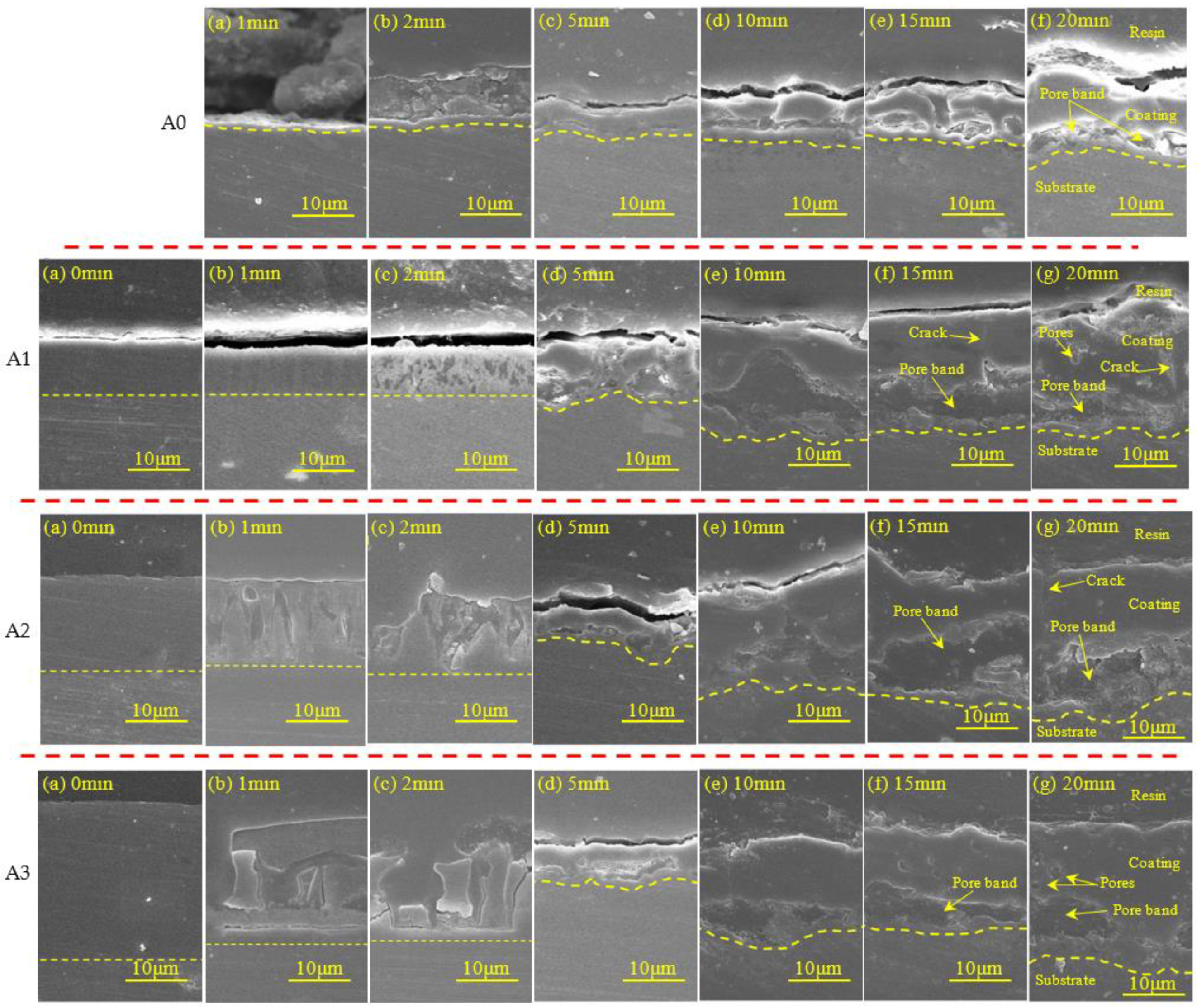


| Sample | 5 min | 10 min | 15 min | 20 min |
|---|---|---|---|---|
| A0 | 3.48 | 5.29 | 8.24 | 8.94 |
| A1 | 2.02 | 4.04 | 5.80 | 7.09 |
| A2 | 3.53 | 2.78 | 4.17 | 5.61 |
| A3 | 3.20 | 4.77 | 6.05 | 7.24 |
| Element at % | ||||
|---|---|---|---|---|
| Sample | Point | Al | O | Si |
| A0 | 1 | 36.32 | 44.85 | 18.83 |
| 2 | 64.56 | 30.26 | 5.18 | |
| A1 | 3 | 61.70 | 26.20 | 12.10 |
| 4 | 55.65 | 38.08 | 6.27 | |
| A2 | 5 | 18.23 | 49.24 | 32.53 |
| 6 | 61.46 | 32.74 | 5.80 | |
| A3 | 7 | 55.68 | 32.95 | 11.37 |
| 8 | 46.11 | 44.14 | 9.75 | |
| Element at % | ||||
|---|---|---|---|---|
| Coating | Time (min) | Al | O | Si |
| A0 | 5 | 53.94 | 38.07 | 7.99 |
| 10 | 49.64 | 39.85 | 10.51 | |
| 15 | 44.37 | 42.40 | 13.23 | |
| 20 | 41.90 | 40.07 | 18.03 | |
| A1 | 5 | 49.31 | 39.40 | 11.29 |
| 10 | 45.75 | 39.56 | 14.69 | |
| 15 | 41.95 | 39.62 | 18.43 | |
| 20 | 38.89 | 39.47 | 22.64 | |
| A2 | 5 | 52.46 | 38.33 | 9.21 |
| 10 | 43.88 | 38.16 | 17.96 | |
| 15 | 41.53 | 39.01 | 19.46 | |
| 20 | 40.61 | 38.13 | 21.26 | |
| A3 | 5 | 50.69 | 39.91 | 9.40 |
| 10 | 48.01 | 39.53 | 12.46 | |
| 15 | 44.78 | 39.79 | 15.43 | |
| 20 | 43.03 | 38.94 | 19.03 | |
| Sample | Ecorr (V) | Icorr (A·cm−2) | Rcorr (mm·a−1) | Rp (Ω·cm2) |
|---|---|---|---|---|
| A0 | –1.473 | 9.73 × 10−5 | 0.9535 | 185.08 |
| A1 | –1.403 | 2.61 × 10−5 | 0.2558 | 689.79 |
| A2 | −1.305 | 5.87 × 10−6 | 0.0576 | 3065.8 |
| A3 | –1.371 | 1.62 × 10−5 | 0.1587 | 1112.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Yang, E.; Yan, Z.; Xie, X.; Wei, W.; Li, W. Effect of Pre-Anodized Film on Micro-Arc Oxidation Process of 6063 Aluminum Alloy. Materials 2022, 15, 5221. https://doi.org/10.3390/ma15155221
Li L, Yang E, Yan Z, Xie X, Wei W, Li W. Effect of Pre-Anodized Film on Micro-Arc Oxidation Process of 6063 Aluminum Alloy. Materials. 2022; 15(15):5221. https://doi.org/10.3390/ma15155221
Chicago/Turabian StyleLi, Linwei, Erhui Yang, Zhibin Yan, Xiaomeng Xie, Wu Wei, and Weizhou Li. 2022. "Effect of Pre-Anodized Film on Micro-Arc Oxidation Process of 6063 Aluminum Alloy" Materials 15, no. 15: 5221. https://doi.org/10.3390/ma15155221
APA StyleLi, L., Yang, E., Yan, Z., Xie, X., Wei, W., & Li, W. (2022). Effect of Pre-Anodized Film on Micro-Arc Oxidation Process of 6063 Aluminum Alloy. Materials, 15(15), 5221. https://doi.org/10.3390/ma15155221






