Microstructural Constituents and Mechanical Properties of Low-Density Fe-Cr-Ni-Mn-Al-C Stainless Steels
Abstract
:1. Introduction
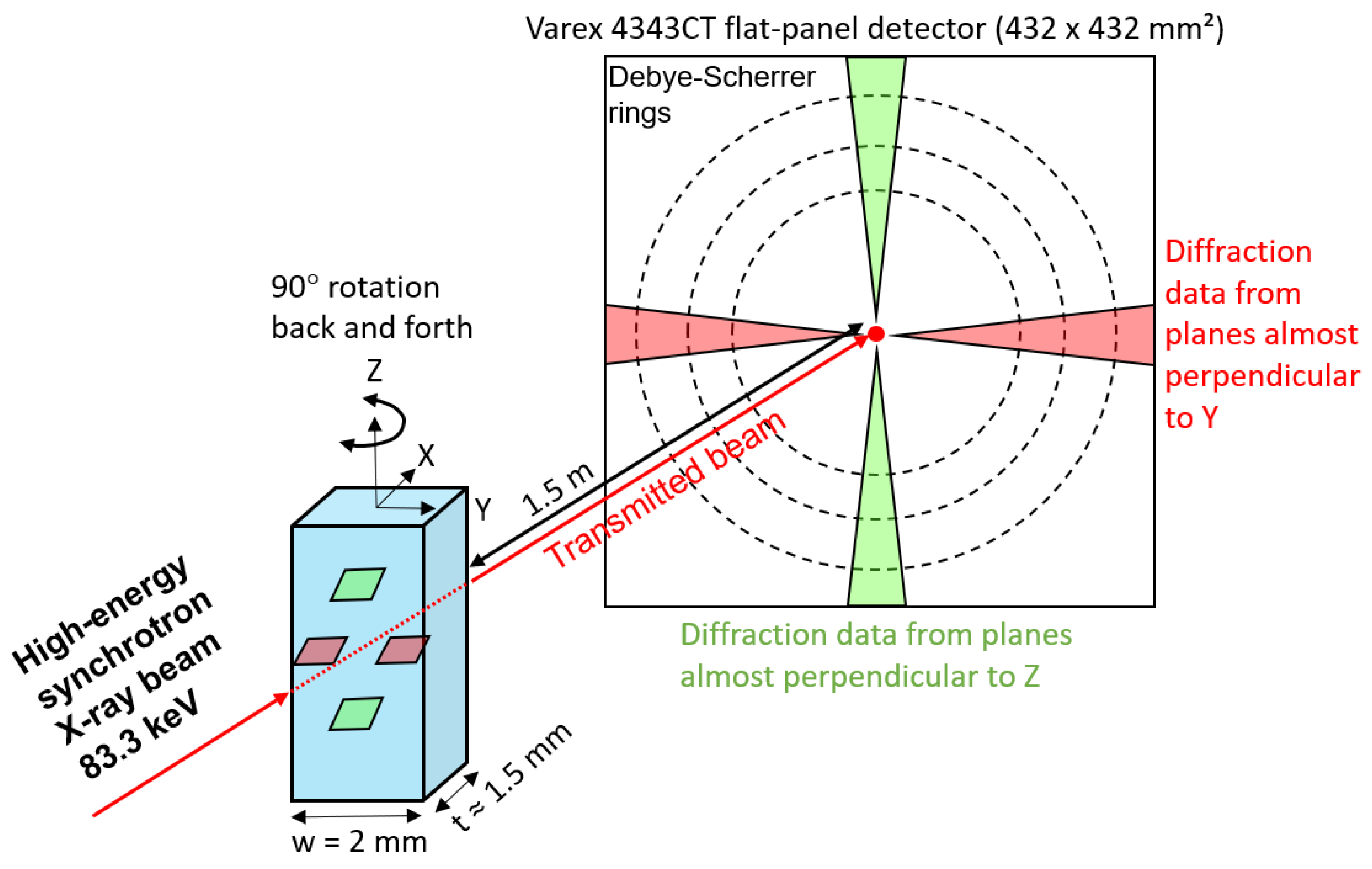
2. Materials and Experimental Procedure
3. Results and Discussion
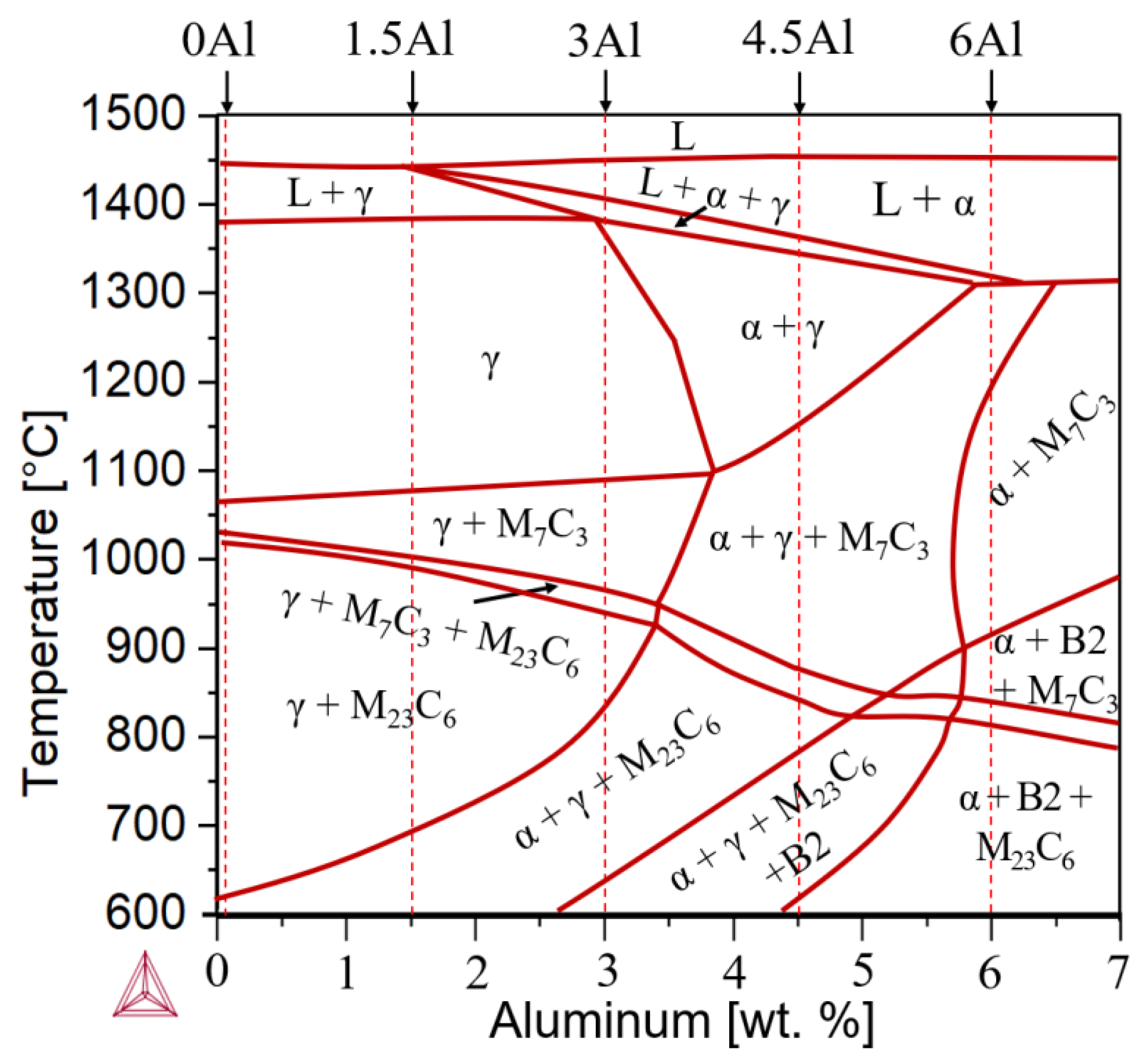
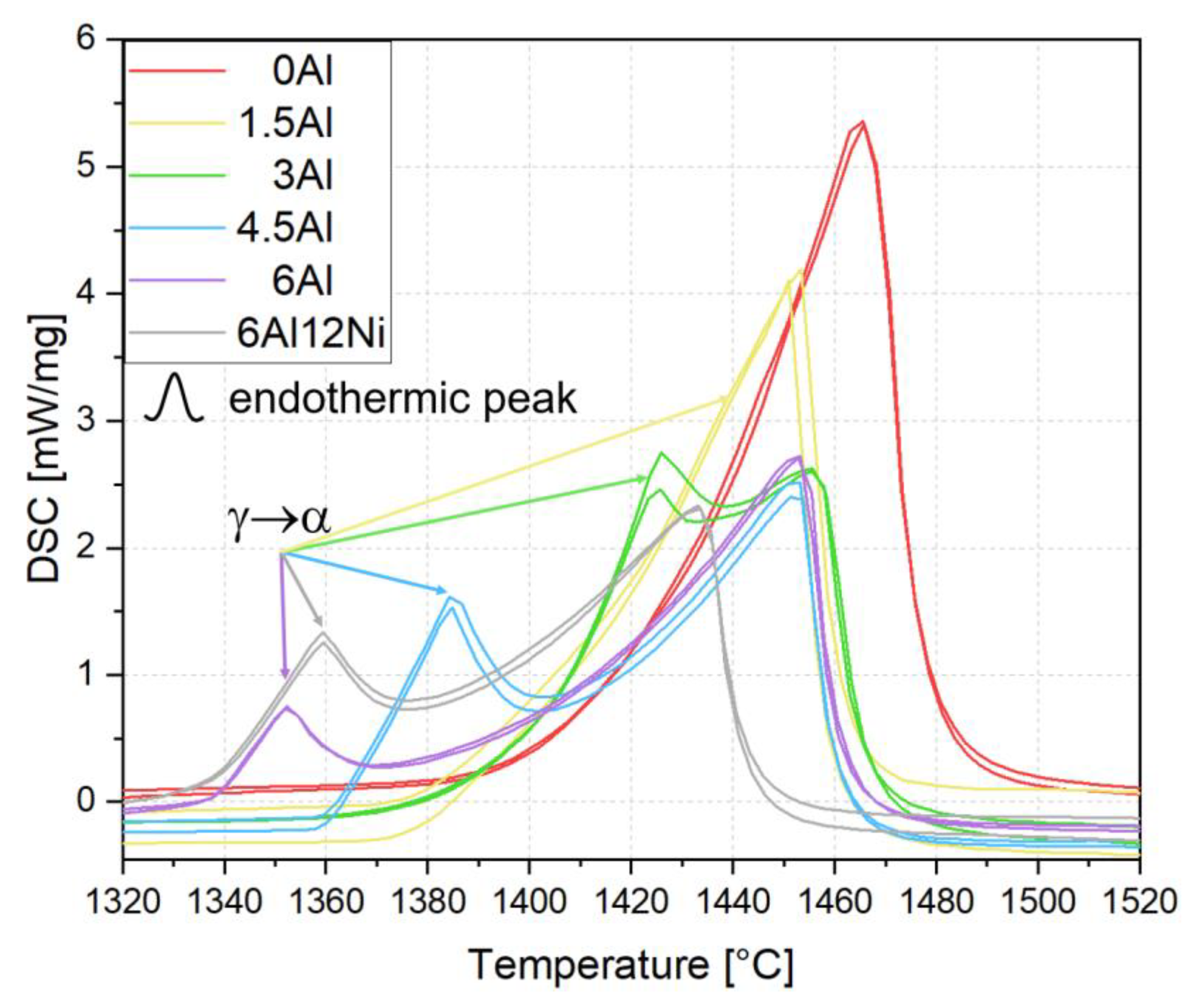
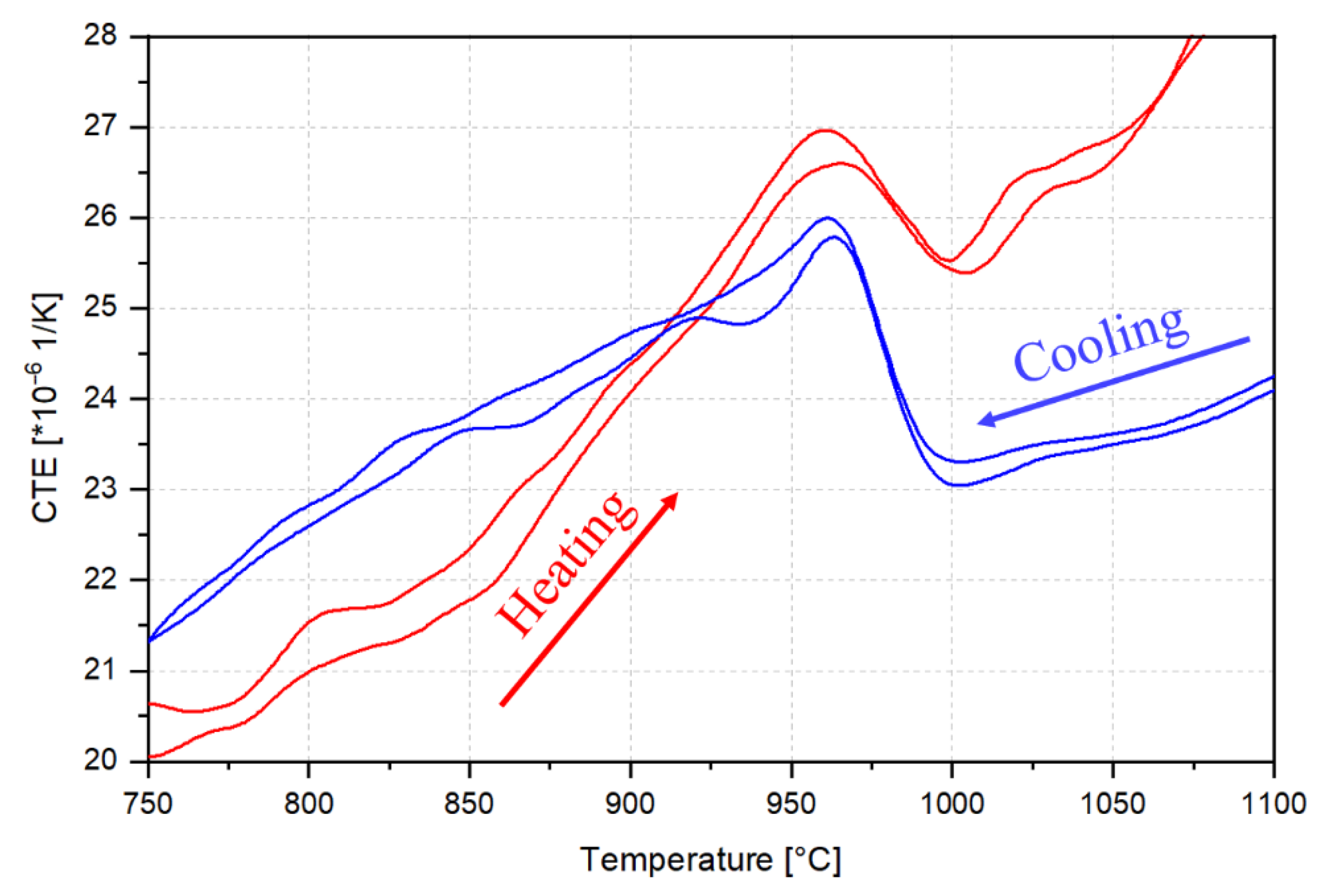
3.1. Thermodynamic Equilibrium Calculations and Solidification Mode
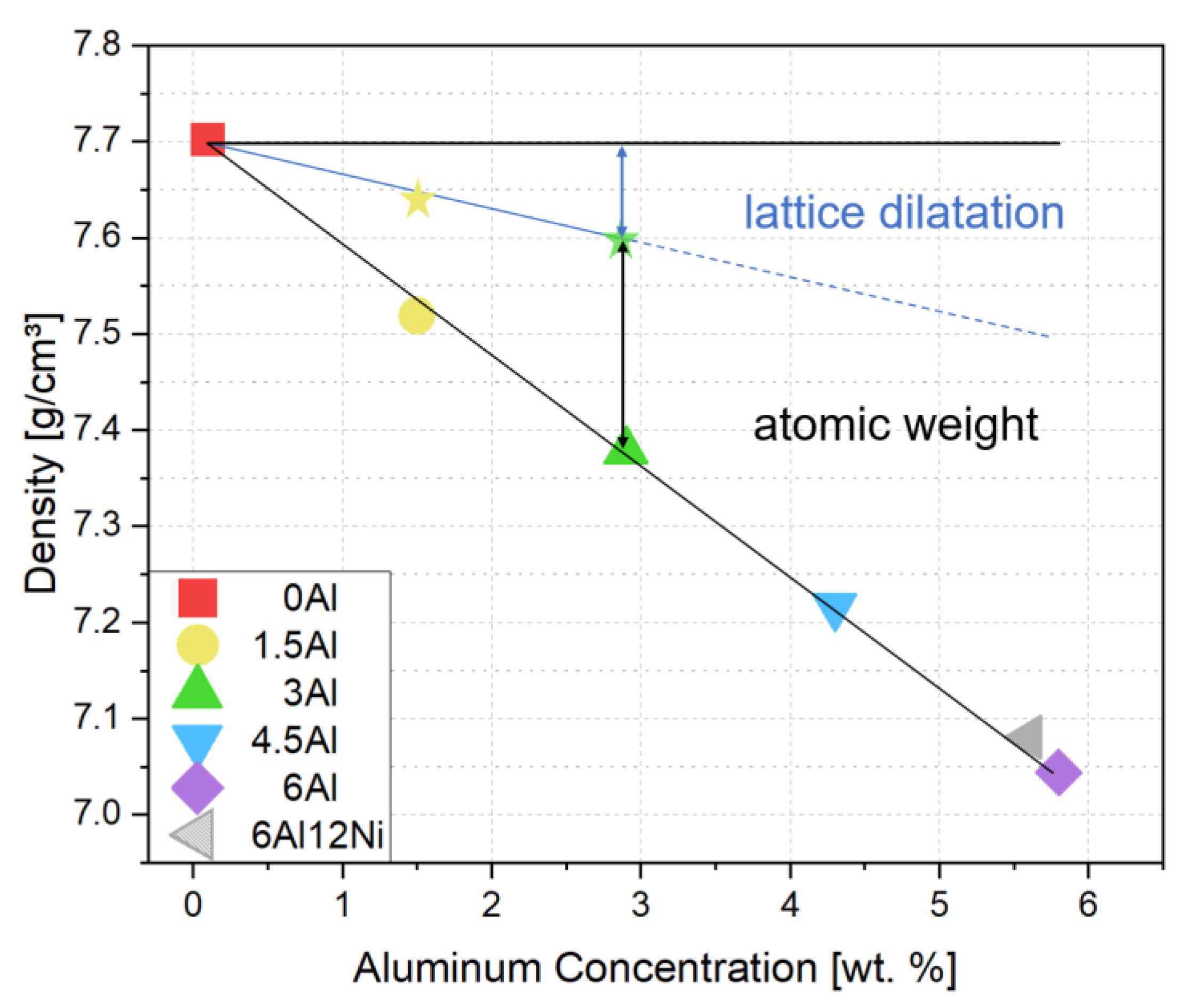
3.2. Contributions to Density Reduction
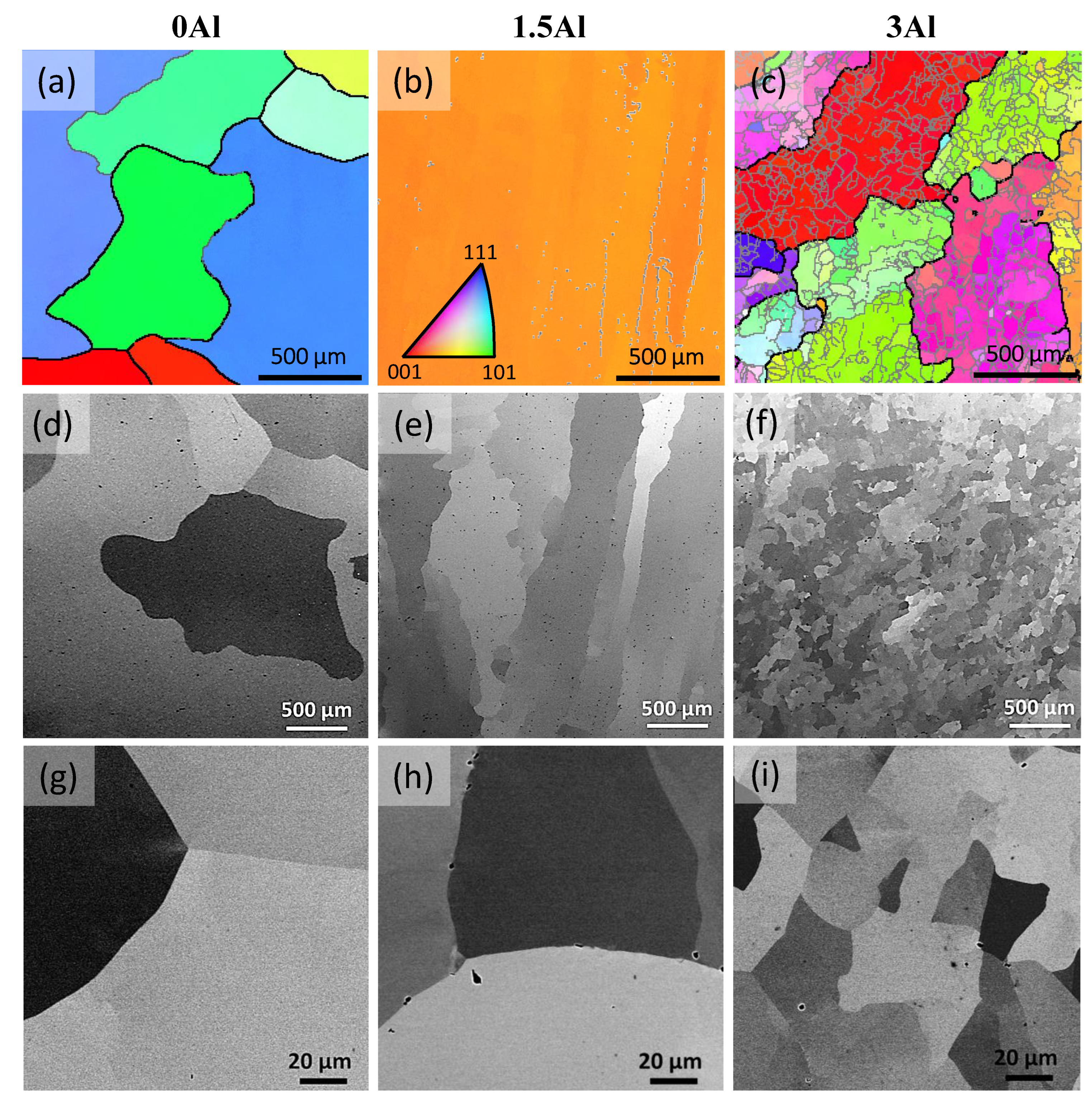
3.3. Microstructural Aspects
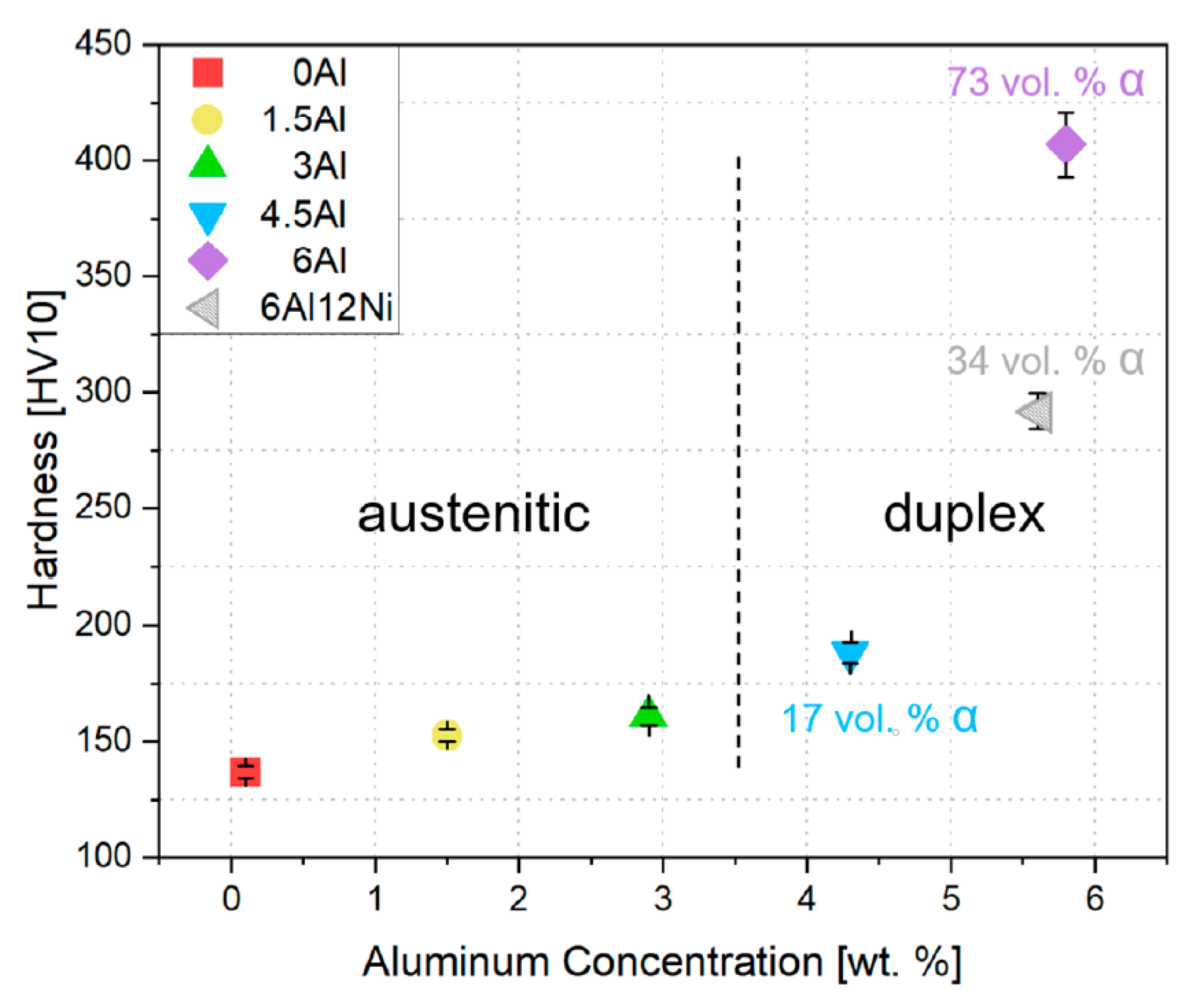
3.4. Hardness
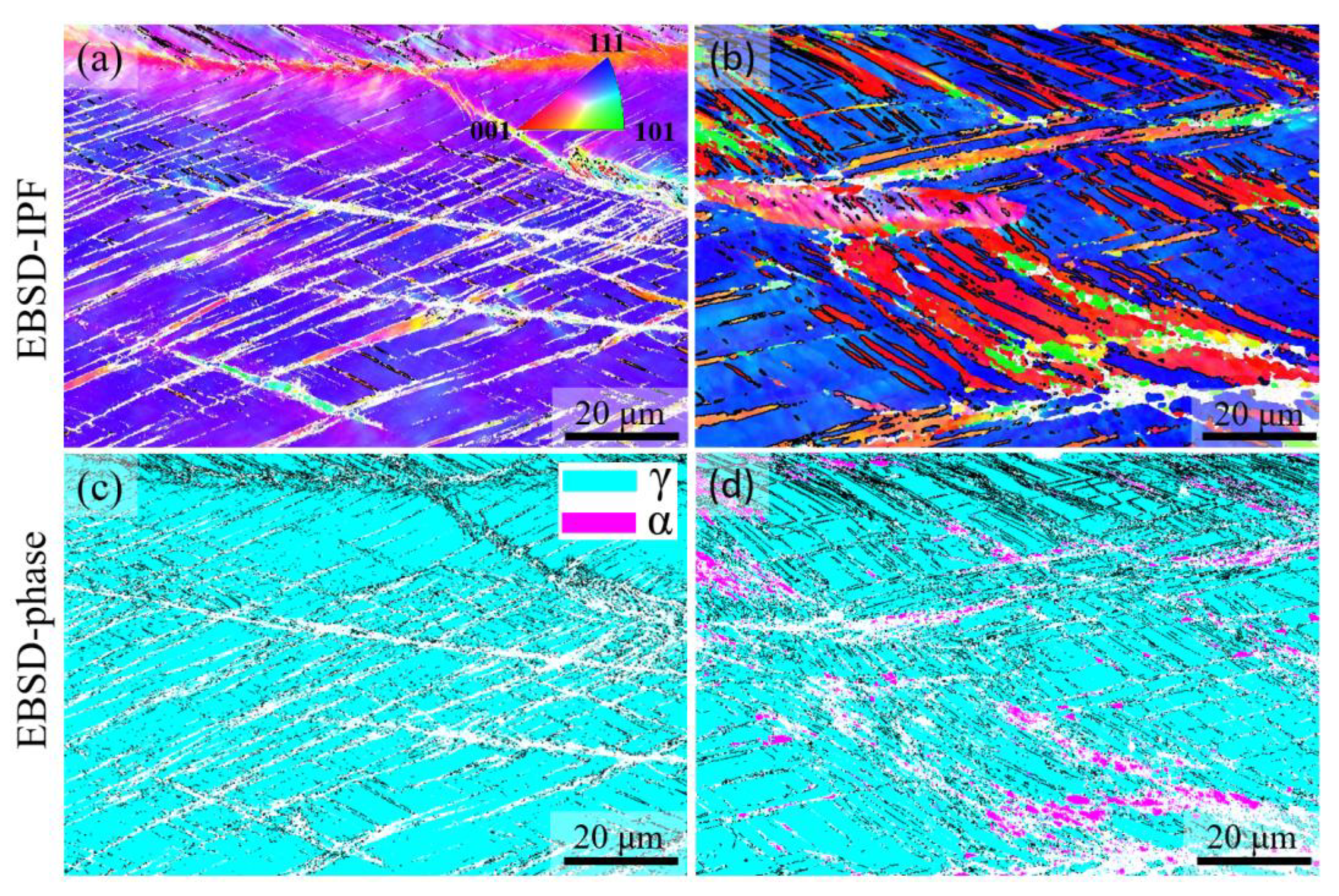
3.5. Tensile Properties, Work Hardening Behavior and Deformation-Induced Processes
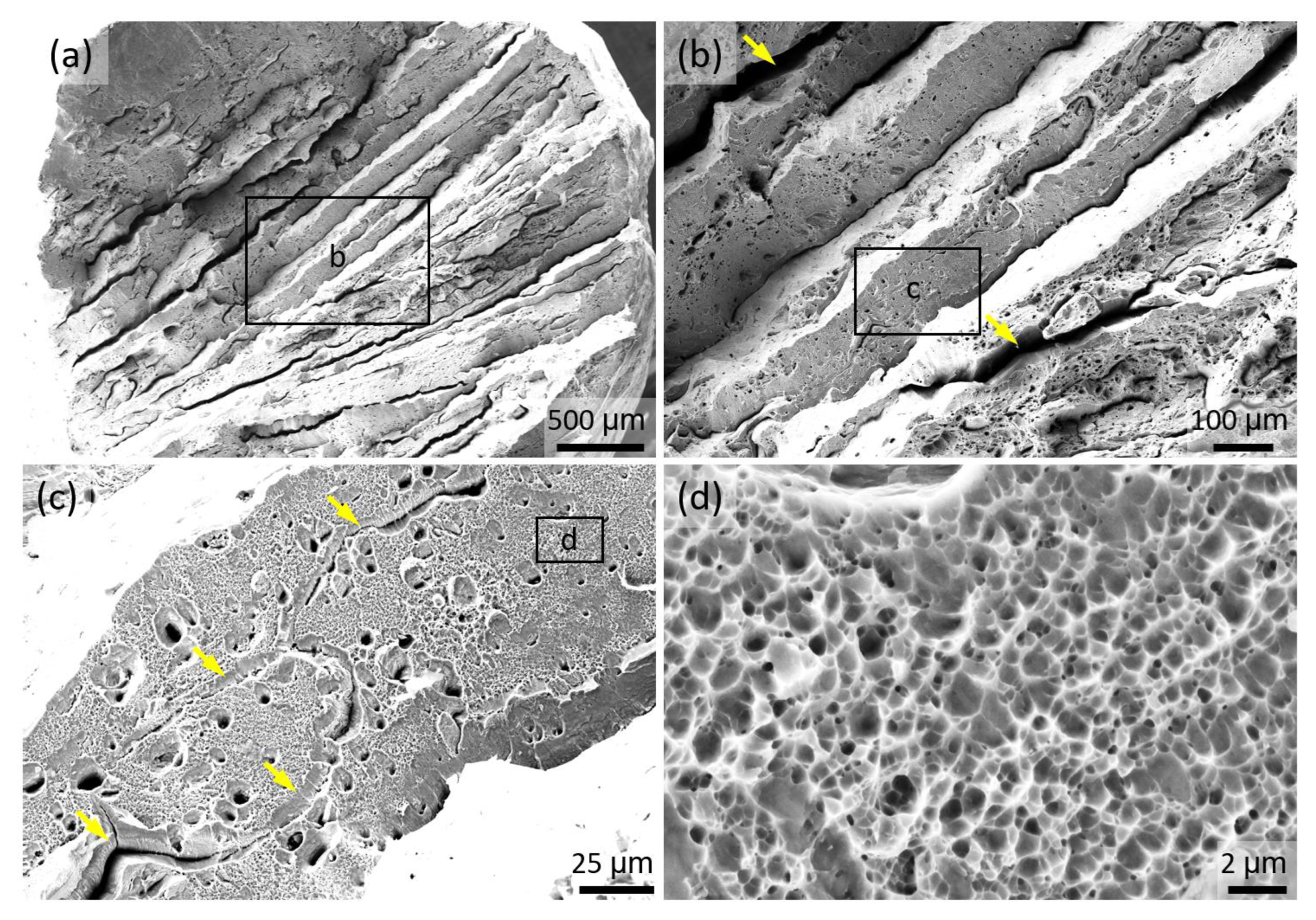
3.6. Fractography
4. Conclusions
- (1)
- Al addition changes the solidification mode from austenitic to ferritic-austenitic. After heat treatment at 1150 °C followed by quenching, the microstructure becomes almost fully austenitic for steels containing up to 3 wt.% Al and duplex for steels with higher Al contents. The fraction of ferrite in duplex steels increases with the Al content. In contrast to the primary austenite formed directly from the liquid phase, the austenite formed from primary ferrite contains low-angle boundaries.
- (2)
- Al addition decreases the density by approximately 1.45% per 1 wt.% Al. A share of nearly 31% arises from the lattice dilatation, the rest from the substitution of heavier atoms by Al.
- (3)
- Al addition only has a mild influence on the hardness of austenite. A dramatic increase in hardness is observed as the microstructure evolves to duplex. The noticeable hardening caused by the presence of ferrite is due to the formation of B2-(Ni,Fe)Al intermetallics in ferrite. The existence of B2 in ferrite was confirmed by SEM examinations and HE-XRD measurements.
- (4)
- Tensile tests indicate hardening without a noticeable change in ductility as the Al concentration is increased to 3 wt.%. The work hardening rate of the austenitic steel containing 3 wt.% Al is initially higher than that of its Al-free counterpart. At higher strains, however, the relative values of work hardening rates are reversed. The initially high work hardening rate of the 3Al steel is correlated with the existence of LAGBs. An additional likely mechanism is the occurrence of a small fraction of deformation-induced martensite in the less stable microstructural regions. For steels with duplex microstructures, the yield and ultimate tensile strengths are higher than the austenitic steels and correlate with the ferrite content. Strengthening occurred at the expense of ductility. Fracture surface examinations after tensile tests revealed dimple formation in austenite and cleavage fracture in ferrite, the latter being responsible for the ductility loss in the presence of high ferrite contents.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhadeshia, H.K.D.H.; Honeycombe, R.W.K. Steels: Microstructure and Properties, 4th ed.; Butterworth-Heinemann: Amsterdam, The Netherlands, 2017; ISBN 978-0-08-100270-4. [Google Scholar]
- Herrmann, J.; Inden, G.; Sauthoff, G. Deformation Behaviour of Iron-Rich Iron-Aluminum Alloys at Low Temperatures. Acta Mater. 2003, 51, 2847–2857. [Google Scholar] [CrossRef]
- Bleck, W.; Moeller, E. (Eds.) Handbuch Stahl: Auswahl, Verarbeitung, Anwendung; Hanser: München, Germany, 2018; ISBN 978-3-446-44962-6. [Google Scholar]
- Chen, G.; Rahimi, R.; Xu, G.; Biermann, H.; Mola, J. Impact of Al Addition on Deformation Behavior of Fe–Cr–Ni–Mn–C Austenitic Stainless Steel. Mater. Sci. Eng. A 2020, 797, 140084. [Google Scholar] [CrossRef]
- Simmons, J.W. Overview: High-Nitrogen Alloying of Stainless Steels. Mater. Sci. Eng. A 1996, 207, 159–169. [Google Scholar] [CrossRef]
- Baddoo, N.R. Stainless Steel in Construction: A Review of Research, Applications, Challenges and Opportunities. J. Constr. Steel Res. 2008, 64, 1199–1206. [Google Scholar] [CrossRef]
- Wang, P.; Kara, S.; Hauschild, M.Z. Role of Manufacturing towards Achieving Circular Economy: The Steel Case. CIRP Ann. 2018, 67, 21–24. [Google Scholar] [CrossRef]
- Holappa, L.; Kekkonen, M.; Jokilaakso, A.; Koskinen, J. A Review of Circular Economy Prospects for Stainless Steelmaking Slags. J. Sustain. Met. 2021, 7, 806–817. [Google Scholar] [CrossRef]
- Morris, D.G.; Muñoz-Morris, M.A. High Creep Strength, Dispersion-Strengthened Iron Aluminide Prepared by Multidirectional High-Strain Forging. Acta Mater. 2010, 58, 6080–6089. [Google Scholar] [CrossRef]
- Saxena, V.K.; Krishna, M.S.G.; Chhaunker, P.S.; Radhakrishnan, V.M. Fatigue and Fracture Behavior of a Nickel-Chromium Free Austenitic Steel. Int. J. Press. Vessel. Pip. 1994, 60, 151–157. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, L.; Xu, Y.; Li, J.; Xiao, X.; Jiang, L. Effect of Aluminum on Microstructure, Mechanical Properties and Pitting Corrosion Resistance of Ultra-Pure 429 Ferritic Stainless Steels. Mater. Des. 2015, 65, 682–689. [Google Scholar] [CrossRef]
- Peckner, D.; Bernstein, I.M. (Eds.) Handbook of Stainless Steels; McGraw-Hill: New York, NY, USA, 1977; ISBN 978-0-07-049147-2. [Google Scholar]
- Davis, J.R. (Ed.) Metals Handbook, 2nd ed.; ASM International: Materials Park, OH, USA, 1998; ISBN 978-0-87170-654-6. [Google Scholar]
- Rahimi, R.; De Cooman, B.C.; Biermann, H.; Mola, J. Microstructure and Mechanical Properties of Al-Alloyed Fe–Cr–Ni–Mn–C Stainless Steels. Mater. Sci. Eng. A 2014, 618, 46–55. [Google Scholar] [CrossRef]
- Frommeyer, G.; Brüx, U. Microstructures and Mechanical Properties of High-Strength Fe-Mn-Al-C Light-Weight TRIPLEX Steels. Steel Res. Int. 2006, 77, 627–633. [Google Scholar] [CrossRef]
- Alvarez-Armas, I.; Degallaix-Moreuil, S. (Eds.) Duplex Stainless Steels; Wiley: Hoboken, NJ, USA, 2009; ISBN 978-1-84821-137-7. [Google Scholar]
- Rahimi, R.; Pekker, P.; Biermann, H.; Volkova, O.; De Cooman, B.C.; Mola, J. Volumetric Changes Associated with B2-(Ni,Fe)Al Dissolution in an Al-Alloyed Ferritic Steel. Mater. Des. 2016, 111, 640–645. [Google Scholar] [CrossRef]
- Huang, S.; Ghosh, G.; Li, X.; Ilavsky, J.; Teng, Z.; Liaw, P.K. Effect of Al on the NiAl-Type B2 Precipitates in Ferritic Superalloys. Met. Mat Trans A 2012, 43, 3423–3427. [Google Scholar] [CrossRef]
- Song, G.; Sun, Z.; Poplawsky, J.D.; Gao, Y.; Liaw, P.K. Microstructural Evolution of Single Ni2TiAl or Hierarchical NiAl/Ni2TiAl Precipitates in Fe-Ni-Al-Cr-Ti Ferritic Alloys during Thermal Treatment for Elevated-Temperature Applications. Acta Mater. 2017, 127, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Song, G.; Sisneros, T.A.; Clausen, B.; Pu, C.; Li, L.; Gao, Y.; Liaw, P.K. Load Partitioning between the Bcc-Iron Matrix and NiAl-Type Precipitates in a Ferritic Alloy on Multiple Length Scales. Sci. Rep. 2016, 6, 23137. [Google Scholar] [CrossRef]
- Song, G.; Sun, Z.; Poplawsky, J.D.; Xu, X.; Chen, M.; Liaw, P.K. Primary and Secondary Precipitates in a Hierarchical-Precipitate-Strengthened Ferritic Alloy. J. Alloy. Compd. 2017, 706, 584–588. [Google Scholar] [CrossRef] [Green Version]
- Harwarth, M.; Chen, G.; Rahimi, R.; Biermann, H.; Zargaran, A.; Duffy, M.; Zupan, M.; Mola, J. Aluminum-Alloyed Lightweight Stainless Steels Strengthened by B2-(Ni,Fe)Al Precipitates. Mater. Des. 2021, 206, 109813. [Google Scholar] [CrossRef]
- Abbasi, M.; Kheirandish, S.; Kharrazi, Y.; Hejazi, J. On the Comparison of the Abrasive Wear Behavior of Aluminum Alloyed and Standard Hadfield Steels. Wear 2010, 268, 202–207. [Google Scholar] [CrossRef]
- Zuidema, B.K.; Subramanyam, D.K.; Leslie, W.C. The Effect of Aluminum on the Work Hardening and Wear Resistance of Hadfield Manganese Steel. Met. Mater. Trans. A 1987, 18, 1629–1639. [Google Scholar] [CrossRef]
- Martin, M.; Weber, S.; Theisen, W.; Michler, T.; Naumann, J. Development of a Stable High-Aluminum Austenitic Stainless Steel for Hydrogen Applications. Int. J. Hydrog. Energy 2013, 38, 5989–6001. [Google Scholar] [CrossRef]
- Saeed-Akbari, A.; Imlau, J.; Prahl, U.; Bleck, W. Derivation and Variation in Composition-Dependent Stacking Fault Energy Maps Based on Subregular Solution Model in High-Manganese Steels. Met. Mater. Trans. A 2009, 40, 3076–3090. [Google Scholar] [CrossRef]
- Wittig, J.E. Temperature Dependent Deformation Mechanisms of a High Nitrogen-Manganese Austenitic Stainless Steel. Steel Res. Int. 2009, 80, 67–70. [Google Scholar] [CrossRef]
- Meric de Bellefon, G.; Gussev, M.N.; Stoica, A.D.; van Duysen, J.C.; Sridharan, K. Examining the Influence of Stacking Fault Width on Deformation Twinning in an Austenitic Stainless Steel. Scr. Mater. 2018, 157, 162–166. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kim, H.; Kim, N.J. Brittle Intermetallic Compound Makes Ultrastrong Low-Density Steel with Large Ductility. Nature 2015, 518, 77–79. [Google Scholar] [CrossRef]
- Tian, X.; Li, H.; Zhang, Y. Effect of Al Content on Stacking Fault Energy in Austenitic Fe–Mn–Al–C Alloys. J. Mater. Sci. 2008, 43, 6214–6222. [Google Scholar] [CrossRef]
- Mahato, B.; Shee, S.K.; Sahu, T.; Ghosh Chowdhury, S.; Sahu, P.; Porter, D.A.; Karjalainen, L.P. An Effective Stacking Fault Energy Viewpoint on the Formation of Extended Defects and Their Contribution to Strain Hardening in a Fe–Mn–Si–Al Twinning-Induced Plasticity Steel. Acta Mater. 2015, 86, 69–79. [Google Scholar] [CrossRef]
- Rahimi, R.; Biermann, H.; Volkova, O.; Mola, J. On the Origin of Subgrain Boundaries during Conventional Solidification of Austenitic Stainless Steels. IOP Conf. Ser. Mater. Sci. Eng. 2018, 373, 012005. [Google Scholar] [CrossRef]
- Ennis, B.L.; Jimenez-Melero, E.; Mostert, R.; Santillana, B.; Lee, P.D. The Role of Aluminium in Chemical and Phase Segregation in a TRIP-Assisted Dual Phase Steel. Acta Mater. 2016, 115, 132–142. [Google Scholar] [CrossRef]
- Krauss, G. Solidification, Segregation, and Banding in Carbon and Alloy Steels. Met. Mater. Trans B 2003, 34, 781–792. [Google Scholar] [CrossRef]
- Suutala, N. Effect of Solidification Conditions on the Solidification Mode in Austenitic Stainless Steels. Met. Mater. Trans. A 1983, 14, 191–197. [Google Scholar] [CrossRef]
- Suutala, N. Effect of Manganese and Nitrogen on the Solidification Mode in Austenitic Stainless Steel Welds. Met. Mater. Trans. A 1982, 13, 2121–2130. [Google Scholar] [CrossRef]
- Chen, G.; Rahimi, R.; Harwarth, M.; Motylenko, M.; Xu, G.; Biermann, H.; Mola, J. Non-Cube-on-Cube Orientation Relationship between M23C6 and Austenite in an Austenitic Stainless Steel. Scr. Mater. 2022, 213, 114597. [Google Scholar] [CrossRef]
- Rahimi, R.; Volkova, O.; Biermann, H.; Mola, J. Thermal Analysis of the Formation and Dissolution of Cr-Rich Carbides in Al-Alloyed Stainless Steels. Adv. Eng. Mater. 2019, 21, 1800658. [Google Scholar] [CrossRef]
- Halliday, D.; Resnick, R.; Walker, J. Fundamentals of Physics, 6th ed.; Wiley: New York, NY, USA, 2001; ISBN 978-0-471-33236-7. [Google Scholar]
- DIN EN ISO 6507-1:2018-07; Metallische Werkstoffe_-Härteprüfung Nach Vickers_-Teil_1: Prüfverfahren (ISO_6507-1:2018). Deutsche Fassung EN_ISO_6507-1:2018; Beuth Verlag GmbH: Berlin, Germany, 2018.
- DIN 50125:2016-12; Prüfung Metallischer Werkstoffe_-Zugproben. Beuth Verlag GmbH: Berlin, Germany, 2016.
- Dastur, P.; Zarei-Hanzaki, A.; Rahimi, R.; Moallemi, M.; Klemm, V.; De Cooman, B.C.; Mola, J. Martensite Reversion Duality Behavior in a Cold-Rolled High Mn Transformation-Induced Plasticity Steel. Met. Mater. Trans. A 2019, 50, 4550–4560. [Google Scholar] [CrossRef]
- Kieffer, J.; Karkoulis, D. PyFAI, a Versatile Library for Azimuthal Regrouping. J. Phys. Conf. Ser. 2013, 425, 202012. [Google Scholar] [CrossRef]
- Ashiotis, G.; Deschildre, A.; Nawaz, Z.; Wright, J.P.; Karkoulis, D.; Picca, F.E.; Kieffer, J. The Fast Azimuthal Integration Python Library: PyFAI. J. Appl. Cryst. 2015, 48, 510–519. [Google Scholar] [CrossRef]
- Frommeyer, G.; Drewes, E.J.; Engl, B. Physical and mechanical properties of iron-aluminium-(Mn, Si) lightweight steels. Rev. Met. Paris 2000, 97, 1245–1253. [Google Scholar] [CrossRef]
- Zuazo, I.; Hallstedt, B.; Lindahl, B.; Selleby, M.; Soler, M.; Etienne, A.; Perlade, A.; Hasenpouth, D.; Massardier-Jourdan, V.; Cazottes, S.; et al. Low-Density Steels: Complex Metallurgy for Automotive Applications. JOM 2014, 66, 1747–1758. [Google Scholar] [CrossRef]
- Rahimi, R.; Ullrich, C.; Klemm, V.; Rafaja, D.; De Cooman, B.C.; Biermann, H.; Mola, J. Influence of Al on the Temperature Dependence of Strain Hardening Behavior and Glide Planarity in Fe–Cr–Ni–Mn–C Austenitic Stainless Steels. Mater. Sci. Eng. A 2016, 649, 301–312. [Google Scholar] [CrossRef]
- Lehnhoff, G.R.; Findley, K.O.; De Cooman, B.C. The Influence of Silicon and Aluminum Alloying on the Lattice Parameter and Stacking Fault Energy of Austenitic Steel. Scr. Mater. 2014, 92, 19–22. [Google Scholar] [CrossRef] [Green Version]
- Mola, J. Considerations in the Design of Formable Austenitic Stainless Steels Based on Deformation-Induced Processes. In Austenitic Stainless Steels—New Aspects; Borek, W., Tanski, T., Brytan, Z., Eds.; InTech: London, UK, 2017; ISBN 978-953-51-3701-6. [Google Scholar]
- Sun, Z.; Liebscher, C.H.; Huang, S.; Teng, Z.; Song, G.; Wang, G.; Asta, M.; Rawlings, M.; Fine, M.E.; Liaw, P.K. New Design Aspects of Creep-Resistant NiAl-Strengthened Ferritic Alloys. Scr. Mater. 2013, 68, 384–388. [Google Scholar] [CrossRef]
- Zhi, X.; Xing, J.; Gao, Y.; Fu, H.; Peng, J.; Xiao, B. Effect of Heat Treatment on Microstructure and Mechanical Properties of a Ti-Bearing Hypereutectic High Chromium White Cast Iron. Mater. Sci. Eng. A 2008, 487, 171–179. [Google Scholar] [CrossRef]
- Carpenter, S.D.; Carpenter, D. X-Ray Diffraction Study of M7C3 Carbide within a High Chromium White Iron. Mater. Lett. 2003, 57, 4456–4459. [Google Scholar] [CrossRef]
- Eshed, E.; Choudhuri, D.; Osovski, S. M7C3: The Story of a Misunderstood Carbide. Acta Mater. 2022, 235, 117985. [Google Scholar] [CrossRef]
- Stallybrass, C.; Schneider, A.; Sauthoff, G. The Strengthening Effect of (Ni,Fe)Al Precipitates on the Mechanical Properties at High Temperatures of Ferritic Fe–Al–Ni–Cr Alloys. Intermetallics 2005, 13, 1263–1268. [Google Scholar] [CrossRef]
- Guo, Z.; Sha, W.; Vaumousse, D. Microstructural Evolution in a PH13-8 Stainless Steel after Ageing. Acta Mater. 2003, 51, 101–116. [Google Scholar] [CrossRef]
- Habibi Bajguirani, H.R. The Effect of Ageing upon the Microstructure and Mechanical Properties of Type 15-5 PH Stainless Steel. Mater. Sci. Eng. A 2002, 338, 142–159. [Google Scholar] [CrossRef]
- Hsiao, C.N.; Chiou, C.S.; Yang, J.R. Aging Reactions in a 17-4 PH Stainless Steel. Mater. Chem. Phys. 2002, 74, 134–142. [Google Scholar] [CrossRef]
- Govindaraj, V.; Hodgson, P.; P Singh, R.; Beladi, H. Precipitation Reactions in 12Cr–3Ni–3Mn–3Cu–0.15Nb Steel. Mater. Sci. Eng. A 2021, 808, 140909. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, P.; Li, D.; Li, Y. Multi-Scale Study on the Heterogeneous Deformation Behavior in Duplex Stainless Steel. J. Mater. Sci. Technol. 2021, 72, 180–188. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Wang, J.J.; Tao, N.R. Tensile Ductility and Deformation Mechanisms of a Nanotwinned 316L Austenitic Stainless Steel. J. Mater. Sci. Technol. 2020, 36, 65–69. [Google Scholar] [CrossRef]
- Bei, H.; Yamamoto, Y.; Brady, M.P.; Santella, M.L. Aging Effects on the Mechanical Properties of Alumina-Forming Austenitic Stainless Steels. Mater. Sci. Eng. A 2010, 527, 2079–2086. [Google Scholar] [CrossRef]
- Kim, J.-K.; De Cooman, B.C. Stacking Fault Energy and Deformation Mechanisms in Fe-XMn-0.6C-YAl TWIP Steel. Mater. Sci. Eng. A 2016, 676, 216–231. [Google Scholar] [CrossRef]
- Hung, C.-Y.; Bai, Y.; Tsuji, N.; Murayama, M. Grain Size Altering Yielding Mechanisms in Ultrafine Grained High-Mn Austenitic Steel: Advanced TEM Investigations. J. Mater. Sci. Technol. 2021, 86, 192–203. [Google Scholar] [CrossRef]
- Jeong, J.S.; Woo, W.; Oh, K.H.; Kwon, S.K.; Koo, Y.M. In Situ Neutron Diffraction Study of the Microstructure and Tensile Deformation Behavior in Al-Added High Manganese Austenitic Steels. Acta Mater. 2012, 60, 2290–2299. [Google Scholar] [CrossRef]
- Dai, Q.-X.; Wang, A.-D.; Cheng, X.-N.; Luo, X.-M. Stacking Fault Energy of Cryogenic Austenitic Steels. Chin. Phys. 2002, 11, 596–600. [Google Scholar] [CrossRef]
- Abbasi, M.; Kheirandish, S.; Kharrazi, Y.; Hejazi, J. The Fracture and Plastic Deformation of Aluminum Alloyed Hadfield Steels. Mater. Sci. Eng. A 2009, 513–514, 72–76. [Google Scholar] [CrossRef]
- Meng, L.; Yang, P.; Xie, Q.; Ding, H.; Tang, Z. Dependence of Deformation Twinning on Grain Orientation in Compressed High Manganese Steels. Scr. Mater. 2007, 56, 931–934. [Google Scholar] [CrossRef]
- Choi, W.S.; De Cooman, B.C.; Sandlöbes, S.; Raabe, D. Size and Orientation Effects in Partial Dislocation-Mediated Deformation of Twinning-Induced Plasticity Steel Micro-Pillars. Acta Mater. 2015, 98, 391–404. [Google Scholar] [CrossRef]
- Seo, E.J.; Kim, J.K.; Cho, L.; Mola, J.; Oh, C.Y.; De Cooman, B.C. Micro-Plasticity of Medium Mn Austenitic Steel: Perfect Dislocation Plasticity and Deformation Twinning. Acta Mater. 2017, 135, 112–123. [Google Scholar] [CrossRef]
- De Cooman, B.C.; Estrin, Y.; Kim, S.K. Twinning-Induced Plasticity (TWIP) Steels. Acta Mater. 2018, 142, 283–362. [Google Scholar] [CrossRef]
- Frommeyer, G.; Brüx, U.; Neumann, P. Supra-Ductile and High-Strength Manganese-TRIP/TWIP Steels for High Energy Absorption Purposes. ISIJ Int. 2003, 43, 438–446. [Google Scholar] [CrossRef] [Green Version]
- Mola, J.; Wendler, M.; Weiß, A.; Reichel, B.; Wolf, G.; De Cooman, B.C. Segregation-Induced Enhancement of Low-Temperature Tensile Ductility in a Cast High-Nitrogen Austenitic Stainless Steel Exhibiting Deformation-Induced A′ Martensite Formation. Met. Mat Trans A 2015, 46, 1450–1454. [Google Scholar] [CrossRef] [Green Version]
- Tyson, W.R.; Ayres, R.A.; Stein, D.F. Anisotropy of Cleavage in B.C.C. Transition Metals. Acta Met. 1973, 21, 621–627. [Google Scholar] [CrossRef]
- Morris, J.W.; Kinney, C.; Pytlewski, K.; Adachi, Y. Microstructure and Cleavage in Lath Martensitic Steels. Sci. Technol. Adv. Mater. 2013, 14, 014208. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, R.D. Effect of Thermomechanical Treatment on the Properties of Fe-11Al and Fe-14Al Alloys. J. Mater. Sci. 2002, 37, 1823–1830. [Google Scholar] [CrossRef]




| Alloy ID | Color | C | Al | Cr | Ni | Mn | Fe |
|---|---|---|---|---|---|---|---|
| 0Al |  | 0.30 | 0.1 | 12.2 | 9.2 | 3.0 | bal. |
| 1.5Al |  | 0.35 | 1.5 | 12.5 | 9.3 | 2.9 | bal. |
| 3Al |  | 0.30 | 2.9 | 11.8 | 8.8 | 3.1 | bal. |
| 4.5Al |  | 0.31 | 4.3 | 12.1 | 9.0 | 3.0 | bal. |
| 6Al |  | 0.32 | 5.8 | 12.4 | 9.2 | 3.0 | bal. |
| 6Al12Ni |  | 0.35 | 5.6 | 11.5 | 12.0 | 3.4 | bal. |
| Assessment Method | 0Al | 1.5Al | 3Al | 4.5Al | 6Al |
|---|---|---|---|---|---|
| Thermo-Calc | A | FA | FA | FA | FA |
| DSC | A | FA | FA | FA | FA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scherbring, S.; Chen, G.; Veltel, B.; Bartzsch, G.; Richter, J.; Vollmer, M.; Blankenburg, M.; Shyamal, S.; Volkova, O.; Niendorf, T.; et al. Microstructural Constituents and Mechanical Properties of Low-Density Fe-Cr-Ni-Mn-Al-C Stainless Steels. Materials 2022, 15, 5121. https://doi.org/10.3390/ma15155121
Scherbring S, Chen G, Veltel B, Bartzsch G, Richter J, Vollmer M, Blankenburg M, Shyamal S, Volkova O, Niendorf T, et al. Microstructural Constituents and Mechanical Properties of Low-Density Fe-Cr-Ni-Mn-Al-C Stainless Steels. Materials. 2022; 15(15):5121. https://doi.org/10.3390/ma15155121
Chicago/Turabian StyleScherbring, Steffen, Guanghui Chen, Bastian Veltel, Gert Bartzsch, Julia Richter, Malte Vollmer, Malte Blankenburg, Saikat Shyamal, Olena Volkova, Thomas Niendorf, and et al. 2022. "Microstructural Constituents and Mechanical Properties of Low-Density Fe-Cr-Ni-Mn-Al-C Stainless Steels" Materials 15, no. 15: 5121. https://doi.org/10.3390/ma15155121
APA StyleScherbring, S., Chen, G., Veltel, B., Bartzsch, G., Richter, J., Vollmer, M., Blankenburg, M., Shyamal, S., Volkova, O., Niendorf, T., Lienert, U., Sahu, P., & Mola, J. (2022). Microstructural Constituents and Mechanical Properties of Low-Density Fe-Cr-Ni-Mn-Al-C Stainless Steels. Materials, 15(15), 5121. https://doi.org/10.3390/ma15155121










