Adsorption of Naphthalene on Clay Minerals: A Molecular Dynamics Simulation Study
Abstract
:1. Introduction
2. Theoretical Models and Methods
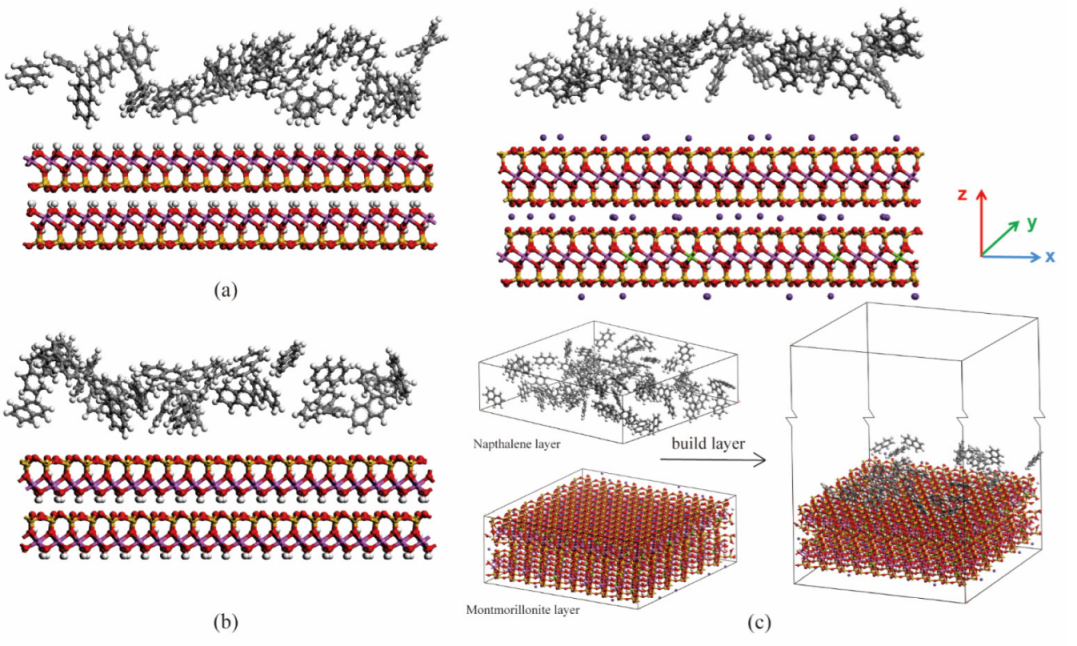
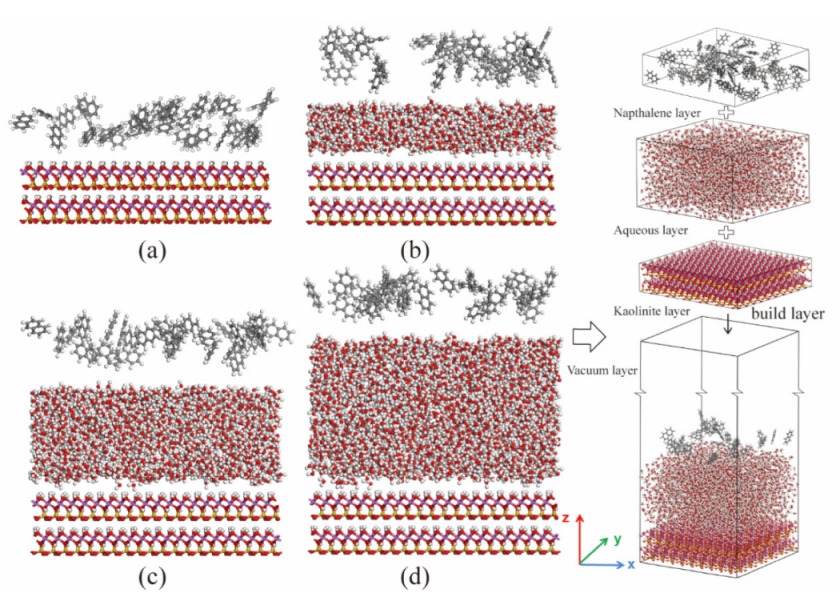
2.1. Model Construction
2.2. Molecular Dynamics Simulation
2.3. Data Analysis
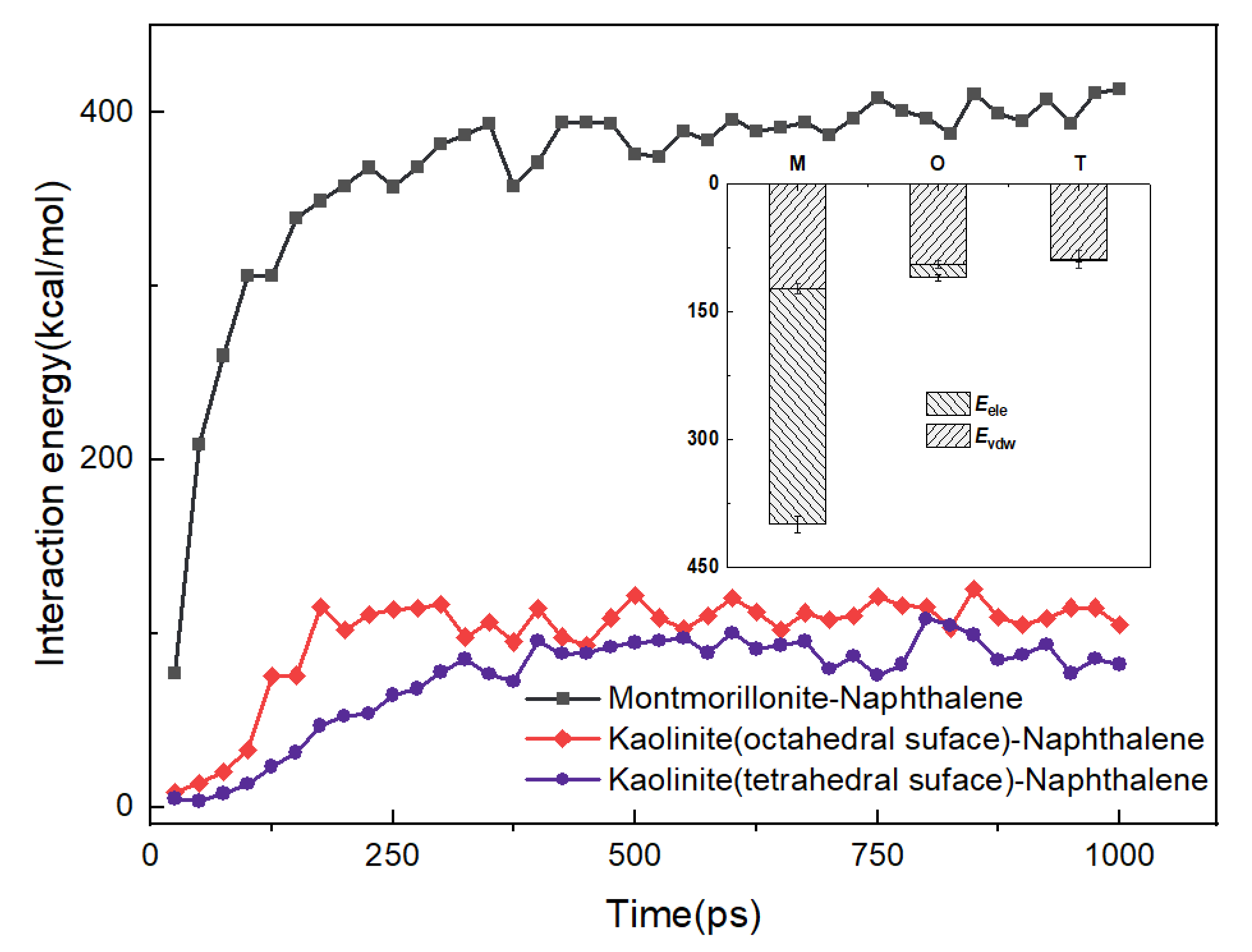
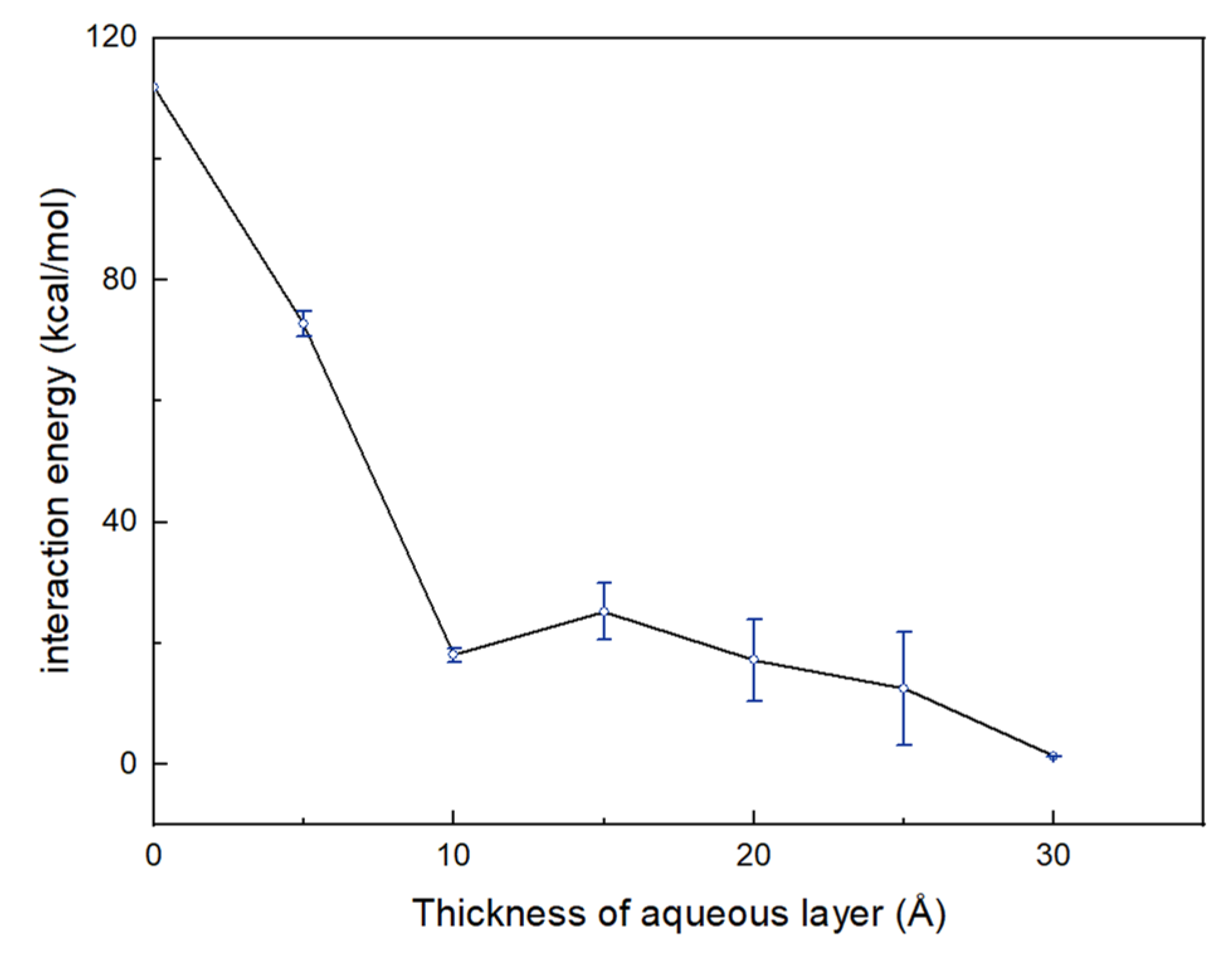
2.3.1. Interaction Energy between Different Layers
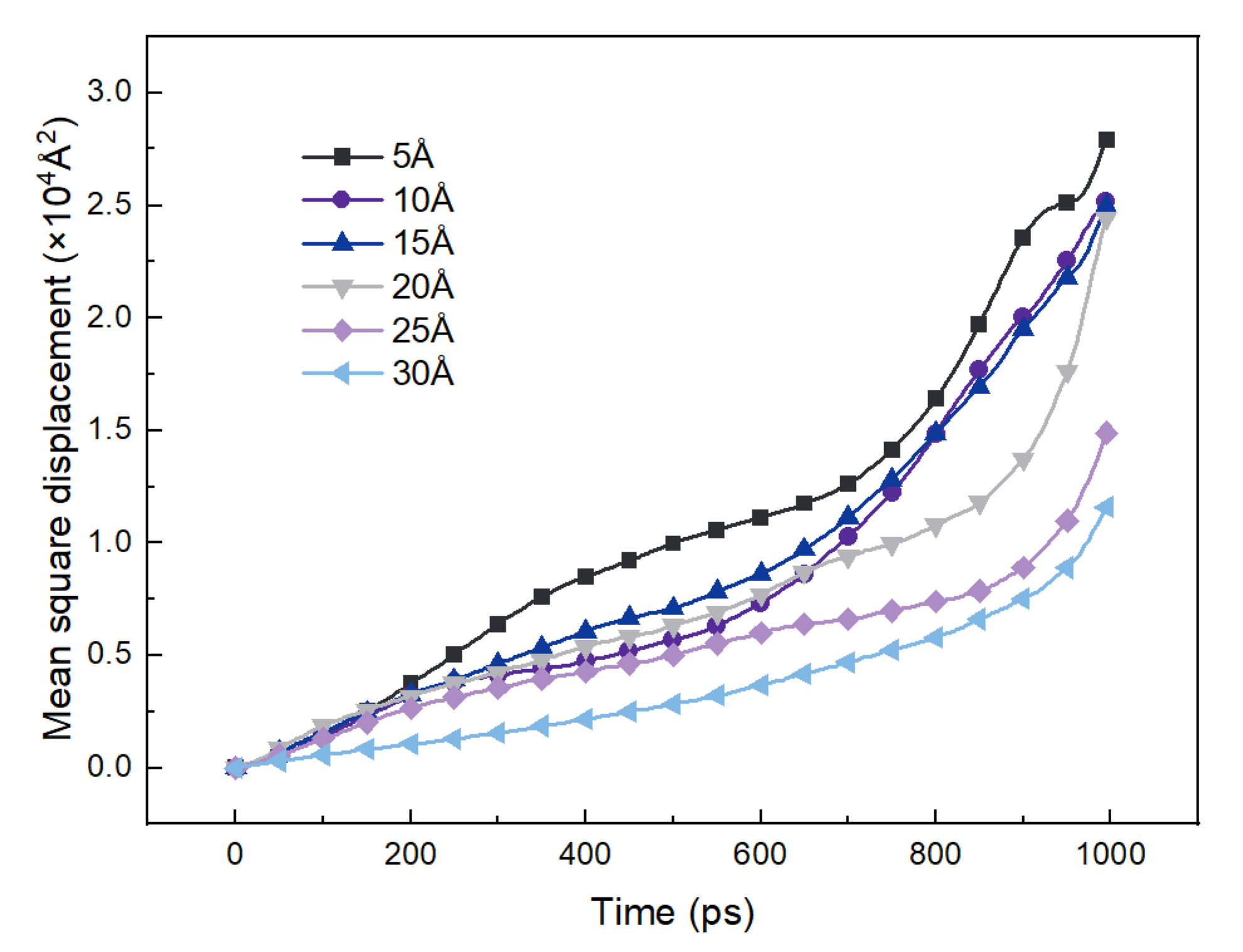
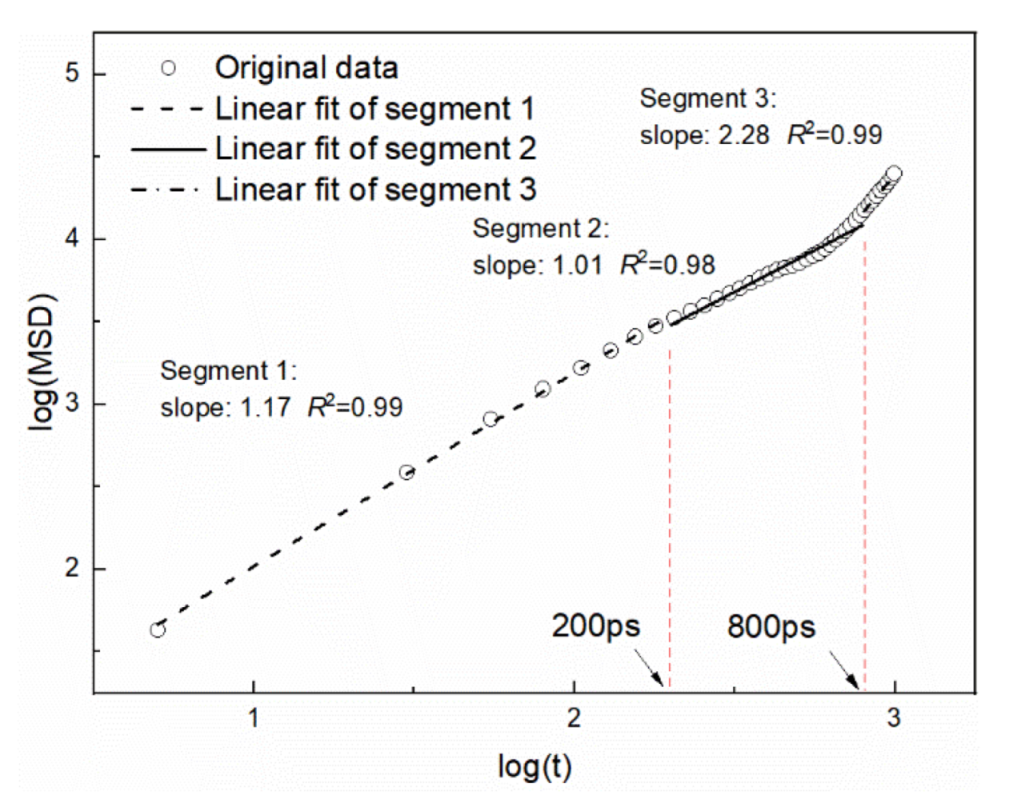
2.3.2. Self-Diffusion Coefficient of Naphthalene
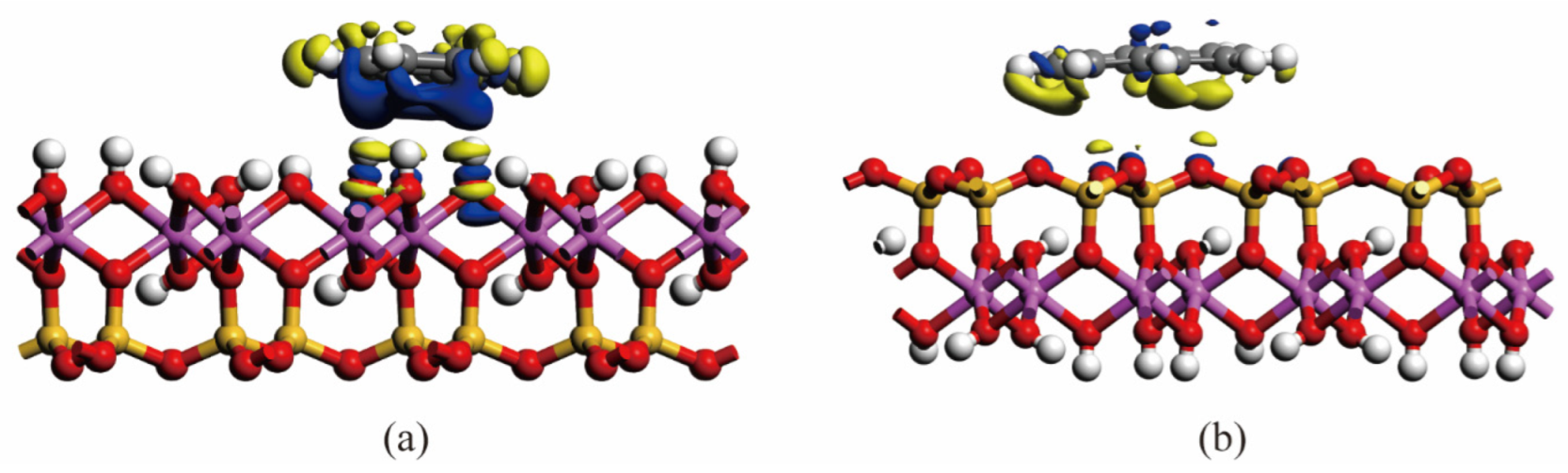
2.3.3. Density Functional Theory (DFT) Calculation
3. Results and Discussions
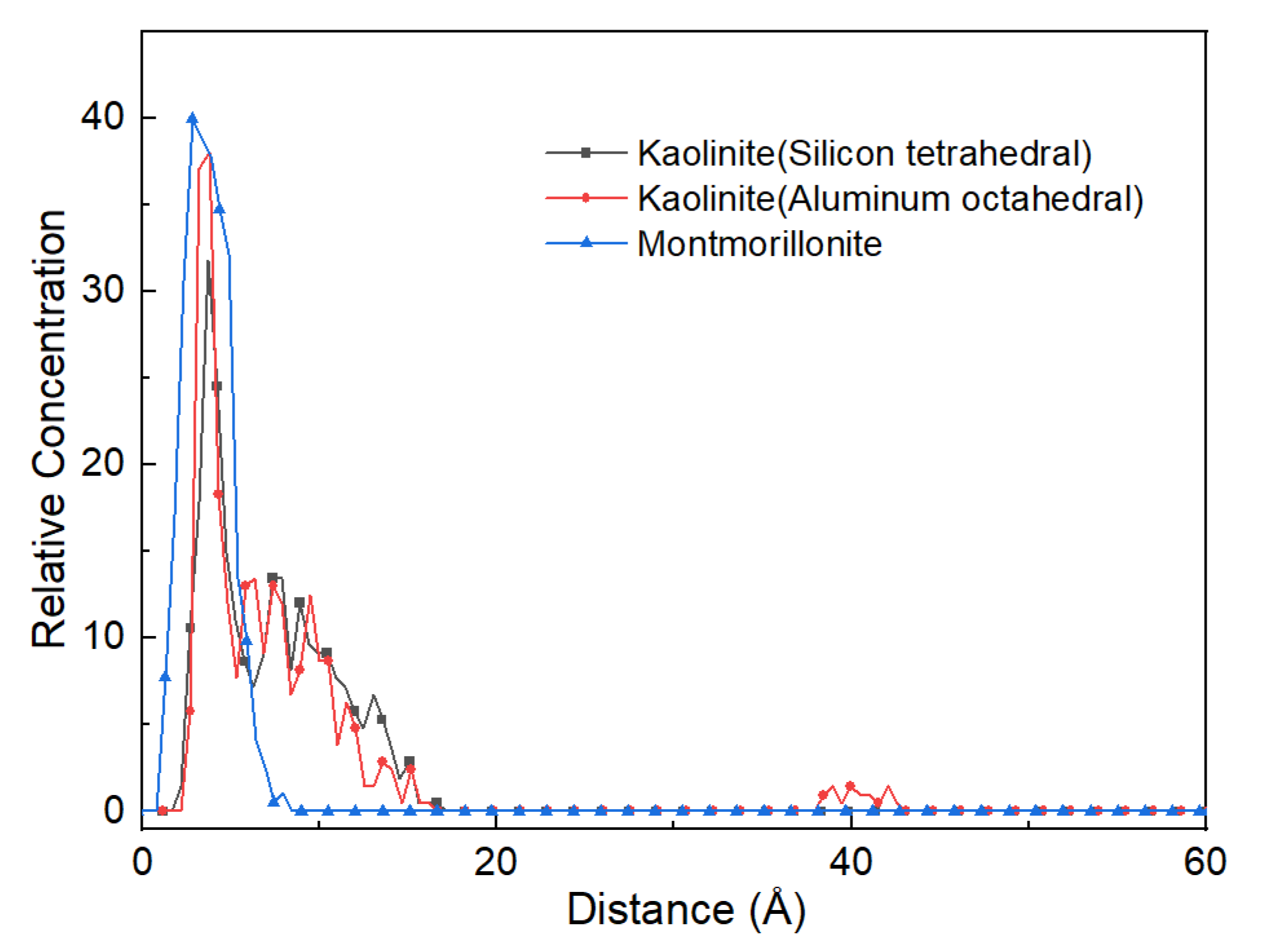
3.1. Effect of Surface of Different Minerals
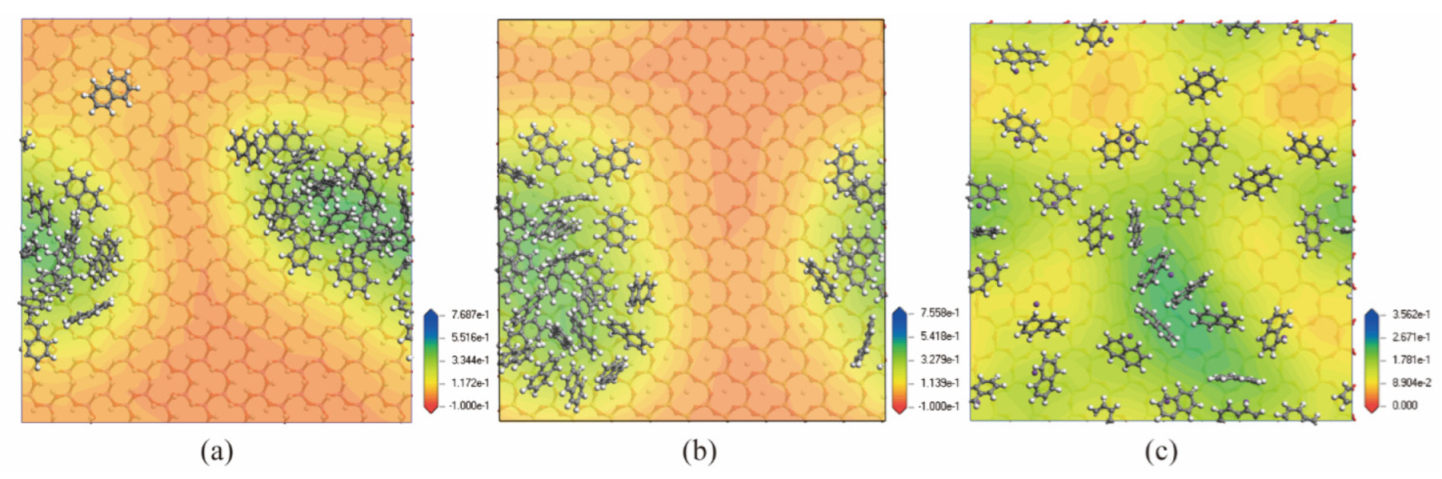
3.2. Effect of Moisture Content
3.3. Competitive Adsorption between Naphthalene and Water
4. Summary and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Titaley, I.A.; Simonich, S.L.M.; Larsson, M. Recent Advances in the Study of the Remediation of Polycyclic Aromatic Compound (PAC)-Contaminated Soils: Transformation Products, Toxicity, and Bioavailability Analyses. Environ. Sci. Technol. Lett. 2020, 7, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Gan, S.; Lau, E.; Ng, H. Remediation of soils contaminated with polycyclic aromatic hydrocarbons (PAHs). J. Hazard. Mater. 2009, 172, 532–549. [Google Scholar] [CrossRef] [PubMed]
- Hussain, K.; Hoque, R.R.; Balachandran, S.; Medhi, S.; Idris, M.G.; Rahman, M.; Hussain, F.L. Monitoring and risk analysis of PAHs in the environment. Handb. Environ. Mater. Manag. 2018, 1, 1–35. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef] [Green Version]
- Hou, D.; O’Connor, D. Green and sustainable remediation: Past, present, and future developments. In Sustainable Remediation of Contaminated Soil and Groundwater; Elsevier: Amsterdam, The Netherlands, 2020; pp. 19–42. [Google Scholar] [CrossRef]
- Hou, D.; Al-Tabbaa, A. Sustainability: A new imperative in contaminated land remediation. Environ. Sci. Policy 2014, 39, 25–34. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, S.; Bao, H.; Chen, D.; Wang, C.; Li, B.; Tong, G.; Yuan, Y.; Xu, B. Improving risk management by using the spatial interaction relationship of heavy metals and PAHs in urban soil. J. Hazard. Mater. 2019, 364, 108–116. [Google Scholar] [CrossRef]
- Kaya, E.; Özcan, A.S.; Gök, Ö.; Özcan, A. Adsorption kinetics and isotherm parameters of naphthalene onto natural-and chemically modified bentonite from aqueous solutions. Adsorption 2013, 19, 879–888. [Google Scholar] [CrossRef]
- Li, S.; Turaga, U.; Shrestha, B.; Anderson, T.A.; Ramkumar, S.; Green, M.J.; Das, S.; Cañas-Carrell, J.E. Mobility of polyaromatic hydrocarbons (PAHs) in soil in the presence of carbon nanotubes. Ecotoxicol. Environ. Saf. 2013, 96, 168–174. [Google Scholar] [CrossRef]
- Frescura, L.M.; de Menezes, B.B.; Duarte, R.; da Rosa, M.B. Application of multivariate analysis on naphthalene adsorption in aqueous solutions. Environ. Sci. Pollut. Res. 2020, 27, 3329–3337. [Google Scholar] [CrossRef]
- Yang, L.; Jin, M.; Tong, C.; Xie, S. Study of dynamic sorption and desorption of polycyclic aromatic hydrocarbons in silty-clay soil. J. Hazard. Mater. 2013, 244–245, 77–85. [Google Scholar] [CrossRef]
- Lamichhane, S.; Krishna, K.B.; Sarukkalige, R. Polycyclic aromatic hydrocarbons (PAHs) removal by sorption: A review. Chemosphere 2016, 148, 336–353. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.-F. Organic pollutant adsorption on clay minerals. In Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2018; Volume 9, pp. 195–253. [Google Scholar]
- Ghosh, D.R.; Keinath, T.M. Effect of clay minerals present in aquifer soils on the adsorption and desorption of hydrophobic organic compounds. Environ. Prog. 1994, 13, 51–59. [Google Scholar] [CrossRef]
- Xing, B. The effect of the quality of soil organic matter on sorption of naphthalene. Chemosphere 1997, 35, 633–642. [Google Scholar] [CrossRef]
- Zhang, J.; Jianhui, Z.; Mengchang, H. Effects of temperature and surfactants on naphthalene and phenanthrene sorption by soil. J. Environ. Sci. 2009, 21, 667–674. [Google Scholar] [CrossRef]
- Osagie, E.I.; Owabor, C.N. Adsorption of naphthalene on clay and sandy soil from aqueous solution. Adv. Chem. Eng. Sci. 2015, 5, 345. [Google Scholar] [CrossRef] [Green Version]
- Bayard, R.; Barna, L.; Mahjoub, B.; Gourdon, R. Investigation of naphthalene sorption in soils and soil fractions using batch and column assays. Environ. Toxicol. Chem. Int. J. 1998, 17, 2383–2390. [Google Scholar] [CrossRef]
- Karaca, G.; Baskaya, H.S.; Tasdemir, Y. Removal of polycyclic aromatic hydrocarbons (PAHs) from inorganic clay mineral: Bentonite. Environ. Sci. Pollut. Res. 2016, 23, 242–252. [Google Scholar] [CrossRef]
- Means, J.C.; Wood, S.G.; Hassett, J.J.; Banwart, W.L. Sorption of polynuclear aromatic hydrocarbons by sediments and soils. Environ. Sci. Technol. 1980, 14, 1524–1528. [Google Scholar] [CrossRef]
- Wang, X.-C.; Zhang, Y.-X.; Chen, R.F. Distribution and partitioning of polycyclic aromatic hydrocarbons (PAHs) in different size fractions in sediments from Boston Harbor, United States. Mar. Pollut. Bull. 2001, 42, 1139–1149. [Google Scholar] [CrossRef]
- Ahangar, A.G. Sorption of PAHs in the soil environment with emphasis on the role of soil organic matter: A review. World Appl. Sci. J. 2010, 11, 759–765. [Google Scholar] [CrossRef] [Green Version]
- Müller, S.; Totsche, K.; Kögel-Knabner, I. Sorption of polycyclic aromatic hydrocarbons to mineral surfaces. Eur. J. Soil Sci. 2007, 58, 918–931. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Y.; Liu, D.; Ning, Y.; Yang, S.; Yang, Z. Adsorption of benzene vapor on natural silicate clay minerals under different moisture contents and binary mineral mixtures. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124072. [Google Scholar] [CrossRef]
- Meleshyn, A.; Tunega, D. Adsorption of phenanthrene on Na-montmorillonite: A model study. Geoderma 2011, 169, 41–46. [Google Scholar] [CrossRef]
- Zhu, S.; Khan, M.A.; Wang, F.; Bano, Z.; Xia, M. Exploration of adsorption mechanism of 2-phosphonobutane-1, 2, 4-tricarboxylic acid onto kaolinite and montmorillonite via batch experiment and theoretical studies. J. Hazard. Mater. 2021, 403, 123810. [Google Scholar] [CrossRef]
- Sawhney, B.L.; Gent, M. Hydrophobicity of clay surfaces: Sorption of 1, 2-dibromoethane and trichloroethene. Clays Clay Miner. 1990, 38, 14–20. [Google Scholar] [CrossRef]
- Lu, N.; Zhang, C. Soil sorptive potential: Concept, theory, and verification. J. Geotech. Geoenviron. Eng. 2019, 145, 04019006. [Google Scholar] [CrossRef]
- Frenkel, D.; Smit, B. Understanding Molecular Simulation: From Algorithms to Applications; Elsevier: Amsterdam, The Netherlands, 2001; Volume 1. [Google Scholar]
- Wu, G.; He, L.; Chen, D. Sorption and distribution of asphaltene, resin, aromatic and saturate fractions of heavy crude oil on quartz surface: Molecular dynamic simulation. Chemosphere 2013, 92, 1465–1471. [Google Scholar] [CrossRef]
- Zhong, J.; Wang, P.; Zhang, Y.; Yan, Y.; Hu, S.; Zhang, J. Adsorption mechanism of oil components on water-wet mineral surface: A molecular dynamics simulation study. Energy 2013, 59, 295–300. [Google Scholar] [CrossRef]
- Wu, G.; Zhu, X.; Ji, H.; Chen, D. Molecular modeling of interactions between heavy crude oil and the soil organic matter coated quartz surface. Chemosphere 2015, 119, 242–249. [Google Scholar] [CrossRef]
- Ren, B.; Lv, K.; Min, F.; Chen, J.; Liu, C. A new insight into the adsorption behavior of NPAM on kaolinite/water interface: Experimental and theoretical approach. Fuel 2021, 303, 121299. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, R.; Ma, J.; Ge, F.; Xu, Y.; Liu, Y. Molecular dynamics simulation study of benzene adsorption to montmorillonite: Influence of the hydration status. Colloids Surf. A Physicochem. Eng. Asp. 2013, 434, 200–206. [Google Scholar] [CrossRef]
- Zhao, N.; Ju, F.; Pan, H.; Tang, Z.; Ling, H. Molecular dynamics simulation of the interaction of water and humic acid in the adsorption of polycyclic aromatic hydrocarbons. Environ. Sci. Pollut. Res. 2020, 27, 25754–25765. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C. Soil and Groundwater Remediation: Fundamentals, Practices, and Sustainability; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Colombano, S.; Davarzani, H.; van Hullebusch, E.D.; Ignatiadis, I.; Huguenot, H.; Zornig, C.; Guyonnet, D. In Situ Thermal Treatments and Enhancements: Theory and Case Study. In Environmental Soil Remediation and Rehabilitation; Springer: Cham, Switzerland, 2020; pp. 149–209. [Google Scholar] [CrossRef]
- Meunier, M.; Robertson, S. Materials Studio 20th Anniversary; Taylor & Francis: Abingdon, UK, 2021; Volume 47, pp. 537–539. [Google Scholar]
- Heinz, H.; Lin, T.J.; Mishra, R.K.; Emami, F.S. Thermodynamically consistent force fields for the assembly of inorganic, organic, and biological nanostructures: The INTERFACE force field. Langmuir 2013, 29, 1754–1765. [Google Scholar] [CrossRef] [PubMed]
- Plimpton, S.; Pollock, R.; Stevens, M. Particle-Mesh Ewald and rRESPA for Parallel Molecular Dynamics Simulations. In Proceedings of the Eighth SIAM Conference on Parallel Processing for Scientific Computing, PPSC, Minneapolis, MI, USA, 14–17 March 1997. [Google Scholar]
- Einstein, A. On the motion of small particles suspended in liquids at rest required by the molecular-kinetic theory of heat. Ann. Phys. 1905, 17, 208. [Google Scholar] [CrossRef] [Green Version]
- Prates Ramalho, J.P.; Dordio, A.V.; Palace Carvalho, A.J. Adsorption of two phenoxyacid compounds on a clay surface: A theoretical study. Adsorption 2013, 19, 937–944. [Google Scholar] [CrossRef]
- Sun, L.; Tanskanen, J.T.; Hirvi, J.T.; Kasa, S.; Schatz, T.; Pakkanen, T.A. Molecular dynamics study of montmorillonite crystalline swelling: Roles of interlayer cation species and water content. Chem. Phys. 2015, 455, 23–31. [Google Scholar] [CrossRef]
- Keiluweit, M.; Kleber, M. Molecular-level interactions in soils and sediments: The role of aromatic π-systems. Environ. Sci. Technol. 2009, 43, 3421–3429. [Google Scholar] [CrossRef]
- Zhao, N.; Ju, F.; Song, Q.; Pan, H.; Ling, H. A simple empirical model for phenanthrene adsorption on soil clay minerals. J. Hazard. Mater. 2022, 429, 127849. [Google Scholar] [CrossRef]
- Tobi, D.; Elber, R.; Thirumalai, D. The dominant interaction between peptide and urea is electrostatic in nature: A molecular dynamics simulation study. Biopolymers 2003, 68, 359–369. [Google Scholar] [CrossRef]
- Wilson, M.J. The origin and formation of clay minerals in soils: Past, present and future perspectives. Clay Miner. 1999, 34, 7–25. [Google Scholar] [CrossRef]
- Underwood, T.; Erastova, V.; Greenwell, H.C. Wetting Effects and Molecular Adsorption at Hydrated Kaolinite Clay Mineral Surfaces. J. Phys. Chem. C 2016, 120, 11433–11449. [Google Scholar] [CrossRef] [Green Version]
- Tunega, D.; Gerzabek, M.H.; Lischka, H. Ab initio molecular dynamics study of a monomolecular water layer on octahedral and tetrahedral kaolinite surfaces. J. Phys. Chem. B 2004, 108, 5930–5936. [Google Scholar] [CrossRef]
- Falciglia, P.P.; Giustra, M.G.; Vagliasindi, F.G. Low-temperature thermal desorption of diesel polluted soil: Influence of temperature and soil texture on contaminant removal kinetics. J. Hazard. Mater. 2011, 185, 392–400. [Google Scholar] [CrossRef]
- Wang, W.; Chen, C.; Xu, W.; Li, C.; Li, Y.-Z. Experimental research on heat transfer characteristics and temperature rise law of in situ thermal remediation of soil. J. Therm. Anal. Calorim. 2021, 147, 3365–3378. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, L. Numerical simulation of heat and mass transfer during the in-situ thermal remediation process of NAPLs-impacted soil. In Proceedings of the 20th International Conference on Soil Mechanics and Geotechnical Engineering, Sydney, Australia, 1–5 May 2022. [Google Scholar]
- Xie, G.; Xiao, Y.; Deng, M.; Zhang, Q.; Huang, D.; Jiang, L.; Yang, Y.; Luo, P. Quantitative Investigation of the Hydration Behavior of Sodium Montmorillonite by Thermogravimetric Analysis and Low-Field Nuclear Magnetic Resonance. Energy Fuels 2019, 33, 9067–9073. [Google Scholar] [CrossRef]
- Wang, H.; Qian, H.; Gao, Y.; Li, Y. Classification and physical characteristics of bound water in loess and its main clay minerals. Eng. Geol. 2020, 265, 105394. [Google Scholar] [CrossRef]



| Parameter | a/Å | b/Å | c/Å | α/° | β/° | γ/° |
| Kaolinite | 5.154 | 8.942 | 7.391 | 91.930 | 105.050 | 89.800 |
| Montmorillonite | 5.192 | 9.015 | 10.023 | 90.000 | 95.735 | 90.000 |
| The thickness of water film | 5 Å | 10 Å | 15 Å | 20 Å | 25 Å | 30 Å |
| Water content/% | 1 | 2 | 3 | 4 | 5 | 6 |
| The thickness of water film | 5 Å | 10 Å | 15 Å | 20 Å | 25 Å | 30 Å |
| E1/2 (kcal/mol) | 71.61 | 18.75 | 28.03 | 13.39 | 7.13 | 1.37 |
| D (10−8 m2/s) | 2.898 | 2.461 | 2.842 | 2.097 | 1.304 | 1.303 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Hu, L. Adsorption of Naphthalene on Clay Minerals: A Molecular Dynamics Simulation Study. Materials 2022, 15, 5120. https://doi.org/10.3390/ma15155120
Chen Z, Hu L. Adsorption of Naphthalene on Clay Minerals: A Molecular Dynamics Simulation Study. Materials. 2022; 15(15):5120. https://doi.org/10.3390/ma15155120
Chicago/Turabian StyleChen, Zhixin, and Liming Hu. 2022. "Adsorption of Naphthalene on Clay Minerals: A Molecular Dynamics Simulation Study" Materials 15, no. 15: 5120. https://doi.org/10.3390/ma15155120
APA StyleChen, Z., & Hu, L. (2022). Adsorption of Naphthalene on Clay Minerals: A Molecular Dynamics Simulation Study. Materials, 15(15), 5120. https://doi.org/10.3390/ma15155120







