Renewal MI Dental Composite Etch and Seal Properties
Abstract
:1. Introduction
- sealing sound drilled cavities.
- forming resin tags and inhibiting enzyme activity with demineralized dentine.
2. Materials and Methods
2.1. Materials
2.1.1. Restorative Materials
2.1.2. Teeth
2.2. Enamel Etching
2.3. Drilled Cavity Restoration and Dye Microleakage
2.3.1. Tooth Restoration
- Renewal MI, Fuji II LC or Fuji IX: directly without cavity preconditioning [23].
- Activa: acid etching for 10 s, then rinse and dry (5 s using an air syringe).
- Z250: acid etching (30 s enamel, 15 s dentine), then rinse and dry. Bonding agent was applied and light-cured for 20 s following manufacturer’s instructions.
2.3.2. Dye Microleakage Evaluation
- 0: None
- 1: Within enamel (only used for cavities with enamel cavosurface)
- 2: In dentine but did not reach the pulpal wall
- 3: Reached the pulpal wall
2.4. Demineralized Dentine Sealing, Enzyme Activity and Remineralization
2.4.1. Dentine Disc Demineralization
2.4.2. Resin Tag Formation
2.4.3. Enzyme Activity
2.4.4. Mineral Precipitation at Set Material/Demineralized Dentine Interfaces
2.5. Statistical Analysis
3. Results
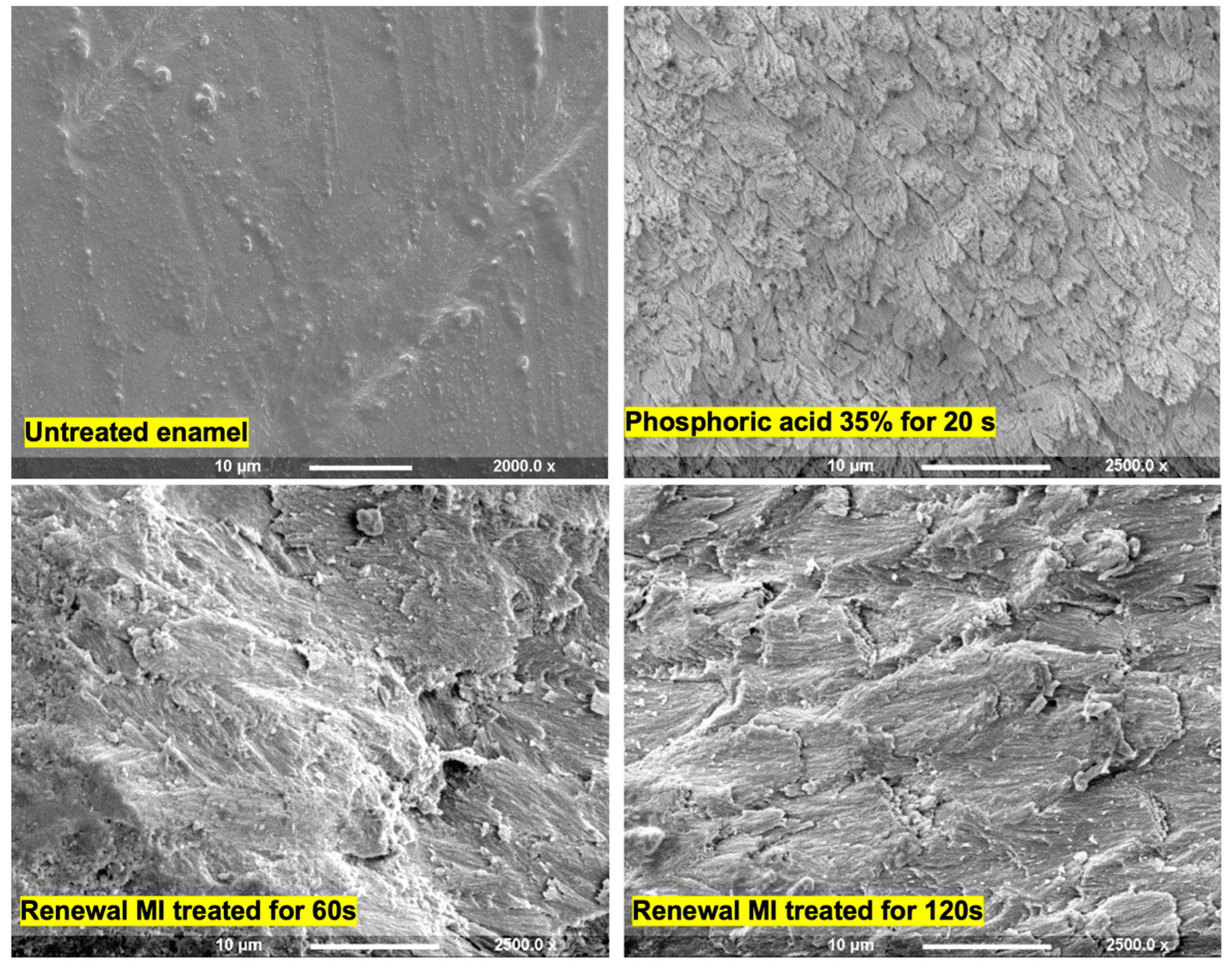
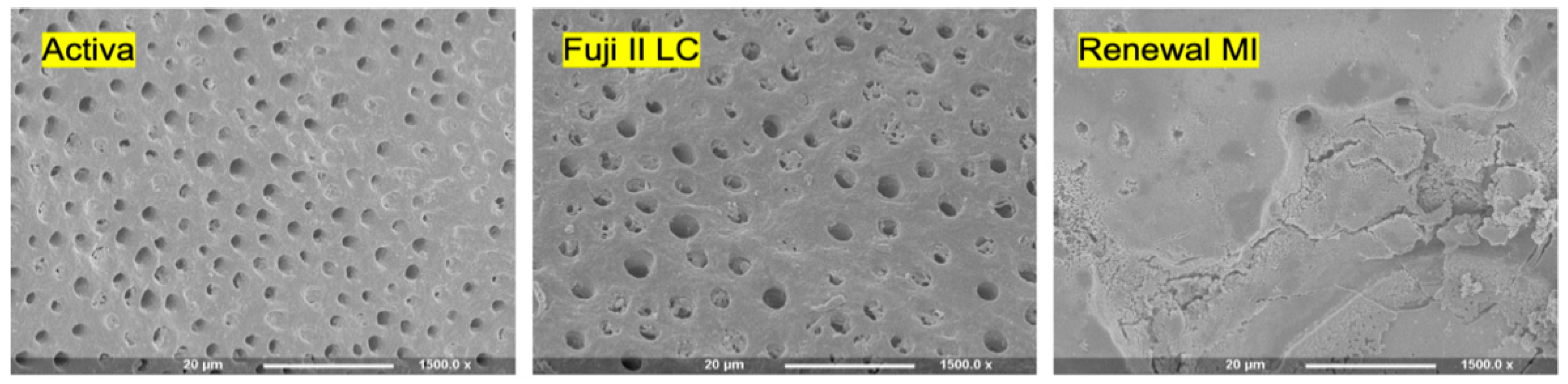
3.1. Enamel Etching
3.2. Dye Microleakage in Sound Cavities
3.3. Sealing of Demineralized Dentine
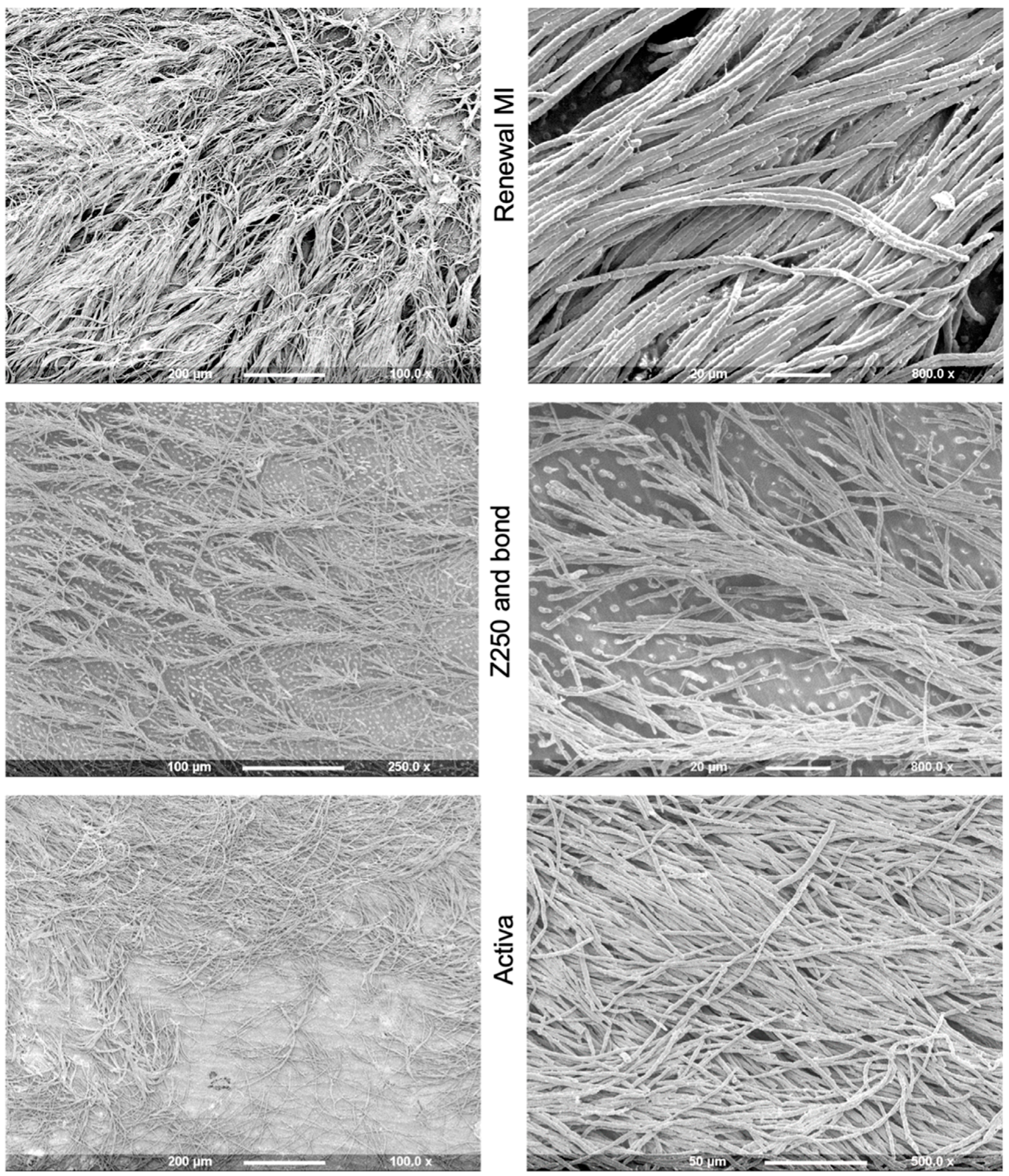
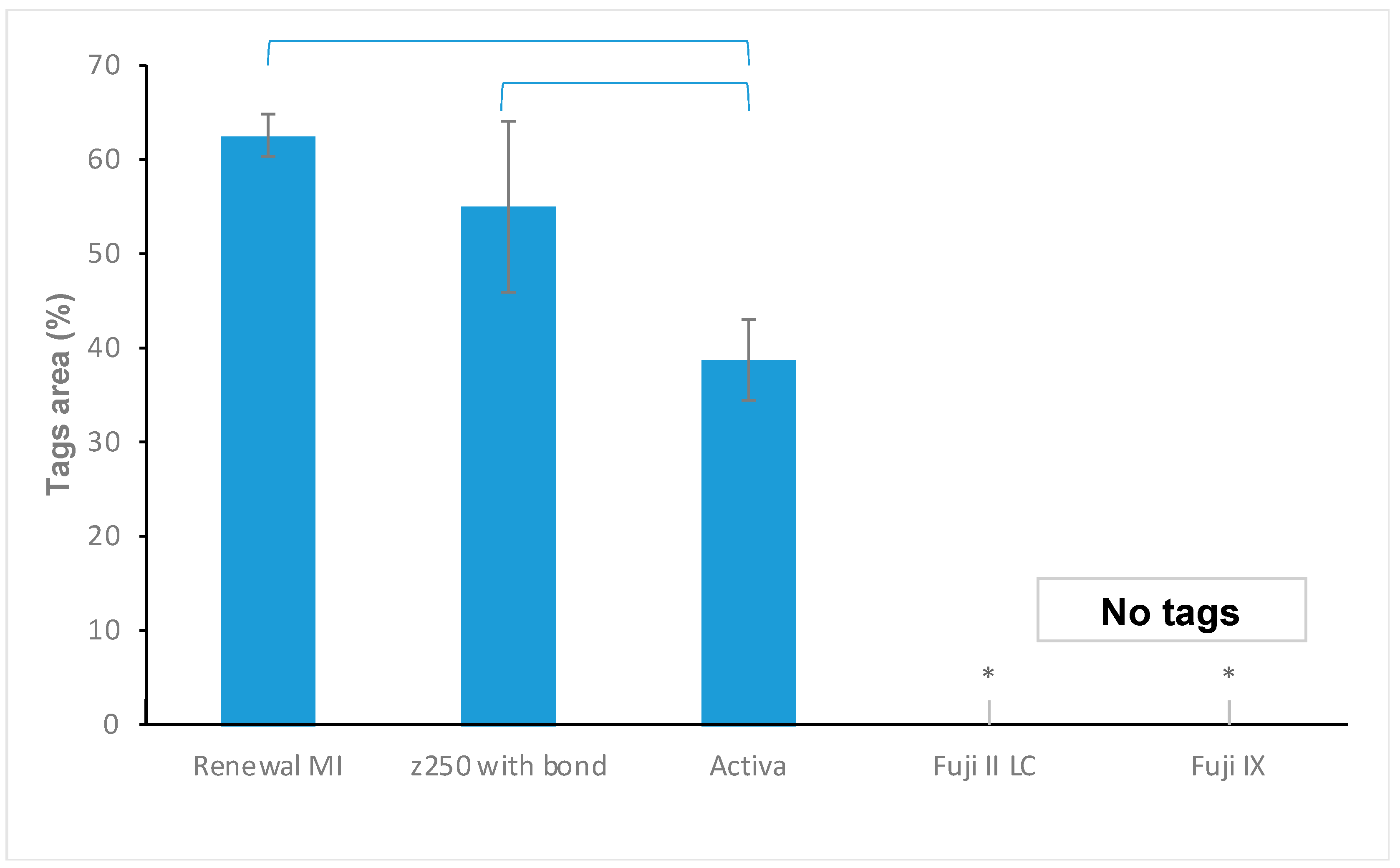
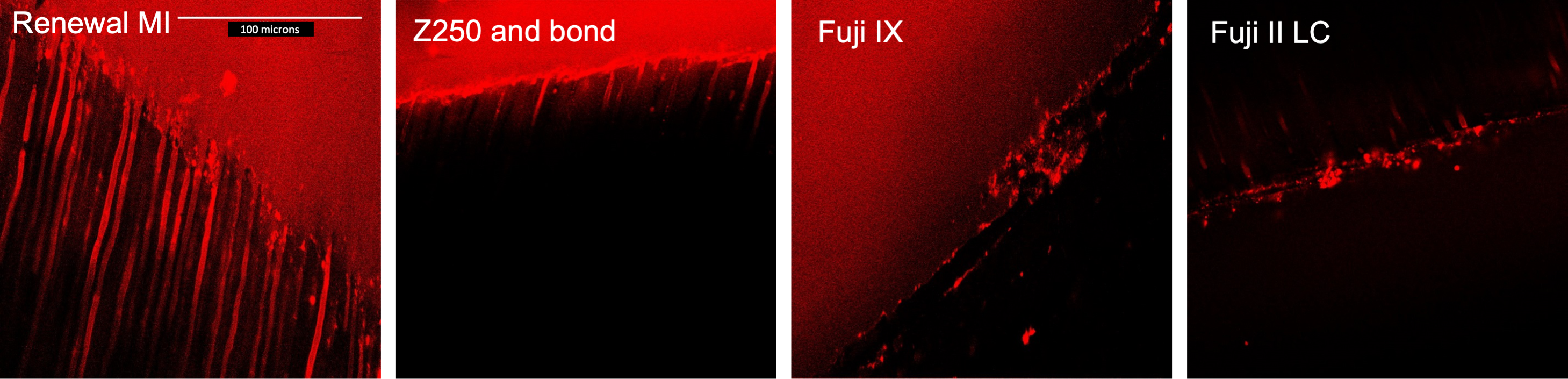
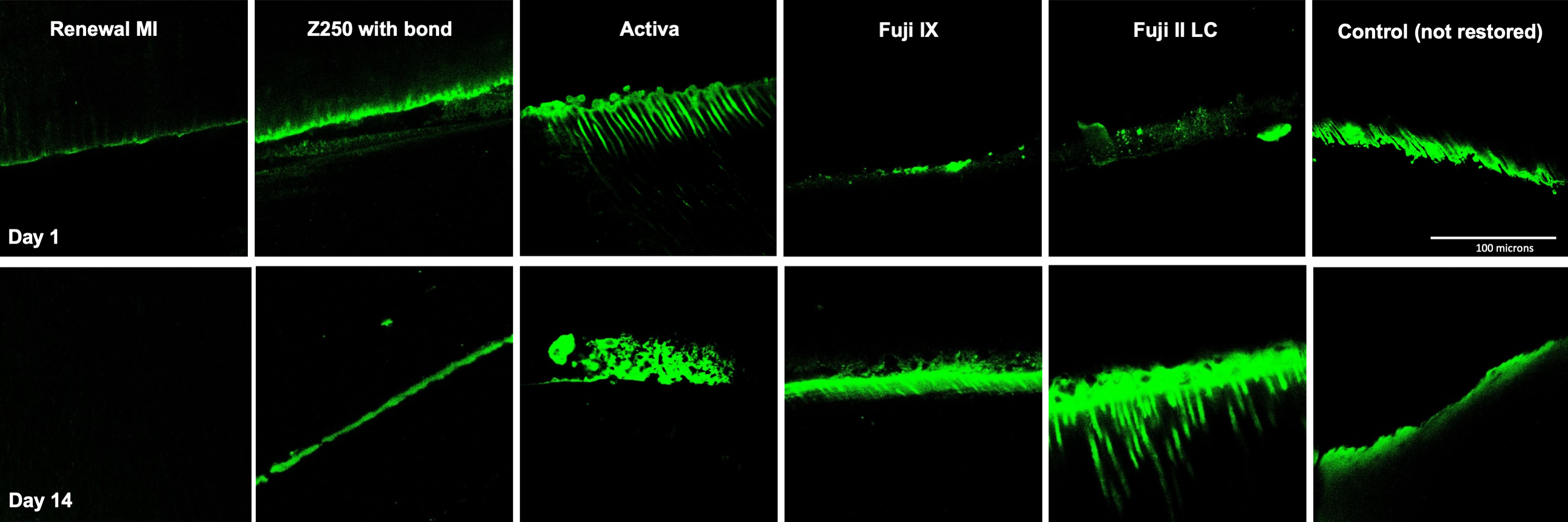
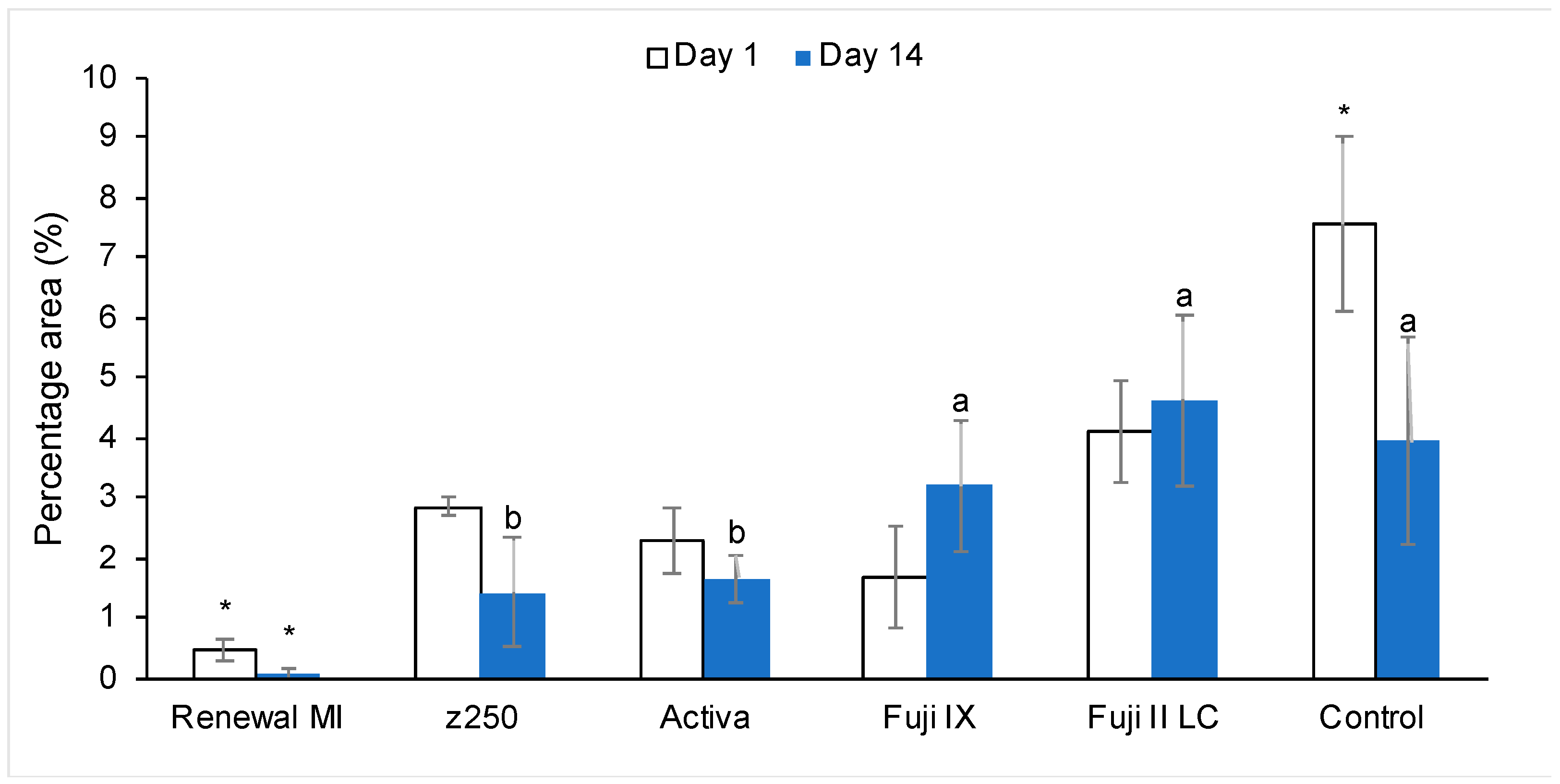
3.3.1. Tags Formation
3.3.2. Enzyme Activity in Restored Demineralized Dentine
3.3.3. Mineral Precipitation at Demineralized Dentine/Set Restoration Interfaces
4. Discussion
4.1. Composition of Renewal MI
4.2. Enamel Etching
4.3. Dye Microleakage in Sound Cavities
4.4. Interactions with Demineralized Dentine
4.4.1. Resin Tags within Demineralized Dentine
4.4.2. Enzyme Activity in Restored Demineralized Dentine
4.5. Mineral Precipitation in Demineralized Dentine
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Larsen, T.; Fiehn, N.E. Dental biofilm infections—An update. Apmis 2017, 125, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Nyvad, B. Ecological Hypothesis of Dentin and Root Caries. Caries Res. 2016, 50, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Jurgensen, N.; Petersen, P.E. Promoting oral health of children through schools—Results from a WHO global survey 2012. Community Dent Health 2013, 30, 204–218. [Google Scholar] [PubMed]
- The Royal College of Surgeons of England. The State of Children’s Oral Health in England; Faculty of Dental Surgery: London, UK, 2015. [Google Scholar]
- Public Health England. National Dental Epidemiology Programme for England: Oral Health Survey of Five-Year-Old Children; PHE Publications: London, UK, 2018.
- Ertugrul, F.; Eltem, R.; Eronat, C. A comparative study of plaque mutans streptococci levels in children receiving glass ionomer cement and amalgam restorations. J. Dent. Child. 2003, 70, 10–14. [Google Scholar]
- Wang, J.; Liu, Z. Influence of amalgam on the growth of mutans streptococcus: An in vivo study. Chin. J. Dent. Res. 2000, 3, 33–37. [Google Scholar]
- FDI World Dental Federation. FDI policy statement on dental amalgam and the Minamata Convention on Mercury: Adopted by the FDI General Assembly: 13 September 2014, New Delhi, India. Int. Dent. J. 2014, 64, 295–296. [Google Scholar] [CrossRef]
- Romero, M.F.; Haddock, F.; Todd, M. Combination of centripetal and successive layering techniques for a stress-reduced posterior direct composite restoration. Gen. Dent. 2017, 65, 72–76. [Google Scholar]
- Rothmund, L.; Reichl, F.X.; Hickel, R.; Styllou, P.; Styllou, M.; Kehe, K.; Yang, Y.; Hogg, C. Effect of layer thickness on the elution of bulk-fill composite components. Dent. Mater. 2017, 33, 54–62. [Google Scholar] [CrossRef]
- Hickel, R.; Roulet, J.F.; Bayne, S.; Heintze, S.D.; Mjor, I.A.; Peters, M.; Rousson, V.; Randall, R.; Schmalz, G.; Tyas, M.; et al. Recommendations for conducting controlled clinical studies of dental restorative materials. Science Committee Project 2/98—FDI World Dental Federation study design (Part I) and criteria for evaluation (Part II) of direct and indirect restorations including onlays and partial crowns. J. Adhes. Dent. 2007, 9 (Suppl. 1), 121–147. [Google Scholar]
- Namgung, C.; Rho, Y.J.; Jin, B.H.; Lim, B.S.; Cho, B.H. A retrospective clinical study of cervical restorations: Longevity and failure-prognostic variables. Oper. Dent. 2013, 38, 376–385. [Google Scholar] [CrossRef]
- Baratieri, L.N.; Ritter, A.V. Four-year clinical evaluation of posterior resin-based composite restorations placed using the total-etch technique. J. Esthet. Restor. Dent. 2001, 13, 50–57. [Google Scholar] [CrossRef]
- Kim, J.H.; Cho, J.; Lee, Y.; Cho, B.H. The Survival of Class V Composite Restorations and Analysis of Marginal Discoloration. Oper. Dent. 2017, 42, E93–E101. [Google Scholar] [CrossRef] [PubMed]
- Loguercio, A.D.; Reis, A.; Barbosa, A.N.; Roulet, J.F. Five-year double-blind randomized clinical evaluation of a resin-modified glass ionomer and a polyacid-modified resin in noncarious cervical lesions. J. Adhes. Dent. 2003, 5, 323–332. [Google Scholar] [PubMed]
- Proffitt, E. Decoding the English standard operating procedures for dentists and the dental industry. Br. Dent. J. 2020, 229, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Ricketts, D.; Lamont, T.; Innes, N.P.T.; Kidd, E.; Clarkson, J.E. Operative caries management in adults and children. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef]
- Chibinski, A.C.; Wambier, L.; Reis, A.; Wambier, D.S. Clinical, mineral and ultrastructural changes in carious dentin of primary molars after restoration. Int. Dent. J. 2016, 66, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Folwaczny, M.; Loher, C.; Mehl, A.; Kunzelmann, K.H.; Hickel, R. Class V lesions restored with four different tooth-colored materials—3-year results. Clin. Oral Investig. 2001, 5, 31–39. [Google Scholar] [CrossRef]
- Van Dijken, J.W.; Pallesen, U. Fracture frequency and longevity of fractured resin composite, polyacid-modified resin composite, and resin-modified glass ionomer cement class IV restorations: An up to 14 years of follow-u. Clin. Oral Investig. 2010, 14, 217–222. [Google Scholar] [CrossRef]
- Bayne, S.C.; Ferracane, J.L.; Marshall, G.W.; Marshall, S.J.; van Noort, R. The Evolution of Dental Materials over the Past Century: Silver and Gold to Tooth Color and Beyond. J. Dent. Res. 2019, 98, 257–265. [Google Scholar] [CrossRef]
- PD ISO/TS 11405:2015; Testing of Adhesion to Tooth Structure. British Standard Institution: London, UK, 2015.
- Dorri, M.; Martinez-Zapata, M.J.; Walsh, T.; Marinho, V.C.C.; Sheiham, A.; Zaror, C. Atraumatic restorative treatment versus conventional restorative treatment for managing dental caries. Cochrane Database Syst. Rev. 2017. [Google Scholar] [CrossRef] [Green Version]
- Alkhouri, N. Optimising Novel Dental Composites for Paediatric Patients. Ph.D. Thesis, Eastman Dental Institute, London, UK, 2020. [Google Scholar]
- Yan, F.; Robert, M.; Li, Y. Statistical methods and common problems in medical or biomedical science research. Int. J. Physiol. Pathophysiol. Pharmacol. 2017, 9, 157–163. [Google Scholar] [PubMed]
- Fleisch, A.F.; Sheffield, P.E.; Chinn, C.; Edelstein, B.L.; Landrigan, P.J. Bisphenol A and related compounds in dental materials. Pediatrics 2010, 126, 760–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kangwankai, K.; Sani, S.; Panpisut, P.; Xia, W.; Ashley, P.; Petridis, H.; Young, A.M. Monomer conversion, dimensional stability, strength, modulus, surface apatite precipitation and wear of novel, reactive calcium phosphate and polylysine-containing dental composites. PLoS ONE 2017, 12, e0187757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehdawi, I.; Neel, E.A.; Valappil, S.P.; Palmer, G.; Salih, V.; Pratten, J.; Spratt, D.A.; Young, A.M. Development of remineralizing, antibacterial dental materials. Acta Biomater. 2009, 5, 2525–2539. [Google Scholar] [CrossRef]
- Labella, R.; Lambrechts, P.; Van Meerbeek, B.; Vanherle, G. Polymerization shrinkage and elasticity of flowable composites and filled adhesives. Dent. Mater. 1999, 15, 128–137. [Google Scholar] [CrossRef]
- Baroudi, K.; Rodrigues, J.C. Flowable Resin Composites: A Systematic Review and Clinical Considerations. J. Clin. Diagn. Res. 2015, 9, ZE18–ZE24. [Google Scholar] [CrossRef]
- Ireland, A.J.; Sherriff, M. An investigation into the use of an anaerobic adhesive with two commercially available orthodontic brackets. Dent. Mater. 2006, 22, 112–118. [Google Scholar] [CrossRef]
- Zhu, J.J.; Tang, A.T.H.; Matinlinna, J.P.; Hägg, U. Acid etching of human enamel in clinical applications: A systematic review. J. Prosthet. Dent. 2014, 112, 122–135. [Google Scholar] [CrossRef]
- Hobson, R.S.; McCabe, J.F. Relationship between enamel etch characteristics and resin-enamel bond strength. Br. Dent. J. 2002, 192, 463–468. [Google Scholar] [CrossRef]
- Gerdolle, D.A.; Mortier, E.; Droz, D. Microleakage and polymerization shrinkage of various polymer restorative materials. J. Dent. Child. 2008, 75, 125–133. [Google Scholar]
- Gupta, A.; Tavane, P.; Gupta, P.K.; Tejolatha, B.; Lakhani, A.A.; Tiwari, R.; Kashyap, S.; Garg, G. Evaluation of Microleakage with Total Etch, Self Etch and Universal Adhesive Systems in Class V Restorations: An In vitro Study. J. Clin. Diagn. Res. 2017, 11, Zc53–Zc56. [Google Scholar] [CrossRef]
- Wahab, F.K.; Shaini, F.J.; Morgano, S.M. The effect of thermocycling on microleakage of several commercially available composite Class V restorations in vitro. J. Prosthet. Dent. 2003, 90, 168–174. [Google Scholar] [CrossRef]
- Diwanji, A.; Dhar, V.; Arora, R.; Madhusudan, A.; Rathore, A.S. Comparative evaluation of microleakage of three restorative glass ionomer cements: An in vitro study. J. Nat. Sci. Biol. Med. 2014, 5, 373–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhadra, D.; Shah, N.C.; Rao, A.S.; Dedania, M.S.; Bajpai, N. A 1-year comparative evaluation of clinical performance of nanohybrid composite with Activa™ bioactive composite in Class II carious lesion: A randomized control study. J. Conserv. Dent. 2019, 22, 92–96. [Google Scholar] [PubMed]
- Van Dijken, J.W.V.; Pallesen, U.; Benetti, A. A randomized controlled evaluation of posterior resin restorations of an altered resin modified glass-ionomer cement with claimed bioactivity. Dent. Mater. 2019, 35, 335–343. [Google Scholar] [CrossRef]
- Mazzoni, A.; Tjaderhane, L.; Checchi, V.; Di Lenarda, R.; Salo, T.; Tay, F.R.; Pashley, D.H.; Breschi, L. Role of dentin MMPs in caries progression and bond stability. J. Dent. Res. 2015, 94, 241–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosa, A.J.; da Silva, E.M.; Tostes, M.A. Scanning electron microscopy analysis of microstructure of the adhesive interface between resin and dentin treated with papain gel. Indian J. Dent. Res. 2015, 26, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Giachetti, L.; Bertini, F.; Scaminaci Russo, D.; Rubino, I. The extension of resin tags in etched dentin: A misinterpretation? Minerva Stomatol. 2005, 54, 139–151. [Google Scholar]
- Mustafa, H.A.; Soares, A.P.; Paris, S.; Elhennawy, K.; Zaslansky, P. The forgotten merits of GIC restorations: A systematic review. Clin. Oral Investig. 2020, 24, 2189–2201. [Google Scholar] [CrossRef]
- Osorio, R.; Yamauti, M.; Sauro, S.; Watson, T.F.; Toledano, M. Experimental resin cements containing bioactive fillers reduce matrix metalloproteinase-mediated dentin collagen degradation. J. Endod. 2012, 38, 1227–1232. [Google Scholar] [CrossRef]
- Kuhn, E.; Reis, A.; Campagnoli, E.B.; Chibinski, A.C.; Carrilho, M.R.; Wambier, D.S. Effect of sealing infected dentin with glass ionomer cement on the abundance and localization of MMP-2, MMP-8, and MMP-9 in young permanent molars in vivo. Int. J. Paediatr. Dent. 2016, 26, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, F.D.; Minciotti, C.L.; Geraldeli, S.; Carrilho, M.R.; Pashley, D.H.; Tay, F.R.; Nader, H.B.; Salo, T.; Tjaderhane, L.; Tersariol, I.L. Cysteine cathepsins in human carious dentin. J. Dent. Res. 2011, 90, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Vidal, C.M.; Tjaderhane, L.; Scaffa, P.M.; Tersariol, I.L.; Pashley, D.; Nader, H.B.; Nascimento, F.D.; Carrilho, M.R. Abundance of MMPs and cysteine cathepsins in caries-affected dentin. J. Dent. Res. 2014, 93, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Scaffa, P.M.; Breschi, L.; Mazzoni, A.; Vidal, C.M.; Curci, R.; Apolonio, F.; Gobbi, P.; Pashley, D.; Tjaderhane, L.; Tersariol, I.L.; et al. Co-distribution of cysteine cathepsins and matrix metalloproteases in human dentin. Arch Oral Biol. 2017, 74, 101–107. [Google Scholar] [CrossRef]
- Femiano, F.; Femiano, R.; Femiano, L.; Jamilian, A.; Rullo, R.; Perillo, L. Dentin caries progression and the role of metalloproteinases: An update. Eur. J. Paediatr. Dent. 2016, 17, 243–247. [Google Scholar]
- Cao, Y.; Mei, M.L.; Xu, J.; Lo, E.C.; Li, Q.; Chu, C.H. Biomimetic mineralisation of phosphorylated dentine by CPP-ACP. J. Dent. 2013, 41, 818–825. [Google Scholar] [CrossRef]

| Material | Type | Manufacturer | Composition | |
|---|---|---|---|---|
| Liquid Phase | Filler | |||
| RENEWAL MI | Single step experimental flowable hybrid composite | Davis, Schottlander and Davis Dental Company, Letchworth, UK | UDMA, PPGDMA, 4META, CQ | Strontium alumino silicate, Monocalcium phosphate, Polylysine |
| Filtek Z250 (Shade B3) | Conventional composite | 3M ESPE, St. Paul, MN, USA | BISGMA, UDMA, other dimethacrylates | Zirconia/silica (0.6 µm) |
| Scotchbond Universal | Combined selective etch, self-etch, total etch | 3M ESPE, St. Paul, MN, USA | BISGMA, HEMA, 10MDP, methacrylate modified polyacid, water, ethanol | Silica |
| Activa kids (Pedo shade double-barrel syringe) | Bioactive RMGIC | Pulpdent, Watertown, MA, USA | UDMA, other methacrylates, modified polyacid | Reactive and unreactive fillers, sodium fluoride |
| Fuji II LC (Shade A2 capsules) | RMGIC | GC America, Alsip, IL, USA | HEMA, UDMA, polyacid, water | Silane treated strontium fluoroaluminosilicate |
| Fuji IX GP (Shade A2 capsules) | GIC | GC America, Alsip, IL, USA | Polyacid, water | Strontium fluoroaluminosilicate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkhouri, N.; Xia, W.; Ashley, P.F.; Young, A.M. Renewal MI Dental Composite Etch and Seal Properties. Materials 2022, 15, 5438. https://doi.org/10.3390/ma15155438
Alkhouri N, Xia W, Ashley PF, Young AM. Renewal MI Dental Composite Etch and Seal Properties. Materials. 2022; 15(15):5438. https://doi.org/10.3390/ma15155438
Chicago/Turabian StyleAlkhouri, Nabih, Wendy Xia, Paul F. Ashley, and Anne M. Young. 2022. "Renewal MI Dental Composite Etch and Seal Properties" Materials 15, no. 15: 5438. https://doi.org/10.3390/ma15155438
APA StyleAlkhouri, N., Xia, W., Ashley, P. F., & Young, A. M. (2022). Renewal MI Dental Composite Etch and Seal Properties. Materials, 15(15), 5438. https://doi.org/10.3390/ma15155438






