3.1. Kinetic Energy Distributions of M+ and MO+ Species in O2 and Ar
Figure 3 and
Figure S1 compare the IEDs of monoatomic ions at different O
2 and Ar pressures. The background gas Ar has a similar mass (40 amu) and kinetic diameter (340 pm, spherical) to O
2 (32 amu, 346 pm elliptical) but is considered to be chemically inert with a comparable geometrical cross section [
17]. Chemical reactions with Ar are known, but none of these known products have yet been detected [
18,
19]. The measured IEDs were the result of both elastic and inelastic collisions during the plume expansion in the respective atmospheric environment. While elastic collisions involve only kinematics, inelastic collisions involve a series of mechanisms ranging from electronic excitation to dissociation and formation of gas molecules, where chemical reactions are triggered, even for Ar [
18,
19]. Elastic and inelastic collisions occur in both O
2 and Ar atmospheres, and to some extent, intra-plume collisions at an early stage of the plume expansion. To simplify the terms involved in this study, we classified the types of collisions into non-reactive collisions (where the plume species do not form chemical bonds with the background gas in measurable quantities, as in Ar) and reactive collisions (where the plume species form chemical bonds with the background gas, such as the formation of LaO
+ in O
2).
The IEDs of the Mn
+ and La
+ ions in O
2 and Ar at different pressures are shown in
Figure 3a–d, and Ca
+ and LaO
+ in S1. Other MO
+ ions such as CaO
+ or MnO
+ were not measurable due to their very low total intensity, even for a vacuum ablation. This reflects the fact that during the initial ablation process and plume formation, the used photon energy of 4.99 eV was able to dissociate most bonds with an energy smaller than the photon energy. In the gas phase, di-atomic species with a bond energy larger than the photon energy can only be dissociated with a multi-photon absorption process, which has a much smaller probability to occur by ns pulses. Hence, more di-atomic species with
Ediss >
Ephoton are measurable as part of the plume species composition for an ablation in vacuum. Additionally, M
+ species with large binding energies tend to recombine easily during the expansion. After the interaction of plume species with the laser beam, gas phase reactions via scattering are the dominant chemical processes in the plasma plume and it should be possible to differentiate between non-reactive and reactive scattering processes depending on the reactivity of the background gas species. The dissociation energy of LaO
+ (
m = 154) with
Ediss = 8.57 eV [
20] is larger than that of O
2, and therefore stable LaO molecules and LaO
+ ions can be formed additionally by reactions in the plume at a higher O
2 background pressure. On the other hand, MnO
+ and CaO
+ have a smaller dissociation energy than O
2 (2.9 eV; 4.1 eV < 5.12 eV) [
21], and their formation at a higher O
2 background pressure is not favoured due to a dissociation via an enhanced scattering rate. Therefore, the pressure evolution of the IED for Mn
+ and Ca
+ in O
2 should reflect the influence of non-reactive collisions in a way similar to an Ar background, where elastic scattering plays a dominant role. In general, a decrease in the total amount of M-species is expected with increasing pressure, and the number of species with a high kinetic energy should be significantly reduced. The influence of non-reactive scattering to the total number of scattering events can be deduced by comparing the corresponding IEDs in O
2 and Ar. For La
+, one would expect to more clearly see the influence of reactive scattering, since the formation of LaO
+ in O
2 would be favoured with increasing O
2 pressure and the number of La
+-species should drop correspondingly [
3,
22].
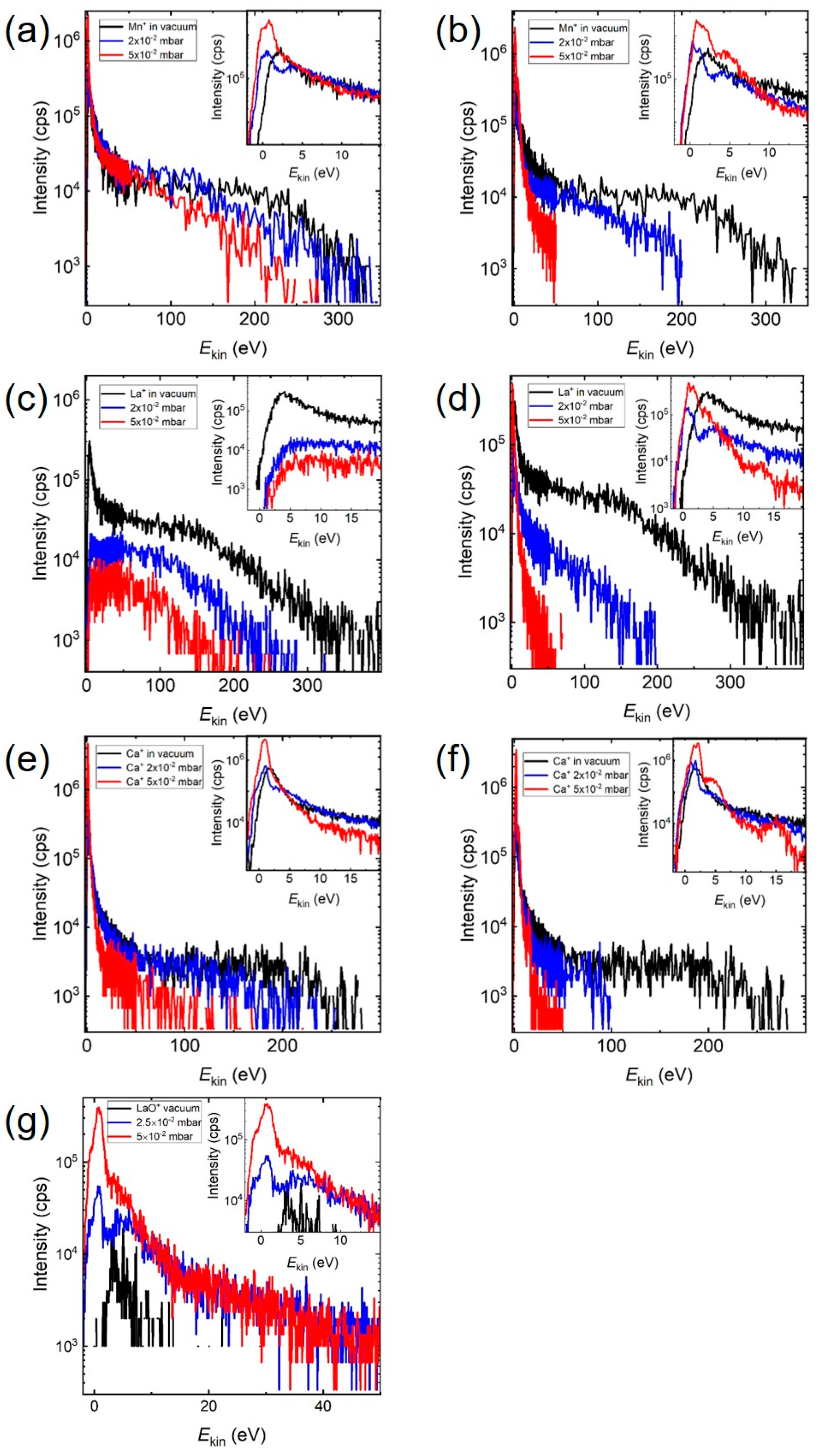
The ablation of
La0.33Ca0.67MnO3 in vacuum Mn
+ species (
m = 55) had a low energy maximum with
Ekin,max < 2 eV and a significant energetic tail up to 400 eV (
Figure 3). With increasing O
2 pressure, these high kinetic energies were reduced to less than 200 eV (
Figure 3a), and in Ar, a substantial reduction to around 50 eV took place (
Figure 3b). In addition, the intensity of the low energy peak decreased at first, in both atmospheres, before increasing to very high intensity values at 5 × 10
−2 mbar (see
Figure 3 inserts). This was also the pressure regime where the mean free path was of the order of the target–substrate distance and smaller, and hence the influence of collision became increasingly more important. Looking at Ca
+ (
m = 40), the high kinetic energies (300 eV in vacuum) were reduced with increasing pressure to <100 eV in O
2 (
Figure 3e) and <50 eV in Ar (
Figure 3f), and
Ekin,vac > 500 eV for La
+ (
m = 139) to less than 200 eV in O
2 (
Figure 3c) and approx. 50 eV in Ar (
Figure 3d). Analogous to Mn
+, the intensity of the low energy Ca
+-peak decreased at first, before increasing to a very high intensity value at 5 × 10
−2 mbar. The situation for La
+ was different; the intensity of the low energy peak decreased with increasing O
2 background pressure. The pressure dependence of the IED for LaO
+ in O
2 (
Figure 3g) showed a different feature. The IED expanded from less than 20 eV in vacuum to 50 eV with increasing O
2 pressure. The overall intensity of the IED as well as the intensity of the low energy LaO
+-peak increased with increasing pressure while the position of the maximum remained largely unchanged with the tendency to be reduced to smaller energies. The latter was different to the low energy part of the IED for the single ionic species where a clear shift of the position for the low energy maximum was observed.
3.2. Dynamic Properties of Positive Ionic Species at Small Kinetic Energies
Another observation for all the measured kinetic energy distributions was the appearance of a structure at lower kinetic energies with increasing background pressure (
Figure 3, insets), in particular for the ablation in Ar. Analysing these distributions more closely by fitting energy-shifted Maxwell–Boltzmann (MB) distributions, we noted that these kinetic energy distributions could be described by two or three MB distributions, indicating that the stopping of these species fell into several kinetic energy windows [
23,
24]. Examples are shown in the
Supplementary Materials Figure S2. One window was the typical
Ekin,max below 5 eV, the second was between 5 and 10 eV, and the third was between 10 and 20 eV. For the ablation in Ar, this was the general appearance; for the ablation in O
2, there was a difference between species with
Ediss larger or smaller than 5.12 eV. For M-species with
Ediss < 5.12 eV, the different energy maxima with increasing pressure was a general feature, whereas for M-species with
Ediss > 5.12 eV, the kinetic energy distribution was smoother but still compatible with several MB-distributions falling into the same energy windows mentioned. The origin of these energy windows is at present not understood, but the appearance of these energy maxima was certainly the outcome of an increase in the scattering rate with increasing pressure, thereby slowing down the plasma species. The scattering-induced excitation and ionisation of plasma species requires discreet energies; the multitude of different species in the plasma have a range of different energetic requirements and the net result is a structured kinetic energy distribution. The smoother energy distribution with increasing pressure in O
2 was most likely the result of the added chemical activity in the plasma as a result of inelastic scattering leading to excitations, ionisation and dissociation, preferably for species with
Ediss < 5.12 eV.
With respect to the low energy peak for Mn
+, Ca
+ and La
+, all three ions showed first a drop and then an increase in intensity with increasing Ar pressure. The same behaviour was observed for Mn
+ and Ca
+ in O
2. This can be seen in
Figure 3. With increasing O
2 pressures, the amount of LaO
+ ions increased while simultaneously the amount of La
+ decreased. In particular, starting at 10
−2 mbar, there was a steep rise in the LaO
+ signal with a simultaneous drop in the La
+ signal. This indicated an increase in the reaction rate of La species (La
+ or La*) with oxygen species (O
+, O* or O
2) at higher pressures. The measured intensities for LaO
+ were mainly for
Ekin < 50 eV at all pressures; above 50 eV, the intensity contribution to the total intensity became negligible. For the vacuum ablation, the LaO
+ signal was very weak, and the measurable kinetic energy distribution reached, at most, 10 eV. Unlike La
+, there was a very large ion intensity at low kinetic energies and an energetic tail up to 350 eV. For the ablation at 5 × 10
−2 mbar O
2, the LaO
+ signal was comparable to La
+ in vacuum at small kinetic energies and the kinetic energy range was up to 50 eV. In addition, the kinetic energy distribution showed a clear structure not seen before. The La
+ signal, on the other hand, dropped significantly at low kinetic energies and the high energy tail reached up to 150 eV, with no distinct maximum to be measured.
3.3. Scattering Kinetics for the Initial Plasma Expansion
To understand this observation, the probable origin of the La
+ and LaO
+ kinetic energy distributions need to be discussed. The ablation of species from a target is the result of photodissociation, photoexcitation and thermal evaporation after the target material absorbs the incoming laser light. For the used wavelength and a pulse length of 20 ns, the large majority of species measured consisted of single ions, a few dimers and a very small number of trimeric species, if any. The plasma composition will also depend on the background gas and respective pressure used [
3]. The initial vapor plume is formed, starts to expand, and continues to absorb the laser light, developing into a highly dense, fast moving vapour cloud with a high pressure and temperature consisting of charged, excited and neutral species. At this stage of cloud formation, the vapour will largely consist of mono-atomic species due to the dominant dissociation mechanisms, and in front of this vapour cloud, a charged layer consisting of electrons is formed, that is responsible for the acceleration of the charged species [
25,
26,
27]. The initial shape of the plasma plume depends on its elemental composition as well as its mass, which is reasonably well described for single elemental plumes by the double layer model [
25,
26,
27]. A good description is still missing for multi-elemental plumes, and plume dynamics—in particular, in the turbulent pressure range between 10
−2 and 10
−1 mbar—becomes very complex [
28,
29]. With a fluence of 2 J/cm
2 used, most of the ions were expected to be single charged [
30], and that dimeric species such as metal–oxygen species could form, however, scattering events and the different dissociation mechanism would result in a mixture of mono-atomic species. Only after the termination of the laser pulse would binary species become more detectable largely due to the thermal evaporation from the target and the expansion of the vapour cloud reducing the effective species density considerably within the timeframe beyond 20 ns. This scenario would account for the observation that the kinetic energy distribution for La
+ for a vacuum ablation had an energetic tail well beyond 300 eV, and a high intensity, since these species experience a relatively long acceleration time in the electric field [
31,
32,
33,
34,
35].
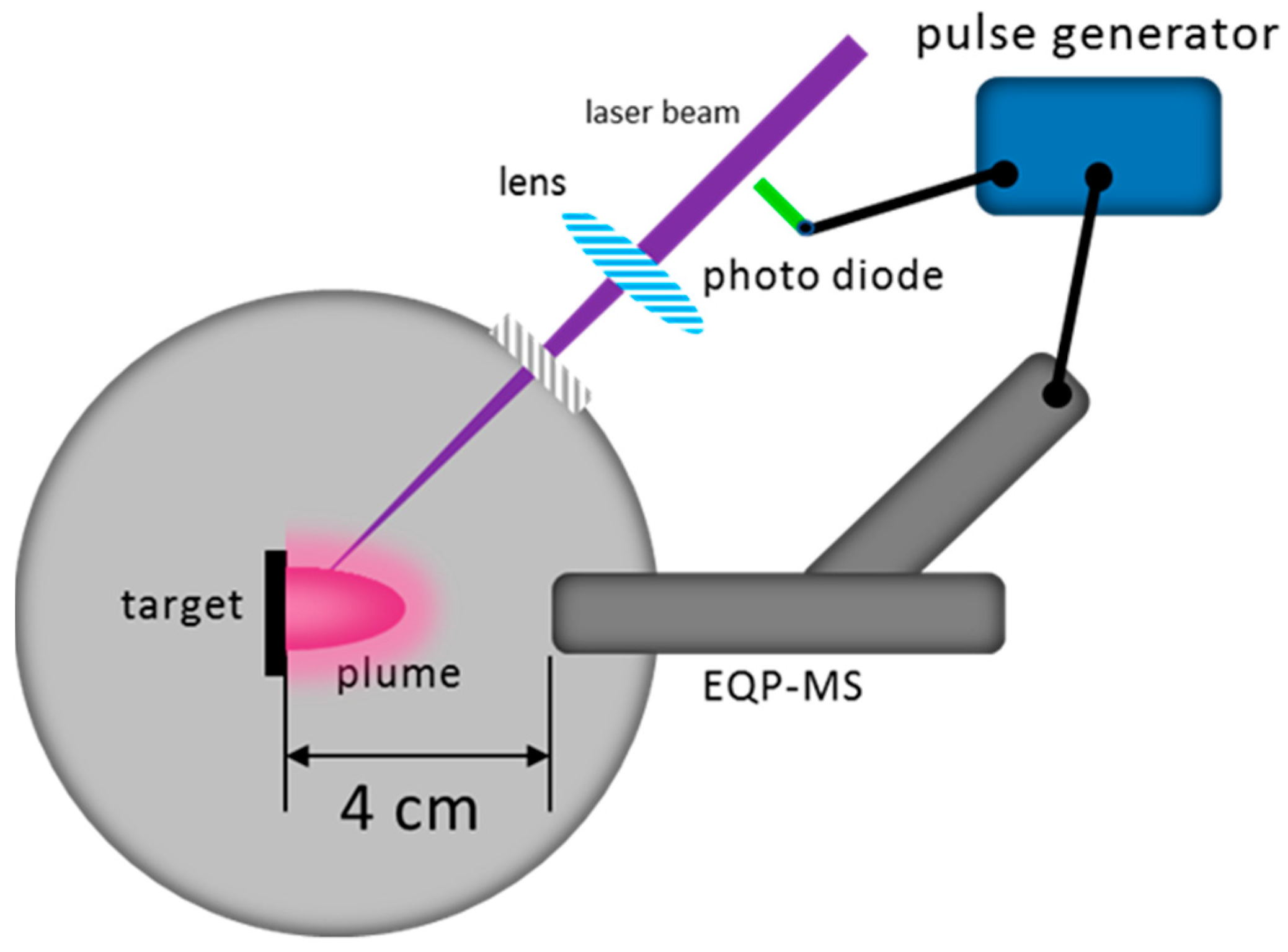
The kinetic energy distribution of neutral species is not well known and was not measured for these experiments. However, it can be safely assumed that they can reach considerable energies due to the almost instantaneous phase explosion when the laser beam interacts with the target material. For a vacuum ablation, fast species reach the position of the mass spectrometer or heater, respectively, within 1 µsec, as measured using time-resolved emission spectroscopy. Those optically monitored species are excited neutral species which can easily reach kinetic energies of 600 eV or more for La I at a fluence of 2 J/cm
2 [
29]. Most other plume species will arrive within 2 to 3 µsec, including all the neutrals. The LaO
+ signal is small because the origin of these species is largely thermal and therefore neutral LaO species are formed before some of them become ionised [
36]. The available acceleration time window for these ionised species is significantly smaller, leading to a kinetic energy distribution with small kinetic energies (
Figure S1c). With the introduction of a background gas, the situation for the scattering kinetics starts to change. A rough estimate of the number of species removed from the target with one pulse approximately corresponds to the number of atoms equivalent to a static pressure in the mbar range (10
21–10
22/m
3). As a consequence of such an initial high local density, the mean free path (mfp) becomes very short (« mm), and even at the time of arrival at the location of the mass spectrometer, the plume density must be large enough to enable a sufficient number of re-excitations via scattering of optically excited species, since the typical lifetime of excited states ranges from ns to several hundred ns. This plume related mfp is still much shorter than a nominal mfp of metres for a pressure of 10
−5 mbar and below, and the number of scattering events until species reach the mass spectrometer is at least 10 to 20. This would also imply that even at a distance 4 cm away from the target, the hydrodynamic conditions are more viscous, rather than molecular. Hence the number of additional scattering events at a relatively low deposition pressure of 5 × 10
−3 mbar is small, but finite, leading to the creation of additional LaO species (ions, excited, neutrals) from La and O species in a reactive atmosphere. As a consequence, the number of LaO
+ species measured in 5 × 10
−3 mbar O
2 increases with an extended kinetic energy range, and at the same time the La
+ signal drops. With increasing pressure, more of the fast species will be stopped, and at the same time, react to LaO species, leading to an overall increase in the number of measured LaO
+ species (
Figure S1c). At this stage a reaction kinetic of the form La
(+,*
) + O
2 ↔ LaO
(+,*
) + O
(+,*
) or La
(+,*
) + O
(+,*
) ↔ LaO
(+,*
) is conceivable. The La
+ kinetic energy distribution at 5 × 10
−2 mbar (
Figure 3c) strongly suggests that mostly La
+ species with a small kinetic energy were involved in the conversion to LaO
+ species. This outlined scenario would also explain why it is difficult to measure a pressure dependence for LaO
+ in Ar. Despite a large bond energy, the readily provided LaO
+ species from the initial ablation process were dissociated or neutralised due to inelastic scattering, in particular, at a higher pressure. Since no new LaO
+ was formed, the signal became too small to be measured at higher pressures.
3.4. Gas Phase Chemistry
For the gas-phase chemistry, it is of interest to verify which kinetic energy range is suitable for the formation of di-atomic species in general, here, in particular LaO+. From the scattering theory a low kinetic energy range is expected to be favourable since the scattering cross section is proportional to Ekin−2. Therefore, La+ (La) species with a low kinetic energy should be the most favourable combination for the formation of LaO+ (LaO) at a high O2 background pressure.
In order to work out the influence of different types of collisions of the IEDs, it is important to consider the gas density, gas molecular mass, and the van der Waals volume of the respective gas environment. As already pointed out, the effective pressure difference between Ar and O
2 for the same nominal pressure is ~40% and the van der Waal’s radii are 188 pm for Ar and 152 pm for O [
37,
38]. A dipole moment should not play a role for a linear molecule such as O
2, assuming elastic and hard-sphere collisions, which is largely correct for Ar. Therefore, at the same nominal pressure, we expect more collisions in Ar than in O
2 and the scattering cross section in Ar is larger, thereby underlining the experimentally known observation of a larger stopping power for Ar, as compared with O
2 [
28]. In addition, since there are predominantly elastic collisions in an Ar gas environment, the chemical reactivity in the plasma is considered to be small.
We turn now to the discussion of the results presented in
Figure 4. First, in
Figure 4a, the comparison of IEDs for Mn
+ in 2.5 × 10
−2 mbar Ar and 5 × 10
−2 mbar O
2 is shown with both IEDs almost overlapping in intensity and kinetic energy range. For both measurements, the number of background molecules were comparable, i.e., the number of potential scattering events, which indicated that the scattering behaviour of Mn
+ in both background gases was very similar. Plotting the total integrated intensity of the IEDs for Mn
+ and Ca
+ in O
2 and Ar (
Figure 4b,c), which corresponded to the total number of species, the change in area with increasing pressure followed a similar pattern for both ions in both background media, with a similar total number of Mn
+ and Ca
+ species for all pressure ranges. After the first initial increase, there was a downturn in the low 10
-2 mbar range, followed by a steep increase at 5 × 10
−2 mbar. The main conclusion was that the majority of slowed-down species, irrespective of the background gas, were indeed pushed to small kinetic energies, which strongly suggested that the expansion of Mn
+ and Ca
+ in O
2 and Ar were mainly influenced by non-reactive collisions. On the other hand, in comparing IEDs for Mn
+ in O
2 and Ar with a comparable number of background species (see
Figure 4a), the total number of species in O
2 were more than twice as large as compared with Mn
+ in Ar. This observation indicated that the observed difference was in part due to non-reactive collisions but also included scattering events as a result of chemical reactions during the ablation in O
2.
This assumption was supported by the pressure dependence of the integrated area of O
+ ions (
Figure 4c). The number of O
+ ions measured in vacuum corresponded to the oxygen derived from the target, and the same was largely correct for
p = 1 × 10
−3 mbar [
3]. The strong dip at
p = 1 × 10
−2 mbar was the consequence of oxygen being scattered more easily, being the lightest element, whereas the recovery of numbers at larger pressures was the result of the background gas starting to be involved in the chemical reactivity of the plasma. This observation confirmed previous observations where most of the oxygen in
La0.6Sr0.4MnO3 films deposited at a higher deposition pressure originated from the background gas [
3]. As for La
+, the corresponding Ar and O
2 pressure dependencies showed a decrease in the number of species with increasing pressure, whereas the number of LaO
+ species increased over the same pressure range by almost two orders. This indicated that the La
+ species was actively involved in the formation of the LaO
+ species with increasing O
2 pressure, and that collisions between La
+ with
Ekin < 50 eV and the O
2 background gas molecules led preferentially to the formation of LaO
+. These measurements further showed that the forward momentum for these heavier species was maintained, which was equally true for La
+ and LaO
+. There the energy maximum moved from ~3 eV for a vacuum ablation to >8 eV at 5 × 10
−2 mbar O
2 (
Figure 4d). At the same time, the energy maximum for LaO
+ changed from ~3 eV to ~1 eV. The pressure dependence of the LaO
+ kinetic energy distribution therefore strongly suggested that slow La
+ species were largely involved in the collision chemistry in the plasma, whereas faster La species seemed to interact via scattering only in a very limited number of cases, and therefore significantly more La
+ species with a broad energy distribution were detected at an elevated background pressure. The low energy peak for the La
+ IED in vacuum and the very confined maximum for the LaO
+ IED at 5 × 10
−2 mbar (
Figure 3d) implied a kinetic energy window of up to 5 eV to be the most effective energy regime to form LaO
+ species defined by a 90% drop in intensity of the La
+ signal. Between 5 and 15 eV, the LaO
+ signal was down by 98% indicating that the majority of LaO
+ species were accounted for.
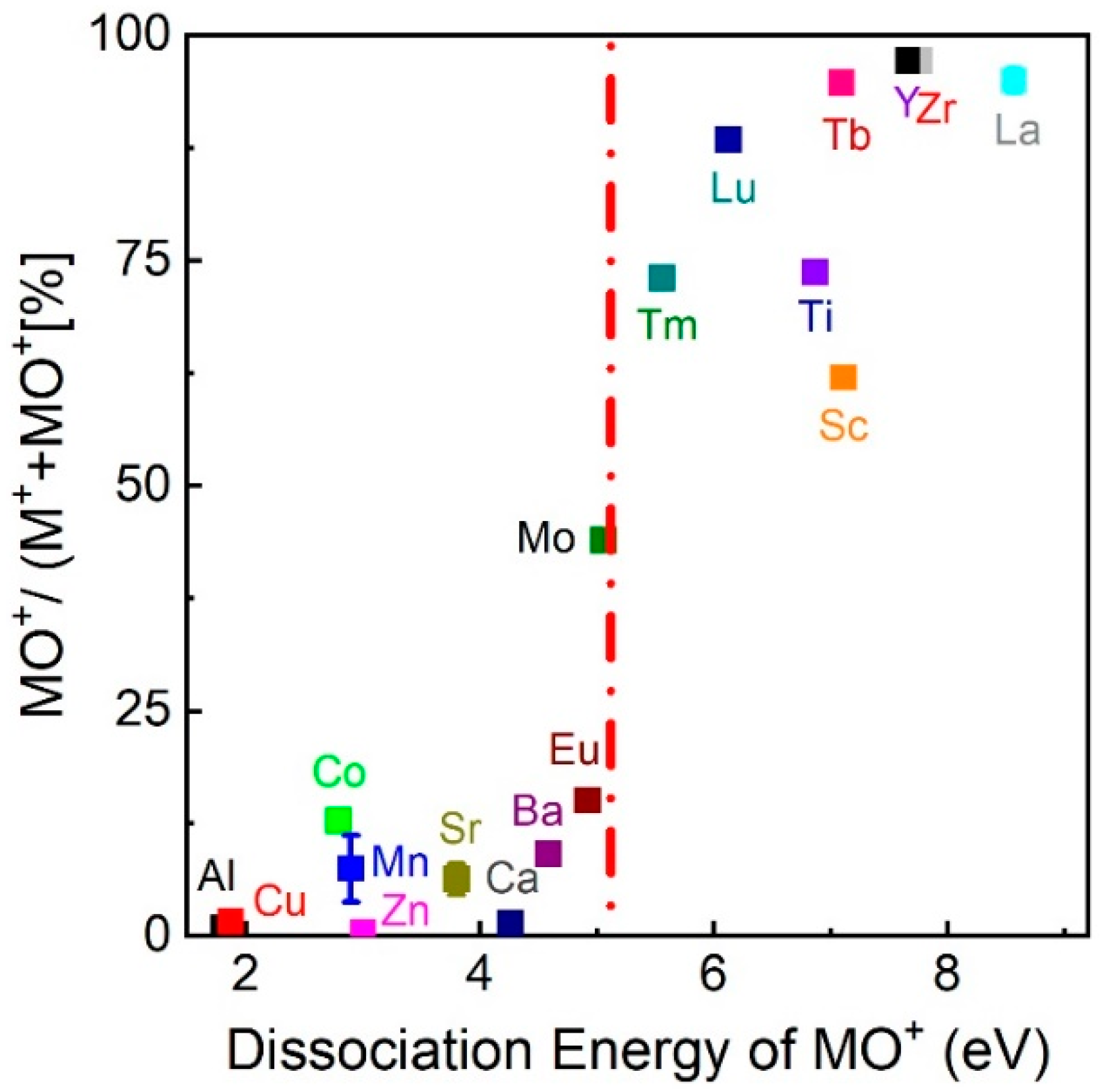
Having identified the low kinetic energy range to be favourable for the formation of LaO
+ at a high O
2 background pressure, we next studied IEDs for a range of different MO
+-ions (M = Zr, Y, Ti, Sc, Lu) with dissociation energies larger than 5.12 eV to confirm whether the plasma processes leading to the formation of these species were comparable.
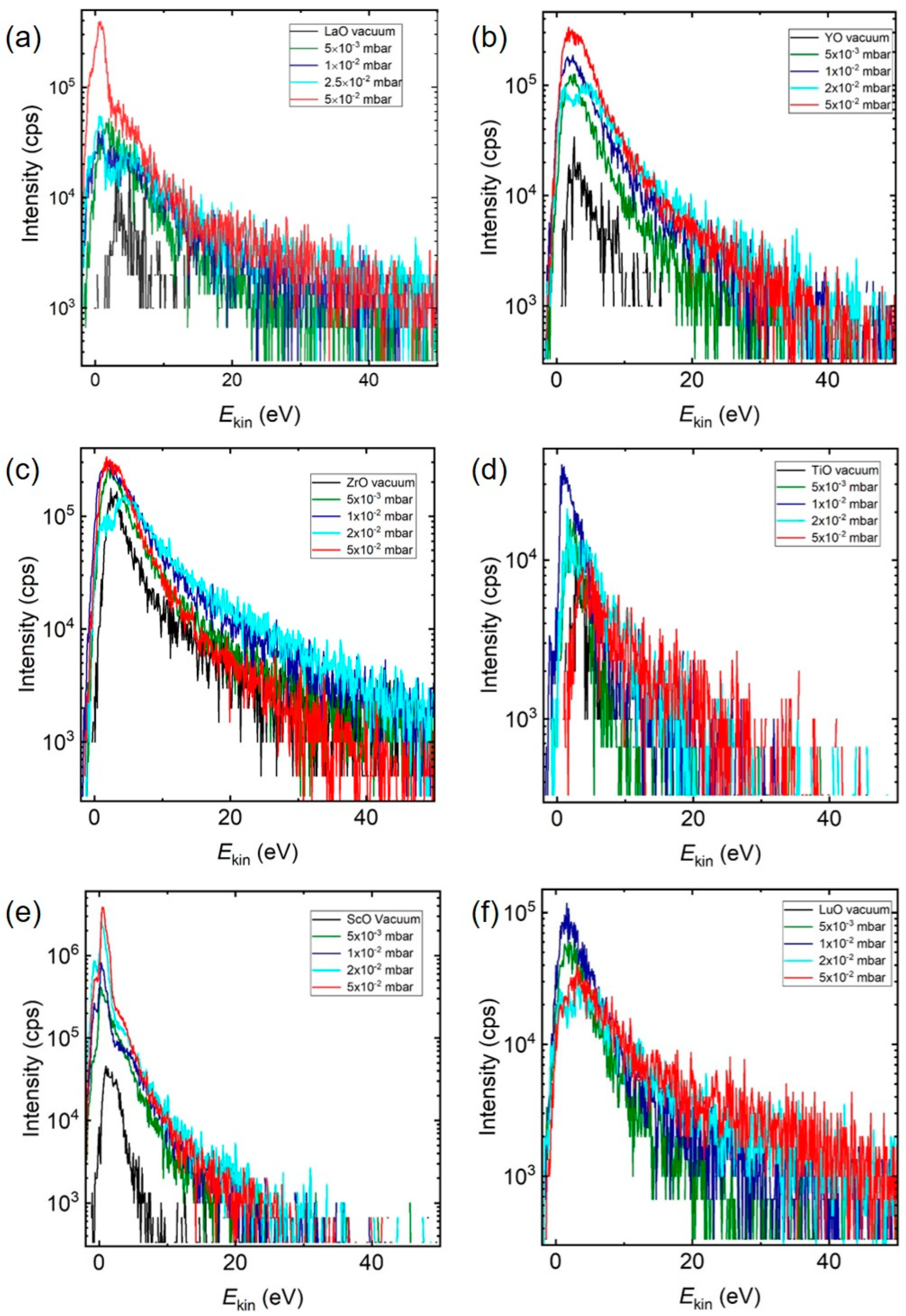
Figure 5 shows the IEDs of MO
+ species selected from
Figure 1 measured at different O
2 pressures (ZrO
+ = 7.76 eV, YO
+ = 7.51 eV, TiO
+ = 6.88 eV, ScO
+ = 7.11 eV, and LuO = 6.89 eV) [
21,
39,
40]. Irrespective of the element and pressure, all MO
+ ions showed a maximum in the IED below 10 eV with an increase in the intensity of
Ekin,max with increasing background pressure. This was the same pattern as observed for La
+/LaO
+, suggesting a preferential reaction between the background gas molecules and low energy ions. Typical for these MO
+ binaries with
Ediss > 5.12 eV were the pressure dependences shown in
Figure 5a–d.
Ekin,max was observed as shifting to smaller energies with increasing pressure, with a simultaneous increase in the number of species measured. Additionally, for a background pressure of a few 10
−2 mbar, the IED developed a double peak structure, indicating a slowing-down of as-ablated, and, during the expansion, created MO species. The pressure dependence shown in
Figure 5e,f was also frequently encountered, but the origin remains unclear at present. However, the increase in total area with increasing pressure was measurable with ~20% for LuO. As already discussed, the chemical reaction cross section decreased with increasing collision energies [
8]. A possible scenario for the gas phase oxidation reaction during the plume expansion in O
2 for other metal species was therefore analogous to La
+:
- (1)
Excited, ionised and ground state species collided with O2 to generate excited, ionised or ground state species of molecular oxygen (O* or O+/−);
- (2)
Ekin of the ions was reduced due to collisions;
- (3)
Low energy ions reacted with O* or O+/− preferentially to form MO+ if Ediss > 5.12 eV.
To study the gas phase reaction dynamics in more detail, complementary measurements, such as time- and species-resolved imaging, need to be conducted [
7,
10,
28].