Fracture Resistance of Monolithic Zirconia Crowns Depending on Different Marginal Thicknesses
Abstract
:1. Introduction
2. Materials and Methods
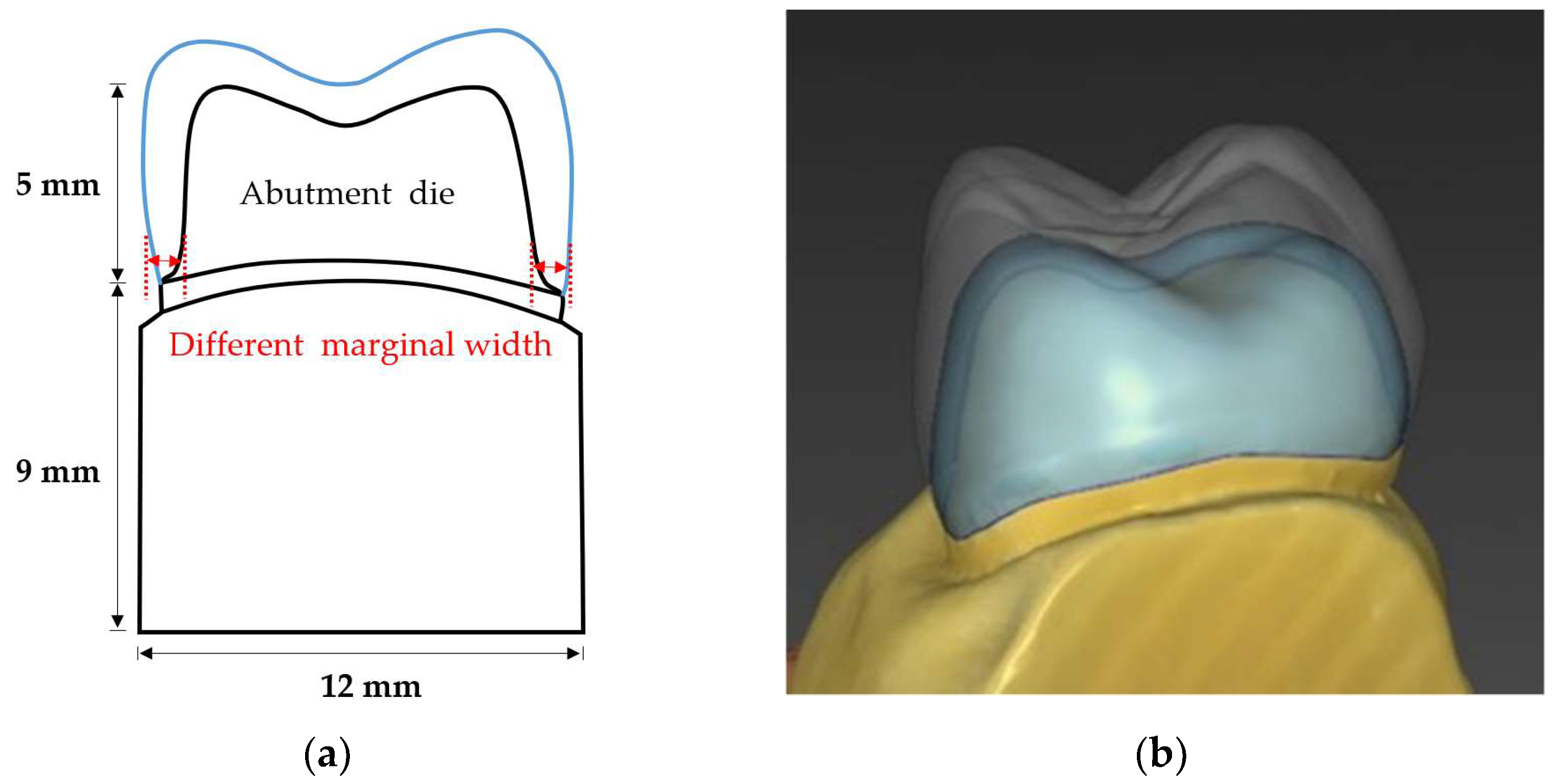
2.1. Abutment Die Preparation
2.2. Crown Design
2.3. Crown Fabrication
2.4. Cementation of Specimens
2.5. Fracture Load Test
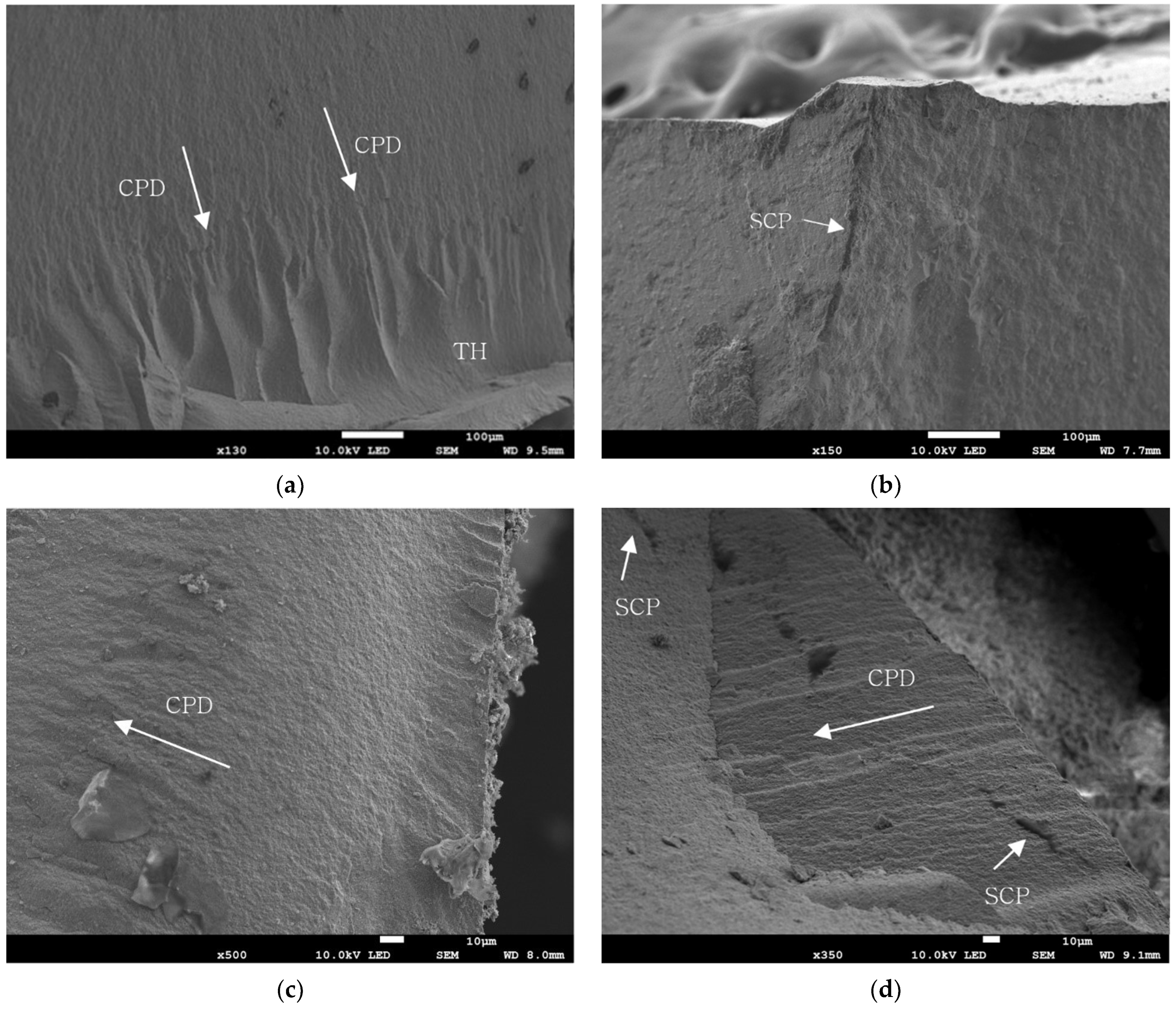
2.6. Evaluation of Fractured Specimen
2.7. Statistical Analysis
3. Results
3.1. Fracture Load Value
3.2. SEM Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suárez, M.J.; Lozano, J.F.; Salido, M.P.; Martínez, F. Three-year clinical evaluation of In-Ceram Zirconia posterior FPDs. Int. J. Prosthodont. 2004, 17, 35–38. [Google Scholar] [PubMed]
- Griggs, J.A. Recent advances in materials for all-ceramic restorations. Dent. Clin. N. Am. 2007, 51, 713–727. [Google Scholar] [CrossRef] [Green Version]
- Raigrodski, A.J.; Hillstead, M.B.; Meng, G.K.; Chung, K.-H. Survival and complications of zirconia-based fixed dental prostheses: A systematic review. J. Prosthet. Dent. 2012, 107, 170–177. [Google Scholar] [CrossRef]
- Ioannidis, A.; Bindl, A. Clinical prospective evaluation of zirconia-based three-unit posterior fixed dental prostheses: Up-to ten-year results. J. Dent. 2016, 47, 80–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papadiochou, S.; Pissiotis, A.L. Marginal adaptation and CAD-CAM technology: A systematic review of restorative material and fabrication techniques. J. Prosthet. Dent. 2018, 119, 545–551. [Google Scholar] [CrossRef]
- Sorrentino, R.; Triulzio, C.; Tricarico, M.G.; Bonadeo, G.; Gherlone, E.F.; Ferrari, M. In vitro analysis of the fracture resistance of CAD–CAM monolithic zirconia molar crowns with different occlusal thickness. J. Mech. Behav. Biomed. Mater. 2016, 61, 328–333. [Google Scholar] [CrossRef]
- Dapieve, K.S.; Silvestri, T.; Rippe, M.P.; Pereira, G.K.R.; Valandro, L.F. Mechanical performance of Y-TZP monolithic ceramic after grinding and aging: Survival estimates and fatigue strength. J. Mech. Behav. Biomed. Mater. 2018, 87, 288–295. [Google Scholar] [CrossRef]
- Sulaiman, T.A.; Abdulmajeed, A.A.; Delgado, A.; Donovan, T.E. Fracture rate of 188695 lithium disilicate and zirconia ceramic restorations after up to 7.5 years of clinical service: A dental laboratory survey. J. Prosthet. Dent. 2020, 123, 807–810. [Google Scholar] [CrossRef]
- Baixauli-López, M.; Roig-Vanaclocha, A.; Amengual-Lorenzo, J.; Agustín-Panadero, R. Prospective study of monolithic zirconia crowns: Clinical behavior and survival rate at a 5-year follow-up. J. Prosthodont. Res. 2021, 65, 284–290. [Google Scholar]
- Sailer, I.; Pjetursson, B.E.; Zwahlen, M.; Hämmerle, C.H. A systematic review of the survival and complication rates of all-ceramic and metal–ceramic reconstructions after an observation period of at least 3 years. Part II: Fixed dental prostheses. Clin. Oral Implant. Res. 2007, 18, 86–96. [Google Scholar] [CrossRef]
- Goodacre, C.J.; Campagni, W.V.; Aquilino, S.A. Tooth preparations for complete crowns: An art form based on scientific principles. J. Prosthet. Dent. 2001, 85, 363–376. [Google Scholar] [CrossRef] [Green Version]
- Manicone, P.F.; Iommetti, P.R.; Raffaelli, L. An overview of zirconia ceramics: Basic properties and clinical applications. J. Dent. 2007, 35, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Winkelmeyer, C.; Wolfart, S.; Marotti, J. Analysis of tooth preparations for zirconia-based crowns and fixed dental prostheses using stereolithography data sets. J. Prosthet. Dent. 2016, 116, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Poon, B.K.; Smales, R.J. Assessment of clinical preparations for single gold and ceramometal crowns. Quintessence Int. 2001, 32, 603–610. [Google Scholar] [PubMed]
- Al-Omari, W.M.; Al-Wahadni, A.M. Convergence angle, occlusal reduction, and finish line depth of full-crown preparations made by dental students. Quintessence Int. 2004, 35, 287–293. [Google Scholar]
- Tiu, J.; Lin, T.; Al-Amleh, B.; Waddell, J.N. Convergence angles and margin widths of tooth preparations by New Zealand dental students. J. Prosthet. Dent. 2016, 116, 74–79. [Google Scholar] [CrossRef]
- Tiu, J.; Al-Amleh, B.; Waddell, J.N.; Duncan, W.J. Reporting numeric values of complete crowns. Part 1: Clinical preparation parameters. J. Prosthet. Dent. 2015, 114, 67–74. [Google Scholar] [CrossRef]
- Mitov, G.; Anastassova-Yoshida, Y.; Nothdurft, F.P.; Von See, C.; Pospiech, P. Influence of the preparation design and artificial aging on the fracture resistance of monolithic zirconia crowns. J. Adv. Prosthodont. 2016, 8, 30–36. [Google Scholar] [CrossRef] [Green Version]
- Abdulazeez, M.I.; Majeed, M.A. Fracture strength of monolithic zirconia crowns with modified vertical preparation: A comparative in vitro study. Eur. J. Dent. 2022, 16, 209–214. [Google Scholar] [CrossRef]
- Haddad, C.; Azzi, K. Influence of the Type and Thickness of Cervical Margins on the Strength of Posterior Monolithic Zirconia Crowns: A Review. Eur. J. Gen. Dent. 2022. [Google Scholar] [CrossRef]
- Alzahrani, A.M.; Beyari, A.M.; Emam, Z.N. The influence of the cervical finish line designs on the fracture resistance of CAD/CAM monolithic zirconia crowns: An in vitro study. Int. J. Health Sci. Res. 2018, 8, 101–110. [Google Scholar]
- Shahmoradi, M.; Wan, B.; Zhang, Z.; Wilson, T.; Swain, M.; Li, Q. Monolithic crowns fracture analysis: The effect of material properties, cusp angle and crown thickness. Dent. Mater. 2020, 36, 1038–1051. [Google Scholar] [CrossRef] [PubMed]
- Juntavee, N.; Kornrum, S. Effect of marginal designs on fracture strength of high translucency monolithic zirconia crowns. Int. J. Dent. 2020, 2020, 8875609. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, V.F.; Sforza, C.; Zanotti, G.; Tartaglia, G.M. Maximal bite forces in healthy young adults as predicted by surface electromyography. J. Dent. 2004, 32, 451–457. [Google Scholar] [CrossRef]
- Varga, S.; Spalj, S.; Lapter Varga, M.; Anic Milosevic, S.; Mestrovic, S.; Slaj, M. Maximum voluntary molar bite force in subjects with normal occlusion. Eur. J. Orthod. 2011, 33, 427–433. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, H.; Aydin, C.; Gul, B.E. Flexural strength and fracture toughness of dental core ceramics. J. Prosthet. Dent. 2007, 98, 120–128. [Google Scholar] [CrossRef]
- Kato, H.; Matsumura, H.; Tanaka, T.; Atsuta, M. Bond strength and durability of porcelain bonding systems. J. Prosthet. Dent. 1996, 75, 163–168. [Google Scholar] [CrossRef]
- Tinschert, J.; Natt, G.; Mautsch, W.; Augthun, M.; Spiekermann, H. Fracture Resistance of Lithium Disilicate-, Alumina-, and Zirconia-Based Three-Unit Fixed Partial Dentures: A Laboratory Study. Int. J. Prosthodont. 2001, 14, 231–238. [Google Scholar]
- Sundh, A.; Molin, M.; Sjögren, G. Fracture resistance of yttrium oxide partially-stabilized zirconia all-ceramic bridges after veneering and mechanical fatigue testing. Dent. Mater. 2005, 21, 476–482. [Google Scholar] [CrossRef]
- Schultheis, S.; Strub, J.R.; Gerds, T.A.; Guess, P.C. Monolithic and bi-layer CAD/CAM lithium–disilicate versus metal–ceramic fixed dental prostheses: Comparison of fracture loads and failure modes after fatigue. Clin. Oral Investig. 2013, 17, 1407–1413. [Google Scholar] [CrossRef]
- Choi, Y.-S.; Kim, S.-H.; Lee, J.-B.; Han, J.-S.; Yeo, I.-S. In vitro evaluation of fracture strength of zirconia restoration veneered with various ceramic materials. J. Adv. Prosthodont. 2012, 4, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.; Bonfante, E.; Silva, N.; Rekow, E.; Thompson, V. Laboratory simulation of Y-TZP all-ceramic crown clinical failures. J. Dent. Res. 2009, 88, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Jian, Y.; Zhang, T.; Wang, X.; Kyaw, L.; Pow, E.H.N.; Zhao, K. Effect of supporting dies’ mechanical properties on fracture behavior of monolithic zirconia molar crowns. Dent. Mater. J. 2022, 41, 249–255. [Google Scholar] [CrossRef]
- Stanford, J.W.; Paffenbarger, G.; Kumpula, J.W.; Sweeney, W. Determination of some compressive properties of human enamel and dentin. J. Am. Dent. Assoc. 1958, 57, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Rosentritt, M.; Plein, T.; Kolbeck, C.; Behr, M.; Handel, G. In vitro fracture force and marginal adaptation of ceramic crowns fixed on natural and artificial teeth. Int. J. Prosthodont. 2000, 13, 387–391. [Google Scholar] [PubMed]
- Aboushelib, M.N.; Elmahy, W.A.; Ghazy, M.H. Internal adaptation, marginal accuracy and microleakage of a pressable versus a machinable ceramic laminate veneers. J. Dent. 2012, 40, 670–677. [Google Scholar] [CrossRef]
- Gherlone, E.F.; Capparé, P.; Pasciuta, R.; Grusovin, M.G.; Mancini, N.; Burioni, R. Evaluation of resistance against bacterial microleakage of a new conical implant-abutment connection versus conventional connections: An in vitro study. New Microbiol. 2016, 39, 49–56. [Google Scholar]
- Tetè, G.; Sacchi, L.; Camerano, C.; Nagni, M.; Capelli, O.; La Rocca, G.; Polizzi, E. Management of the delicate phase of the temporary crown: An in vitro study. J. Biol. Regul. Homeost. Agents 2020, 34 (Suppl. S3), 67–78. [Google Scholar]
- Aboushelib, M.N.; Feilzer, A.J.; Kleverlaan, C.J. Bridging the gap between clinical failure and laboratory fracture strength tests using a fractographic approach. Dent. Mater. 2009, 25, 383–391. [Google Scholar] [CrossRef]
- Cappare, P.; Ferrini, F.; Mariani, G.; Nagni, M.; Cattoni, F. Implant rehabilitation of edentulous jaws with predominantly monolithic zirconia compared to metal-acrylic prostheses: A 2-year retrospective clinical study. J. Biol. Regul. Homeost. Agents 2021, 35 (Suppl. S1), 99–112. [Google Scholar]

| Mechanical Property | Value |
|---|---|
| Tensile modulus [MPa] | 492–583 |
| Maximum tensile strength [MPa] | 26–34 |
| Elongation at break [%] | 5–6 |
| Flexural modulus [MPa] | 173–191 |
| Flexural strength [MPa] | 21–33 |
| Shore D hardness | 77–85 |
| Viscosity [Pas] | 0.7–0.8 |
| Material | Value [Wt. %] | Test Method |
|---|---|---|
| ZrO2 | 88–90 | ICP |
| Y2O3 | 7.0–8.0 | |
| SiO2 | ≤0.01 | |
| Fe2O3 | ≤0.001 | |
| CaO | ≤0.007 | |
| Na2O | ≤0.004 |
| Mechanical Property | Value | Test Method |
|---|---|---|
| Bending strength | 1100 ± 50 [Mpa] | 3-point-bending ISO 6872 |
| Sintered density | ≥6.04 [g/cm3] | Archimedes’ method |
| Transparency | ≥46 [%] | Spectrophotometer |
| Thermal expansion coefficient | 10.5 × 10−6 [K−1] | ASTM test method E 289 |
| Fracture Strength [N] | Control Group (1.0 mm) | Group A (0.8 mm) | Group B (0.6 mm) | Group C (0.4 mm) |
|---|---|---|---|---|
| Mean | 3090.91 | 2645.39 | 2256.85 | 1957.8 |
| Standard deviation | 527.77 | 329.21 | 454.15 | 522.14 |
| Groups | Mean Difference [N] | p-Value | Lower Control Limit | Upper Control Limit |
|---|---|---|---|---|
| Control–Group A | 445.52 | 0.2346 | −135.386 | 1026.426 |
| Control–Group B | 834.06 * | 0.0018 | 253.154 | 1414.966 |
| Control–Group C | 1133.11 * | <0.001 | 552.204 | 1714.016 |
| Group A–Group B | 388.54 | 0.4200 | −192.366 | 969.446 |
| Group A–Group C | 687.59 * | 0.0130 | 106.684 | 1268.496 |
| Group B–Group C | 299.05 | 0.9556 | −281.856 | 879.956 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-H.; Yeo, M.-Y.; Choi, S.-Y.; Park, E.-J. Fracture Resistance of Monolithic Zirconia Crowns Depending on Different Marginal Thicknesses. Materials 2022, 15, 4861. https://doi.org/10.3390/ma15144861
Kim S-H, Yeo M-Y, Choi S-Y, Park E-J. Fracture Resistance of Monolithic Zirconia Crowns Depending on Different Marginal Thicknesses. Materials. 2022; 15(14):4861. https://doi.org/10.3390/ma15144861
Chicago/Turabian StyleKim, Seung-Han, Mi-Yeon Yeo, Sun-Young Choi, and Eun-Jin Park. 2022. "Fracture Resistance of Monolithic Zirconia Crowns Depending on Different Marginal Thicknesses" Materials 15, no. 14: 4861. https://doi.org/10.3390/ma15144861
APA StyleKim, S.-H., Yeo, M.-Y., Choi, S.-Y., & Park, E.-J. (2022). Fracture Resistance of Monolithic Zirconia Crowns Depending on Different Marginal Thicknesses. Materials, 15(14), 4861. https://doi.org/10.3390/ma15144861






