Study on Green Degradation Process of Polyurethane Foam Based on Integral Utilization and Performance of Recycled Polyurethane Oil-Absorbing Foam
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Glycolysis
2.2.2. Synthetic Recycled PU Foam
2.2.3. Amino Silane Modified Recycled PU Foam
2.3. Characterization
2.4. Oil-Absorbing Capacity
2.5. Reusable Performance
3. Results and Discussion
3.1. Degradation and Characterization of Polyurethane Foams
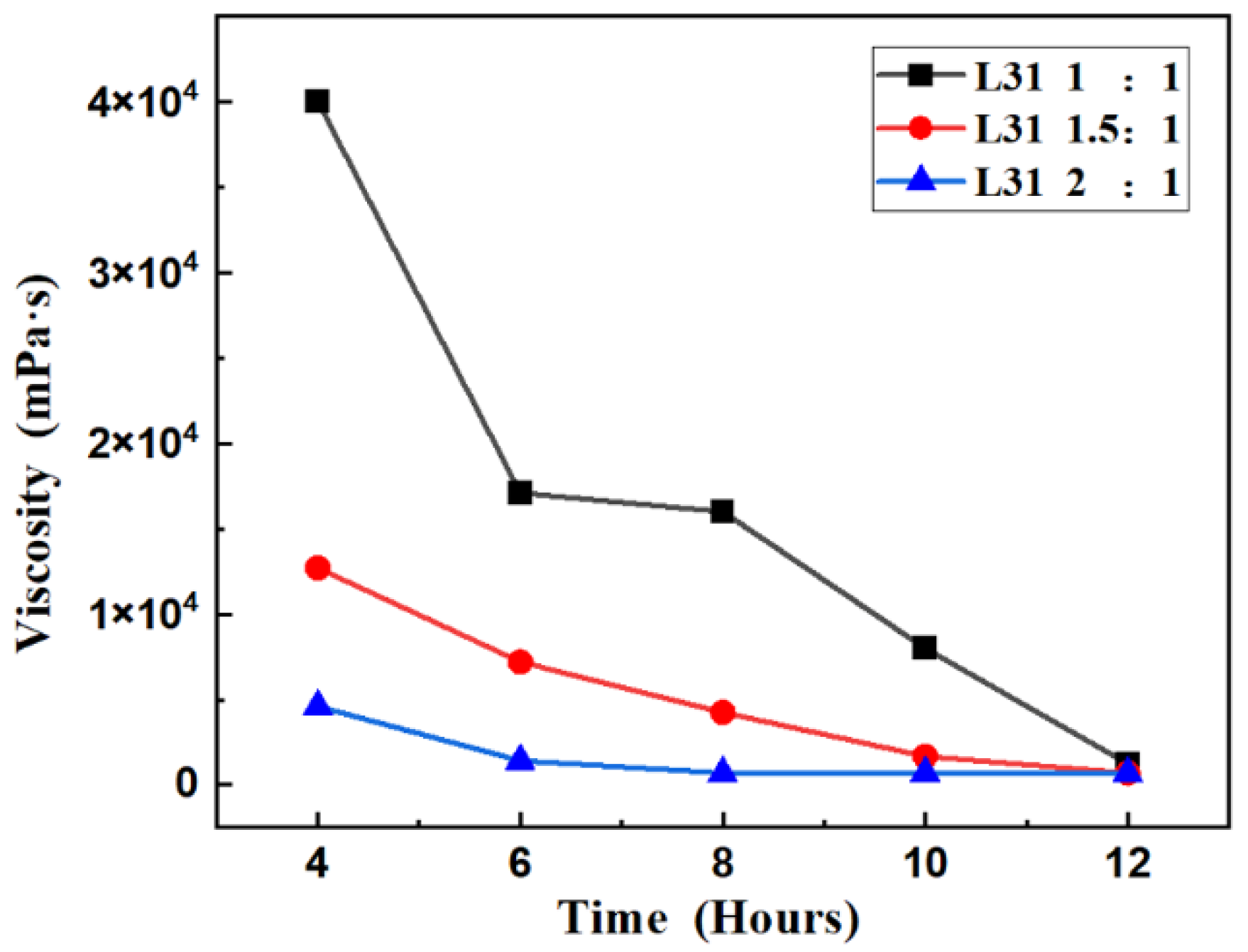
3.1.1. Degradation Performance of Tirblock Polyether (PEG-PPG-PEG) and Polyether Polyols (PPG)
3.1.2. Molecular Weight and Composition Analysis of Degradation Products
3.2. Study on Recycled PU Foam and Its Oil Absorption Performance
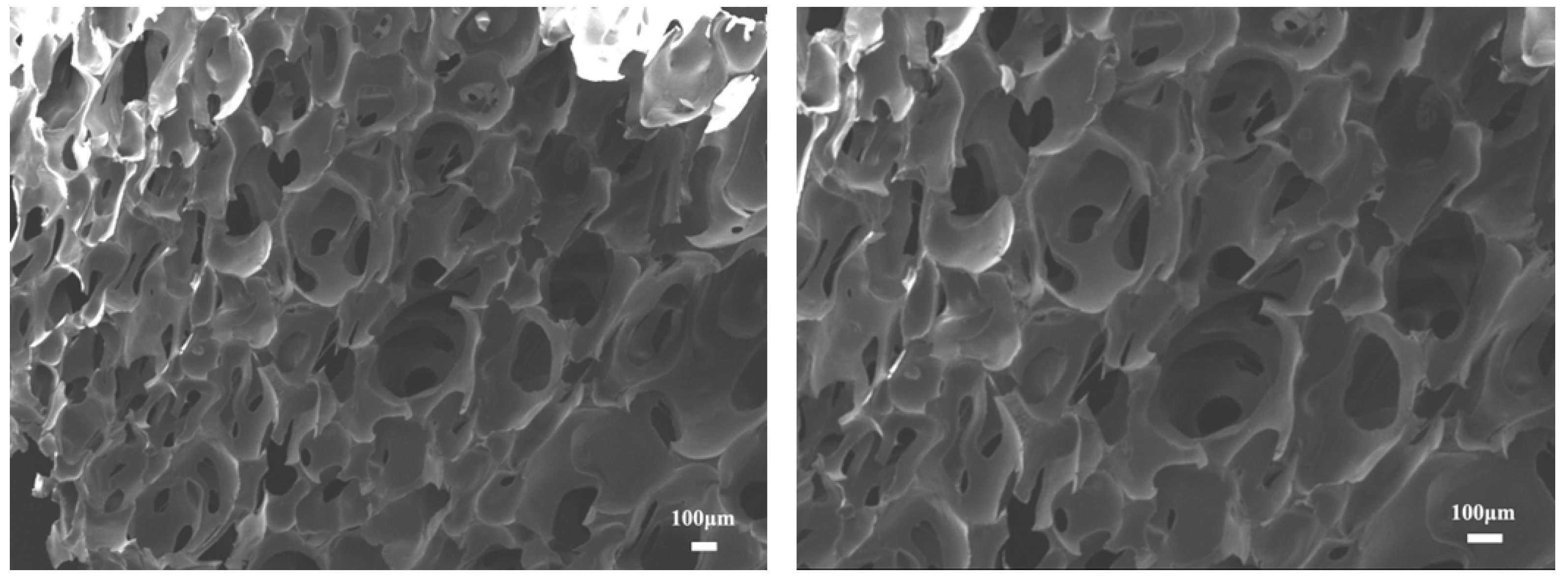
3.2.1. Micromorphology of Recycled PU Foam
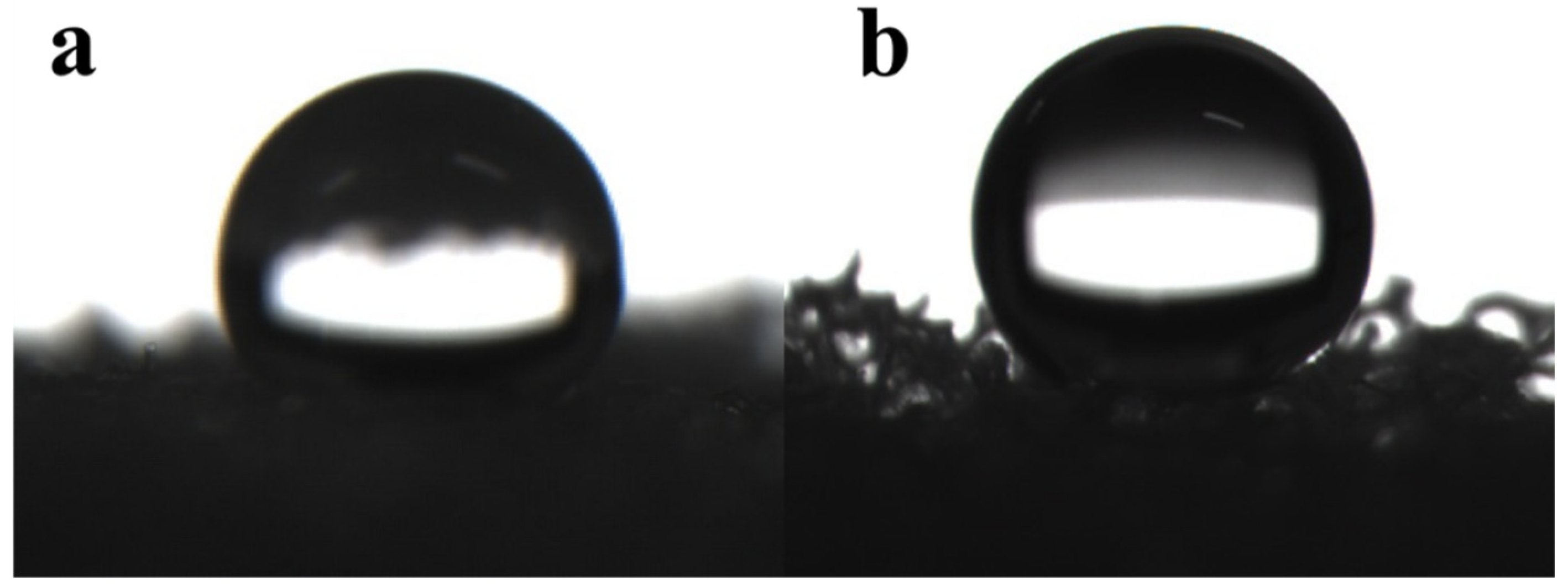
3.2.2. Hydrophobic Performance of Recycled PU Oil-Absorbing Foam
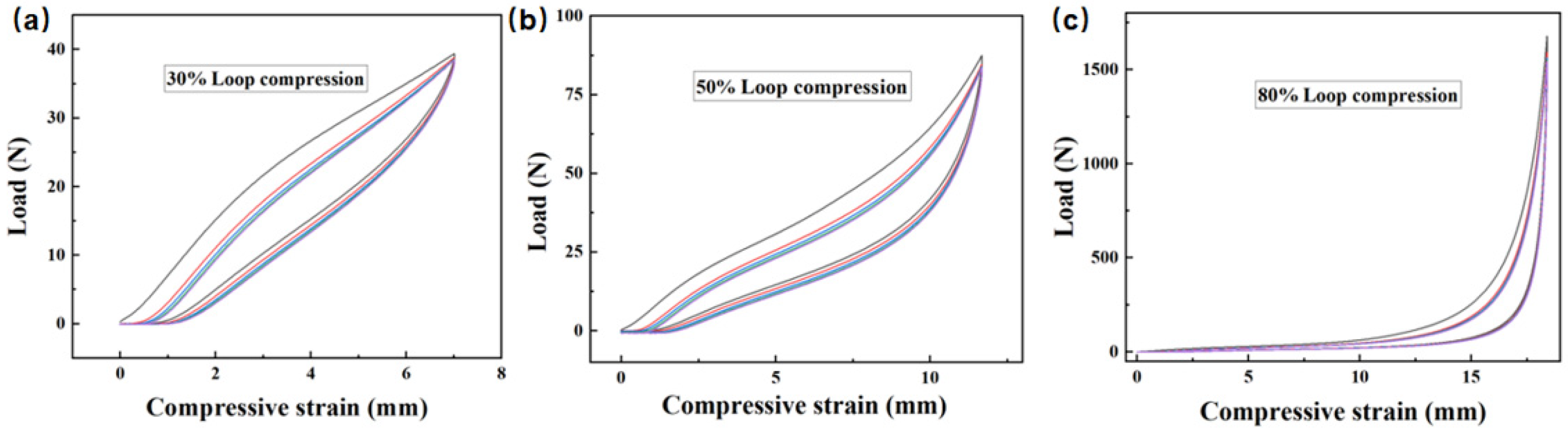
3.2.3. Compression Performance of Recycled Oil-Absorbing PU Foam
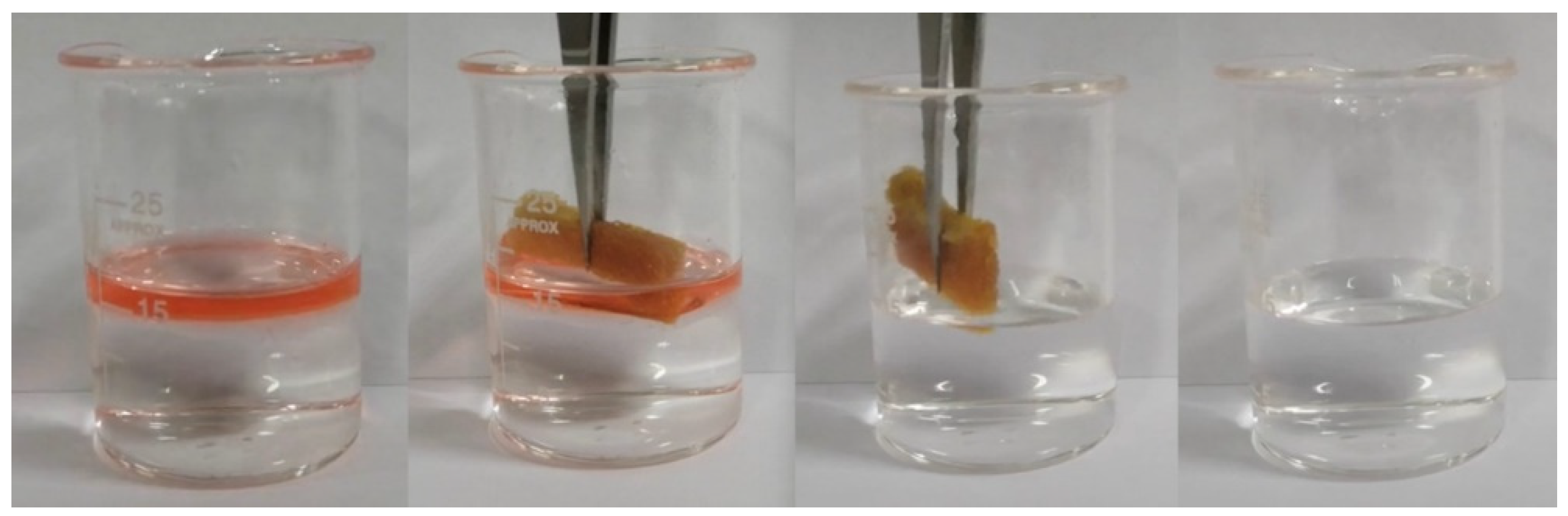
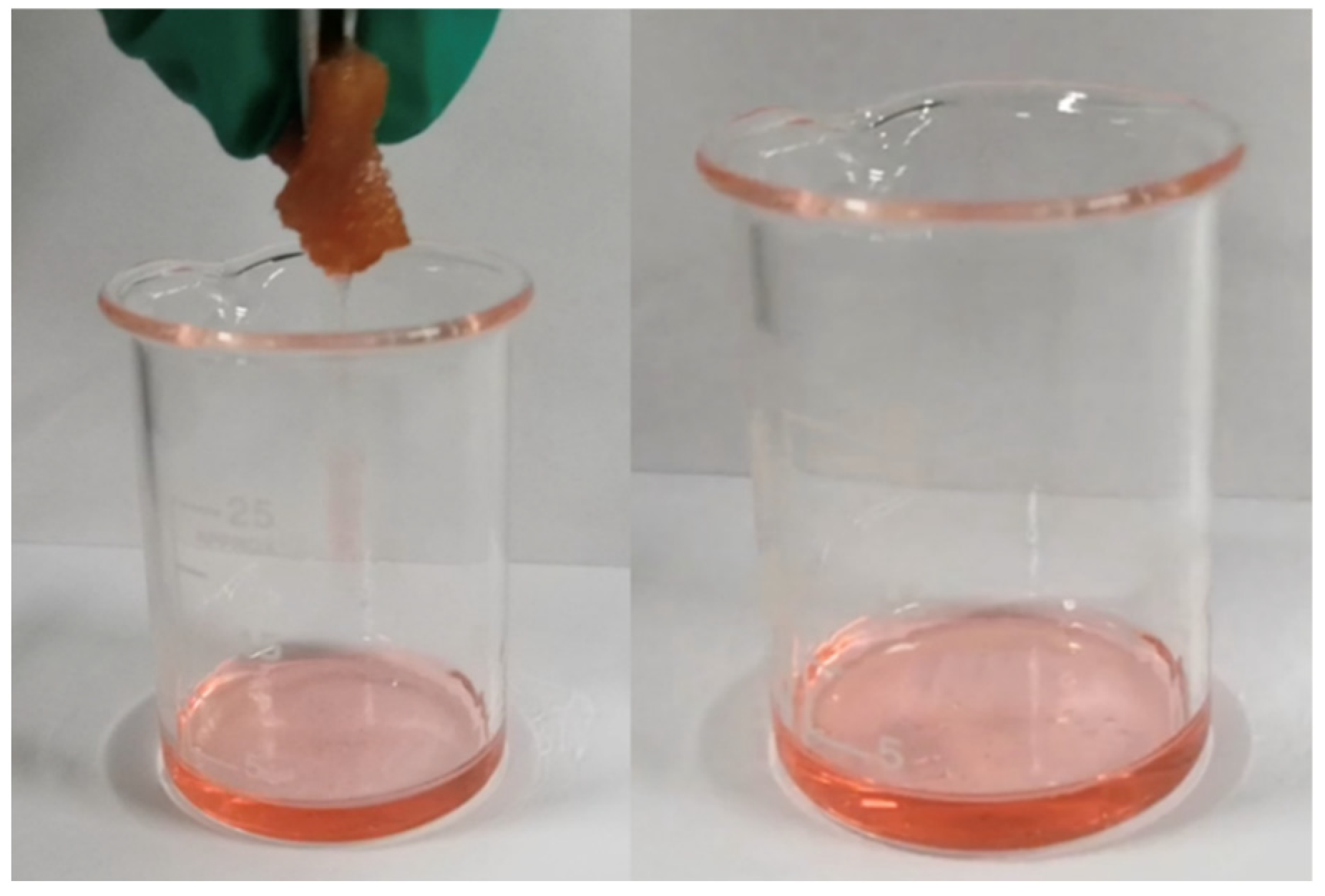
3.2.4. Oil-Absorbing Capacity of Recycled Oil-Absorbing PU Foam
3.2.5. Reusable Performance
3.2.6. Oil–Water Separation Selection Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, A.; Kumar, R.; Gupta, A.; Agrawal, P.R.; Dwivedi, N.; Mondal, D.P.; Srivastava, A.K.; Dhakate, S.R. Enhanced Electromagnetic Interference Shielding Properties of Phenolic Resin Derived Lightweight Carbon Foam Decorated with Electrospun Zinc Oxide Nanofibers. Mater. Today Commun. 2022, 30, 103055. [Google Scholar] [CrossRef]
- Pietras, D.; Linul, E.; Sadowski, T.; Rusinek, A. Out-of-Plane Crushing Response of Aluminum Honeycombs in-Situ Filled with Graphene-Reinforced Polyurethane Foam. Compos. Struct. 2020, 249, 112548. [Google Scholar] [CrossRef]
- Sebaey, T.A.; Rajak, D.K.; Mehboob, H. Internally Stiffened Foam-Filled Carbon Fiber Reinforced Composite Tubes under Impact Loading for Energy Absorption Applications. Compos. Struct. 2021, 255, 112910. [Google Scholar] [CrossRef]
- Nikje, M.M.A.; Garmarudi, A.B.; Idris, A.B. Polyurethane Waste Reduction and Recycling: From Bench to Pilot Scales. Des. Monomers Polym. 2011, 14, 395–421. [Google Scholar] [CrossRef]
- Simón, D.; Borreguero, A.M.; de Lucas, A.; Rodríguez, J.F. Recycling of Polyurethanes from Laboratory to Industry, a Journey towards the Sustainability. Waste Manag. 2018, 76, 147–171. [Google Scholar] [CrossRef]
- Behrendt, G.; Naber, B.W. The Chemical Recycling of Polyurethanes. J. Univ. Chem. Technol. Met. 2009, 44, 3–23. [Google Scholar]
- Del Amo, J.; Simon, D.; Ramos, M.J.; Rodriguez, J.F.; De Lucas, A.; Borreguero, A.M. Scaled-up and Economic Assessment Approach of the Split-Phase Glycolysis Process for the Recycling of Flexible Polyurethane Foam Wastes. J. Mater. Cycles Waste Manag. 2022, 24, 1059–1071. [Google Scholar] [CrossRef]
- Modesti, M.; Simioni, F.; Munari, R.; Baldoin, N. Recycling of Flexible Polyurethane Foams with a Low Aromatic Amine Content. React. Funct. Polym. 1995, 26, 157–165. [Google Scholar] [CrossRef]
- Simón, D.; García, M.T.; de Lucas, A.; Borreguero, A.M.; Rodríguez, J.F. Glycolysis of Flexible Polyurethane Wastes Using Stannous Octoate as the Catalyst: Study on the Influence of Reaction Parameters. Polym. Degrad. Stab. 2013, 98, 144–149. [Google Scholar] [CrossRef]
- Simón, D.; Borreguero, A.M.; de Lucas, A.; Rodríguez, J.F. Glycolysis of Flexible Polyurethane Wastes Containing Polymeric Polyols. Polym. Degrad. Stab. 2014, 109, 115–121. [Google Scholar] [CrossRef]
- Molero, C.; de Lucas, A.; Romero, F.; Rodríguez, J.F. Influence of the Use of Recycled Polyols Obtained by Glycolysis on the Preparation and Physical Properties of Flexible Polyurethane. J. Appl. Polym. Sci. 2008, 109, 617–626. [Google Scholar] [CrossRef]
- Datta, J.; Haponiuk, J.T. Influence of Glycols on the Glycolysis Process and the Structure and Properties of Polyurethane Elastomers. J. Elastomers Plast. 2011, 43, 529–541. [Google Scholar] [CrossRef]
- Molero, C.; de Lucas, A.; Rodríguez, J.F. Recovery of Polyols from Flexible Polyurethane Foam by “Split-Phase” Glycolysis: Glycol Influence. Polym. Degrad. Stab. 2006, 91, 221–228. [Google Scholar] [CrossRef]
- Molero, C.; de Lucas, A.; Rodríguez, J.F. Activities of Octoate Salts as Novel Catalysts for the Transesterification of Flexible Polyurethane Foams with Diethylene Glycol. Polym. Degrad. Stab. 2009, 94, 533–539. [Google Scholar] [CrossRef]
- Simón, D.; Borreguero, A.M.; de Lucas, A.; Rodríguez, J.F. Valorization of Crude Glycerol as a Novel Transesterification Agent in the Glycolysis of Polyurethane Foam Waste. Polym. Degrad. Stab. 2015, 121, 126–136. [Google Scholar] [CrossRef]
- Datta, J.; Kopczyńska, P.; Simón, D.; Rodríguez, J.F. Thermo-Chemical Decomposition Study of Polyurethane Elastomer Through Glycerolysis Route with Using Crude and Refined Glycerine as a Transesterification Agent. J. Polym. Environ. 2018, 26, 166–174. [Google Scholar] [CrossRef] [Green Version]
- Vanbergen, T.; Verlent, I.; De Geeter, J.; Haelterman, B.; Claes, L.; De Vos, D. Recycling of Flexible Polyurethane Foam by Split-Phase Alcoholysis: Identification of Additives and Alcoholyzing Agents to Reach Higher Efficiencies. ChemSusChem 2020, 13, 3835–3843. [Google Scholar] [CrossRef]
- Kiss, G.; Rusu, G.; Peter, F.; Tanase, I.; Bandur, G. Recovery of Flexible Polyurethane Foam Waste for Efficient Reuse in Industrial Formulations. Polymers 2020, 12, 1533. [Google Scholar] [CrossRef]
- Zhang, T.; Kong, L.; Dai, Y.; Yue, X.; Rong, J.; Qiu, F.; Pan], J. Enhanced Oils and Organic Solvents Absorption by Polyurethane Foams Composites Modified with MnO2 Nanowires. Chem. Eng. J. 2017, 309, 7–14. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, L.; Zhang, Y.; Wang, A. Junping. Mussel and Fish Scale-Inspired Underwater Superoleophobic Kapok Membranes for Continuous and Simultaneous Removal of Insoluble Oils and Soluble Dyes in Water. J. Mater. Chem. Mater. Energy Sustain. 2015, 3, 18475–18482. [Google Scholar] [CrossRef]
- Ben Moshe, S.; Rytwo, G. Thiamine-Based Organoclay for Phenol Removal from Water. Appl. Clay Sci. 2018, 155, 50–56. [Google Scholar] [CrossRef]
- Zhang, R.-Z.; Quan, S.; Xia, M.; Wang, Q.; Zhang, W.; Yang, J.-M. Effect of Surface Charge Status of Amorphous Porous Coordination Polymer Particles on the Adsorption of Organic Dyes from an Aqueous Solution. J. Colloid Interface Sci. 2018, 525, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.A.; Mohamed, M.; Deyab, N. A Simple Method for Developing Efficient Room Temperature Reduced Graphene Oxide-Coated Polyurethane Sponge and Cotton for Oil-Water Separation. Sep. Sci. Technol. 2022. [Google Scholar] [CrossRef]



| Glycolysis Agent | L31 | |||
|---|---|---|---|---|
| Mass Ratio | 1:1 | 1.5:1 | 2:1 | |
| Time | ||||
| 4 h | A1 | B1 | C1 | |
| 6 h | A2 | B2 | C2 | |
| 8 h | A3 | B3 | C3 | |
| 10 h | A4 | B4 | C4 | |
| 12 h | A5 | B5 | C5 | |
| Glycolysis Agent | L31 | HSH-210 | ||||
|---|---|---|---|---|---|---|
| Mass Ratios | 1:1 | 1.5:1 | 2:1 | 3:1 | 4:1 | |
| Time | ||||||
| 2 h | Split * | Split * | Split | Split * | Split * | |
| 4 h | Split | Split | Split | Split * | Split * | |
| 6 h | Split | Split | Single-phase | Split * | Split * | |
| 8 h | Split | Single-phase | Single-phase | Split * | Split | |
| 10 h | Split | Single-phase | Single-phase | Split | Split | |
| 12 h | Single-phase | Single-phase | Single-phase | Split | Single-phase | |
| Oils | Gasoline | Diesel Oil | Canola Oil |
|---|---|---|---|
| K1 (before modification) | 4.7 | 4.3 | 3.1 |
| K2 (after modification) | 6.2 | 6.7 | 4.8 |
| Times | 5 | 10 | 15 | 20 | 25 |
|---|---|---|---|---|---|
| K | 4.9 | 5.28 | 5.21 | 4.8 | 4.7 |
| WCA | 143.6° | 145.7° | 146.4° | 145.5° | 144.6° |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, S.; Gong, D.; Zhou, Y.; Zhang, C.; Li, Y.; Zhang, C.; Sheng, Y. Study on Green Degradation Process of Polyurethane Foam Based on Integral Utilization and Performance of Recycled Polyurethane Oil-Absorbing Foam. Materials 2022, 15, 4269. https://doi.org/10.3390/ma15124269
Peng S, Gong D, Zhou Y, Zhang C, Li Y, Zhang C, Sheng Y. Study on Green Degradation Process of Polyurethane Foam Based on Integral Utilization and Performance of Recycled Polyurethane Oil-Absorbing Foam. Materials. 2022; 15(12):4269. https://doi.org/10.3390/ma15124269
Chicago/Turabian StylePeng, Shu, Depeng Gong, Youliang Zhou, Chaocan Zhang, Yinchun Li, Chunyang Zhang, and Yitian Sheng. 2022. "Study on Green Degradation Process of Polyurethane Foam Based on Integral Utilization and Performance of Recycled Polyurethane Oil-Absorbing Foam" Materials 15, no. 12: 4269. https://doi.org/10.3390/ma15124269
APA StylePeng, S., Gong, D., Zhou, Y., Zhang, C., Li, Y., Zhang, C., & Sheng, Y. (2022). Study on Green Degradation Process of Polyurethane Foam Based on Integral Utilization and Performance of Recycled Polyurethane Oil-Absorbing Foam. Materials, 15(12), 4269. https://doi.org/10.3390/ma15124269






