The Effects of Ru4+ Doping on LiNi0.5Mn1.5O4 with Two Crystal Structures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Synthesis
2.2. Characterization
2.3. Preparation of Electrodes and Construction of Cells
2.4. Electrochemical Measurements
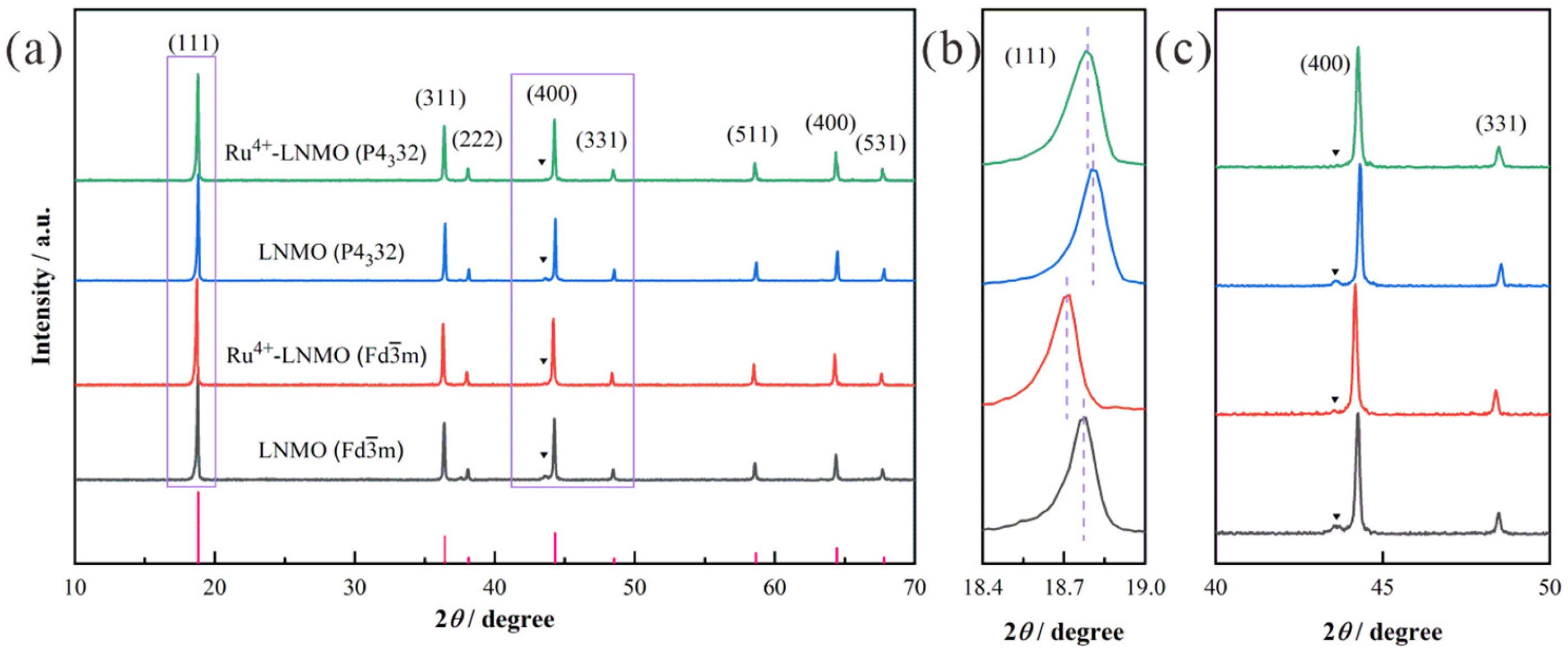
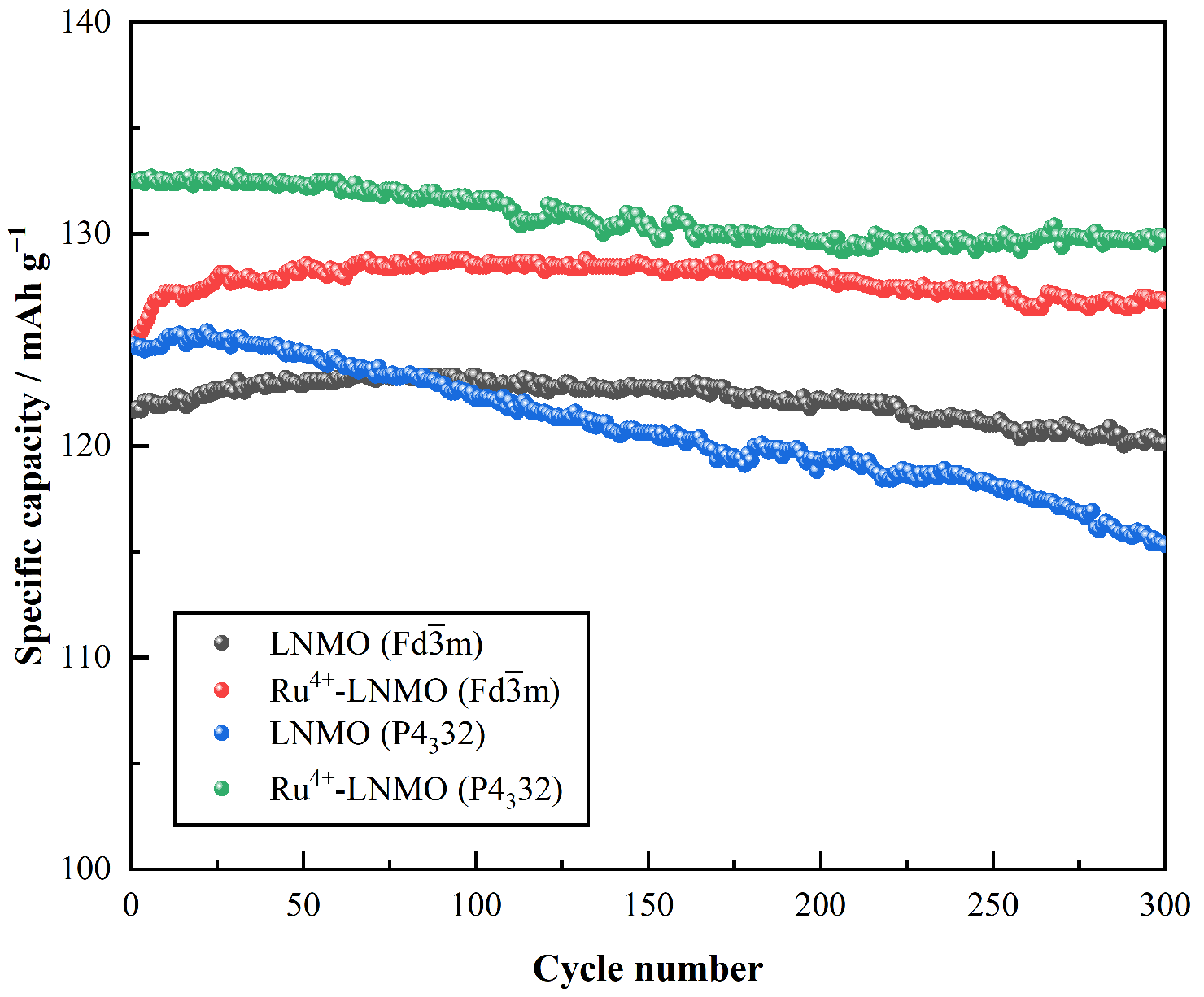
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Risthaus, T.; Wang, J.; Friesen, A.; Wilken, A.; Berghus, D.; Winter, M.; Li, J. Synthesis of spinel LiNi0.5Mn1.5O4 with secondary plate morphology as cathode material for lithium ion batteries. J. Power Sources 2015, 293, 137–142. [Google Scholar] [CrossRef]
- Liu, H.; Kloepsch, R.; Wang, J.; Winter, M.; Li, J. Truncated octahedral LiNi0.5Mn1.5O4 cathode material for ultralong-life lithium-ion battery: Positive (100) surfaces in high-voltage spinel system. J. Power Sources 2015, 300, 430–437. [Google Scholar] [CrossRef]
- Zhong, Q.; Bonakdarpour, A.; Zhang, M.; Gao, Y.; Dahn, J.R. ChemInform Abstract: Synthesis and Electrochemistry of LiNixMn20−xO4. ChemInform 1997, 144, 205–213. [Google Scholar] [CrossRef]
- Liu, D.; Zhu, W.; Trottier, J.; Gagnon, C.; Barray, F.; Guerfi, A.; Mauger, A.; Groult, H.; Julien, C.M.; Goodenough, J.B.; et al. Spinel materials for high-voltage cathodes in Li-ion batteries. RSC Adv. 2014, 4, 154–167. [Google Scholar] [CrossRef]
- Song, J.; Shin, D.W.; Lu, Y.; Amos, C.D.; Manthiram, A.; Goodenough, J.B. Role of oxygen vacancies on the performance of Li[Ni0.5−xMn1.5+x]O4 (x = 0, 0.05, and 0.08) spinel cathodes for lithium-ion batteries. Chem. Mater. 2012, 24, 3101–3109. [Google Scholar] [CrossRef]
- Deng, Y.-F.; Zhao, S.-X.; Xu, Y.-H.; Nan, C.-W. Effect of temperature of Li2O–Al2O3–TiO2–P2O5 solid-state electrolyte coating process on the performance of LiNi0.5Mn1.5O4 cathode materials. J. Power Sources 2015, 296, 261–267. [Google Scholar] [CrossRef]
- Xiao, J.; Chen, X.; Sushko, P.V.; Sushko, M.L.; Kovarik, L.; Feng, J.; Deng, Z.; Zheng, J.; Graff, G.L.; Nie, Z.; et al. High-performance LiNi0.5Mn1.5O4 spinel controlled by Mn3+ concentration and site disorder. Adv. Mater. 2012, 24, 2109–2116. [Google Scholar] [CrossRef]
- Patoux, S.; Daniel, L.; Bourbon, C.; Lignier, H.; Pagano, C.; LE Cras, F.; Jouanneau, S.; Martinet, S. High voltage spinel oxides for Li-ion batteries: From the material research to the application. J. Power Sources 2009, 189, 344–352. [Google Scholar] [CrossRef]
- Santhanam, R.; Rambabu, B. Research progress in high voltage spinel LiNi0.5Mn1.5O4 material. J. Power Sources 2010, 195, 5442–5451. [Google Scholar] [CrossRef]
- Kunduraci, M.; Al-Sharab, J.F.; Amatucci, G.G. High-power nanostructured LiMn2−xNixO4 high-voltage lithium-ion battery electrode materials: Electrochemical impact of electronic conductivity and morphology. Chem. Mater. 2006, 18, 3585–3592. [Google Scholar] [CrossRef]
- Li, B.; Xing, L.; Xu, M.; Lin, H.; Li, W. New solution to instability of spinel LiNi0.5Mn1.5O4 as cathode for lithium ion battery at elevated temperature. Electrochem. Commun. 2013, 34, 48–51. [Google Scholar] [CrossRef]
- Park, O.K.; Cho, Y.; Lee, S.; Yoo, H.-C.; Song, H.-K.; Cho, J. Who will drive electric vehicles, olivine or spinel? Energy Environ. Sci. 2011, 4, 1621–1633. [Google Scholar] [CrossRef]
- Jarry, A.; Gottis, S.; Yu, Y.S.; Roque-Rosell, J.; Kim, C.; Cabana, J.; Kerr, J.; Kostecki, R. The formation mechanism of fluorescent metal complexes at the LixNi0.5Mn1.5O4-delta/carbonate ester electrolyte interface. J. Am. Chem. Soc. 2015, 137, 3533–3539. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Q.; Wen, L.; Liu, Y.M. Spinel LiNi0.5Mn1.5O4 and its derivatives as cathodes for high-voltage Li-ion batteries. J. Solid State Electrochem. 2010, 14, 2191–2202. [Google Scholar] [CrossRef]
- Zhu, X.; Li, X.; Zhu, Y.; Jin, S.; Wang, Y.; Qian, Y. LiNi0.5Mn1.5O4 nanostructures with two-phase intergrowth as enhanced cathodes for lithium-ion batteries. Electrochim. Acta 2014, 121, 253–257. [Google Scholar] [CrossRef]
- Kim, J.-H.; Huq, A.; Chi, M.; Pieczonka, N.P.W.; Lee, E.; Bridges, C.A.; Tessema, M.M.; Manthiram, A.; Persson, K.A.; Powell, B.R. Integrated nano-domains of disordered and ordered spinel phases in LiNi0.5Mn1.5O4 for Li-ion batteries. Chem. Mater. 2014, 26, 4377–4386. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Park, K.-S.; Song, J.; Goodenough, J.B. Influence of thermal history on the electrochemical properties of Li[Ni0.5Mn1.5]O4. J. Power Sources 2013, 243, 260–266. [Google Scholar] [CrossRef]
- Kim, J.H.; Myung, S.T.; Yoon, C.S.; Kang, S.G.; Sun, Y.K. Comparative study of LiNi0.5Mn1.5O4-δ and LiNi0.5Mn1.5O4 cathodes having two crystallographic structures: Fdm and P4332. Chem. Mater. 2004, 16, 906–914. [Google Scholar] [CrossRef]
- Wang, J.F.; Chen, D.; Wu, W.; Wang, L.; Liang, G.C. Effects of Na+ doping on crystalline structure and electrochemical performances of LiNi0.5Mn1.5O4 cathode material. Trans. Nonferr. Metal. Soc. 2017, 27, 2239–2248. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, L.; Lu, S.; Lun, W. A new strategy to diminish the 4 V voltage plateau of LiNi0.5Mn1.5O4. Mater. Res. Bull. 2013, 48, 4960–4962. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, H.; Tan, M.; Hu, Y.; Shu, X.; Zhang, M.; Chen, B.; Liu, X. Er-Doped LiNi0.5Mn1.5O4 Cathode Material with Enhanced Cycling Stability for Lithium-Ion Batteries. Materials 2017, 10, 859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.; Zhang, J.; Zhang, X.; Du, Y.; Zhang, K. Study on oxygen deficiency in spinel LiNi0.5Mn1.5O4 and its Fe and Cr-doped compounds. J. Alloys Compd. 2017, 725, 580–586. [Google Scholar] [CrossRef]
- Lan, L.; Li, S.; Li, J.; Lu, L.; Lu, Y.; Huang, S.; Xu, S.; Pan, C.; Zhao, F. Enhancement of the electrochemical performance of the spinel structure LiNi0.5-xGaxMn1.5O4 cathode material by Ga doping. Nanoscale Res. Lett. 2018, 13, 251. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tan, T.A.; Yang, P.; Lai, M.O.; Lu, L. High-rate performances of the Ru-doped spinel LiNi0.5Mn1.5O4: Effects of doping and particle size. J. Phys. Chem. C. 2011, 115, 6102–6110. [Google Scholar] [CrossRef]
- Wang, S.; Li, P.; Shao, L.; Wu, K.; Lin, X.; Shui, M.; Long, N.; Wang, D.; Shu, J. Preparation of spinel LiNi0.5Mn1.5O4 and Cr-doped LiNi0.5Mn1.5O4 cathode materials by tartaric acid assisted sol-gel method. Ceram. Int. 2015, 41, 1347–1353. [Google Scholar] [CrossRef]
- Sun, J.; Li, P.; Wang, K.; Tan, Y.; Xue, B.; Niu, J. Effect of Al3+ and Al2O3 co-modification on electrochemical characteristics of the 5-V cathode material LiNi0.5Mn1.5O4. Ionics 2020, 26, 3725–3736. [Google Scholar] [CrossRef]
- Zhou, D.; Li, J.; Chen, C.; Lin, F.; Wu, H.; Guo, J. A hydrothermal synthesis of Ru-doped LiMn1.5Ni0.5O4 cathode materials for enhanced electrochemical performance. RSC Adv. 2021, 11, 12549–12558. [Google Scholar] [CrossRef]
- Zhou, D.; Li, J.; Chen, C.; Chen, C.; Wu, H.; Lin, F.; Guo, J. Ruthenium doped LiMn1.5Ni0.5O4 microspheres with enhanced electrochemical performance as lithium-ion battery cathode. J. Mater. Sci. Mater. Electron. 2021, 32, 23786–23797. [Google Scholar] [CrossRef]
- Chae, J.S.; Jo, M.R.; Kim, Y.-I.; Han, D.-W.; Park, S.-M.; Kang, Y.-M.; Roh, K.C. Kinetic favorability of Ru-doped LiNi0.5Mn1.5O4 for high-power lithium-ion batteries. J. Ind. Eng. Chem. 2015, 21, 731–735. [Google Scholar] [CrossRef]
- Oh, S.H.; Chung, K.Y.; Jeon, S.H.; Kim, C.S.; Cho, W.I.; Cho, B.W. Structural and electrochemical investigations on the LiNi0.5−xMn1.5−yMx+yO4 (M = Cr, Al, Zr) compound for 5 V cathode material. J. Alloys Compd. 2009, 469, 244–250. [Google Scholar] [CrossRef]
- Manthiram, A.; Chemelewski, K.; Lee, E.-S. A perspective on the high-voltage LiMn1.5Ni0.5O4 spinel cathode for lithium-ion batteries. Energy Environ. Sci. 2014, 7, 1339–1350. [Google Scholar] [CrossRef]
- Yi, T.-F.; Mei, J.; Zhu, Y.-R. Key strategies for enhancing the cycling stability and rate capacity of LiNi0.5Mn1.5O4 as high-voltage cathode materials for high power lithium-ion batteries. J. Power Sources 2016, 316, 85–105. [Google Scholar] [CrossRef]
- Cabana, J.; Zheng, H.; Shukla, A.K.; Kim, C.; Battaglia, V.S.; Kunduraci, M. Comparison of the performance of LiNi1/2Mn3/2O4 with different microstructures. J. Electrochem. Soc. 2011, 158, A997–A1004. [Google Scholar] [CrossRef]
- Chen, Z.; Qiu, S.; Cao, Y.; Ai, X.; Xie, K.; Hong, X.; Yang, H. Surface-oriented and nanoflake-stacked LiNi0.5Mn1.5O4 spinel for high-rate and long-cycle-life lithium ion batteries. J. Mater. Chem. 2012, 22, 17768–17772. [Google Scholar] [CrossRef]
- Kim, J.-S.; Kim, K.; Cho, W.; Shin, W.H.; Kanno, R.; Choi, J.W. A Truncated Manganese Spinel Cathode for Excellent Power and Lifetime in Lithium-Ion Batteries. Nano Lett. 2012, 12, 6358–6365. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, F.; Yang, J.; Chen, J. LiNi0.5Mn1.5O4 Porous Nanorods as high-rate and long-life cathodes for Li-ion batteries. Nano. Lett. 2013, 13, 2822–2825. [Google Scholar] [CrossRef]
- Hirayama, M.; Sonoyama, N.; Ito, M.; Minoura, M.; Mori, D.; Yamada, A.; Tamura, K.; Mizuki, J.I.; Kanno, R. Characterization of electrode/electrolyte interface with X-Ray reflectometry and epitaxial-film LiMn2O4 electrode. J. Electrochem. Soc. 2007, 154, A1065–A1072. [Google Scholar] [CrossRef]
- Benedek, R.; Thackeray, M.M. Simulation of the surface structure of lithium manganese oxide spinel. Phys. Rev. B 2011, 83, 195439. [Google Scholar] [CrossRef] [Green Version]
- Fang, C.C.M.; Parker, S.; De With, G. Atomistic Simulation of the Surface Energy of Spinel MgAl2O4. J. Am. Ceram. Soc. 2000, 83, 2082–2084. [Google Scholar] [CrossRef]
- Huang, F.; Gilbert, B.; Zhang, H.; Banfield, J. Reversible, Surface-Controlled Structure Transformation in Nanoparticles Induced by an Aggregation State. Phys. Rev. Lett. 2004, 92, 155501. [Google Scholar] [CrossRef] [Green Version]
- Lafont, U.; Locati, C.; Kelder, E. Nanopowders of spinel-type electrode materials for Li-ion batteries. Solid State Ionics 2006, 177, 3023–3029. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Su, B.; Xue, W.; Zhang, J. The Effects of Ru4+ Doping on LiNi0.5Mn1.5O4 with Two Crystal Structures. Materials 2022, 15, 4273. https://doi.org/10.3390/ma15124273
Li X, Su B, Xue W, Zhang J. The Effects of Ru4+ Doping on LiNi0.5Mn1.5O4 with Two Crystal Structures. Materials. 2022; 15(12):4273. https://doi.org/10.3390/ma15124273
Chicago/Turabian StyleLi, Xinli, Ben Su, Wendong Xue, and Junnan Zhang. 2022. "The Effects of Ru4+ Doping on LiNi0.5Mn1.5O4 with Two Crystal Structures" Materials 15, no. 12: 4273. https://doi.org/10.3390/ma15124273
APA StyleLi, X., Su, B., Xue, W., & Zhang, J. (2022). The Effects of Ru4+ Doping on LiNi0.5Mn1.5O4 with Two Crystal Structures. Materials, 15(12), 4273. https://doi.org/10.3390/ma15124273




