Development of Efficient Photocatalyst MIL-68(Ga)_NH2 Metal-Organic Framework for the Removal of Cr(VI) and Cr(VI)/RhB from Wastewater under Visible Light
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents
2.2. Synthesis of MIL-68(Ga)_NH2
2.3. Synthesis of MIL-68(Ga)
2.4. Measurements
2.5. Photocatalytic Degradation Experiments
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Phoebe, Z.R.; Heather, J.S. Inorganic nano-adsorbents for the removal of heavy metals and arsenic: A review. RSC Adv. 2015, 5, 29885–29907. [Google Scholar]
- Zhang, S.; Wang, Z.; Chen, H.; Kai, C.; Jiang, M. Polyethylenimine functionalized Fe3O4/steam-exploded rice straw composite as an efficient adsorbent for Cr(VI) removal. Appl. Surf. Sci. 2018, 440, 1277–1285. [Google Scholar] [CrossRef]
- Qu, Y.; Zhou, W.; Jiang, L.; Fu, H.G. Novel heterogeneous CdS nanoparticles/NiTiO3 nanorods with enhanced visible-light-driven photocatalytic activity. RSC Adv. 2013, 3, 18305. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Ramjan, M.M.; Suresh, P.; Muthuraj, V. Fabrication of highly efficient visible light driven Ag/CeO2 photocatalyst for degradation of organic pollutants. J. Alloys Compd. 2016, 664, 149–160. [Google Scholar] [CrossRef]
- Chen, F.Y.; Yu, C.L.; Wei, L.F. Fabrication and characterization of ZnTiO3/Zn2Ti3O8/ZnO ternary photocatalyst for synergetic removal of aqueous organic pollutants and Cr(VI) ions. Sci. Total Environ. 2020, 706, 136026. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.W.; Wang, B.; Zhang, J.; Hao, C.; Yang, P.; Hao, A.; Zhai, J. Factors influencing the photocatalytic activity of rutile TiO2 nanorods with different aspect ratios for dye degradation and Cr(VI) photoreduction. Phys. Chem. Chem. Phys. 2015, 17, 18670–18676. [Google Scholar] [CrossRef]
- Lin, Z.; Zhang, Y.X.; Meng, F.L.; Jia, Y.; Luo, T.; Yu, X.Y.; Wang, J.; Liu, J.H.; Huang, X.J. Facile synthesis of porous single crystalline ZnO nanoplates and their application in photocatalytic reduction of Cr(VI) in the presence of phenol. J. Hazard. Mater. 2014, 276, 400–407. [Google Scholar]
- Wang, J.C.; Ren, J.; Yao, H.C.; Zhang, L.; Wang, J.S.; Zang, S.Q.; Han, L.F.; Li, Z.J. Synergistic photocatalysis of Cr(VI) reduction and 4-Chlorophenol degradation over hydroxylated α-Fe2O3 under visible light irradiation. J. Hazard. Mater. 2016, 311, 11–19. [Google Scholar] [CrossRef]
- Zhu, M.; Kurniawan, T.A.; Yanping, S.; Othman, M.H.D.; Avtar, R.; Fu, D.; Hwang, G.H. Fabrication, characterization, and application of ternary magnetic recyclable Bi2WO6/BiOI@Fe3O4 composite for photodegradation of tetracycline in aqueous solutions. J. Environ. Manag. 2020, 270, 110839. [Google Scholar]
- Zhu, M.; Kurniawan, T.A.; You, Y.; Othman, M.H.D.; Avtar, R. 2D Graphene oxide (GO) doped p-n type BiOI/Bi2WO6 as a novel composite for photodegradation of bisphenol A (BPA) in aqueous solutions under UV-vis irradiation. Mater. Sci. Eng. C 2020, 108, 110420. [Google Scholar]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [Green Version]
- Adil, K.; Belmabkhout, Y.; Pillai, R.S.; Cadiau, A.; Bhatt, P.M.; Assen, A.H.; Maurin, G.; Eddaoudi, M. Gas/vapour separation using ultra-microporous metal-organic frameworks: Insights into the structure/separation relationship. Chem. Soc. Rev. 2017, 46, 3402–3430. [Google Scholar] [CrossRef] [PubMed]
- Stassen, I.; Burtch, N.; Talin, A.; Falcaro, P.; Allendorf, M.; Ameloot, R. An updated roadmap for the integration of metal-organic frameworks with electronic devices and chemical sensors. Chem. Soc. Rev. 2017, 46, 3185–3241. [Google Scholar] [CrossRef] [PubMed]
- Medishetty, R.; Zareba, K.; Mayer, D.; Samoc, M.; Fischer, R.A. Nonlinear optical properties, upconversion and lasing in metal-organic frameworks. Chem. Soc. Rev. 2017, 46, 4976–5004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.B.; Liu, X.Q.; Zhou, H.C. Design and fabrication of mesoporous heterogeneous basic catalysts. Chem. Soc. Rev. 2015, 44, 5092–5147. [Google Scholar] [CrossRef]
- Partha, M.G.M.; Srinivasan, N. Novel Photocatalysts for the Decomposition of Organic Dyes Based on Metal-Organic Framework Compounds. J. Phys. Chem. B 2006, 110, 13759–13768. [Google Scholar]
- Zhang, T.; Lin, W. Metal-organic frameworks for artificial photosynthesis and photocatalysis. Chem. Soc. Rev. 2014, 43, 5982–5993. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X. Multifunctional Metal-Organic Frameworks for Photocatalysis. Small 2015, 11, 3097–3112. [Google Scholar] [CrossRef]
- Liang, Z.; Qu, C.; Xia, D. Atomically Dispersed Metal Sites in MOF-Based Materials for Electrocatalytic and Photocatalytic Energy Conversion. Angew. Chem. Int. Ed. 2018, 57, 9604–9633. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Asiri, A.M.; Garcia, H. Metal-Organic Framework (MOF) Compounds: Photocatalysts for Redox Reactions and Solar Fuel Production. Angew. Chem. Int. Ed. 2016, 55, 5414–5445. [Google Scholar] [CrossRef]
- Alvaro, M.; Carbonell, E.; Ferrer, B.; Xamena, F.X.L.; Garcia, H. Semiconductor Behavior of a Metal-Organic Framework (MOF). Chem. Eur. J. 2007, 13, 5106–5112. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.H.; Zhang, X.G.; Feng, Y.; Zhang, X.F. Modified metal-organic frameworks as photocatalysts. Appl. Catal. B Environ. 2018, 231, 317–342. [Google Scholar] [CrossRef]
- Wang, C.C.; Lu, J.R.; Lv, X.L.; Zhang, Y.Q.; Guo, G.S. Photocatalytic organic pollutants degradation in metal-organic frameworks. Energy Environ. Sci. 2014, 7, 2831–2867. [Google Scholar] [CrossRef]
- Wang, C.C.; Zhang, Y.Q.; Li, J.; Wang, P. Photocatalytic CO2 reduction in metal-organic frameworks: A mini review. J. Mol. Struct. 2015, 1083, 127–136. [Google Scholar] [CrossRef]
- Zhu, J.J.; Li, P.Z.; Guo, W.H.; Zhao, Y.L.; Zou, R.Q. Titanium-based metal-organic frameworks for photocatalytic applications. Coord. Chem. Rev. 2018, 359, 80–101. [Google Scholar] [CrossRef]
- Du, J.J.; Yuan, Y.P.; Sun, J.X.; Peng, F.M.; Jiang, X.; Qiu, L.G.; Xie, A.J.; Shen, Y.H.; Zhu, J.F. New photocatalysts based on MIL-53 metal–organic frameworks for the decolorization of methylene blue dye. J. Hazard. Mater. 2011, 190, 945–951. [Google Scholar] [CrossRef]
- Jing, F.F.; Liang, R.W.; Xiong, J.H.; Chen, R.; Zhang, S.Y.; Li, Y.H.; Wu, L. MIL-68(Fe) as an efficient visible-light-driven photocatalyst for the treatment of a simulated waste-water contain Cr(VI) and Malachite Green. Appl. Catal. B Environ. 2017, 206, 9–15. [Google Scholar] [CrossRef]
- Xu, W.T.; Ma, L.; Ke, F.; Peng, F.M.; Xu, G.S. Meta-organic frameworks MIL-88A hexagonalmicrorods as a new photocatalyst for efficient decolorization of methylene blue dye. Dalton Trans. 2014, 43, 3792. [Google Scholar] [CrossRef]
- Shen, L.J.; Liang, R.W.; Wu, L. Multifunctional NH2-mediated zirconium metal-organic framework as an efficient visible-light-driven photocatalyst for selective oxidation of alcohols and reduction of aqueous Cr(VI). Dalton Trans. 2013, 42, 13649–13657. [Google Scholar] [CrossRef]
- Liang, R.W.; Shen, L.J.; Jing, F.F.; Wu, W.M.; Qin, N.; Lin, R.; Wu, L. NH2-mediated indium metal-organic framework as a novel visible-light-driven photocatalyst for reduction of the aqueous Cr(VI). Appl. Catal. B Environ. 2015, 162, 245–251. [Google Scholar] [CrossRef]
- Pi, Y.H.; Li, X.Y.; Xia, Q.B. Formation of willow leaf-like structures composed of NH2-MIL-68(In) on a multifunctional multiwalled carbon nanotube backbone for enhanced photocatalytic reduction of Cr(VI). Nano Res. 2017, 10, 3543–3556. [Google Scholar] [CrossRef]
- Jiang, D.N.; Xu, P.; Wang, H.; Zeng, G.M.; Huang, D.L.; Chen, M.; Lai, C.; Zhang, C.; Wan, J.; Xue, W.J. Strategies to improve metal organic frameworks photocatalyst’s performance for degradation of organic pollutants. Coord. Chem. Rev. 2019, 376, 449–466. [Google Scholar] [CrossRef]
- Barthelet, K.; Marrot, J.; Riou, D. VIII(OH){O2C–C6H4–CO2}.(HO2C–C6H4–CO2H)x(DMF)y(H2O)z (or MIL-68), a new vanadocarboxylate with a large pore hybrid topology: Reticular synthesis with infinite inorganic building blocks? Chem. Commun. 2004, 5, 520–521. [Google Scholar] [CrossRef] [PubMed]
- Fateeva, A.; Horcaiada, P.; Devic, T.; Serre, C.; Marrot, J.; Grenèche, J.M.; Morcrette, M.; Tarascon, J.M.; Maurin, G.; Férey, G. Synthesis, Structure, Characterization, and Redox Properties of the Porous MIL-68(Fe) Solid. Eur. J. Inorg. Chem. 2010, 2010, 3789–3794. [Google Scholar] [CrossRef]
- Yang, Q.Y.; Vaeseb, S.; Vishnuvarthan, M.; Ragon, F.; Serre, C.; Vimont, A.; Daturi, M.; Weireld, G.D.; Maurin, G. Probing the adsorption performance of the hybrid porous MIL-68(Al): A synergic combination of experimental and modelling tools. J. Mater. Chem. 2012, 22, 10210–10220. [Google Scholar] [CrossRef]
- Volkringer, C.; Meddouri, M.; Loiseau, T.; Guillou, N.; Marrot, G. The Kagomé Topology of the Gallium and Indium Metal-Organic Framework Types with a MIL-68 Structure: Synthesis, XRD, Solid-State NMR Characterizations, and Hydrogen Adsorption. Inorg. Chem. 2008, 47, 11892–11901. [Google Scholar] [CrossRef]
- Shen, L.J.; Liang, R.W.; Liang, W. Electronic effects of ligand substitution on metal-organic framework photocatalysts: The case study of UiO-66. Phys. Chem. Chem. Phys. 2015, 17, 117–121. [Google Scholar] [CrossRef]
- Yang, C.; Cheng, J.H.; Chen, Y.C. Experimental investigation on the water stability of amino-modified indium metal-organic frameworks. RSC Adv. 2016, 6, 61703–61706. [Google Scholar] [CrossRef]
- Walton, K.S.; Snurr, R.Q. Applicability of the BET Method for Determining Surface Areas of Microporous Metal-Organic Frameworks. J. Am. Chem. Soc. 2007, 129, 8552–8556. [Google Scholar] [CrossRef]
- Rouquerol, J. Is the bet equation applicable to microporous adsorbents? Stud. Surf. Sci. Catal. 2007, 160, 49–56. [Google Scholar]
- Ani, I.; Nursia, H.; Roslina, R. Kinetic and regeneration studies of photocatalytic magnetic separable beads for chromium (VI) reduction under sunlight. J. Hazard. Mater. 2011, 186, 629–635. [Google Scholar]
- Boultif, A.; Louër, D. Powder pattern indexing with the dichotomy method. J. Appl. Crystallogr. 2004, 37, 724–731. [Google Scholar] [CrossRef]
- Kandiah, M.; Nilsen, M.H.; Usseglio, S.; Jakobsen, S.; Olsbye, U.; Tilset, M.; Larabi, C.; Quadrelli, E.; Bonino, F.; Lillerud, K.P. Synthesis and Stability of Tagged UiO-66 Zr-MOFs. Chem. Mater. 2010, 22, 6632–6640. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds. J. Chem. Educ. 1979, 5, A209. [Google Scholar]
- Banerjee, R.; Furukawa, H.; Britt, D.; Knobler, C.; Keeffe, M.O.; Yaghi, O.M. Control of Pore Size and Functionality in Isoreticular Zeolitic Imidazolate Frameworks and their Carbon Dioxide Selective Capture Properties. J. Am. Chem. Soc. 2009, 131, 3875–3877. [Google Scholar] [CrossRef] [PubMed]
- Mowat, J.P.S.; Miller, S.R.; Griffin, J.M.; Seymour, V.R.; Ashbrook, S.E.; Thompson, S.P.; Fairen-Jimenez, D.; Banu, A.M.; Düren, T.; Wright, P.A. Structural Chemistry, Monoclinic-to-Orthorhombic Phase Transition, and CO2 Adsorption Behavior of the Small Pore Scandium Terephthalate, Sc2(O2CC6H4CO2)3, and Its Nitro- And Amino-Functionalized Derivatives. Inorg. Chem. 2011, 50, 10844–10858. [Google Scholar] [CrossRef] [PubMed]
- Garibay, S.J.; Cohen, S.M. Isoreticular synthesis and modification of frameworks with the UiO-66 topology. Chem. Commun. 2010, 46, 7700–7702. [Google Scholar] [CrossRef] [Green Version]
- Kurihara, M.; Nishihara, H. Azo- and quinone-conjugated redox complexes-photo- and proton-coupled intramolecular reactions based on d-π interaction. Coord. Chem. Rev. 2002, 226, 125–135. [Google Scholar] [CrossRef]
- Wang, X.; Pehkonen, S.; Ray, A.K. Removal of Aqueous Cr(VI) by a Combination of Photocatalytic Reduction and Coprecipitation. Ind. Eng. Chem. Res. 2004, 43, 1665–1672. [Google Scholar] [CrossRef]
- Gimknez, J.M.; Aguado, A. Photocatalytic reduction of chromium(VI) with titania powders in a flow system. Kinet. Catal. Act. J. Mol. Catal. A-Chem. 1996, 105, 67–78. [Google Scholar] [CrossRef]
- Guo, Y.D.; Li, C.X.; Guo, Y.Q.; Wang, X.G.; Li, X.M. Ultrasonic-assisted synthesis of mesoporous g-C3N4/Na-bentonite composites and its application for efficient photocatalytic simultaneous removal of Cr(VI) and RhB. Colloids Surf. A 2019, 578, 123624. [Google Scholar] [CrossRef]
- Guo, Y.Q.; Guo, Y.D.; Tang, D.D.; Liu, Y.Y.; Wang, X.G.; Li, P.; Wang, G.H. Sol-gel synthesis of new ZnFe2O4/Na-bentonite composites for simultaneous oxidation of RhB and reduction of Cr(VI) under visible light irradiation. J. Alloy. Compd. 2019, 781, 1101–1109. [Google Scholar] [CrossRef]
- Bai, X.; Jia, J.; Du, Y.Y.; Hu, X.Y.; Li, J.L.; Liu, E.Z.; Fan, J. Multi-level traped electrons system in enhancing photocatalytic activity of TiO2 nanosheets for simultaneous reduction of Cr (VI) and RhB degradation. Appl. Surf. Sci. 2020, 503, 144298. [Google Scholar] [CrossRef]
- Lu, D.Z.; Fang, P.F.; Wu, W.H.; Ding, J.Q.; Jiang, L.L.; Zhao, X.N.; Li, C.H.; Yang, M.C.; Li, Y.Z.; Wang, D.H. Solvothermal-Assisted Synthesis for Self-Assembling TiO2 Nanorods on Large Graphitic Carbon Nitride Sheets with Their Anti-Recombination in Photocatalytic Removal of Cr(VI) and Rhodamin B Under Visible Light Irradiation. Nanoscale 2017, 9, 3231–3245. [Google Scholar] [CrossRef]
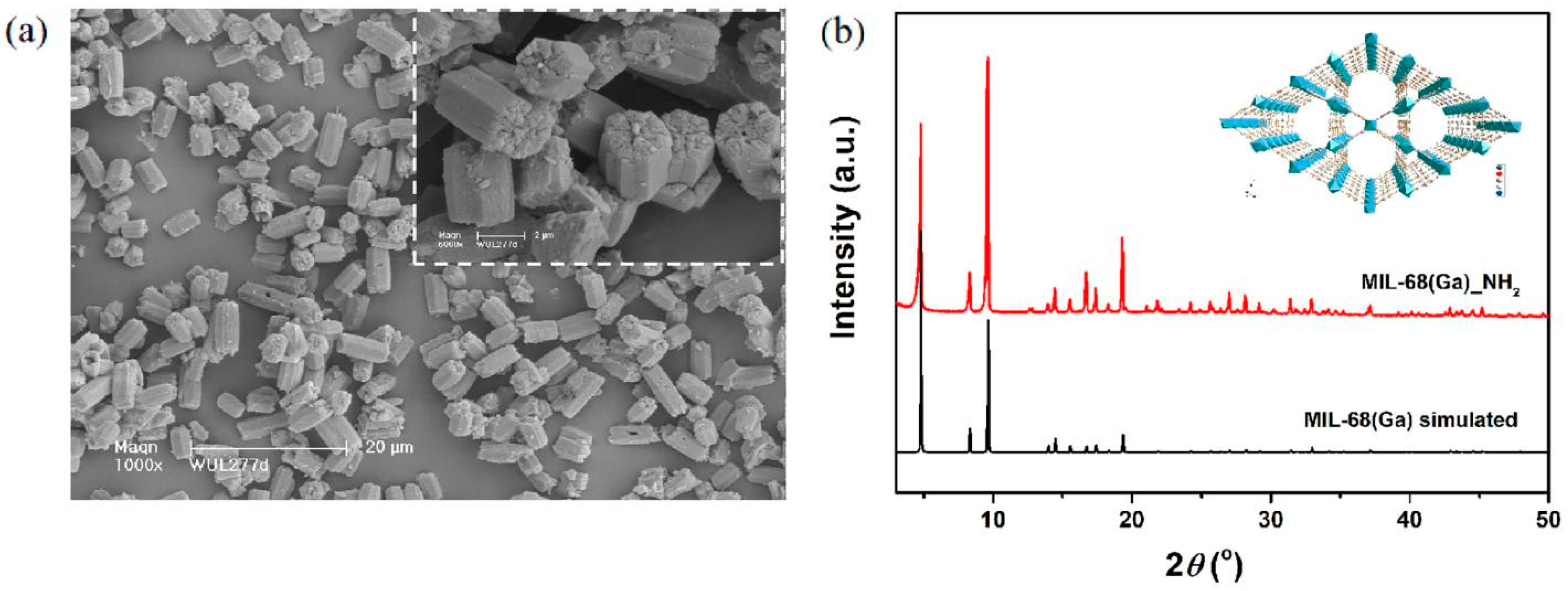
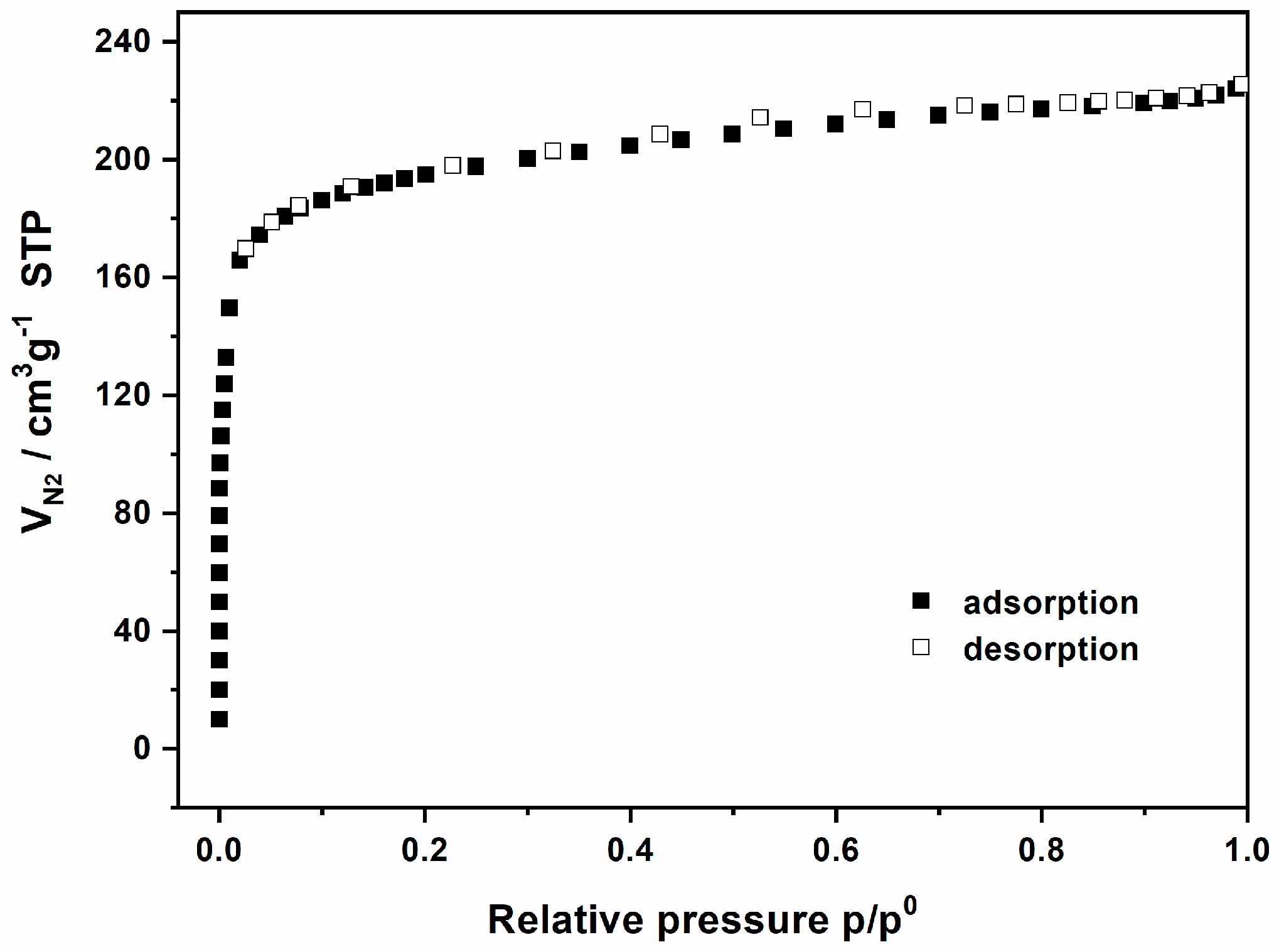
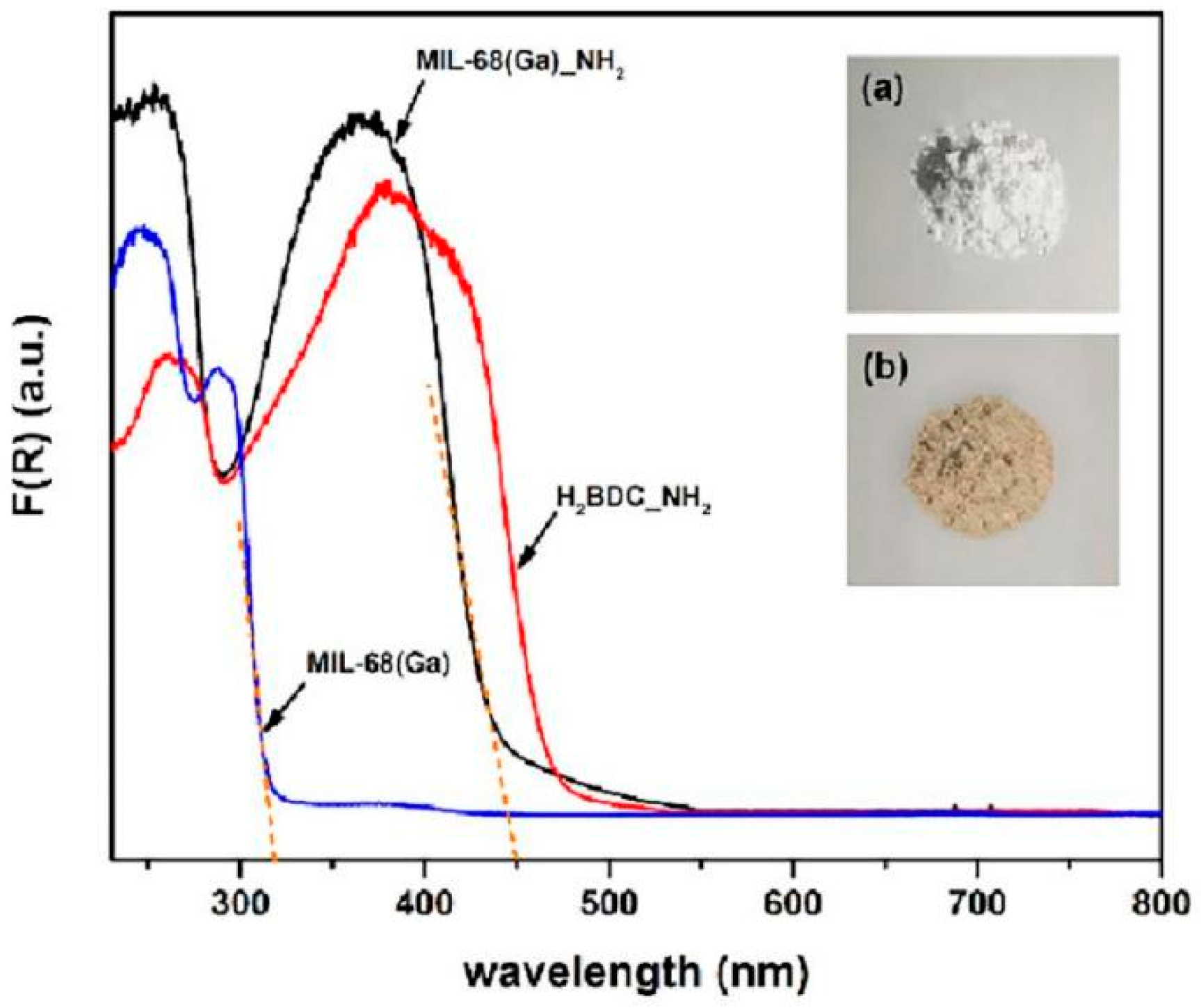
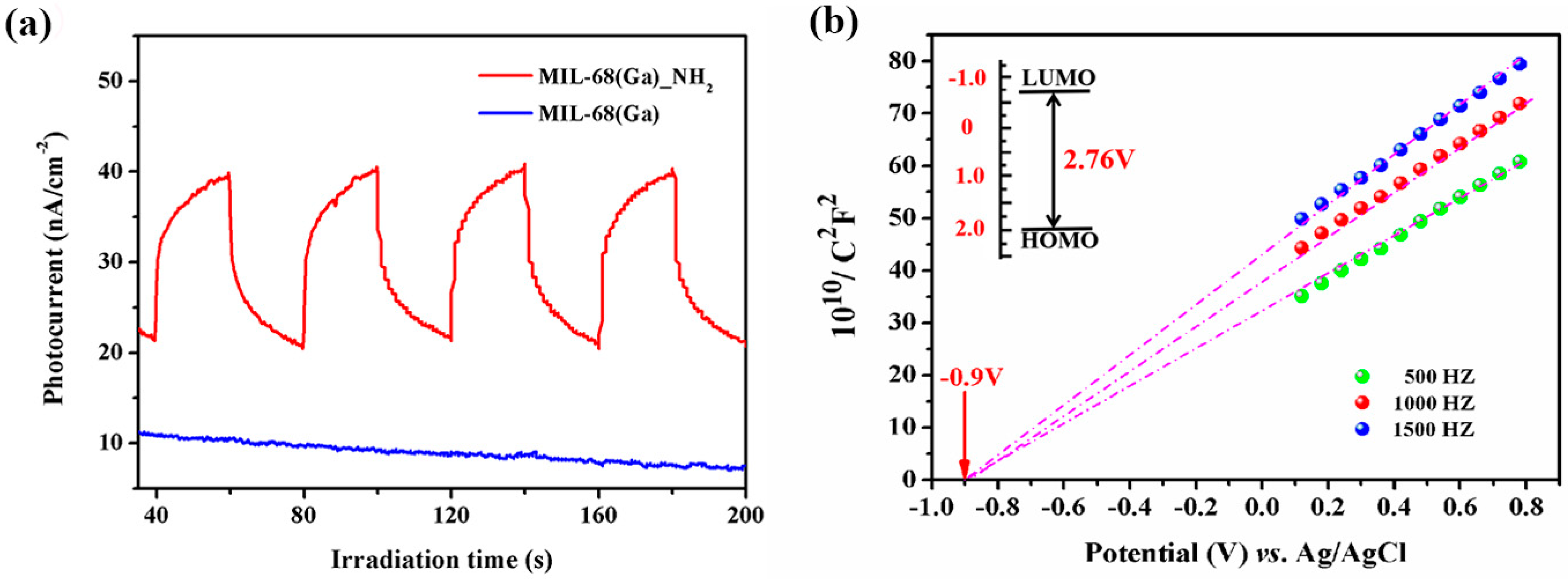
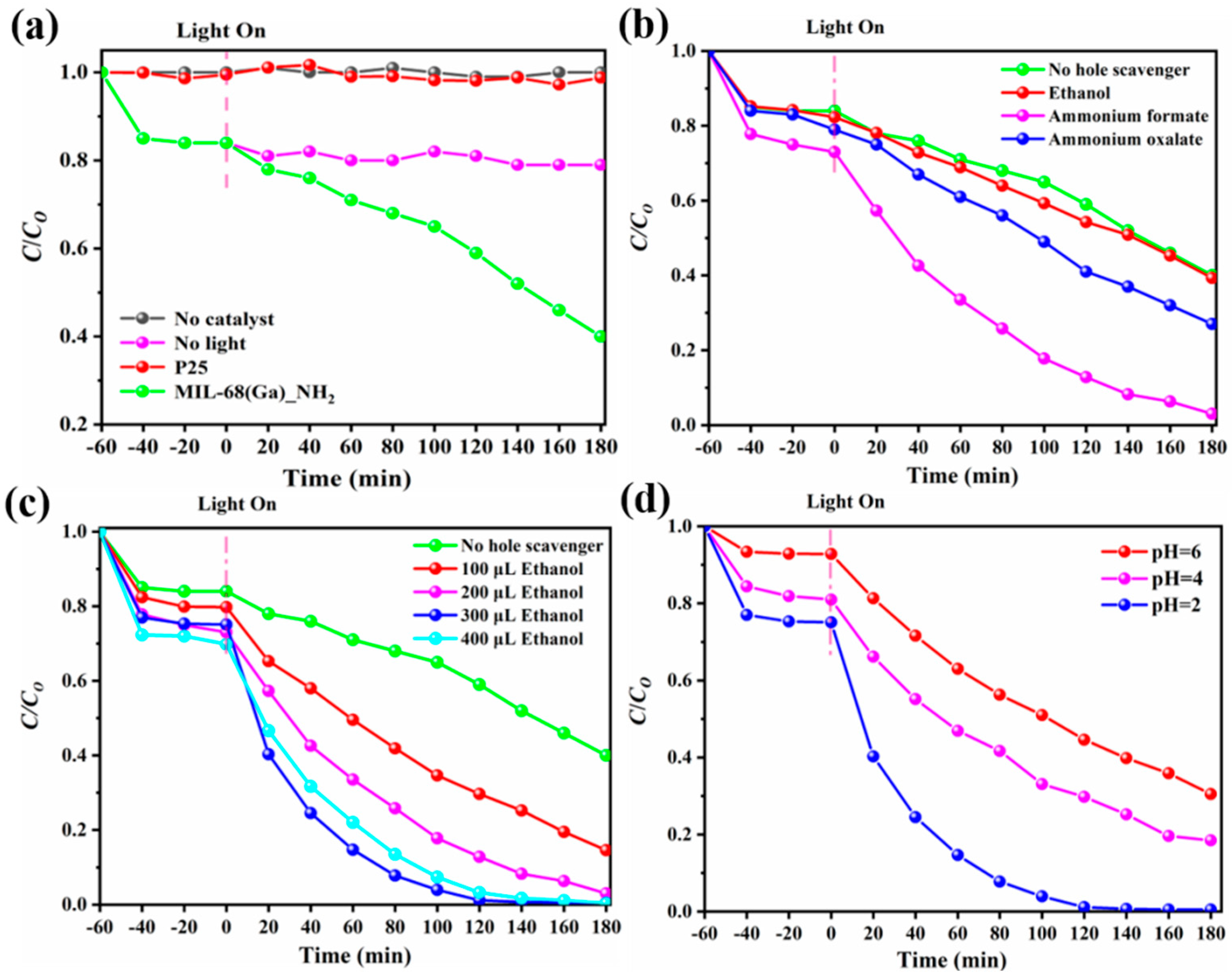

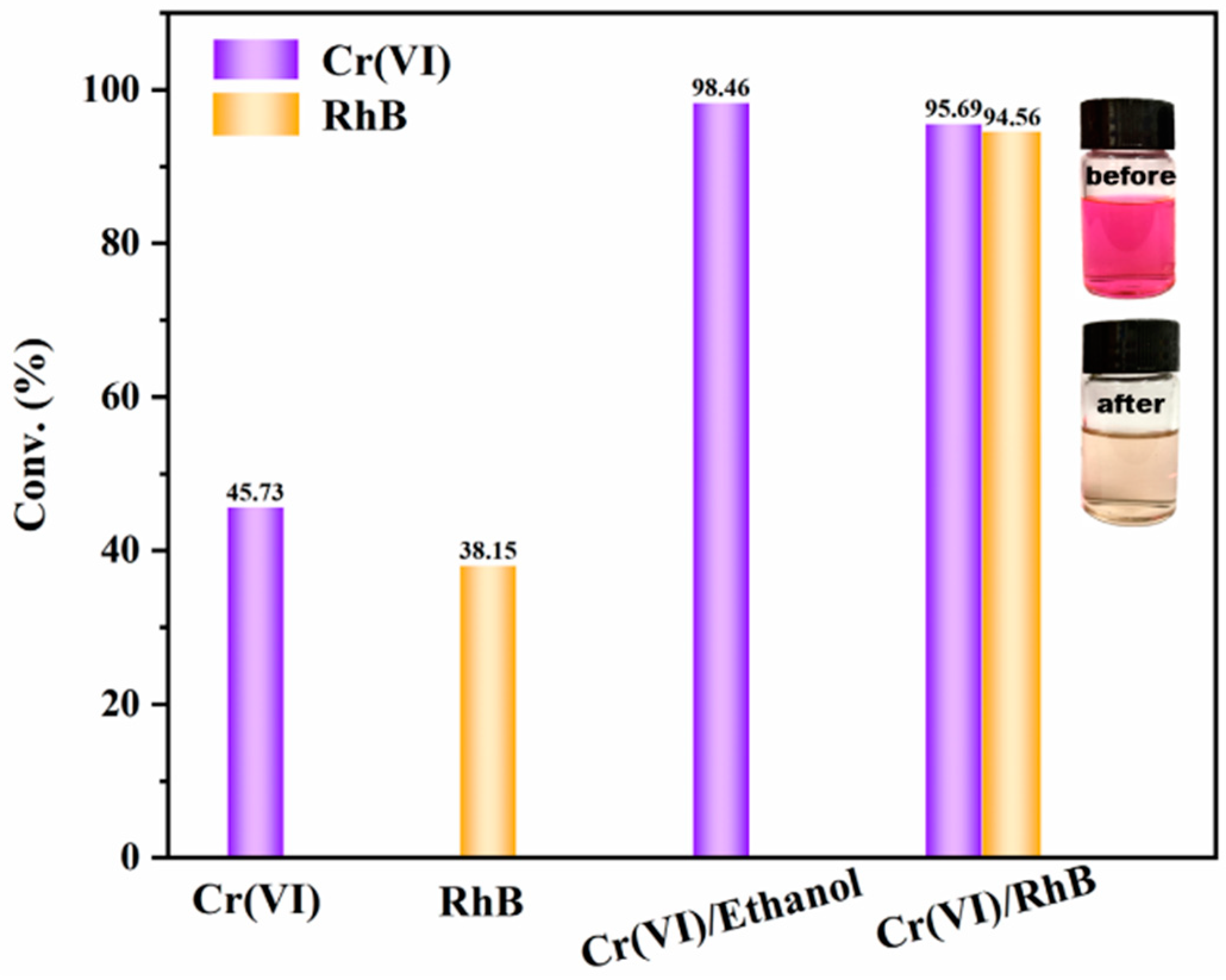

| Func. | a (Å) | b (Å) | c (Å) | V (Å3) | System | Space | F(30) |
|---|---|---|---|---|---|---|---|
| — | 21.176 | 36.703 | 6.742 | 5240 | Orthorhombic | Cmcm | — |
| –NH2 | 36.699 | 21.223 | 6.750 | 5257 | Orthorhombic | Cmcm | 41.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Qin, D.; Fang, F.; Wang, W.; Zhao, W. Development of Efficient Photocatalyst MIL-68(Ga)_NH2 Metal-Organic Framework for the Removal of Cr(VI) and Cr(VI)/RhB from Wastewater under Visible Light. Materials 2022, 15, 3761. https://doi.org/10.3390/ma15113761
Wu L, Qin D, Fang F, Wang W, Zhao W. Development of Efficient Photocatalyst MIL-68(Ga)_NH2 Metal-Organic Framework for the Removal of Cr(VI) and Cr(VI)/RhB from Wastewater under Visible Light. Materials. 2022; 15(11):3761. https://doi.org/10.3390/ma15113761
Chicago/Turabian StyleWu, Lei, Doudou Qin, Fan Fang, Weifeng Wang, and Wenying Zhao. 2022. "Development of Efficient Photocatalyst MIL-68(Ga)_NH2 Metal-Organic Framework for the Removal of Cr(VI) and Cr(VI)/RhB from Wastewater under Visible Light" Materials 15, no. 11: 3761. https://doi.org/10.3390/ma15113761
APA StyleWu, L., Qin, D., Fang, F., Wang, W., & Zhao, W. (2022). Development of Efficient Photocatalyst MIL-68(Ga)_NH2 Metal-Organic Framework for the Removal of Cr(VI) and Cr(VI)/RhB from Wastewater under Visible Light. Materials, 15(11), 3761. https://doi.org/10.3390/ma15113761





