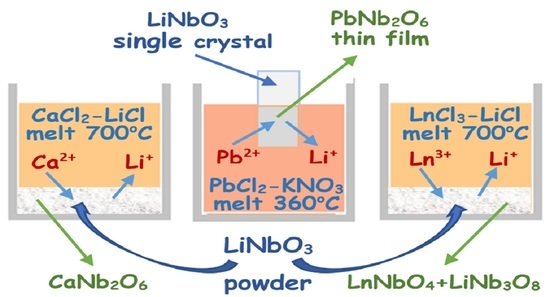
Molten Chlorides as the Precursors to Modify the Ionic Composition and Properties of LiNbO3 Single Crystal and Fine Powders
Abstract
:1. Introduction
2. Materials and Methods
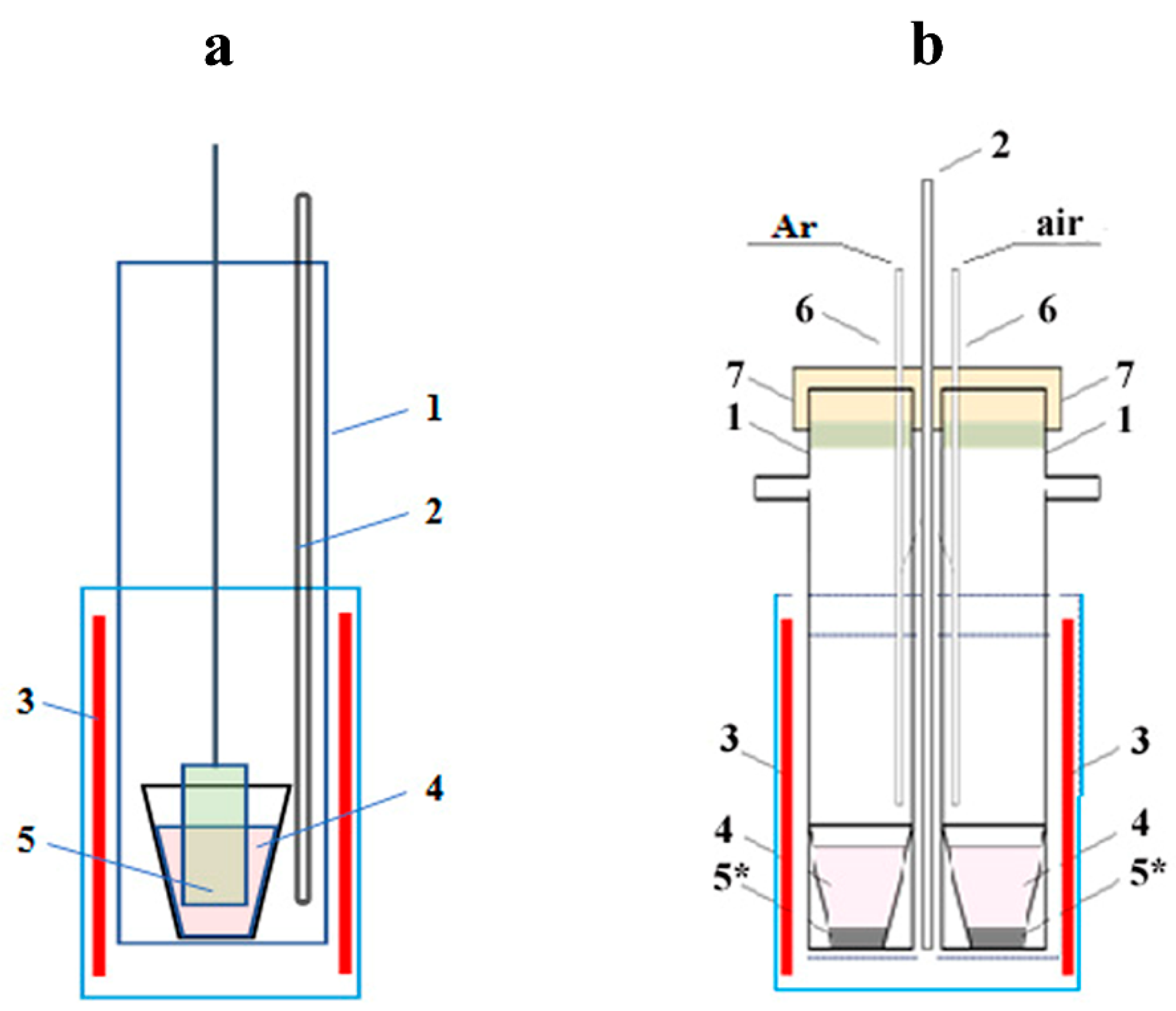
2.1. Initial Materials and Molten Salt Reaction Media
2.2. Technique of Ionic Modifying Lithium Niobate
2.3. Examination Methods of the Reaction Products
3. Results and Discussion
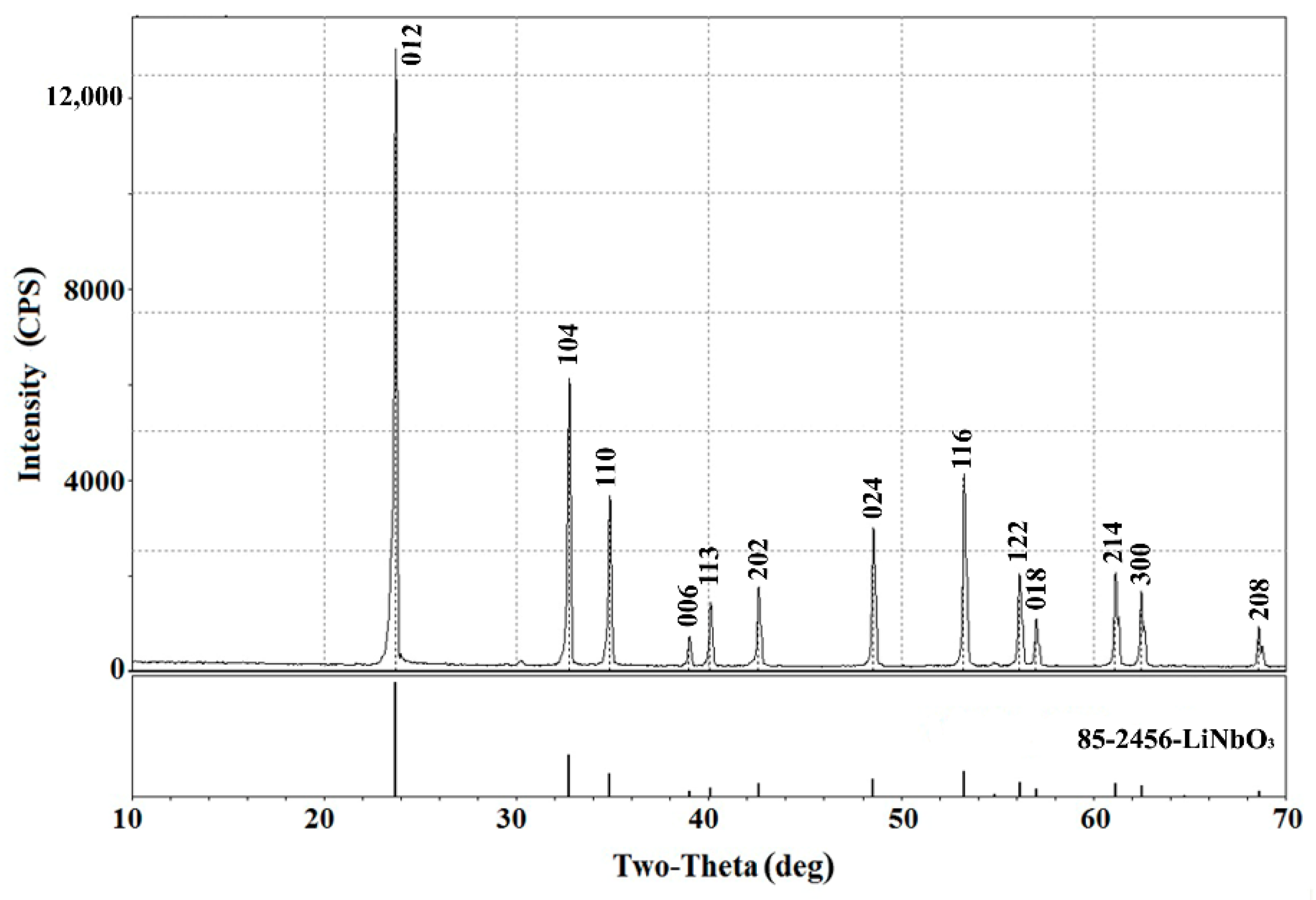
3.1. Formation of the Lead Metaniobate Film on the Y-Z Face of LiNbO3 Single Crystal
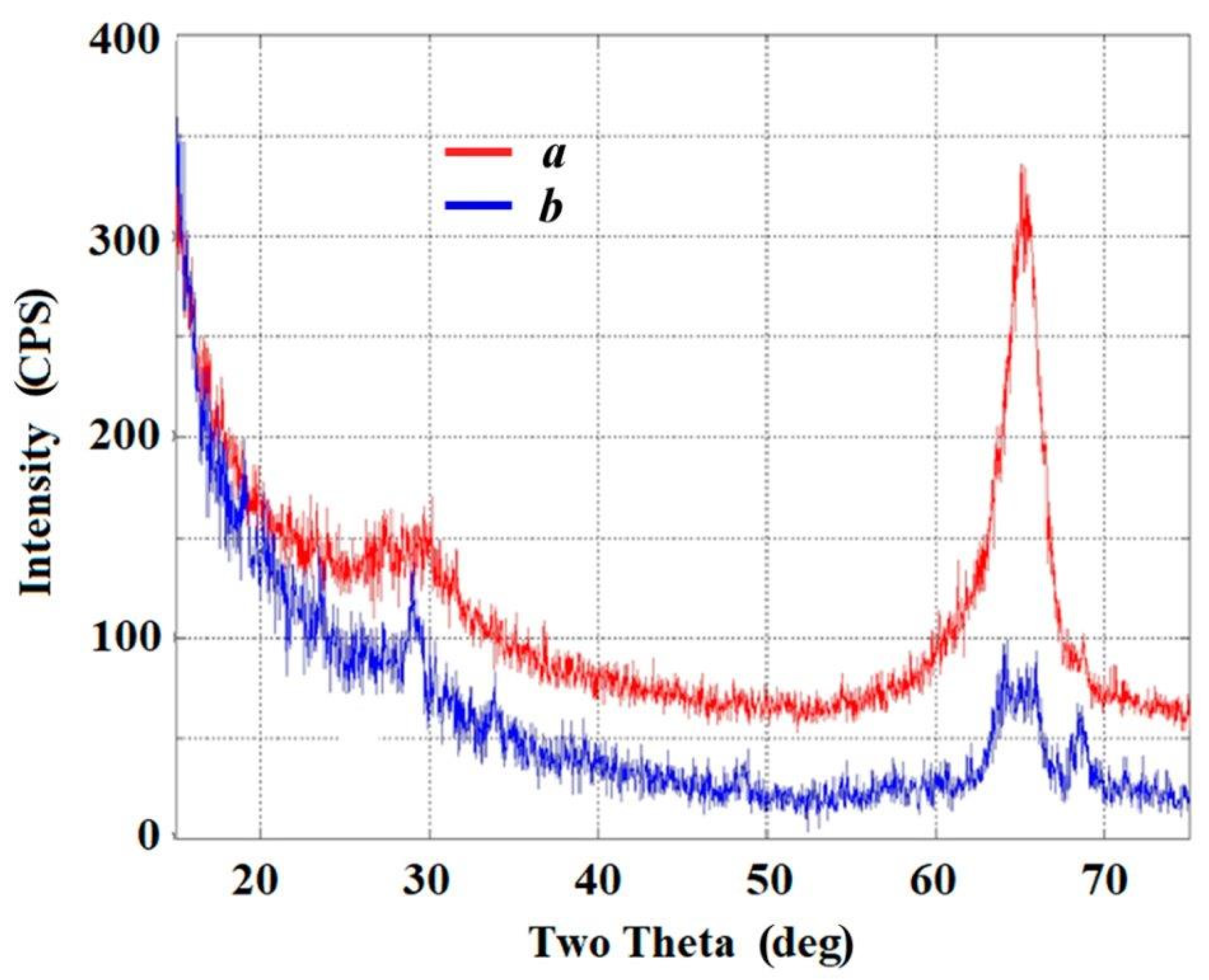
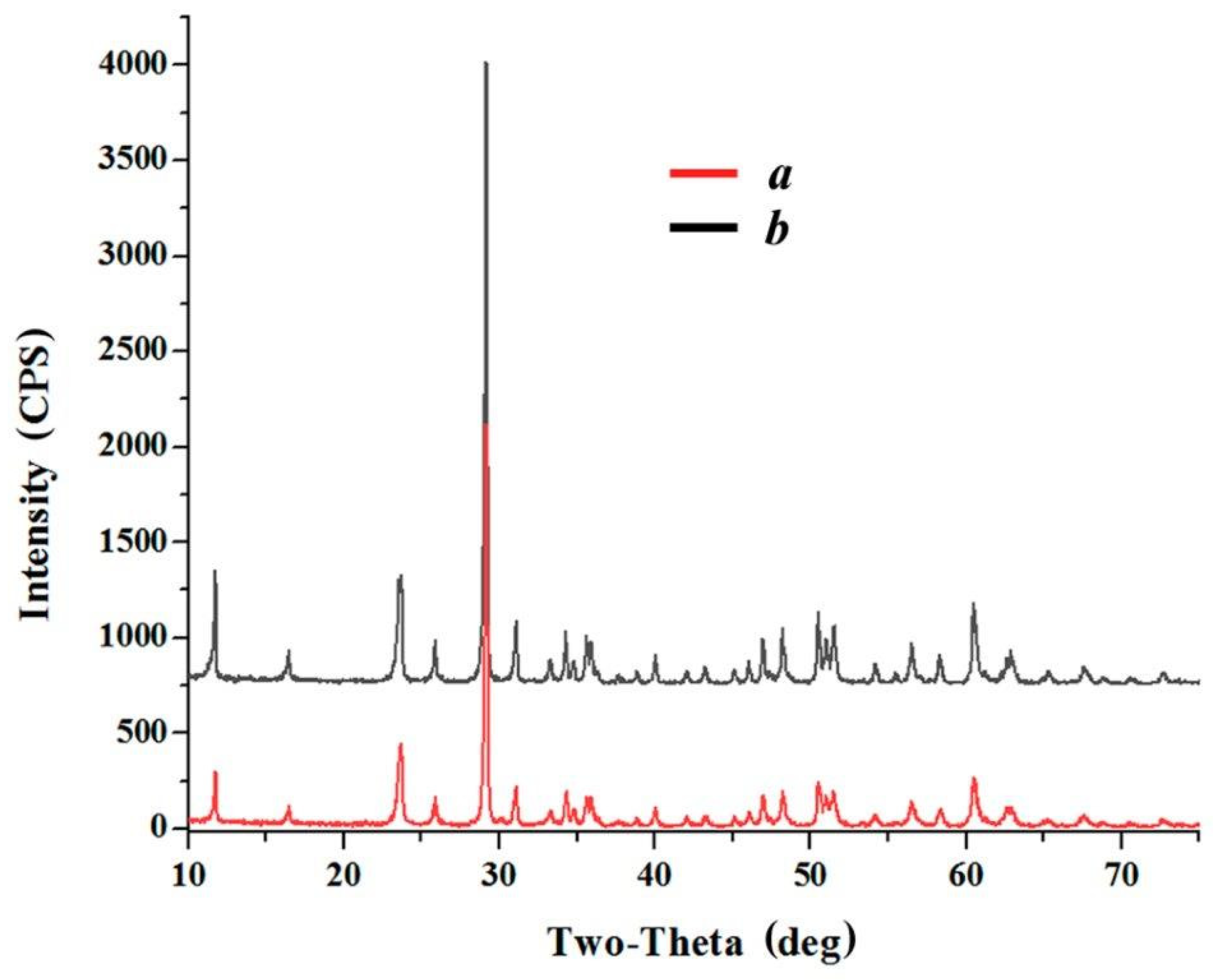
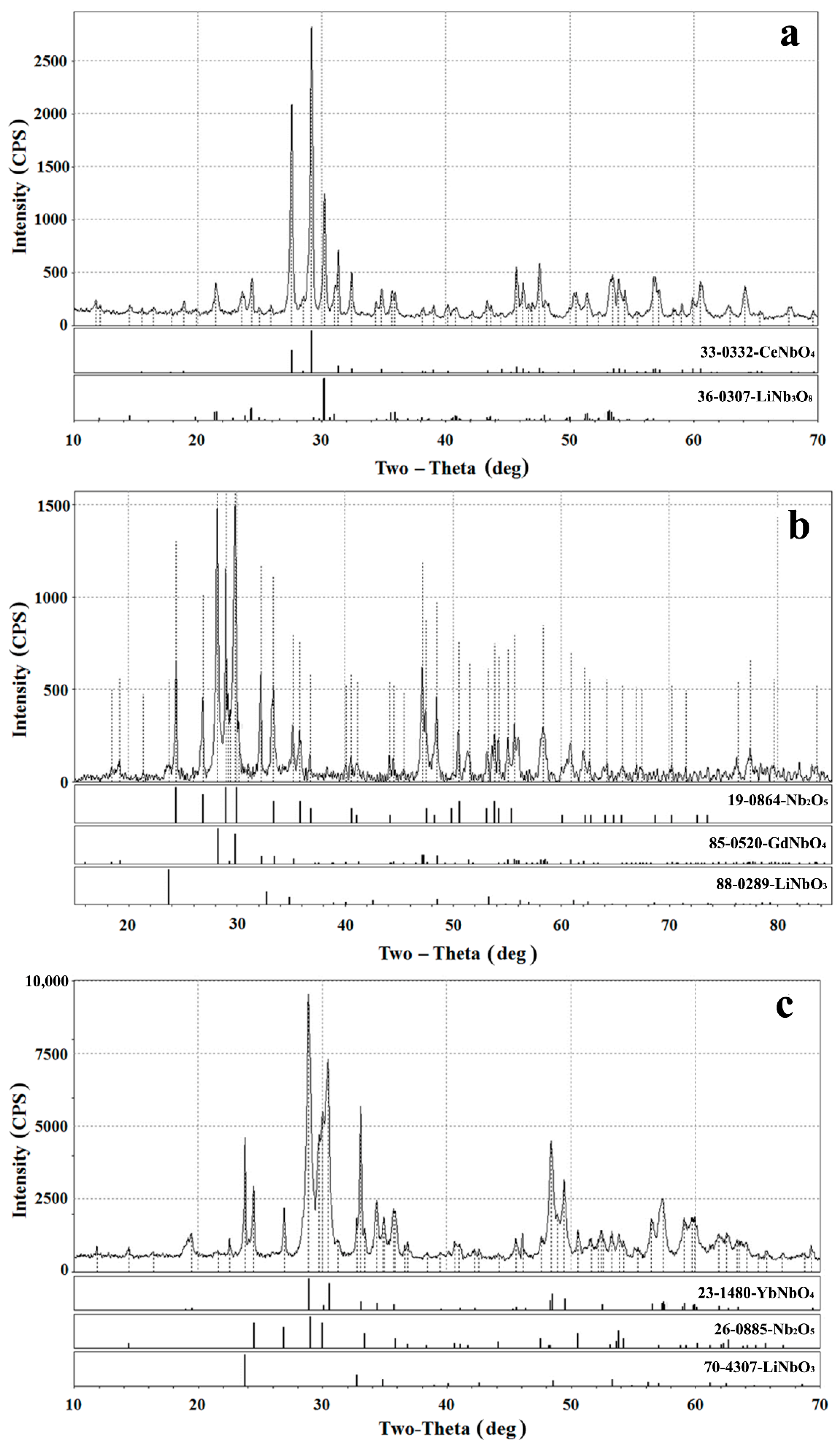
3.2. Modifying Composition of the LiNbO3 Fine Powders in Calcium-Containing Chloride Melts
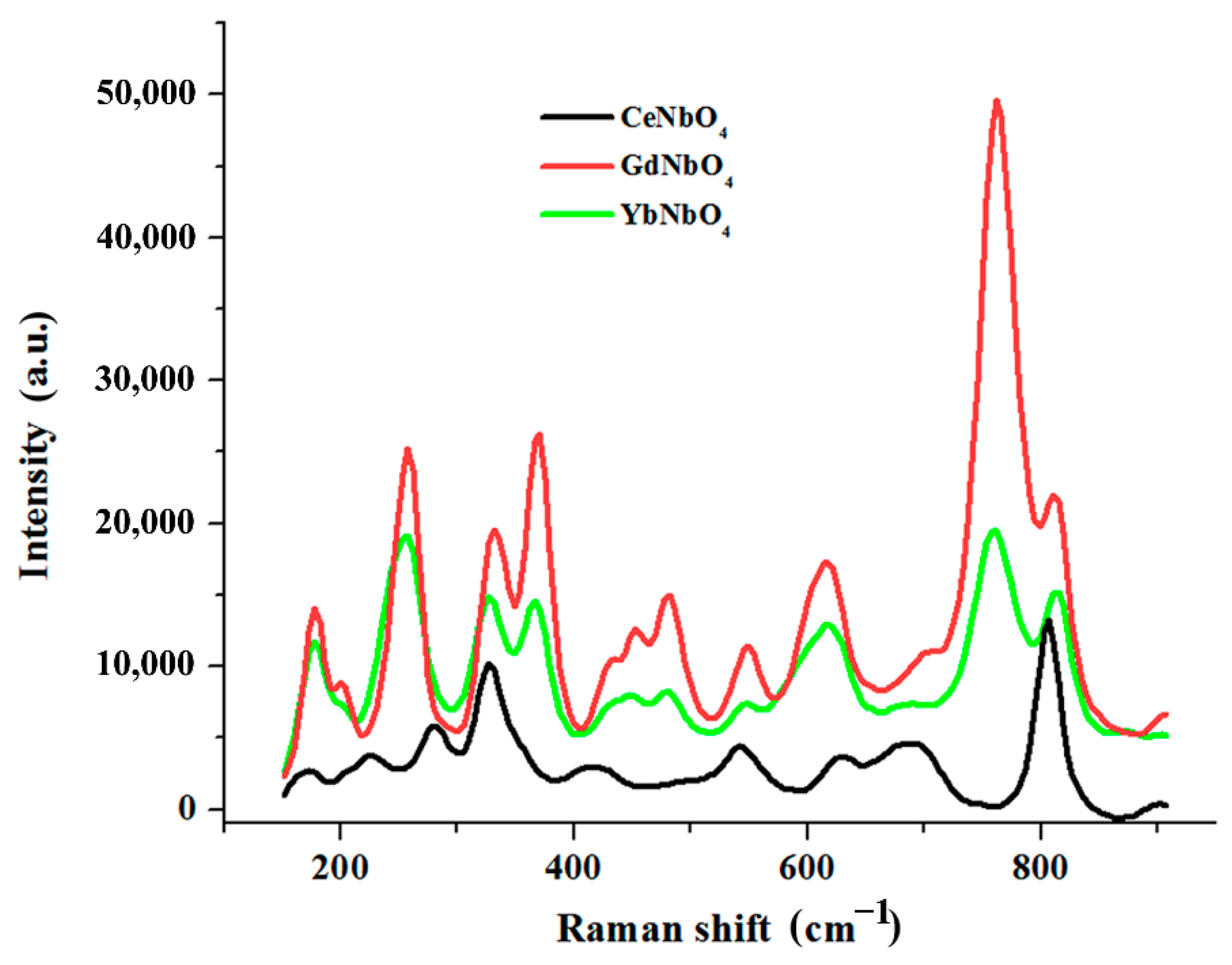
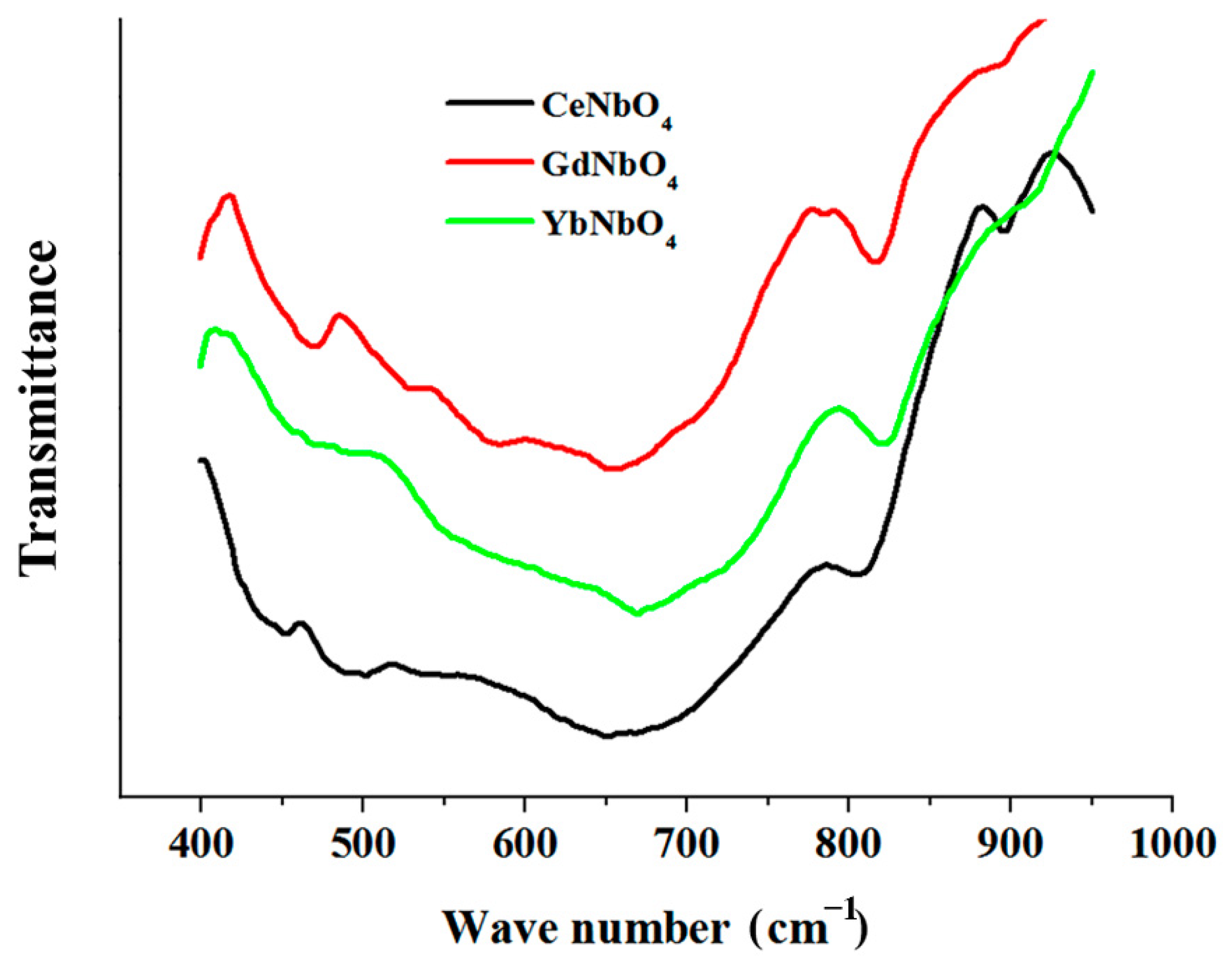
3.3. Modifying Composition of the LiNbO3 Fine Powders in Chloride Melts Containing Rare-Earth Ions
4. Conclusions
- hetero-epitaxial cation exchange at the interface PbCl2-containing melt/single crystal of lithium niobate;
- the formation of solid solution with cation vacancies as an intermediate product of the reaction of heterovalent substitution of lithium ion by calcium one in nanocrystalline LiNbO3 powders;
- the formation of tetragonal cerium, gadolinium, and ytterbium orthoniobates with crystal structures like scheelite one as the result of the interaction of the lithium metaniobate with the rare-earth trichlorides.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuz’minov, Y.S. Lithium Niobate and Tantalate: Materials for Nonlinear Optics; Nauka: Moscow, Russia, 1975. (In Russian) [Google Scholar]
- Lisiecki, R.; Macalik, B.; Kowalski, R.; Komar, J.; Ryba-Romanowski, W. Effect of Temperature on Luminescence of LiNbO3 Crystals Single-Doped with Sm3+, Tb3+, or Dy3+ Ions. Crystals 2020, 10, 1034. [Google Scholar] [CrossRef]
- Skvortsov, L.A.; Stepantsov, E.S. Laser damage resistance of a lithium niobate-tantalate bicrystal system. Quantum Electron. 1993, 23, 981–982. [Google Scholar] [CrossRef]
- Bach, F.; Mero, M.; Chou, M.-H.; Petrov, V. Laser induced damage studies of LiNbO3 using 1030-nm, ultrashort pulses at 10–1000 kHz. Opt. Mater. Express 2017, 7, 240–252. [Google Scholar] [CrossRef]
- Colomban, P. Proton conductors and their applications: A tentative historical overview of the early researches. Solid State Ion. 2019, 334, 125–144. [Google Scholar] [CrossRef]
- Rice, C.E.; Jackel, J.L. HNbO3 and HTaO3: New Cubic Perovskites Prepared from LiNbO3 and LiTaO3 via Ion Exchange. J. Solid State Chem. 1982, 41, 308–314. [Google Scholar] [CrossRef]
- Jackel, J.L.; Rice, C.E.; Veselka, J. Proton Exchange for High-Index Waveguides in LiNbO3. Appl. Phys. Lett. 1982, 41, 607–608. [Google Scholar] [CrossRef]
- Korkishko, Y.N.; Fedorov, V.A. Chapter 8.6: Refractive indices of different crystal phases in proton-exchanged LiNbO3 waveguides. In Properties of Lithium Niobate, EMIS Data Reviews Series № 28; Wong, K.K., Ed.; INSPEC, IEE: London, UK, 2002; pp. 146–152. Available online: https://www.researchgate.net/publication/265479355 (accessed on 8 April 2022).
- Lin, J.; Bo, F.; Cheng, Y.; Xu, J. Advances in on-chip photonic devices based on lithium niobate on insulator. Photonics Res. 2020, 8, 1910–1936. [Google Scholar] [CrossRef]
- Coll, M.; Fontcuberta, J.; Althammer, M.; Bibes, M.; Boschker, H.; Calleja, A.; Cheng, G.; Cuoco, M.; Dittmann, R.; Dkhil, B.; et al. M Towards Oxide Electronics: A Roadmap. Appl. Surf. Sci. 2019, 482, 1–93. [Google Scholar] [CrossRef]
- Sun, D.; Zhang, Y.; Wang, D.; Song, W.; Liu, X.; Pang, J.; Geng, D.; Sang, Y.; Liu, H. Microstructure and domain engineering of lithium niobate crystal films for integrated photonic applications. Light Sci. Appl. 2020, 9, 197. [Google Scholar] [CrossRef]
- Hreniak, D.; Speghini, A.; Bettinelli, M.; Strek, W. Spectroscopic investigations of nanostructured LiNbO3 doped with Eu3+. J. Lumin. 2006, 119, 219–223. [Google Scholar] [CrossRef]
- Günter, P.; Huignard, J.-P. Photorefractive Materials and Their Applications 2. Materials; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Schlarb, U.; Betzler, K. Refractive indices of lithium niobate as a function of temperature, wavelength, and composition: A generalized fit. Phys. Rev. B 1993, 48, 15613–15620. [Google Scholar] [CrossRef]
- Bausá, L.E.; Ramirez, M.O.; Montoya, E. Optical performance of Yb3+ in LiNbO3 laser crystal. Phys. Status Solidi A 2004, 201, 289–297. [Google Scholar] [CrossRef]
- Kondo, Y.; Fukuda, T.; Yamashita, Y.; Yokoyama, K.; Arita, K.; Watanabe, M.; Furukawa, Y.; Kitamura, K.; Nakajima, H. An increase of more than 30% inelectrooptic coefficient of Fe-doped and Ce-doped stoichiometric LiNbO3 crystals. Jpn. J. Appl. Phys. 2000, 39, 1477–1480. [Google Scholar] [CrossRef]
- Antonycheva, E.A.; Syuy, A.V.; Sidorov, N.V.; Yanichev, A.A. Photorefractive light scattering in LiNbO3: Cu crystals. Tech. Phys. 2010, 55, 877–879. [Google Scholar] [CrossRef]
- Volk, T.; Wöhlecke, M.; Rubinina, N.; Reichert, A.; Razumovski, N. Optical-damage-resistant impurities (Mg, Zn, In, Sc) in lithium niobate. Ferroelectrics 1996, 183, 291–300. [Google Scholar] [CrossRef]
- Herreros, B.; Lifante, G. LiNbO3 optical waveguides by Zn diffusion from vapor phase. Appl. Phys. Lett. 1995, 66, 1449–1451. [Google Scholar] [CrossRef]
- Schiller, F.; Herreros, B.; Lifante, G. Optical characterization of vapor Zn-diffused waveguides in lithium niobate. J. Am. Opt. Soc. A 1997, 14, 425–429. [Google Scholar] [CrossRef]
- Fedorov, V.A.; Korkishko Yu, N.; Vereda, F.; Lifante, G.; Cusso, F. Structural characterisation of vapour Zn-diffused waveguides in lithium niobate. J. Cryst. Growth 1998, 194, 94–100. [Google Scholar] [CrossRef]
- Zhou, Q.; Lam, K.H.; Zheng, H.; Qiu, W.; Shunga, K.K. Piezoelectric single crystal ultrasonic transducers for biomedical applications. Prog. Mater. Sci. 2014, 66, 87–111. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Dai, J.Y.; Zhang, C.; Zhang, Z.T.; Feng, G.P. Bandwidth improvement of LiNbO3 ultrasonic transducers by half-concaved inversion layer approach. Rev. Sci. Instrum. 2012, 83, 114903. [Google Scholar] [CrossRef] [Green Version]
- Hendee, W.R.; Ritenour, E.R. Ultrasound Transducers. In Medical Imaging Physics, 4th ed.; Wiley-Liss. Inc.: New York, NY, USA, 2002; pp. 317–330. [Google Scholar] [CrossRef]
- Lach, M.; Platte, M.; Ries, A. Piezoelectric Materials for Ultrasonic Probes. 1996. Available online: http://www.ndt.net/article/platte2/platte2.htm (accessed on 2 April 2022).
- Castilla, H.; Bélanger, P.; Zednik, R.J. High temperature characterization of piezoelectric lithium niobate using electrochemical impedance spectroscopy resonance method. J. Appl. Phys. 2017, 122, 244103. [Google Scholar] [CrossRef] [Green Version]
- Zhukov, R.N.; Kushnerev, K.S.; Kiselev, D.A.; Ilina, T.S.; Kubasov, I.V.; Kislyuk, A.M.; Malinkovich, M.D.; Parkhomenko, Y.N. Enhancement of piezoelectric properties of lithium niobate thin films by different annealing parameters. Mod. Electron. Mater. 2020, 6, 47–52. [Google Scholar] [CrossRef]
- Kazys, R.; Vaskeliene, V. High Temperature Ultrasonic Transducers: A Review. Sensors 2021, 21, 3200. [Google Scholar] [CrossRef]
- Kimura, T. Molten Salt Synthesis of Ceramic Powders. In Advances in Ceramics—Synthesis and Characterization, Processing and Specific Applications; Sikalidis, C., Ed.; InTech: Rijeka, Croatia, 2011; pp. 75–100. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Deng, J.; Chen, J.; Xing, X. Topochemical molten salt synthesis for functional perovskite compounds. Chem. Sci. 2016, 7, 855–865. [Google Scholar] [CrossRef] [Green Version]
- Khokhlov, V.; Modenov, D.; Dokutovich, V.; Kochedykov, V.; Zakir’yanova, I.; Vovkotrub, E.; Beketov, I. Lithium oxide solution in chloride melts as a medium to prepare LiCoO2 nanoparticles. MRS Commun. 2014, 4, 15–18. [Google Scholar] [CrossRef]
- Khokhlov, V.A.; Dokutovich, V.N.; Viugin, N.A.; Bobrova, K.O. Structural and Morphological Peculiarities of the Lithium Niobate and Lithium Tantalate Powders Synthesized in Chloride Melts. Russ. Metall. 2019, 2019, 90–96. [Google Scholar] [CrossRef]
- Kochedykov, V.A.; Khokhlov, V.A. Refractive Indices and Molar Refractivities of Molten Rare Earth Trichlorides and Their Mixtures with Alkali Chlorides. J. Chem. Eng. Data 2017, 62, 44–51. [Google Scholar] [CrossRef]
- Sahu, K.R.; De, U. Dielectric Properties of Rhombohedral PbNb2O6. J. Solid State Phys. 2013, 2013, 451563. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, F.; Almeida, B.G.; Caldelas, P.; Mendes, J.A. Structural Characterization of Lead Metaniobate Thin Films Deposited by Pulsed Laser Ablation. Mat. Sci. Forum 2006, 514–516, 207–211. [Google Scholar] [CrossRef]
- Souk, J.H.; Segmüller, A.; Angilello, J. Oriented growth of ultrathin tungsten films on sapphire substrates. J. Appl. Phys. 1987, 62, 509–512. [Google Scholar] [CrossRef]
- Chakraborty, K.R.; Sahu, K.R.; De, A.; De, U. Structural Characterization of Orthorhombic and Rhombohedral Lead Meta-Niobate Samples. Integr. Ferroelectr. 2010, 120, 102–113. [Google Scholar] [CrossRef]
- Cardoso, F.; Almeida, B.G.; Caldelas, P.; Mendes, J.A.; Barbosa, J. Structural and Electrical Characterization of Lead Metaniobate Thin Films Deposited by Pulsed Laser Ablation. Ferroelectrics 2006, 335, 201–209. [Google Scholar] [CrossRef]
- Sahu, K.R.; Chakraborty, K.R.; De, U. Crystallographic phases in PbNb2O6 and Piezoelectricity. Mater. Today Proc. 2019, 11, 869–874. [Google Scholar] [CrossRef]
- Ilhan, M.; Ekmekçi, M.K.; Demir, A.; Demirer, H. Synthesis and Optical Properties of Novel Red-Emitting PbNb2O6: Eu3+ Phosphors. J. Fluoresc. 2016, 26, 1637–1643. [Google Scholar] [CrossRef]
- Fontana, M.D.; Bourson, P. Microstructure and defects probed by Raman spectroscopy in lithium niobate crystals and devices. Appl. Phys. Rev. 2015, 2, 040602. [Google Scholar] [CrossRef]
- Sanna, S.; Neufeld, S.; Rüsing, M.; Berth, G.; Zrenner, A.; Schmidt, W.G. Raman scattering efficiency in LiTaO3 and LiNbO3 crystals. Phys. Rev. B 2015, 91, 224302. [Google Scholar] [CrossRef]
- Lengyel, K.; Péter, Á.; Kovács, L.; Corradi, G.; Pálfalvi, L.; Hebling, J.; Unferdorben, M.; Dravecz, G.; Hajdara, I.; Szaller, Z.; et al. Growth, defect structure, and THz application of stoichiometric lithium niobate. Appl. Phys. Rev. 2015, 2, 040601. [Google Scholar] [CrossRef] [Green Version]
- Bartasyte, A.; Plausinaitiene, V.; Abrutis, A.; Stanionyte, S.; Margueron, S.; Boulet, P.; Kobata, T.; Uesu, Y.; Gleize, J. Identification of LiNbO3, LiNb3O8 and Li3NbO4 Phases in Thin Films Synthesized with Different Deposit Techniques by Means of XRD and Raman Spectroscopy. J. Phys. Condens. Matter 2013, 25, 205901. [Google Scholar] [CrossRef]
- Mirsaneh, M.; Hayden, B.E.; Miao, S.; Pokorny, J.; Perini, S.; Furman, E.; Lanagan, M.T.; Ubic, R.; Reaney, I.M. High throughput synthesis and characterization of the PbnNb2O5+n (0.5 <n <4.1) system on a single chip. Acta Mater. 2011, 59, 2201–2209. [Google Scholar] [CrossRef]
- Sá, P.; Barbosa, J.; Gomes, I.T.; Mendes, J.A.; Ventura, J.; Araújo, J.P.; Almeida, B.G. Synthesis, structural and magnetic characterization of lead-metaniobate/cobalt-ferrite nanocomposite films deposited by pulsed laser ablation. Appl. Phys. A 2015, 118, 275–281. [Google Scholar] [CrossRef]
- Capper, P.; Irvine, S.; Joyce, T. Epitaxial Crystal Growth: Methods and Materials. In Springer Handbook of Electronic and Photonic Materials; Kasap, S., Capper, P., Eds.; Springer Int. Publ.: Cham, Switzerland, 2017; pp. 309–341. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Zheng, H.; Jun, Y.; Alivisatos, A.P. Hetero-Epitaxial Anion Exchange Yields Single-Crystalline Hollow Nanoparticles. J. Am. Chem. Soc. 2009, 131, 13943–13945. [Google Scholar] [CrossRef] [Green Version]
- Bohnstedt-Kupletskaya, E.M.; Burova, T.A. Fersmite, a New Calcium Niobate from Pegmatites of the Vishnevye Mts., the Central Urals. Compt. Rend. (Dokl.) L’Académie Sci. L’URSS 1946, 52, 69–71. [Google Scholar]
- Cummings, J.P.; Simonsen, S.H. The Crystal Structure of Calcium Niobate (CaNb2O6). Am. Mineral. J. Earth Planet. Mater. 1970, 55, 90–97. [Google Scholar]
- Mathai, K.C.; Vidya, S.; John, A.; Solomon, S.; Thomas, J.K. Structural, Optical, and Compactness Characteristics of Nanocrystalline CaNb2O6 Synthesized through an Autoigniting Combustion Method. Adv. Cond. Matter Phys. 2014, 2014, 735878. [Google Scholar] [CrossRef] [Green Version]
- Cho, I.-S.; Bae, S.T.; Kim, D.H.; Hong, K.S. Effects of crystal and electronic structures of ANb2O6 (A = Ca, Sr, Ba) metaniobate compounds on their photocatalytic H2 evolution from pure water. Int. J. Hydrogen Energy 2010, 35, 12954–12960. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, P. Effects of lanthanides on structural and dielectric properties of NdNbO4-LnNbO4 ceramics. Ceram. Intern. 2018, 44, 1935–1941. [Google Scholar] [CrossRef]
- Siqueira, K.P.F.; Moreira, R.L.; Dias, A. Synthesis and crystal structure of lanthanide orthoniobates studied by vibrational spectroscopy. Chem. Mater. 2010, 22, 2668–2674. [Google Scholar] [CrossRef]



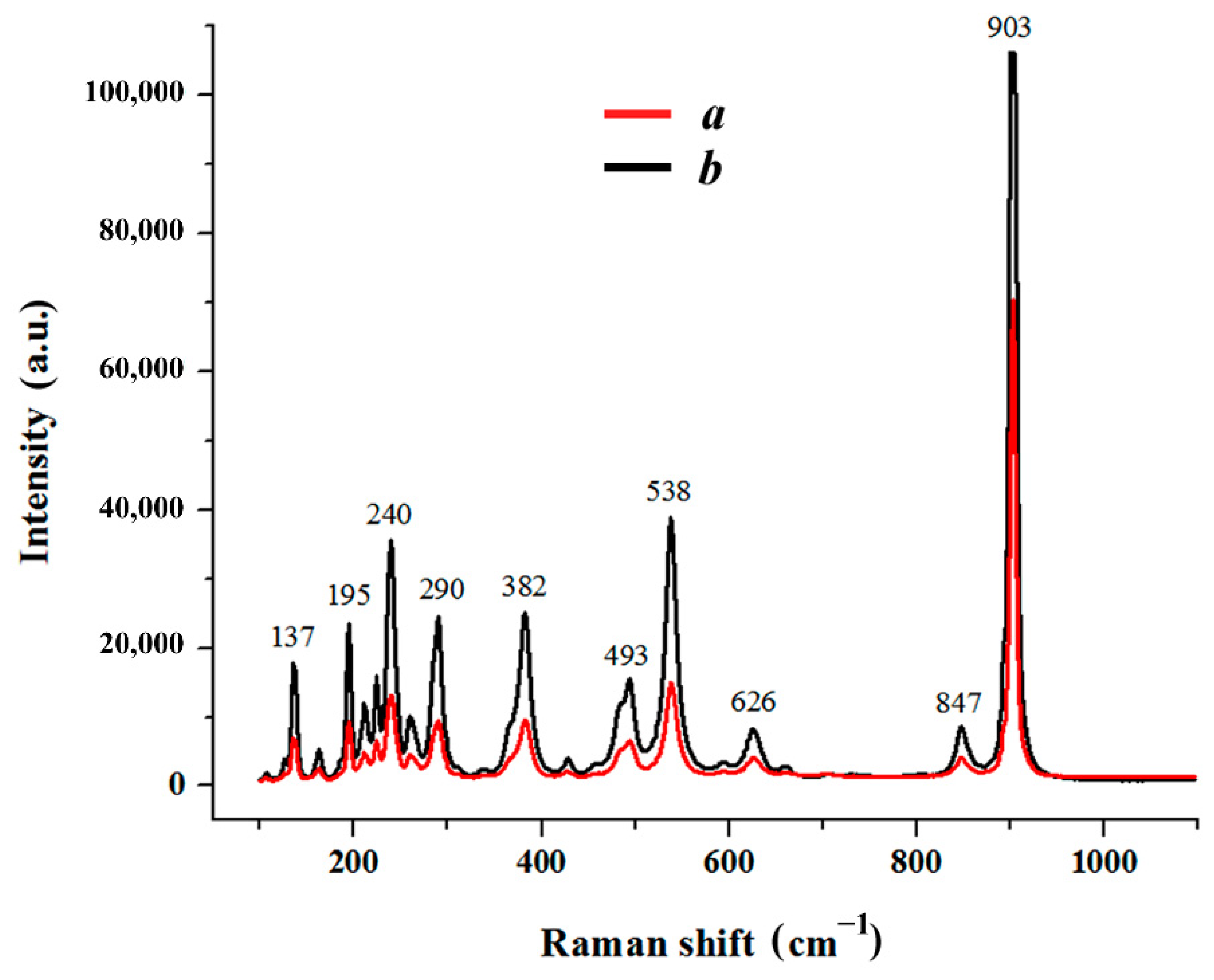
| FWHM (Full Width at Half Maximum)/cm−1 | Normalized Intensity (I/I874) | Peak Maximum Position/cm−1 | |||
|---|---|---|---|---|---|
| Initial Sample | Modified Sample | Initial Sample | Modified Sample | Initial Sample | Modified Sample |
| 24 | 24 | 0.37 | 0.59 | 237 | 237 |
| 32 | 34 | 0.41 | 0.60 | 269 | 268 |
| 34 | 36 | 0.40 | 0.50 | 327 | 325 |
| 33 | 33 | 0.19 | 0.24 | 370 | 370 |
| 33 | 34 | 0.53 | 0.56 | 430 | 431 |
| 24 | 26 | 0.05 | 0.08 | 477 | 479 |
| 35 | 36 | 0.60 | 0.78 | 583 | 583 |
| 29 | 37 | 0.12 | 0.37 | 620 | 621 |
| 112 | 92 | 0.06 | 0.09 | 649 | 659 |
| 40 | 40 | 1 | 1 | 874 | 874 |
| [K]/mg∙dm−3 | [Li]/mg∙dm−3 | [Nb]/mg∙dm−3 | [Pb]/mg∙dm−3 |
|---|---|---|---|
| 7522 | 0.035 | ˂0.001 | 1169 |
| [O]/at. % | [Nb]/at. % | [Pb]/at. % |
|---|---|---|
| 72.0 | 27.0 | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viugin, N.A.; Khokhlov, V.A.; Zakiryanova, I.D.; Dokutovich, V.N.; Antonov, B.D. Molten Chlorides as the Precursors to Modify the Ionic Composition and Properties of LiNbO3 Single Crystal and Fine Powders. Materials 2022, 15, 3551. https://doi.org/10.3390/ma15103551
Viugin NA, Khokhlov VA, Zakiryanova ID, Dokutovich VN, Antonov BD. Molten Chlorides as the Precursors to Modify the Ionic Composition and Properties of LiNbO3 Single Crystal and Fine Powders. Materials. 2022; 15(10):3551. https://doi.org/10.3390/ma15103551
Chicago/Turabian StyleViugin, Nikolay A., Vladimir A. Khokhlov, Irina D. Zakiryanova, Vasiliy N. Dokutovich, and Boris D. Antonov. 2022. "Molten Chlorides as the Precursors to Modify the Ionic Composition and Properties of LiNbO3 Single Crystal and Fine Powders" Materials 15, no. 10: 3551. https://doi.org/10.3390/ma15103551
APA StyleViugin, N. A., Khokhlov, V. A., Zakiryanova, I. D., Dokutovich, V. N., & Antonov, B. D. (2022). Molten Chlorides as the Precursors to Modify the Ionic Composition and Properties of LiNbO3 Single Crystal and Fine Powders. Materials, 15(10), 3551. https://doi.org/10.3390/ma15103551