Layered Perovskites BaM2In2O7 (M = La, Nd): From the Structure to the Ionic (O2−, H+) Conductivity
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Material Characterization
3.2. Oxygen-Ionic Conductivity
3.3. Protonic Conductivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ruddlesden, S.N.; Popper, P. The compound Sr3Ti2O7 and its structure. Acta Cryst. 1958, 11, 54–55. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, K.; Tsuji, Y.; Hatamachi, T.; Toda, K.; Kodama, T.; Sato, M.; Kitayama, Y. Photocatalytic water splitting on hydrated layered perovskite tantalate A2SrTa2O7·nH2O (A = H, K, and Rb). Phys. Chem. Chem. Phys. 2004, 6, 1064–1069. [Google Scholar] [CrossRef]
- Liang, Z.; Tang, K.; Shao, Q.; Lia, G.; Zeng, S.; Zheng, H. Synthesis, crystal structure, and photocatalytic activity of a new two-layer Ruddlesden–Popper phase, Li2CaTa2O7. J. Solid State Chem. 2008, 181, 964–970. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Wang, L.; Hao, Q.; Zhu, X.; Chen, X.; Tang, K. Preparation of interlayer surface tailored protonated double-layered perovskite H2CaTa2O7 with n-alcohols, and their photocatalytic activity. RSC Adv. 2014, 4, 4047–4054. [Google Scholar] [CrossRef]
- Wang, C.; Jin, Y.; Lv, Y.; Ju, G.; Chen, L.; Li, X.; Hu, Y. A bifunctional phosphor Sr3Sn2O7:Eu3+: Red luminescence and photochromism properties. J. Lumin. 2017, 192, 337–342. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, B.; Liu, Q.; Ding, N.; Yang, H.; Wang, L.; Zhang, Q. Novel layered perovskite Sr3Ti2O7:Eu3+ phosphor with high-efficiency luminescence enhanced by charge compensation. J. Alloys Compd. 2016, 657, 27–31. [Google Scholar] [CrossRef]
- Battle, P.D.; Rosseinsky, M.J. Synthesis, structure, and magnetic properties of n=2 Ruddlesden–Popper manganates. Curr. Opin. Solid State Mater. Sci. 1999, 4, 163–170. [Google Scholar] [CrossRef]
- Ling, C.D.; Millburn, J.E.; Mitchell, J.F.; Argyriou, D.N.; Linton, J. Interplay of spin and orbital ordering in the layered colossal magnetoresistance manganite La2–2xSr1+2xMn2O7 (0.5 ≤ x ≤ 1.0). Phys. Rev. B 2000, 62, 15096–15111. [Google Scholar] [CrossRef] [Green Version]
- Medvedeva, J.E.; Anisimov, V.I.; Mryasov, O.N.; Freeman, A.J. The role of Coulomb correlation in magnetic and transport properties of doped manganites: La0.5Sr0.5MnO3 and LaSr2Mn2O7. J. Phys. Condens. Matter 2002, 14, 4533–4542. [Google Scholar] [CrossRef] [Green Version]
- Taşarkuyu, E.; Coşkun, A.; Irmak, A.E.; Aktürk, S.; Ünlüa, G.; Samancıoğlu, Y.; Yücel, A.; Sarıkürkçü, C.; Aksoy, S.; Acet, M. Effect of high temperature sintering on the structural and the magnetic properties of La1.4Ca1.6Mn2O7. J. Alloys Compd. 2011, 509, 3717–3722. [Google Scholar] [CrossRef]
- Dudric, R.; Goga, F.; Neumann, M.; Mican, S.; Tetean, R. Magnetic properties and magnetocaloric effect in La1.4–xCexCa1.6Mn2O7 perovskites synthesized by sol–gel method. J. Mater. Sci. 2012, 47, 3125–3130. [Google Scholar] [CrossRef]
- Denbri, F.; Mahamdioua, N.; Meriche, F.; Altintas, S.P.; Terzioglu, C. Investigation of magneto-transport properties of the codoped La1.6-xPrxCa1.4-xBaxMn2O7 (x = 0.2 and 0.4) double-layered manganite. J. Mater. Sci. Mater. Electron. 2021, 32, 18808–18824. [Google Scholar] [CrossRef]
- Boulahya, K.; Muñoz-Gil, D.; Gómez-Herrero, A.; Azcondo, M.T.; Amador, U. Eu2SrCo1.5Fe0.5O7 a new promising Ruddlesden–Popper member as cathode component for intermediate temperature solid oxide fuel cells. J. Mater. Chem. A 2019, 7, 5601–5611. [Google Scholar] [CrossRef]
- Wang, Q.; Hou, J.; Fan, Y.; Xi, X.; Li, J.; Lu, Y.; Huo, G.; Shao, L.; Fu, X.; Luo, J. Pr2BaNiMnO7-δ double-layered Ruddlesden–Popper perovskite oxides as efficient cathode electrocatalysts for low temperature proton conducting solid oxide fuel cells. J. Mater. Chem. A 2020, 8, 7704–7712. [Google Scholar] [CrossRef]
- Muñoz Gil, D.; Boulahya, K.; Santoyo, M.S.; Azcondo, M.T.; Amador, U. Superior Performance as Cathode Material for Intermediate-Temperature Solid Oxide Fuel Cells of the Ruddlesden−Popper n = 2 Member Eu2SrCo0.50Fe1.50O7−δ with Low Cobalt Content. Inorg. Chem. 2021, 60, 3094–3105. [Google Scholar] [CrossRef]
- Kananke-Gamage, C.C.W.; Ramezanipou, F. Variation of the electrocatalytic activity of isostructural oxides Sr2LaFeMnO7 and Sr2LaCoMnO7 for hydrogen and oxygen-evolution reactions. Dalton Trans. 2021, 50, 14196–14206. [Google Scholar] [CrossRef]
- Tarasova, N.; Galisheva, A.; Animitsa, I.; Korona, D.; Kreimesh, H.; Fedorova, I. Protonic transport in layered perovskites BaLanInnO3n+1 (n = 1, 2) with Ruddlesden-Popper structure. Appl. Sci. 2022, 12, 4082. [Google Scholar] [CrossRef]
- Kim, S.; Kim, G.; Manthiram, A. A review on infiltration techniques for energy conversion and storage devices: From fundamentals to applications. Sustain. Energy Fuels 2021, 5, 5024–503721. [Google Scholar] [CrossRef]
- Klyndyuk, A.I.; Chizhova, E.A.; Kharytonau, D.S.; Medvedev, D.A. Layered oxygen-deficient double perovskites as promising cathode materials for solid oxide fuel cells. Materials 2022, 15, 141. [Google Scholar] [CrossRef]
- Hanif, M.B.; Motola, M.; Qayyum, S.; Rauf, S.; Khalid, A.; Li, C.-J.; Li, C.-X. Recent advancements, doping strategies and the future perspective of perovskite-based solid oxide fuel cells for energy conversion. Chem. Eng. J. 2022, 42815, 132603. [Google Scholar] [CrossRef]
- Shim, J.H. Ceramics breakthrough. Nat. Energy 2018, 3, 168–169. [Google Scholar] [CrossRef]
- Meng, Y.; Gao, J.; Zhao, Z.; Amoroso, J.; Tong, J.; Brinkman, K.S. Review: Recent progress in low-temperature proton-conducting ceramics. J. Mater. Sci. 2019, 54, 9291–9312. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Sengodan, S.; Kim, S.; Kwon, O.; Bud, Y.; Kim, G. Proton conducting oxides: A review of materials and applications for renewable energy conversion and storage. Renew. Sustain. Energy Rev. 2019, 109, 606–618. [Google Scholar] [CrossRef]
- Medvedev, D.A. Current drawbacks of proton-conducting ceramic materials: How to overcome them for real electrochemical purposes. Curr. Opin. Green Sustain. Chem. 2021, 32, 100549. [Google Scholar] [CrossRef]
- Bello, I.T.; Zhai, S.; He, Q.; Cheng, C.; Dai, Y.; Chen, B.; Zhang, Y.; Ni, M. Materials development and prospective for protonic ceramic fuel cells. Int. J. Energy Res. 2022, 46, 2212–2240. [Google Scholar] [CrossRef]
- Irvine, J. Roadmap on inorganic perovskites for energy applications. J. Phys. Energy 2021, 3, 031502. [Google Scholar] [CrossRef]
- Titov, Y.A.; Belyavina, N.M.; Markiv, V.Y.; Slobodyanik, M.S.; Krayevska, Y.A.; Yaschuk, V.P. Synthesis and crystal structure of BaLn2In2O7. Rep. Natl. Acad. Sci. Ukr. 2010, 1, 148–153. [Google Scholar]
- Caldes, M.; Michel, C.; Rouillon, T.; Hervieu, M.; Raveau, B. Novel indates Ln2BaIn2O7, n = 2 members of the Ruddlesden–Popper family (Ln = La, Nd). J. Mater. Chem. 2002, 12, 473–476. [Google Scholar] [CrossRef]
- Fujii, K.; Esaki, Y.; Omoto, K.; Yashima, M.; Hoshikawa, A.; Ishigaki, T.; Hester, J.R. New Perovskite-Related Structure Family of Oxide-Ion Conducting Materials NdBaInO4. Chem. Mater. 2014, 26, 2488–2491. [Google Scholar] [CrossRef]
- Fujii, K.; Shiraiwa, M.; Esaki, Y.; Yashima, M.; Kim, S.J.; Lee, S. Improved oxide-ion conductivity of NdBaInO4 by Sr doping. J. Mater. Chem. A 2015, 3, 11985. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, T.; Yan, Y.; Sakai, T.; Ida, S. Oxide ion conductivity in doped NdBaInO4. Solid State Ionics 2016, 288, 262–265. [Google Scholar] [CrossRef]
- Yang, X.; Liu, S.; Lu, F.; Xu, J.; Kuang, X. Acceptor Doping and Oxygen Vacancy Migration in Layered Perovskite NdBaInO4-Based Mixed Conductors. J. Phys. Chem. C 2016, 120, 6416–6426. [Google Scholar] [CrossRef]
- Fijii, K.; Yashima, M. Discovery and development of BaNdInO4—A brief review. J. Ceram. Soc. Jpn. 2018, 126, 852–859. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Shiraiwa, M.; Nagao, M.; Fujii, K.; Tanaka, I.; Yashima, M.; Baque, L.; Basbus, J.F.; Mogni, L.V.; Skinner, S.J. Protonic Conduction in the BaNdInO4 Structure Achieved by Acceptor Doping. Chem. Mater. 2021, 33, 2139–2146. [Google Scholar] [CrossRef]
- Tarasova, N.; Galisheva, A.; Animitsa, I. Ba2+/Ti4+- co-doped layered perovskite BaLaInO4: The structure and ionic (O2−, H+) conductivity. Int. J. Hydrogen Energy 2021, 46, 16868–16877. [Google Scholar] [CrossRef]
- Tarasova, N.A.; Galisheva, A.O.; Animitsa, I.E.; Lebedeva, E.L. Oxygen-Ion and Proton Transport in Sc-Doped Layered Perovskite BaLaInO4. Russ. J. Electrochem. 2021, 57, 1008–1014. [Google Scholar] [CrossRef]
- Tarasova, N.A.; Galisheva, A.O.; Animitsa, I.E.; Dmitrieva, A.A. The Effect of Donor Doping on the Ionic (O2−, H+) Transport in Novel Complex Oxides BaLaIn1–xNbxO4+x with the Ruddlesden–Popper Structure. Russ. J. Electrochem. 2021, 57, 962–969. [Google Scholar] [CrossRef]
- Tarasova, N.; Animitsa, I.; Galisheva, A. Effect of acceptor and donor doping on the state of protons in block-layered structures based on BaLaInO4. Solid State Commun. 2021, 323, 14093. [Google Scholar] [CrossRef]
- Tarasova, N.; Animitsa, I.; Galisheva, A. Spectroscopic and transport properties of Ba- and Ti-doped BaLaInO4. J. Raman Spec. 2021, 52, 980–987. [Google Scholar] [CrossRef]
- Tarasova, N.; Animitsa, I.; Galisheva, A. Effect of doping on the local structure of new block-layered proton conductors based on BaLaInO4. J. Raman Spec. 2021, 51, 2290–2297. [Google Scholar] [CrossRef]
- Tarasova, N.; Animitsa, I. Materials AIILnInO4 with Ruddlesden-Popper structure for electrochemical applications: Relationship between ion (oxygen-ion, proton) conductivity, water uptake and structural changes. Materials 2022, 15, 114. [Google Scholar] [CrossRef] [PubMed]
- Scherban, T.; Villeneuve, R.; Abello, L.; Lucazeau, G. Raman scattering study of acceptor-doped BaCeO3. Solid State Ion. 1993, 61, 93–98. [Google Scholar] [CrossRef]
- Chemarin, C.; Rosman, N.; Pagnier, T.; Lucazeau, G. High-Pressure Raman Study of Mixed Perovskites BaCexZr1-xO3 (0≤x≤1). J. Solid State Chem. 2000, 149, 298–307. [Google Scholar] [CrossRef]
- Paul, B.; Chatterjee, S.; Gop, S.; Roy, A.; Grover, V.; Shukla, R.; Tyagi, A.K. Evolution of lattice dynamics in ferroelectric hexagonal REInO3 (RE = Ho, Dy, Tb, Gd, Eu, Sm) perovskites. Mater. Res. Express 2016, 3, 075703. [Google Scholar] [CrossRef]
- Kamba, S.; SamoukPina, F.; Kadlec, F.; Pokorny, J.; Petzelt, J.; Reaney, I.M.; Wise, P.L. Composition dependence of the lattice vibrations in Srn+1TinO3n+1 Ruddlesden–Popper homologous series. J. Eur. Ceram. Soc. 2003, 23, 2639–2645. [Google Scholar] [CrossRef]
- Iliev, M.N.; Popov, V.N.; Litvinchuk, A.P.; Abrashev, M.V.; Backstrom, J.; Sun, Y.Y.; Mena, R.L.; Chu, C.W. Comparative Raman studies of Sr2RuO4; Sr3Ru2O7 and Sr4Ru3O10. Physica B 2005, 358, 138–152. [Google Scholar] [CrossRef] [Green Version]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]

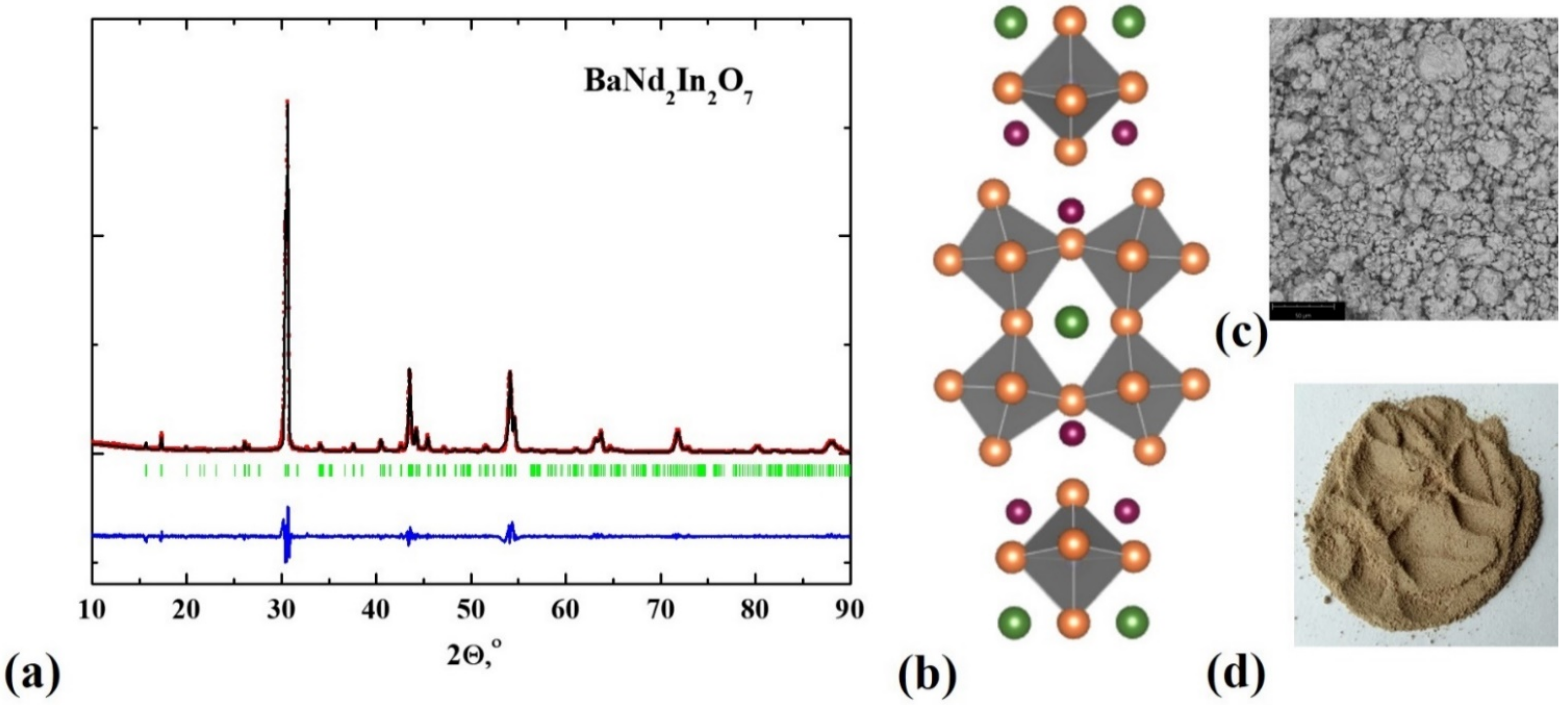
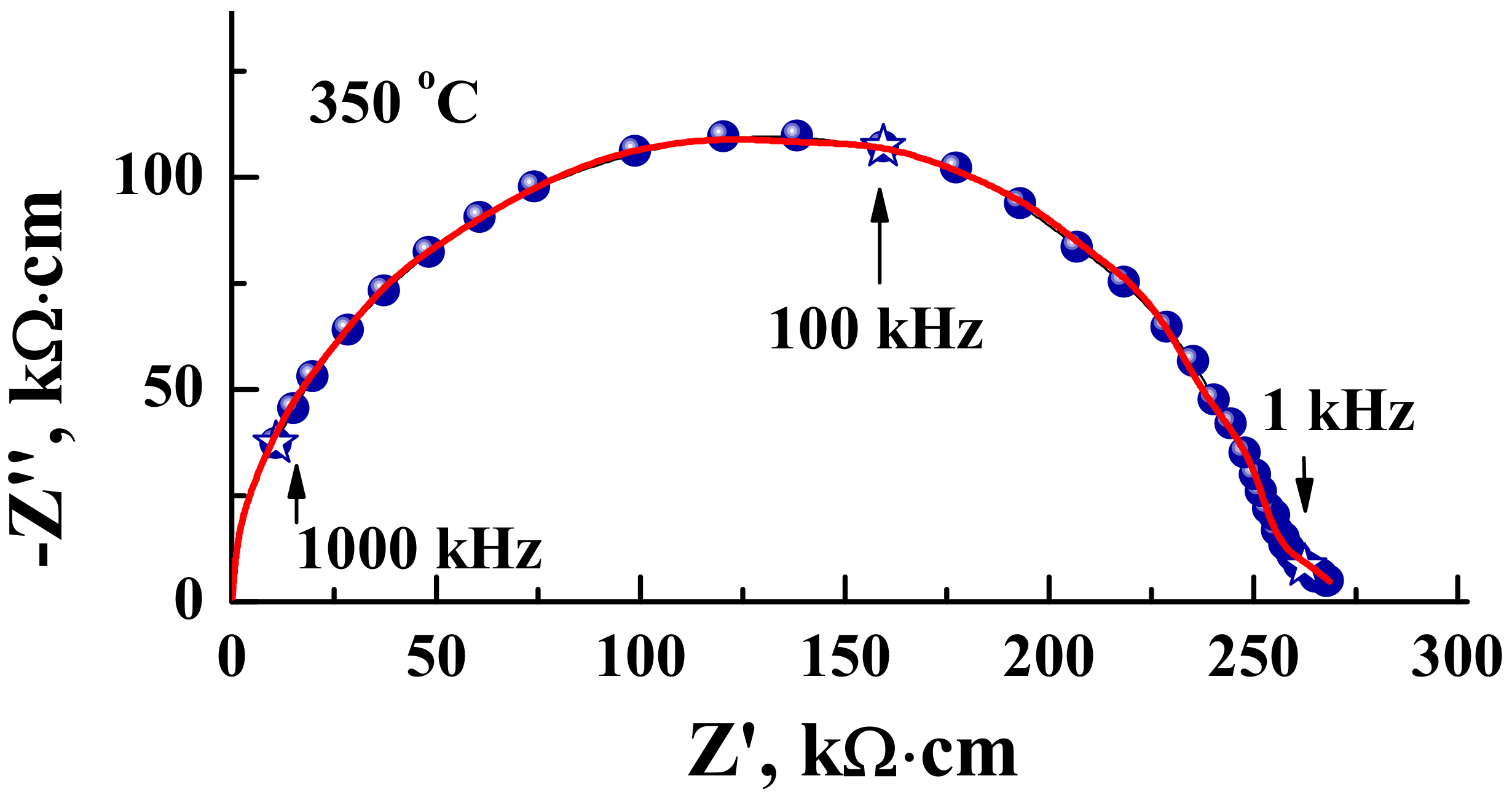

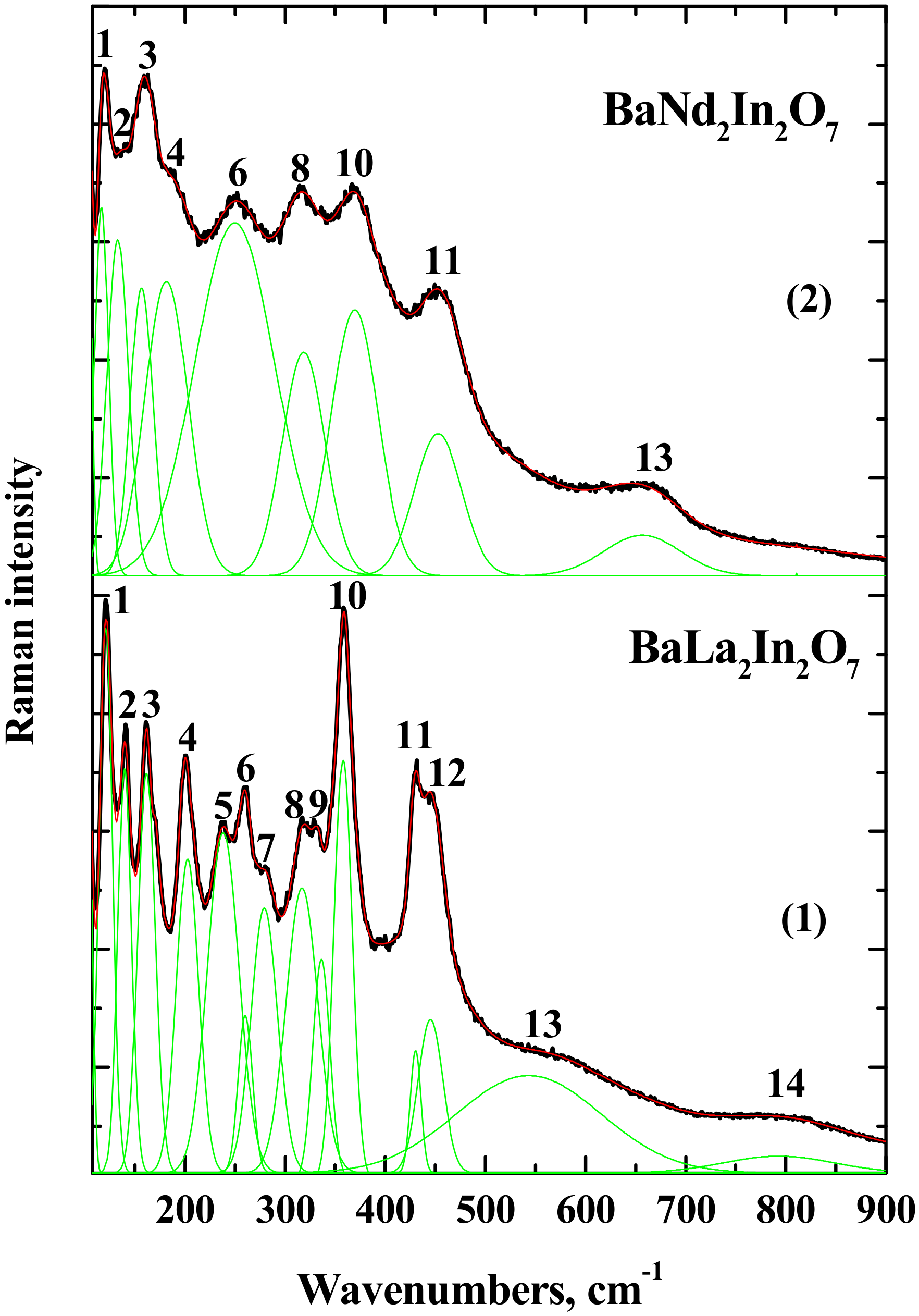



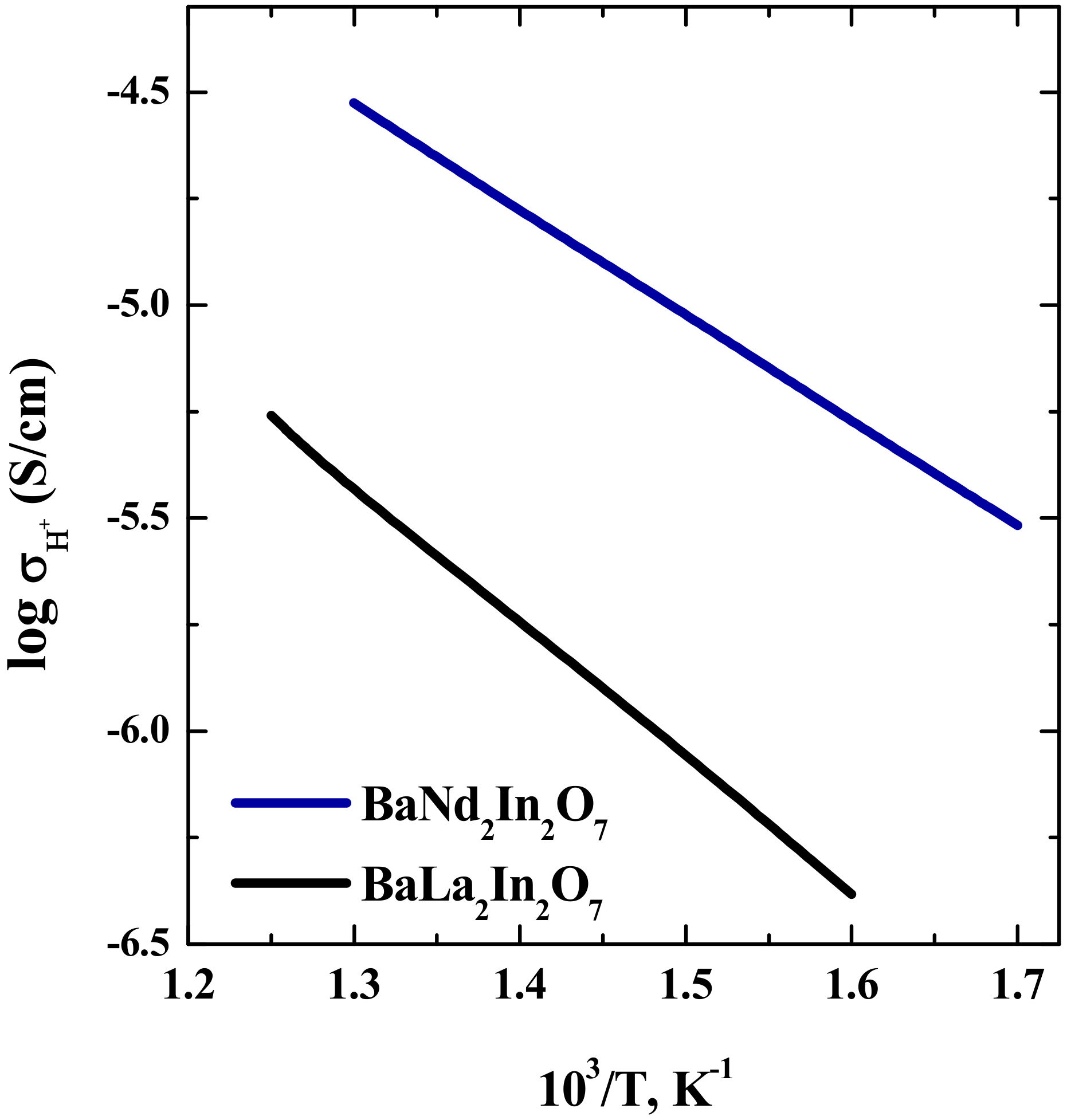
| Composition | Unit Cell Parameters | This Work | Titov et al. [27] | Raveau et al. [28] |
|---|---|---|---|---|
| BaNd2In2O7 | a, Å | 5.8916(9) | 5.8969(8) | 5.8940(3) |
| c, Å | 20.469(0) | 20.439(3) | 20.467(1) | |
| V, Å3 | 710.52 | 710.76 | 711.02 | |
| BaLa2In2O7 | a, Å | 5.914(9) | 5.915(2) | 5.9141(3) |
| c, Å | 20.846(5) | 20.861(1) | 20.831(2) | |
| V, Å3 | 729.34 | 729.92 | 729.73 |
| Element | Content of the Elements, Atomic % | |
|---|---|---|
| BaNd2In2O7 | BaLa2In2O7 | |
| Ba | 8.81 (8.33) | 8.42 (8.33) |
| La/Nd | 16.23 (16.67) | 16.37 (16.67) |
| In | 16.95 (16.67) | 16.71 (16.67) |
| O | 58.01 (58.33) | 58.50 (58.33) |
| No of Band | BaLa2In2O7 | BaNd2In2O7 |
|---|---|---|
| 1 | 120 | 118 |
| 2 | 140 | 133 |
| 3 | 161 | 157 |
| 4 | 203 | 181 |
| 5 | 237 | - |
| 6 | 260 | 250 |
| 7 | 279 | - |
| 8 | 316 | 319 |
| 9 | 336 | - |
| 10 | 358 | 370 |
| 11 | 430 | 453 |
| 12 | 445 | - |
| 13 | 545 | 660 |
| 14 | 800 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarasova, N.; Galisheva, A.; Animitsa, I.; Belova, K.; Egorova, A.; Abakumova, E.; Medvedev, D. Layered Perovskites BaM2In2O7 (M = La, Nd): From the Structure to the Ionic (O2−, H+) Conductivity. Materials 2022, 15, 3488. https://doi.org/10.3390/ma15103488
Tarasova N, Galisheva A, Animitsa I, Belova K, Egorova A, Abakumova E, Medvedev D. Layered Perovskites BaM2In2O7 (M = La, Nd): From the Structure to the Ionic (O2−, H+) Conductivity. Materials. 2022; 15(10):3488. https://doi.org/10.3390/ma15103488
Chicago/Turabian StyleTarasova, Nataliia, Anzhelika Galisheva, Irina Animitsa, Ksenia Belova, Anastasia Egorova, Ekaterina Abakumova, and Dmitry Medvedev. 2022. "Layered Perovskites BaM2In2O7 (M = La, Nd): From the Structure to the Ionic (O2−, H+) Conductivity" Materials 15, no. 10: 3488. https://doi.org/10.3390/ma15103488
APA StyleTarasova, N., Galisheva, A., Animitsa, I., Belova, K., Egorova, A., Abakumova, E., & Medvedev, D. (2022). Layered Perovskites BaM2In2O7 (M = La, Nd): From the Structure to the Ionic (O2−, H+) Conductivity. Materials, 15(10), 3488. https://doi.org/10.3390/ma15103488








