Alterations to Titanium Surface Depending on the Fluorides and Abrasives in Toothpaste
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Slurry Preparation and Toothbrush Abrasion Test
2.3. Titanium Surface Analysis
2.4. Scanning Electron Microscopy Observation
2.5. Data Analysis
3. Results
3.1. Observation of Abrasives in Toothpaste
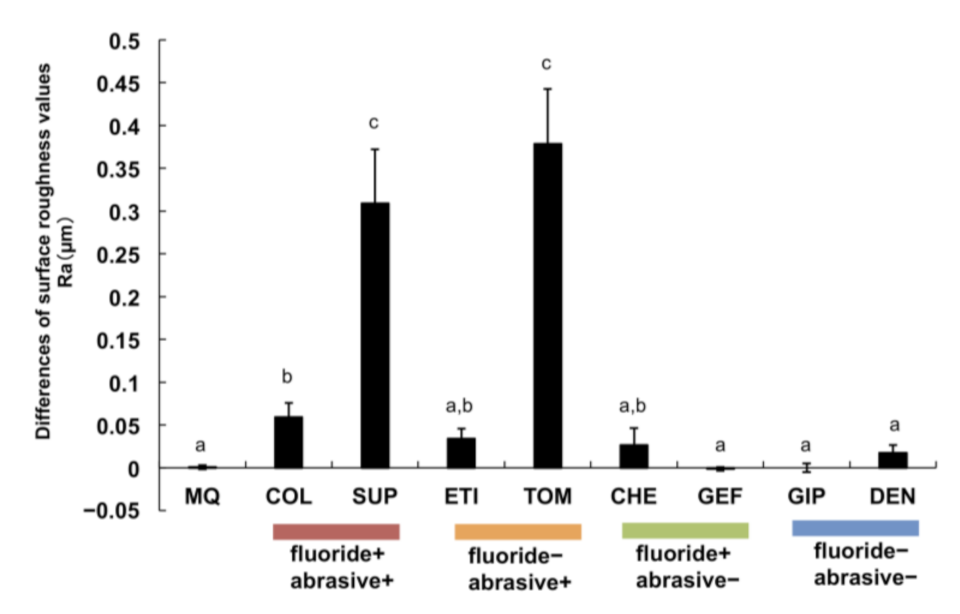
3.2. Variations in Titanium Surface Roughness after Brushing with Toothpastes
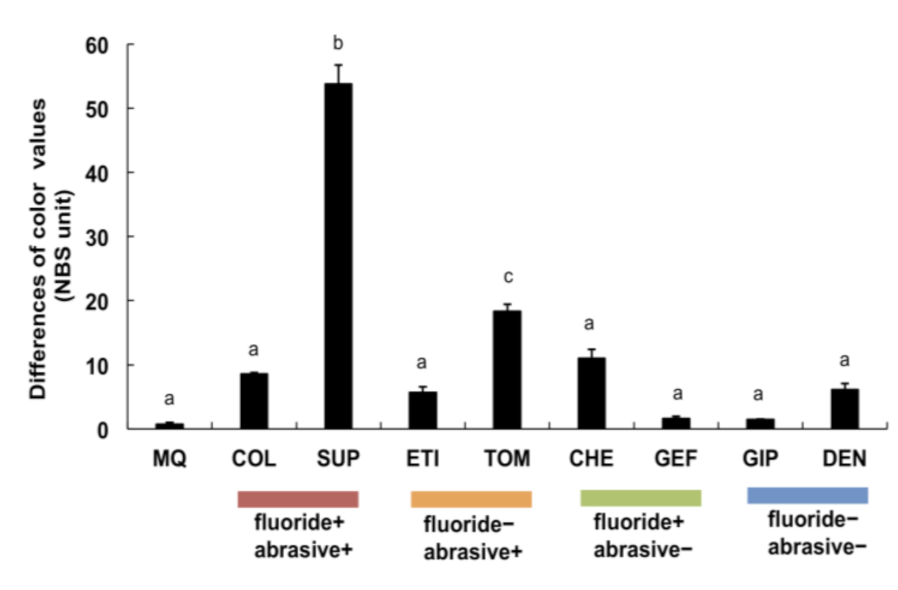
3.3. Alterations in Titanium Color after Brushing with Toothpastes
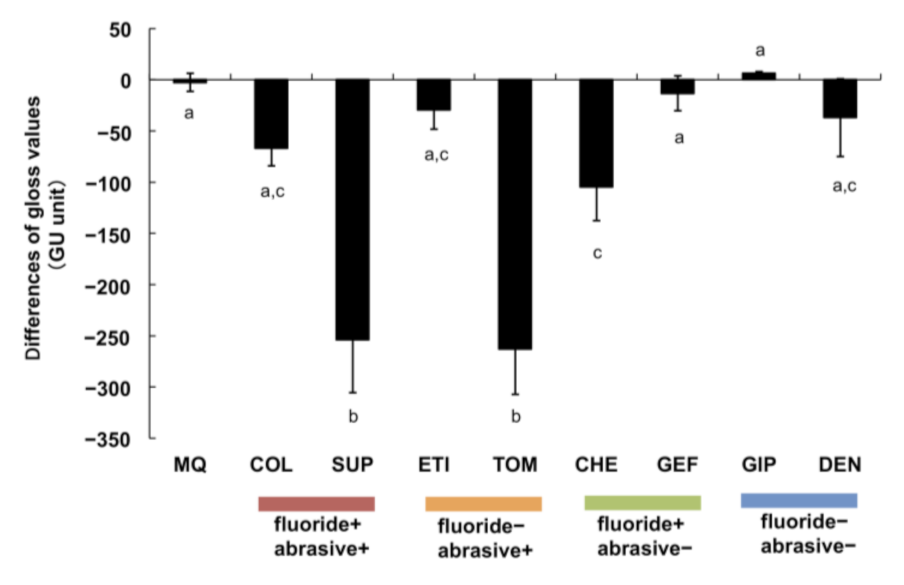
3.4. Variations in Titanium Gloss after Brushing with Toothpastes
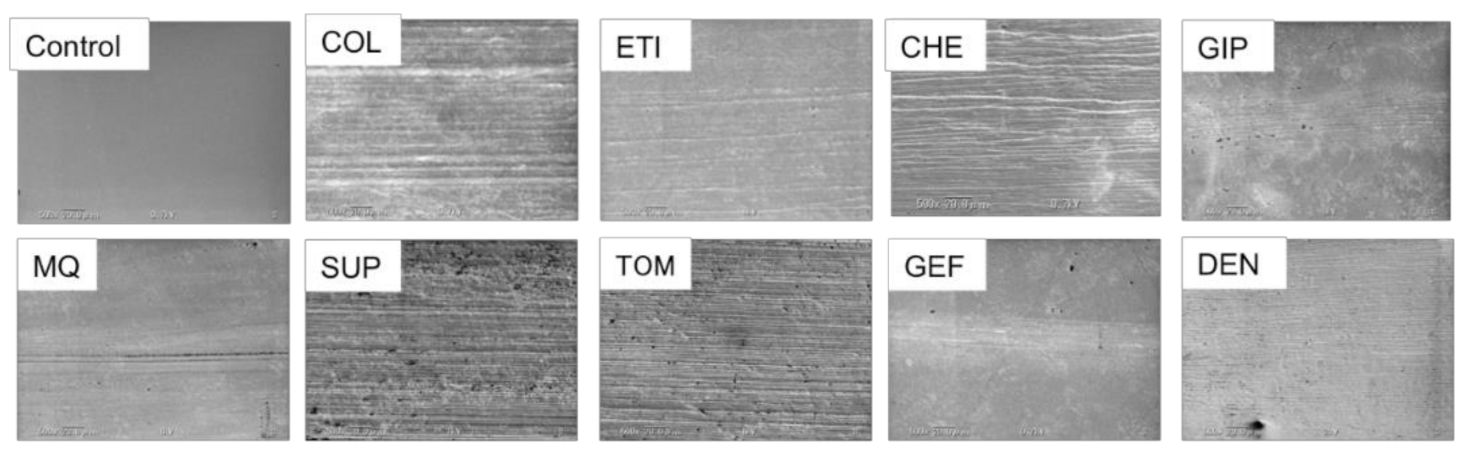
3.5. Titanium Surface Observations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanawa, T.; Asami, K.; Asaoka, K. Repassivation of titanium and surface oxide film regenerated in simulated bioliquid. J. Biomed. Mater. Res. 1998, 40, 530–538. [Google Scholar] [CrossRef]
- Ohkubo, C.; Sato, Y.; Nishiyama, Y.; Suzuki, Y. Titanium removable denture based on a one-metal rehabilitation concept. Dent. Mater. J. 2017, 36, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Ottria, L.; Lauritano, D.; Andreasi Bassi, M.; Palmieri, A.; Candotto, V.; Tagliabue, A.; Tettamanti, L. Mechanical, chemical and biological aspects of titanium and titanium alloys in implant dentistry. J. Biol. Regul. Homeost. Agents 2018, 32, 81–90. [Google Scholar] [PubMed]
- Brantley, W.A. Evolution, clinical applications, and prospects of nickel-titanium alloys for orthodontic purposes. J. World Fed. Orthod. 2020, 9, S19–S26. [Google Scholar] [CrossRef]
- Koizumi, H.; Takeuchi, Y.; Imai, H.; Kawai, T.; Yoneyama, T. Application of titanium and titanium alloys to fixed dental prostheses. J. Prosthodont. Res. 2019, 63, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Reclaru, L.; Meyer, J.M. Effects of fluorides on titanium and other dental alloys in dentistry. Biomaterials 1998, 19, 85–92. [Google Scholar] [CrossRef]
- Noguchi, T.; Takemoto, S.; Hattori, M.; Yoshinari, M.; Kawada, E.; Oda, Y. Discoloration and dissolution of titanium and titanium alloys with immersion in peroxide- or fluoride-containing solutions. Dent. Mater. J. 2008, 27, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Milošev, I.; Barbara, K.; Selih, V.S. The effect of fluoride ions on the corrosion behavior of Ti metal, and Ti6-Al-7Nb and Ti-6Al-4V alloys in artificial saliva. Acta Chim. Slov. 2013, 60, 543–555. [Google Scholar] [PubMed]
- Peñarrieta-Juanito, G.; Sordi, M.B.; Henriques, B.; Dotto, M.E.R.; Teughels, W.; Silva, F.S.; Magini, R.S.; Souza, J.C.M. Surface damage of dental implant systems and ions release after exposure to fluoride and hydrogen peroxide. J. Periodontal. Res. 2019, 54, 46–52. [Google Scholar] [CrossRef]
- Nakagawa, M.; Matsuya, S.; Shiraishi, T.; Ohta, M. Effect of fluoride concentration and pH on corrosion behavior of titanium for dental use. J. Dent. Res. 1999, 78, 1568–1572. [Google Scholar] [CrossRef] [PubMed]
- Kimura, E.; Nomura, T.; Mizoguchi, T.; Yoshinari, M. Influence of fluoride-containing pastes on corrosion resistance of titanium. J. Jpn. Soc. Oral Implant. 2014, 27, 54–60. [Google Scholar]
- Chen, W.Q.; Zhang, S.M.; Qiu, J. Surface analysis and corrosion behavior of pure titanium under fluoride exposure. J. Prosthet. Dent. 2020, 124, 239.e1–239.e8. [Google Scholar] [CrossRef]
- Golvano, I.; Garcia, I.; Conde, A.; Tato, W.; Aginagalde, A. Influence of fluoride content and pH on corrosion and tribocorrosion behaviour of Ti13Nb13Zr alloy in oral environment. J. Mech. Behav. Biomed. Mater. 2015, 49, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Whelton, H.P.; Spencer, A.J.; Do, L.G.; Rugg-Gunn, A.J. Fluoride revolution and dental caries: Evolution of Policies for global use. J. Dent. Res. 2019, 98, 837–846. [Google Scholar] [CrossRef]
- ISO 11609:2017; Dentistry–Dentifrices–Requirements, Test Methods and Marking. ISO Central Secretariat: Geneva, Switzerland. Available online: https://www.iso.org/standard/70956.html (accessed on 18 October 2021).
- Griffin, S.O.; Regnier, E.; Griffin, P.M.; Huntley, V. Effectiveness of fluoride in preventing caries in adults. J. Dent. Res. 2007, 86, 410–415. [Google Scholar] [CrossRef]
- Fais, L.M.; Fernandes-Filho, R.B.; Pereira-da-Silva, M.A.; Vaz, L.G.; Adabo, G.L. Titanium surface topography after brushing with fluoride and fluoride-free toothpaste simulating 10 years of use. J. Dent. 2012, 40, 265–275. [Google Scholar] [PubMed]
- Barros, R.N.; de Gouvêa, C.V.D. Prophylactic agents and bacterial adherence to titanium. Clin. Oral. Implants Res. 2011, 22, 1221–1226. [Google Scholar] [CrossRef]
- Acharya, B.L.G.; Nadiger, R.; Shetty, B.; Gururaj, G.; Kumar, K.N.; Darshan, D.D. Brushing-induced surface roughness of two nickel based alloys and a titanium based alloy: A comparative study—In vitro study. J. Int. Oral Health 2014, 6, 36–49. [Google Scholar]
- Sampaio, M.; Buciumeanu, M.; Henriques, B.; Silva, F.S.; Souza, J.C.M.; Gomes, J.R. Comparison between PEEK and Ti6Al4V concerning micro-scale abrasion wear on dental applications. J. Mech. Behav. Biomed. Mater. 2016, 60, 212–219. [Google Scholar] [PubMed]
- Lazaridou, D.; Belli, R.; Petschelt, A.; Lohbauer, U. Are resin composites suitable replacements for amalgam? A study of two-body wear. Clin. Oral Investig. 2015, 19, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, E.Ҫ. Investigation of three-body wear behavior and hardness of experimental titanium alloys for dental applications in oral environment. Mater. Sci. Eng. Technol. 2020, 51, 47–53. [Google Scholar] [CrossRef]
- ISO/TS 14569-1; Dental Materials–Guidance on Testing of Wear Resistance–Part 1: Wear by Tooth Brushing. ISO Central Secretariat: Geneva, Switzerland. Available online: https://www.iso.org/standard/31417.html (accessed on 18 October 2021).
- Hossain, A.; Okawa, S.; Miyakawa, O. Effect of toothbrushing on titanium surface: An approach to understanding surface properties of brushed titanium. Dent. Mater. 2006, 22, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Tsuruta, S.; Kani, T.; Ozeki, Y.; Koyama, K.; Hasegawa, J. Wear effect of titanium prosthesis on fluorine concentration with the experimental dentifrice. Aichi-Gakuin J. Dent. Sci. 2001, 39, 175–180. [Google Scholar]
- Marinho, V.C.C.; Chong, L.Y.; Worthington, H.V.; Walsh, T. Fluoride mouthrinses for preventing dental caries in children and adolescents. Cochrane Database Syst. Rev. 2016, 7, CD002284. [Google Scholar]
- Rugg-Gunn, A. Preventing the preventable—The enigma of dental caries. Br. Dent. J. 2001, 191, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, D.; Clarkson, J.E. Fluoride varnish for caries prevention: Efficacy and implementation. Caries Res. 2016, 50, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Wachi, T.; Shuto, T.; Shinohara, Y.; Matono, Y.; Makihira, S. Corrosion of titanium and release from titanium exposed to tooth surface embrocation of fluoride products. J. Jpn. Soc. Oral Implant. 2016, 29, 12–19. [Google Scholar]
- Nakagawa, M.; Matsuya, S.; Udoh, K. Corrosion behavior of pure titanium and titanium alloys in fluoride-containing solutions. Dent. Mater. J. 2001, 20, 305–314. [Google Scholar] [CrossRef]
- Ide, K.; Hattori, M.; Yoshinari, M.; Kawada, E.; Oda, Y. The influence of albumin on corrosion resistance of titanium in fluoride solution. Dent. Mater. J. 2003, 22, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, S.; Hattori, M.; Yoshinari, M.; Kawada, E.; Oda, Y. Corrosion behavior and surface characterization of titanium in solution containing fluoride and albumin. Biomaterials 2005, 26, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, A.; Mayanagi, G.; Sasaki, K.; Takahashi, N. Corrosive effects of fluoride on titanium under artificial biofilm. J. Prosthodont. Res. 2018, 62, 104–109. [Google Scholar] [CrossRef]
- Mine, Y.; Makihira, S.; Imari, N.; Shuto, T.; Nikawa, H. Effects of commercial dental rinses and toothpastes on the surface properties of titanium. J. Jpn. Soc. Oral Implant. 2012, 25, 22–30. [Google Scholar]
- Gomi, Y.; Nagasaki, M.; Misawa, E.; Kajiyama, S.; Saito, M.; Nagano, T.; Inoue, K.; Gomi, K. Influence of various fluoridated toothpastes and trial manufacture fluorine-free toothpaste on titanium surface roughness. J. Jpn. Soc. Periodontol. 2014, 56, 49–56. [Google Scholar] [CrossRef][Green Version]
- Hossain, A.; Okawa, S.; Miyakawa, O. Surface texture and composition of titanium brushed with toothpaste slurries of different pHs. Dent. Mater. 2007, 23, 186–192. [Google Scholar] [CrossRef]
- Faria, A.C.L.; Bordin, A.R.V.; Pedrazzi, V.; Rodrigues, R.C.S.; Ribeiro, R.F. Effect of whitening toothpaste on titanium and titanium alloy surfaces. Braz. Oral. Res. 2012, 26, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Fais, L.M.G.; Carmello, J.C.; Spolidorio, D.M.P.; Adabo, G.L. Streptococcus mutans adhesion to titanium after brushing with fluoride and fluoride-free toothpaste simulating 10 years of use. Int. J. Oral Maxillofac. Implants 2013, 28, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Ruiz, R.; Romanos, G. Potential causes of titanium particle and ion release in implant dentistry: A systematic review. Int. J. Mol. Sci. 2018, 19, 3585. [Google Scholar] [CrossRef]
- Olmedo, D.G.; Nalli, G.; Verdú, S.; Paparella, M.L.; Cabrini, R.L. Exfoliative cytology and titanium dental implants: A pilot study. J. Periodontol. 2013, 84, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Høl, P.J.; Kristoffersen, E.K.; Gjerdet, N.R.; Pellowe, A.S. Novel nanoparticulate and ionic titanium antigens for hypersensitivity testing. Int. J. Mol. Sci. 2018, 19, 1101. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Q.; Pang, Z.; Gong, M.; Tang, L. Titanium ions promote exogenous calcium-dependent calcium influx in activated Jurkat T cells: A possible mechanism to explain its immunostimulatory properties. Mediators Inflamm. 2018, 2018, 3286905. [Google Scholar] [CrossRef] [PubMed]
- Wachi, T.; Shuto, T.; Shinohara, Y.; Matono, Y.; Makihira, S. Release of titanium ions from an implant surface and their effect on cytokine production related to alveolar bone resorption. Toxicology 2015, 327, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Berryman, Z.; Bridger, L.; Hussaini, H.M.; Rich, A.M.; Atieh, M.; Tawse-Smith, A. Titanium particles: An emerging risk factor for peri-implant bone loss. Saudi Dent. J. 2020, 32, 283–292. [Google Scholar] [CrossRef] [PubMed]
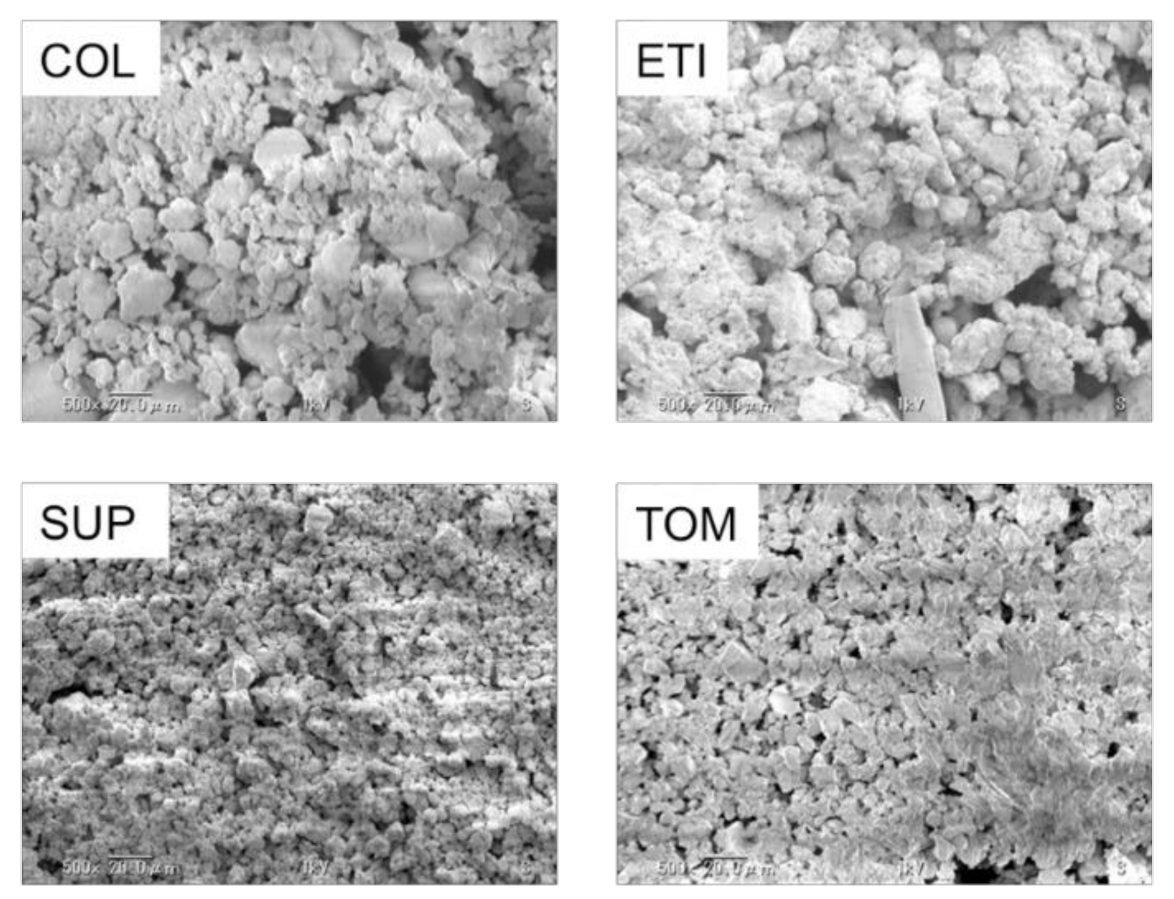
| Products | Manufacturers | Abbreviations | Fluorides | Abrasives | pH | Slurry pH | State |
|---|---|---|---|---|---|---|---|
| Total 12 h Protection Super Smile | Colgate Cosmetechs | COL SUP | NaF (1450 ppm) MFP (900-999 ppm) | Silicic anhydride Calcium carbonate Magnesium carbonate | 6.8 7.9 | 7.4 8.3 | Paste Paste |
| Etiquette Lion Aa Fluoride-Free Antiplaque &Whitening Toothpaste | Lion Tom’s OF MAINE | ETI TOM | — — | Silicic anhydride Calcium carbonate Silicic anhydride | 6.6 7.8 | 6.8 8.0 | Paste Paste |
| Check-up Gel Gel Coat F | Lion Weltec | CHE GEF | NaF (950 ppm) NaF (950 ppm) | — — | 8.2 8.4 | 8.6 8.8 | Gel Gel |
| Gel Coat IP Dennovate Implant Teethgel | Weltec Hakusui Trading | GIP DEN | — — | — — | 7.1 6.4 | 7.5 6.8 | Gel Gel |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shuto, T.; Mine, Y.; Makihira, S.; Nikawa, H.; Wachi, T.; Kakimoto, K. Alterations to Titanium Surface Depending on the Fluorides and Abrasives in Toothpaste. Materials 2022, 15, 51. https://doi.org/10.3390/ma15010051
Shuto T, Mine Y, Makihira S, Nikawa H, Wachi T, Kakimoto K. Alterations to Titanium Surface Depending on the Fluorides and Abrasives in Toothpaste. Materials. 2022; 15(1):51. https://doi.org/10.3390/ma15010051
Chicago/Turabian StyleShuto, Takahiro, Yuichi Mine, Seicho Makihira, Hiroki Nikawa, Takanori Wachi, and Kazutoshi Kakimoto. 2022. "Alterations to Titanium Surface Depending on the Fluorides and Abrasives in Toothpaste" Materials 15, no. 1: 51. https://doi.org/10.3390/ma15010051
APA StyleShuto, T., Mine, Y., Makihira, S., Nikawa, H., Wachi, T., & Kakimoto, K. (2022). Alterations to Titanium Surface Depending on the Fluorides and Abrasives in Toothpaste. Materials, 15(1), 51. https://doi.org/10.3390/ma15010051






