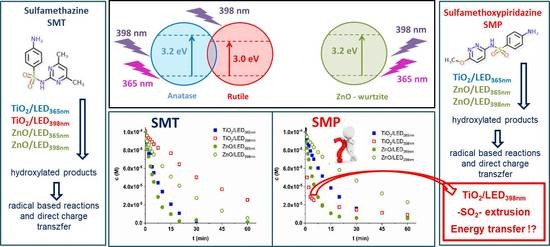
Wavelength Dependence of the Transformation Mechanism of Sulfonamides Using Different LED Light Sources and TiO2 and ZnO Photocatalysts
Abstract
:1. Introduction
2. Materials and Analytical Methods
2.1. Photochemical Experiments
2.2. Analytical Methods
2.3. Chemicals and Solvents Used
3. Results and Discussion
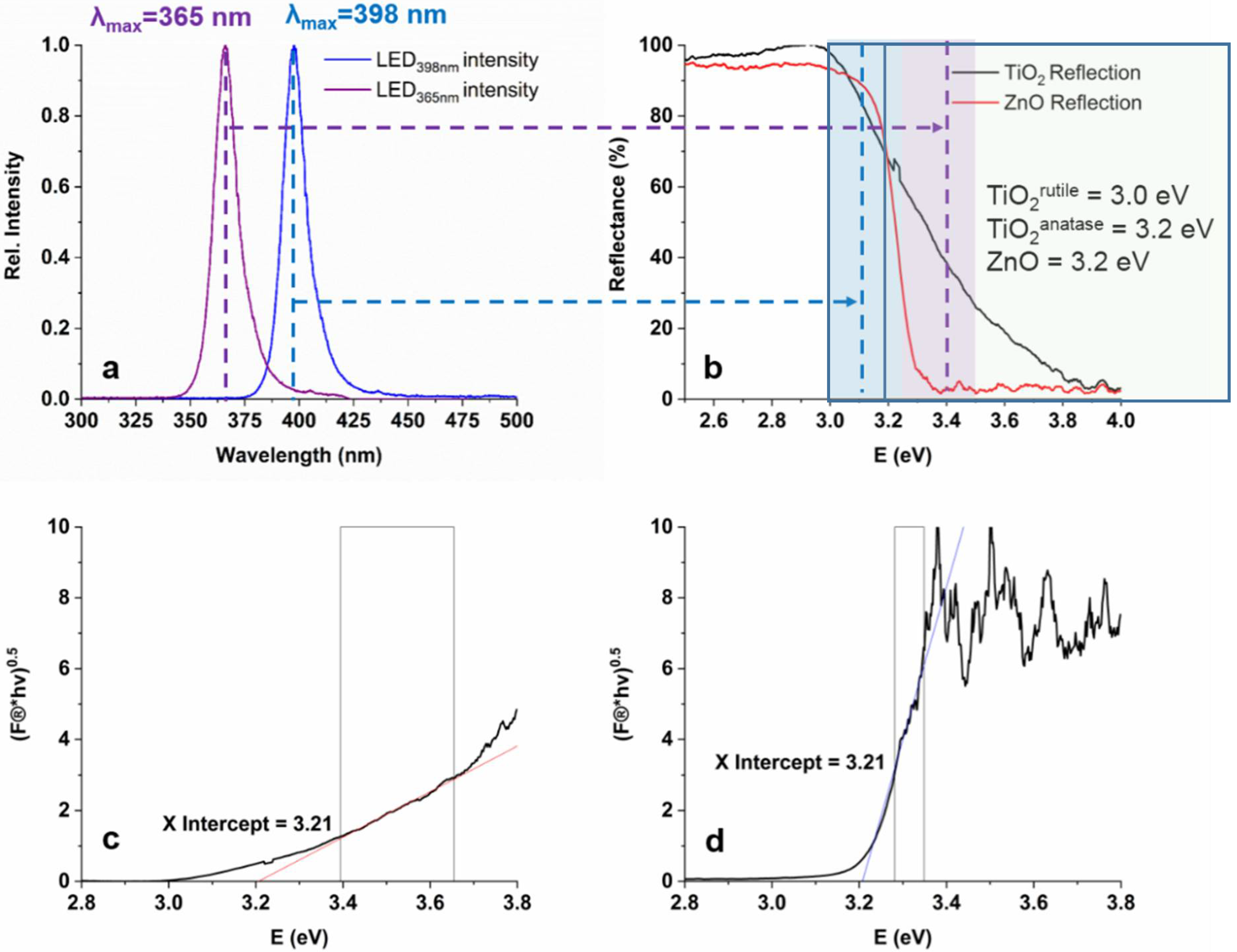
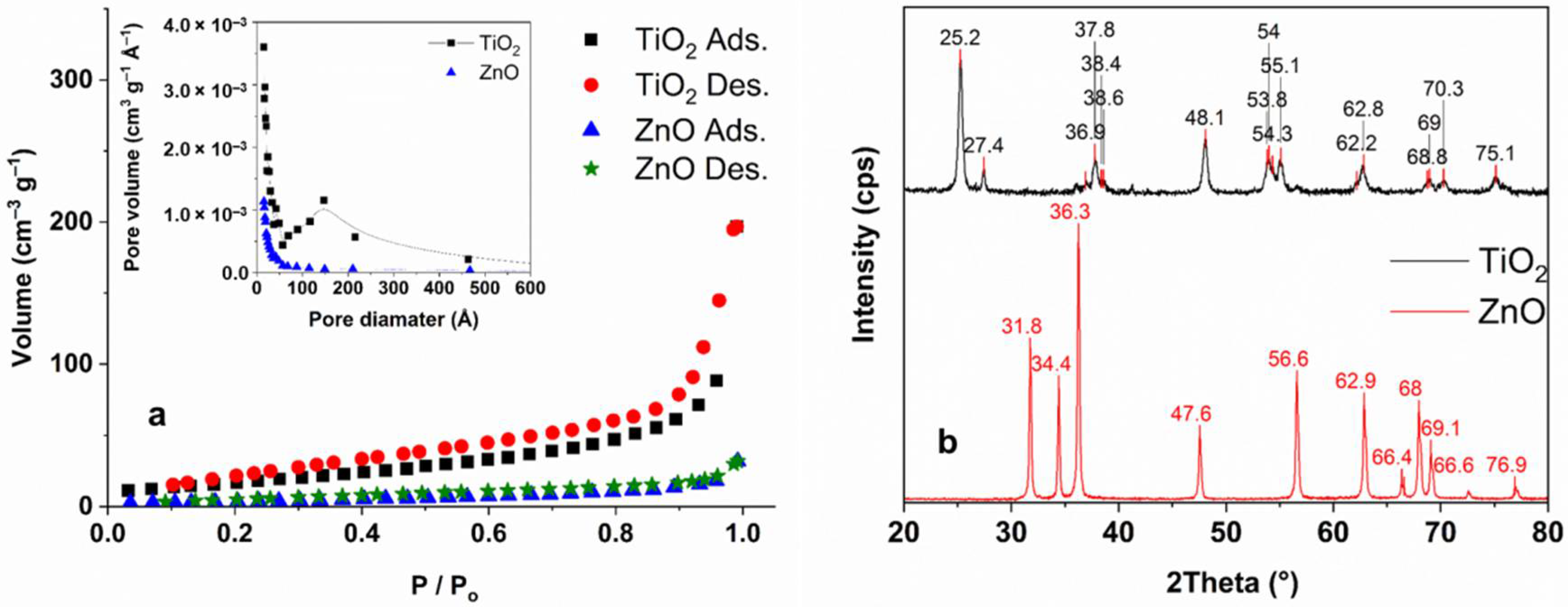
3.1. The Characterization of the Light Sources and the Photocatalysts
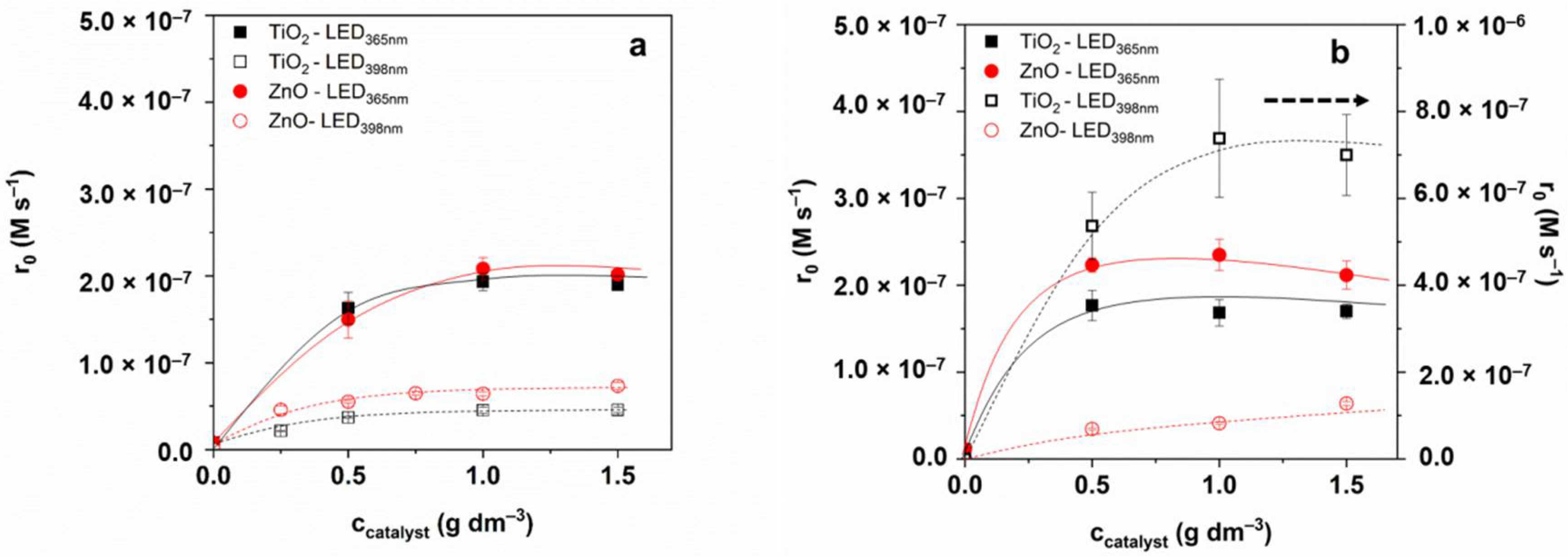
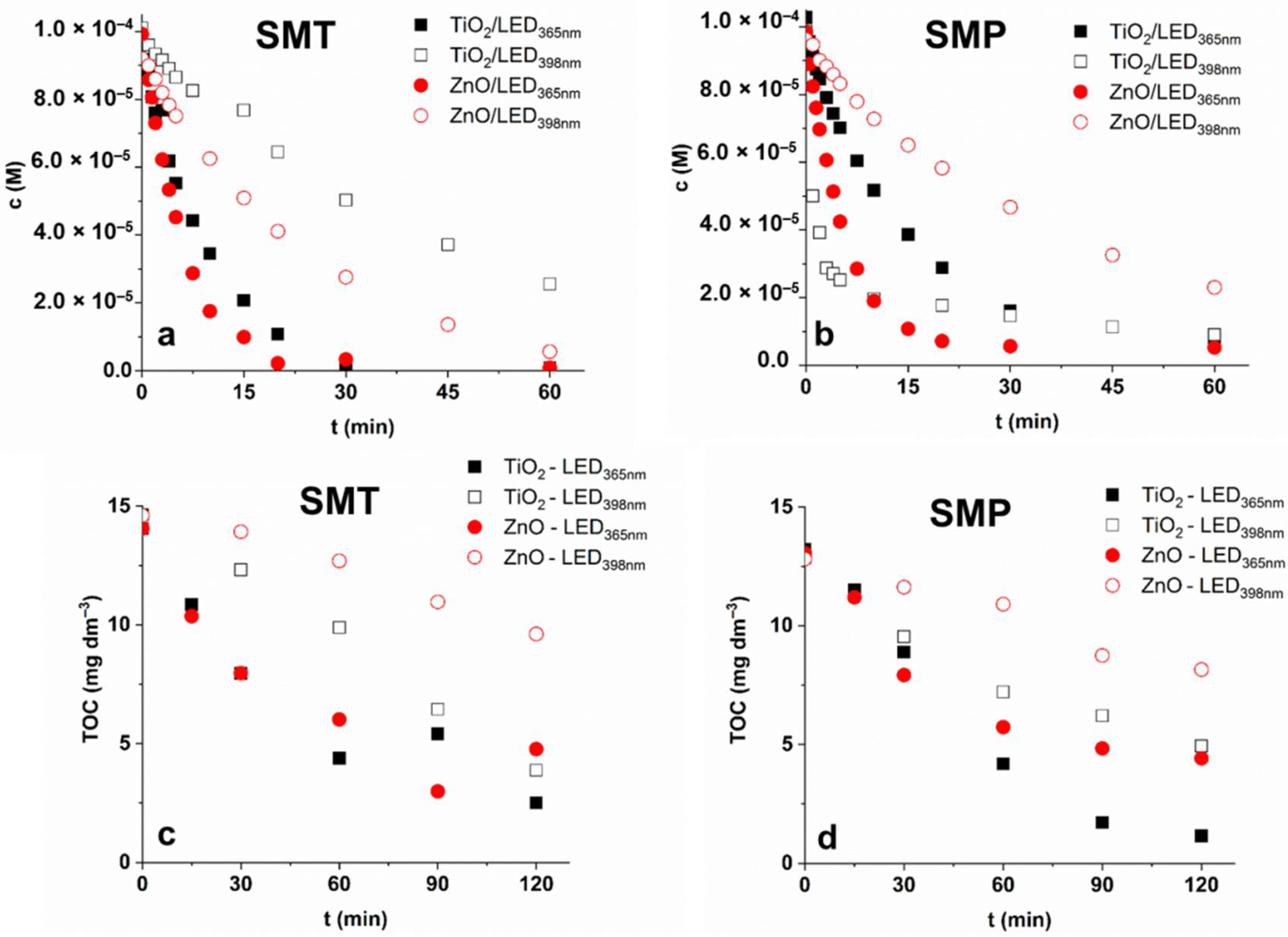
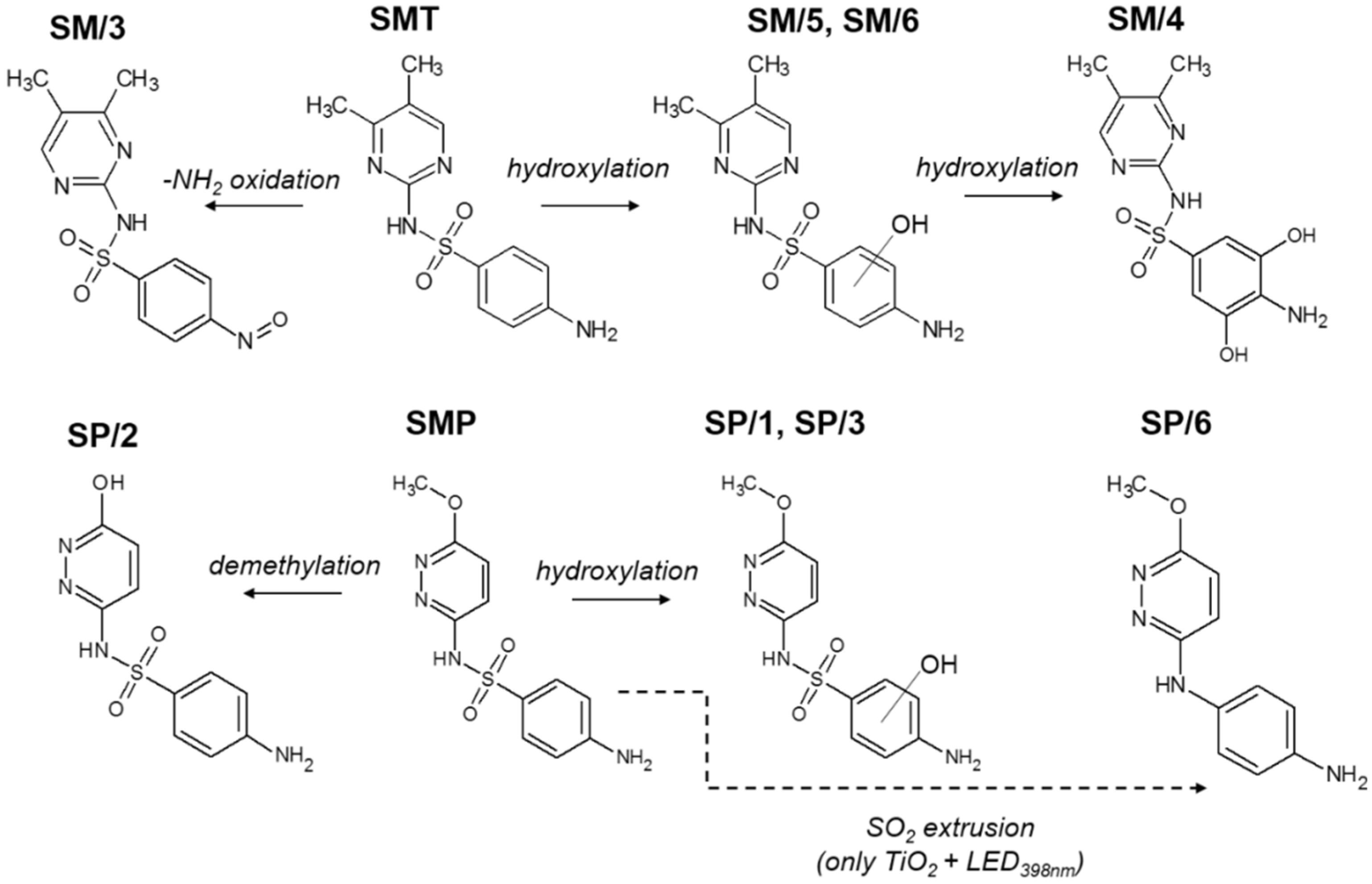
3.2. Transformation and Mineralization of Sulfonamides
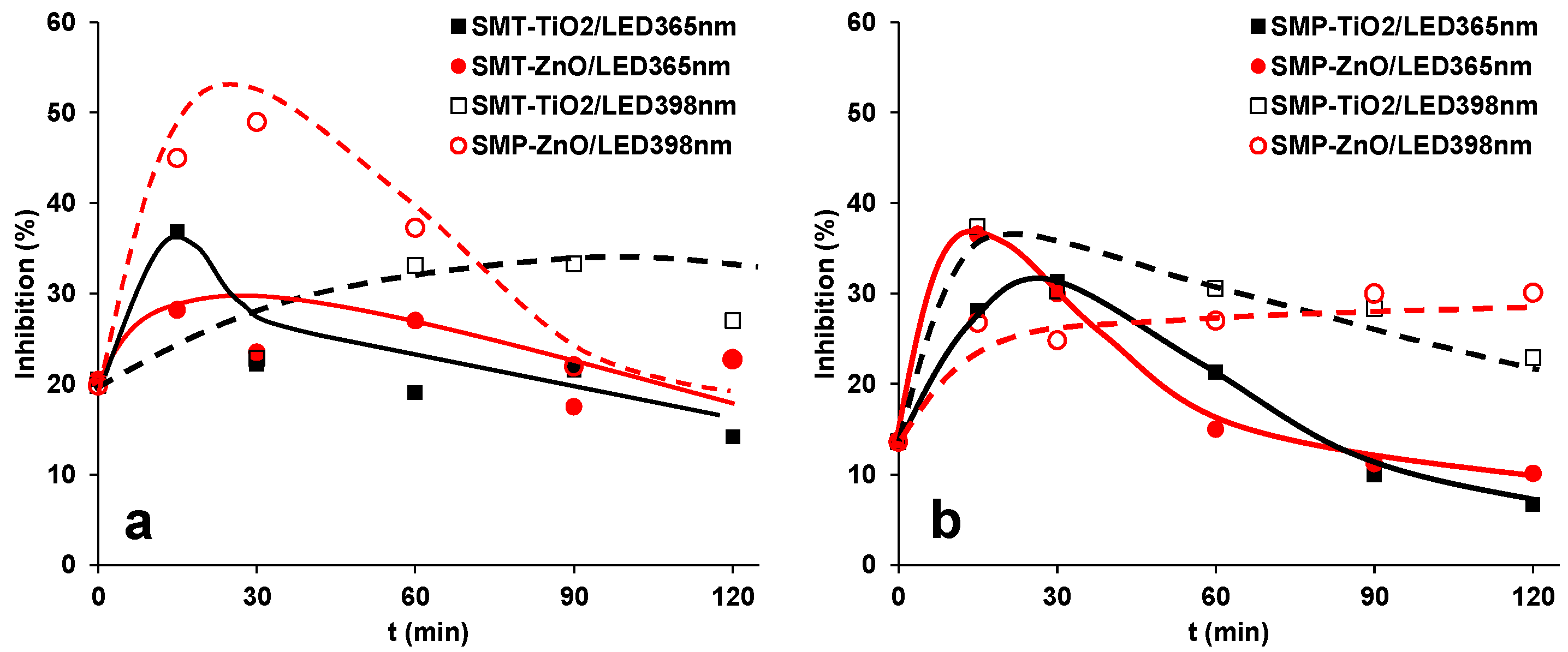
3.3. Mineralization of Sulfonamides and Ecotoxicity Assays
3.4. Transformation of Coumarin—The Comparison of •OH Formation Efficiency
3.5. Transformation of 1,4-BQ—The Comparison of Charge Separation Efficiency
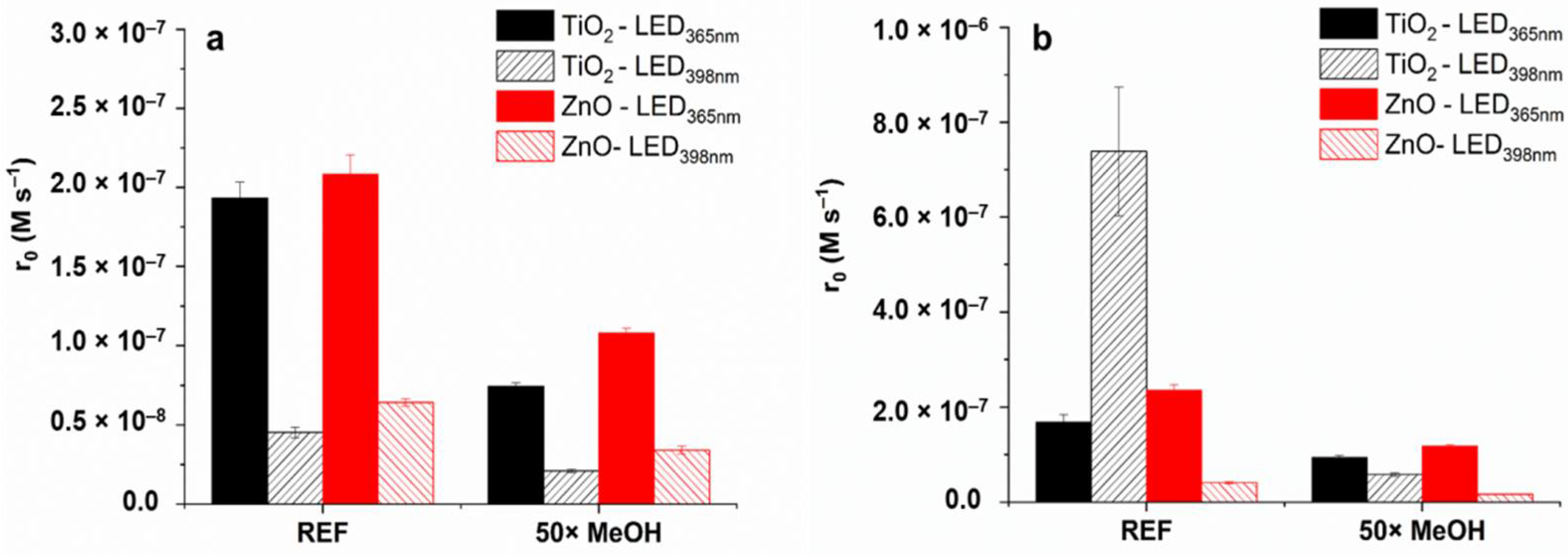
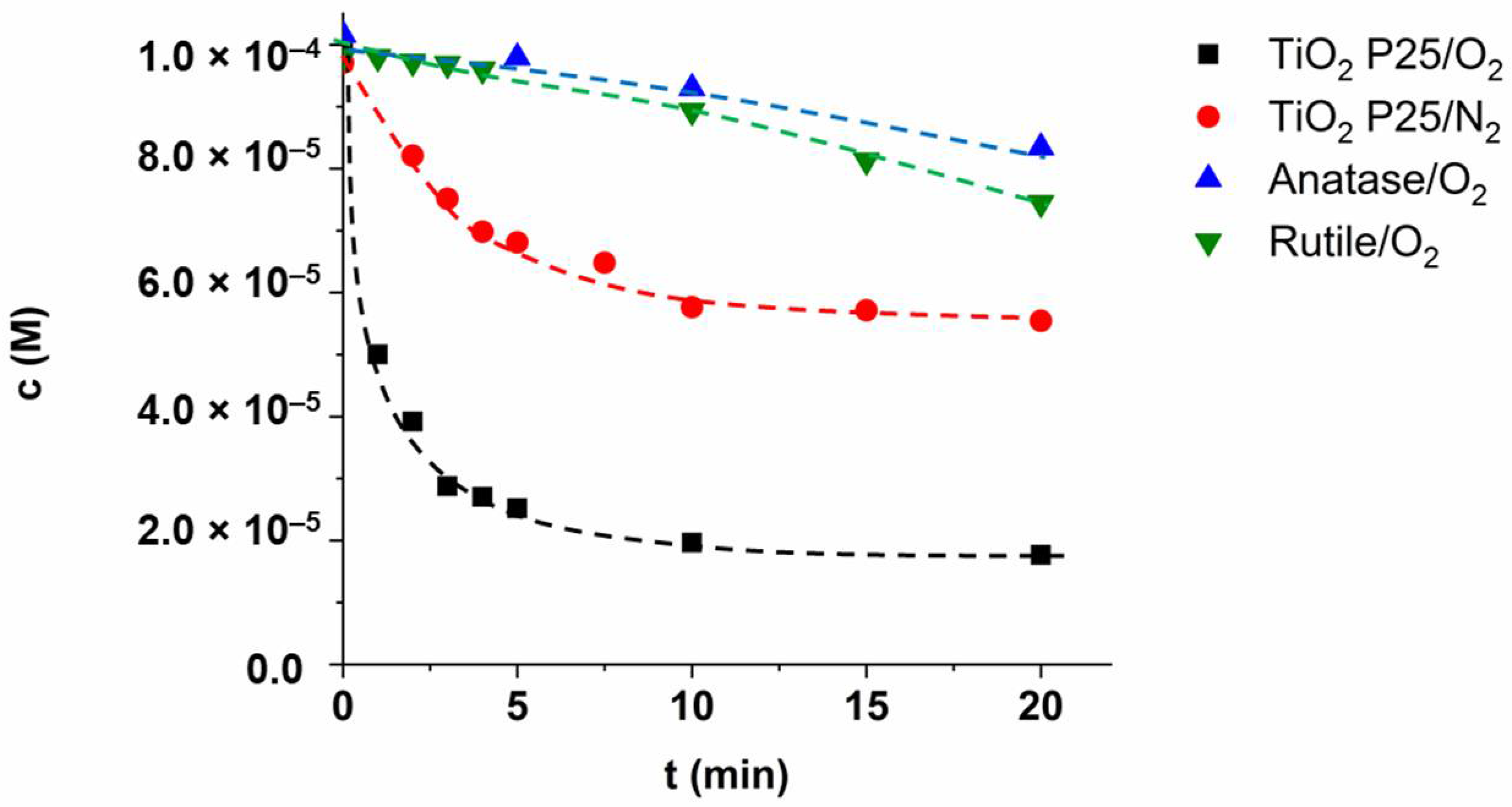
3.6. Reaction Mechanism—Effect of Radical Scavenger, Dissolved O2, and the Quality of TiO2
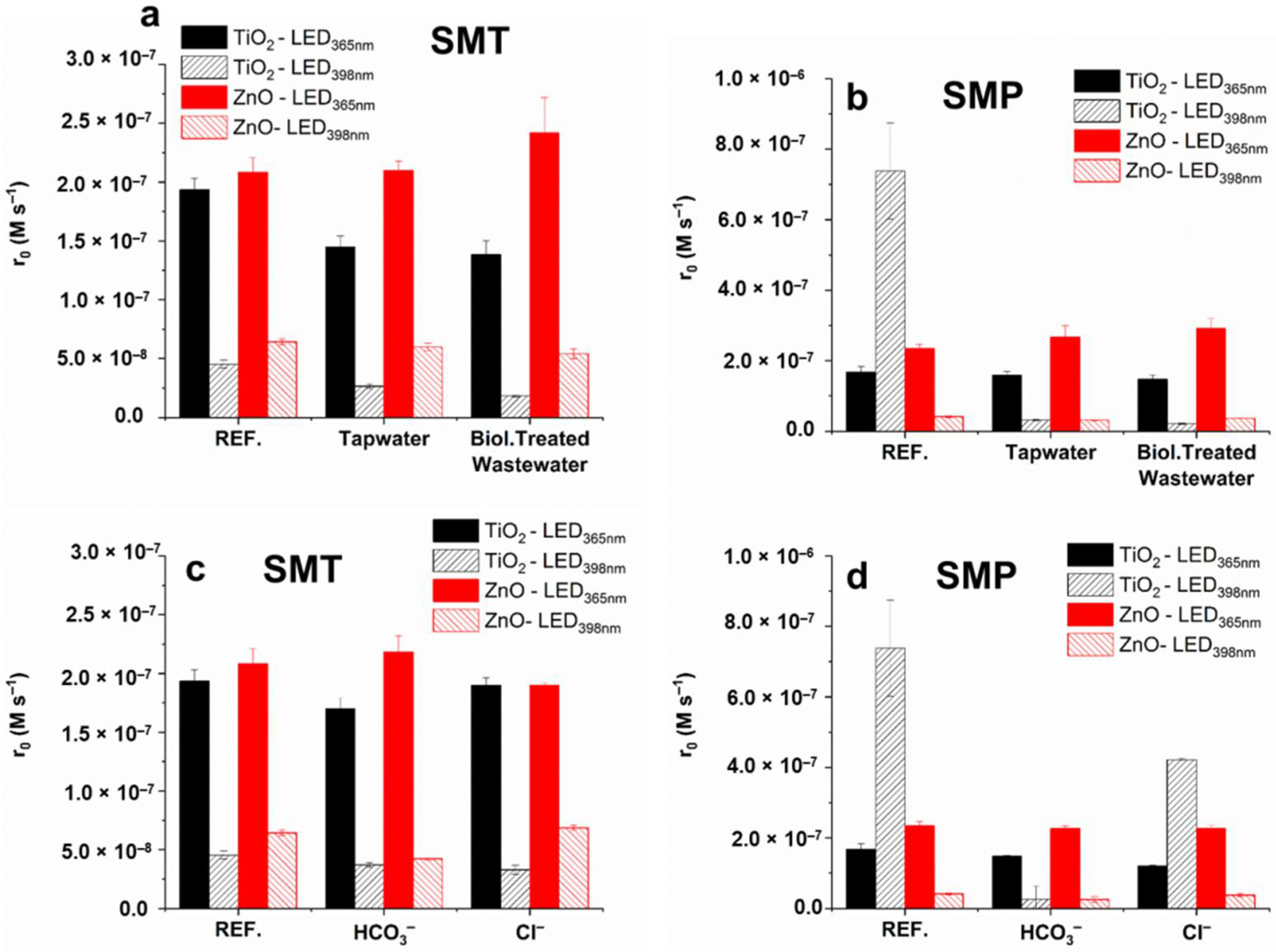
3.7. Effect of Matrices on the Removal of Sulfonamides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Falowo, A.B.; Akimoladun, O.F. Veterinary drug residues in meat and meat products: Occurrence, detection and implications. In Veterinary Medicine and Pharmaceuticals; Bekoe, S.O., Saravanan, M., Eds.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef] [Green Version]
- Tacic, A.; Nikolic, V.; Nikolic, L.; Savic, I. Antimicrobial Sulfonamide Drugs. Adv. Technol. 2017, 6, 58–71. [Google Scholar] [CrossRef] [Green Version]
- Klauson, D.; Romero Sarcos, N.; Krichevskaya, M.; Kattel, E.; Dulova, N.; Dedova, T.; Trapido, M. Advanced Oxidation Processes for Sulfonamide Antibiotic Sulfamethizole Degradation: Process Applicability Study at ppm Level and Scale-down to ppb Level. J. Environ. Chem. Eng. 2019, 7, 103287. [Google Scholar] [CrossRef]
- Wang, J.; Chu, L.; Wojnárovits, L.; Takács, E. Occurrence and Fate of Antibiotics, Antibiotic Resistant Genes (ARGs) and Antibiotic Resistant Bacteria (ARB) in Municipal Wastewater Treatment Plant: An Overview. Sci. Total Environ. 2020, 744, 140997. [Google Scholar] [CrossRef]
- Mendiola-Alvarez, S.Y.; Palomino-Cabello, C.; Hernández-Ramírez, A.; Turnes-Palomino, G.; Guzmán-Mar, J.L.; Hinojosa-Reyes, L. Coupled Heterogeneous Photocatalysis Using a P-TiO2-AFe2O3 Catalyst and K2S2O8 for the Efficient Degradation of a Sulfonamide Mixture. J. Photochem. Photobiol. A Chem. 2020, 394, 112485. [Google Scholar] [CrossRef]
- Li, P.; Guo, M.; Wang, Q.; Li, Z.; Wang, C.C.C.; Chen, N.; Wang, C.C.C.; Wan, C.; Chen, S. Controllable Synthesis of Cerium Zirconium Oxide Nanocomposites and Their Application for Photocatalytic Degradation of Sulfonamides. Appl. Catal. B Environ. 2019, 259, 118107. [Google Scholar] [CrossRef]
- Estrada-Flórez, S.E.; Serna-Galvis, E.A.; Torres-Palma, R.A. Photocatalytic vs. Sonochemical Removal of Antibiotics in Water: Structure-Degradability Relationship, Mineralization, Antimicrobial Activity, and Matrix Effects. J. Environ. Chem. Eng. 2020, 8, 104359. [Google Scholar] [CrossRef]
- Wu, C.H.; Kuo, C.Y.; Dong, C.D.; Chen, C.W.; Lin, Y.L. Removal of Sulfonamides from Wastewater in the UV/TiO2 System: Effects of PH and Salinity on Photodegradation and Mineralization. Water Sci. Technol. 2019, 79, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, J.; Su, H.; Zhao, Y.; Zeng, X.; Hu, M.; Xiao, W.; Mao, X. H-Bonding Effect of Oxyanions Enhanced Photocatalytic Degradation of Sulfonamides by g-C3N4 in Aqueous Solution. J. Hazard. Mater. 2019, 366, 259–267. [Google Scholar] [CrossRef]
- Song, Y.; Tian, J.; Gao, S.; Shao, P.; Qi, J.; Cui, F. Photodegradation of Sulfonamides by G-C3N4 under Visible Light Irradiation: Effectiveness, Mechanism and Pathways. Appl. Catal. B Environ. 2017, 210, 88–96. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, Q.; Zheng, X.; Tan, C.; Zhuo, M.; Chen, T.; Wang, F.; Liu, H.; Liu, Y.; Feng, Y.; et al. Phosphate-Modified m-Bi2O4 Enhances the Absorption and Photocatalytic Activities of Sulfonamide: Mechanism, Reactive Species, and Reactive Sites. J. Hazard. Mater. 2020, 384, 121443. [Google Scholar] [CrossRef]
- Wang, W.; Tian, J.; Zhu, Z.; Zhu, C.; Liu, B.; Hu, C. Insight into Quinolones and Sulfonamides Degradation, Intermediate Product Identification and Decomposition Pathways with the Assistance of Bi2MoO6/Bi2WO6/MWCNTs Photocatalyst. Process Saf. Environ. Prot. 2021, 147, 527–546. [Google Scholar] [CrossRef]
- Zhu, W.; Sun, F.; Goei, R.; Zhou, Y. Facile Fabrication of RGO-WO3 Composites for Effective Visible Light Photocatalytic Degradation of Sulfamethoxazole. Appl. Catal. B Environ. 2017, 207, 93–102. [Google Scholar] [CrossRef]
- Yang, C.C.; Huang, C.L.; Cheng, T.C.; Lai, H.T. Inhibitory Effect of Salinity on the Photocatalytic Degradation of Three Sulfonamide Antibiotics. Int. Biodeterior. Biodegrad. 2015, 102, 116–125. [Google Scholar] [CrossRef]
- Kaniou, S.; Pitarakis, K.; Barlagianni, I.; Poulios, I. Photocatalytic Oxidation of Sulfamethazine. Chemosphere 2005, 60, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Baran, W.; Sochacka, J.; Wardas, W. Toxicity and Biodegradability of Sulfonamides and Products of Their Photocatalytic Degradation in Aqueous Solutions. Chemosphere 2006, 65, 1295–1299. [Google Scholar] [CrossRef]
- Ahmed, S.; Rasul, M.G.; Brown, R.; Hashib, M.A. Influence of Parameters on the Heterogeneous Photocatalytic Degradation of Pesticides and Phenolic Contaminants in Wastewater: A Short Review. J. Environ. Manag. 2011, 92, 311–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younis, S.A.; Kim, K.H. Heterogeneous Photocatalysis Scalability for Environmental Remediation: Opportunities and Challenges. Catalysts 2020, 11, 1109. [Google Scholar] [CrossRef]
- Bellardita, M.; Ceccato, R.; Dirè, S.; Loddo, V.; Palmisano, L.; Parrino, F. Energy Transfer in Heterogeneous Photocatalysis. Subst. Int. J. Hist. Chem. 2019, 3, 49–57. [Google Scholar] [CrossRef]
- Nosaka, Y.; Daimon, T.; Nosaka, A.Y.; Murakami, Y. Singlet Oxygen Formation in Photocatalytic TiO2 Aqueous Suspension. Phys. Chem. Chem. Phys. 2004, 6, 2917–2918. [Google Scholar] [CrossRef]
- Jańczyk, A.; Krakowska, E.; Stochel, G.; Macyk, W. Singlet Oxygen Photogeneration at Surface Modified Titanium Dioxide. J. Am. Chem. Soc. 2006, 128, 15574–15575. [Google Scholar] [CrossRef] [PubMed]
- Buchalska, M.; Łabuz, P.; Bujak, Ł.; Szewczyk, G.; Sarna, T.; Maćkowski, S.; Macyk, W. New Insight into Singlet Oxygen Generation at Surface Modified Nanocrystalline TiO2– the Effect of near-Infrared Irradiation. J. Chem. Soc. Dalt. Trans. 2013, 42, 9468–9475. [Google Scholar] [CrossRef]
- Ciriminna, R.; Parrino, F.; De Pasquale, C.; Palmisano, L.; Pagliaro, M. Photocatalytic Partial Oxidation of Limonene to 1,2 Limonene Oxide. Chem. Commun. 2018, 54, 1008–1011. [Google Scholar] [CrossRef]
- Parrino, F.; Fidalgo, A.; Palmisano, L.; Ilharco, L.M.; Pagliaro, M.; Ciriminna, R. Polymers of Limonene Oxide and Carbon Dioxide: Polycarbonates of the Solar Economy. ACS Omega 2018, 3, 4884–4890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parrino, F.; Di Paola, A.; Loddo, V.; Pibiri, I.; Bellardita, M.; Palmisano, L. Photochemical and Photocatalytic Isomerization of Trans-Caffeic Acid and Cyclization of Cis-Caffeic Acid to Esculetin. Appl. Catal. B Environ. 2016, 182, 347–355. [Google Scholar] [CrossRef]
- Srikant, V.; Clarke, D.R. On the Optical Band Gap of Zinc Oxide. J. Appl. Phys. 1998, 83, 5447–5451. [Google Scholar] [CrossRef]
- Kafizas, A.; Wang, X.; Pendlebury, S.R.; Barnes, P.; Ling, M.; Sotelo-Vazquez, C.; Quesada-Cabrera, R.; Li, C.; Parkin, I.P.; Durrant, J.R. Where Do Photogenerated Holes Go in Anatase:Rutile TiO2? A Transient Absorption Spectroscopy Study of Charge Transfer and Lifetime. J. Phys. Chem. A 2016, 120, 715–723. [Google Scholar] [CrossRef]
- Rohilla, S.; Gupta, A.; Kumar, V.; Kumari, S.; Petru, M.; Amor, N.; Noman, M.T.; Dalal, J. Excellent Uv-light Triggered Photocatalytic Performance of Zno.SiO2 Nanocomposite for Water Pollutant Compound Methyl Orange Dye. Nanomaterials 2021, 11, 2548. [Google Scholar] [CrossRef] [PubMed]
- Ohno, T.; Sarukawa, K.; Tokieda, K.; Matsumura, M. Morphology of a TiO2 Photocatalyst (Degussa, P-25) Consisting of Anatase and Rutile Crystalline Phases. J. Catal. 2001, 203, 82–86. [Google Scholar] [CrossRef]
- Datye, A.K.; Riegel, G.; Bolton, J.R.; Huang, M.; Prairie, M.R. Microstructural Characterization of a Fumed Titanium Dioxide Photocatalyst. J. Solid State Chem. 1995, 115, 236–239. [Google Scholar] [CrossRef]
- Bickley, R.I.; Gonzalez-Carreno, T.; Lees, J.S.; Palmisano, L.; Tilley, R.J.D. A Structural Investigation of Titanium Dioxide Photocatalysts. J. Solid State Chem. 1991, 92, 178–190. [Google Scholar] [CrossRef]
- Hurum, D.C.; Agrios, A.G.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Explaining the Enhanced Photocatalytic Activity of Degussa P25 Mixed-Phase TiO2 Using EPR. J. Phys. Chem. B 2003, 107, 4545–4549. [Google Scholar] [CrossRef]
- MacDonald, I.R.; Howe, R.F.; Zhang, X.; Zhou, W. In Situ EPR Studies of Electron Trapping in a Nanocrystalline Rutile. J. Photochem. Photobiol. A Chem. 2010, 216, 238–243. [Google Scholar] [CrossRef]
- MacDonald, I.R.; Rhydderch, S.; Holt, E.; Grant, N.; Storey, J.M.D.; Howe, R.F. EPR Studies of Electron and Hole Trapping in Titania Photocatalysts. Catal. Today 2012, 182, 39–45. [Google Scholar] [CrossRef]
- Jiang, X.; Manawan, M.; Feng, T.; Qian, R.; Zhao, T.; Zhou, G.; Kong, F.; Wang, Q.; Dai, S.; Pan, J.H. Anatase and Rutile in Evonik Aeroxide P25: Heterojunctioned or Individual Nanoparticles? Catal. Today 2018, 300, 12–17. [Google Scholar] [CrossRef]
- Ohtani, B.; Prieto-Mahaney, O.O.; Li, D.; Abe, R. What Is Degussa (Evonic) P25? Crystalline Composition Analysis, Reconstruction from Isolated Pure Particles and Photocatalytic Activity Test. J. Photochem. Photobiol. A Chem. 2010, 216, 179–182. [Google Scholar] [CrossRef] [Green Version]
- Prieto-Mahaney, O.O.; Murakami, N.; Abe, R.; Ohtani, B. Correlation between Photoeatalytic Activities and Structural and Physical Properties of Titanium(IV) Oxide Powders. Chem. Lett. 2009, 38, 238–239. [Google Scholar] [CrossRef]
- Ide, Y.; Inami, N.; Hattori, H.; Saito, K.; Sohmiya, M.; Tsunoji, N.; Komaguchi, K.; Sano, T.; Bando, Y.; Golberg, D.; et al. Remarkable Charge Separation and Photocatalytic Efficiency Enhancement through Interconnection of TiO2 Nanoparticles by Hydrothermal Treatment. Angew. Chem. Int. Ed. 2016, 55, 3600–3605. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, G.; Bahnemann, D.W. Photoelectrocatalytic Materials for Environmental Applications. J. Mater. Chem. 2009, 19, 5089–5121. [Google Scholar] [CrossRef]
- Deng, X.; Chen, Y.; Wen, J.; Xu, Y.; Zhu, J.; Bian, Z. Polyaniline-TiO2 Composite Photocatalysts for Light-Driven Hexavalent Chromium Ions Reduction. Sci. Bull. 2020, 65, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Tang, B.; Chen, H.; Peng, H.; Wang, Z.; Huang, W. Graphene Modified TiO2 Composite Photocatalysts: Mechanism, Progress and Perspective. Nanomaterials 2018, 8, 105. [Google Scholar] [CrossRef] [Green Version]
- Qin, R.; Meng, F.; Khan, M.W.; Yu, B.; Li, H.; Fan, Z.; Gong, J. Fabrication and Enhanced Photocatalytic Property of TiO2-ZnO Composite Photocatalysts. Mater. Lett. 2019, 240, 84–87. [Google Scholar] [CrossRef]
- Kumari, S.; Malik, S.; Kumar, S.; Dalal, J.; Dahiya, S.; Ohlan, A.; Punia, R.; Maan, A.S. Excellent Photoelectrical Properties of ZnO Thin Film Based on ZnO /Epoxy-Resin Ink for UV-Light Detectors. AIP Conf. Proc. 2019, 2142, 120004. [Google Scholar] [CrossRef]
- Dossin Zanrosso, C.; Piazza, D.; Lansarin, M.A. PVDF/ZnO Composite Films for Photocatalysis: A Comparative Study of Solution Mixing and Melt Blending Methods. Polym. Eng. Sci. 2020, 60, 1146–1157. [Google Scholar] [CrossRef]
- Mirikaram, N.; Pérez-molina, Á.; Morales-torres, S.; Salemi, A.; Maldonado-hódar, F.J.; Pastrana-martínez, L.M. Photocatalytic Perfomance of Zno-graphene Oxide Composites towards the Degradation of Vanillic Acid under Solar Radiation and Visibleled. Nanomaterials 2021, 11, 1576. [Google Scholar] [CrossRef]
- Raha, S.; Mohanta, D.; Ahmaruzzaman, M. Novel CuO/Mn3O4/ZnO Nanocomposite with Superior Photocatalytic Activity for Removal of Rabeprazole from Water. Sci. Rep. 2021, 11, 15187. [Google Scholar] [CrossRef] [PubMed]
- Thangadurai, P.; Beura, R.; Kumar, J.S. Nanomaterials with different morphologies for photocatalysis. In Green Photocatalysts; Naushad, M., Rajendran, S., Lichfouse, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; ISBN 9783540228608. [Google Scholar]
- Yang, H.; Li, G.; An, T.; Gao, Y.; Fu, J. Photocatalytic Degradation Kinetics and Mechanism of Environmental Pharmaceuticals in Aqueous Suspension of TiO2: A Case of Sulfa Drugs. Catal. Today 2010, 153, 200–207. [Google Scholar] [CrossRef]
- Mirzaei, A.; Yerushalmi, L.; Chen, Z.; Haghighat, F.; Guo, J. Enhanced Photocatalytic Degradation of Sulfamethoxazole by Zinc Oxide Photocatalyst in the Presence of Fluoride Ions: Optimization of Parameters and Toxicological Evaluation. Water Res. 2018, 132, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Náfrádi, M.; Hernadi, K.; Kónya, Z.; Alapi, T. Investigation of the Efficiency of BiOI/BiOCl Composite Photocatalysts Using UV, Cool and Warm White LED Light Sources-Photon Efficiency, Toxicity, Reusability, Matrix Effect, and Energy Consumption. Chemosphere 2021, 280, 130636. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.A.; Sayed, M.; Khan, S.; Shah, N.S.; Dionysiou, D.D.; Boczkaj, G. Advanced oxidation processes for the treatment of contaminants of emerging concern. In Contaminants of Emerging Concern in Water and Wastewater; Hernandez-Maldonado, A., Blaney, L., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128135617. [Google Scholar]
- Bertagna Silva, D.; Buttiglieri, G.; Babić, S. State-of-the-Art and Current Challenges for TiO2/UV-LED Photocatalytic Degradation of Emerging Organic Micropollutants. Environ. Sci. Pollut. Res. 2021, 28, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.K.; Tayade, R.J. New Generation Energy-Efficient Light Source for Photocatalysis: LEDs for Environmental Applications. Ind. Eng. Chem. Res. 2014, 53, 2073–2084. [Google Scholar] [CrossRef]
- Tokode, O.; Prabhu, R.; Lawton, L.A.; Robertson, P.K.J. UV LED Sources for Heterogeneous Photocatalysis. Handb. Environ. Chem. 2015, 35, 159–179. [Google Scholar] [CrossRef] [Green Version]
- Eskandarian, M.R.; Choi, H.; Fazli, M.; Rasoulifard, M.H. Effect of UV-LED Wavelengths on Direct Photolytic and TiO2 Photocatalytic Degradation of Emerging Contaminants in Water. Chem. Eng. J. 2016, 300, 414–422. [Google Scholar] [CrossRef]
- Biancullo, F.; Moreira, N.F.F.; Ribeiro, A.R.; Manaia, C.M.; Faria, J.L.; Nunes, O.C.; Castro-Silva, S.M.; Silva, A.M.T. Heterogeneous Photocatalysis Using UVA-LEDs for the Removal of Antibiotics and Antibiotic Resistant Bacteria from Urban Wastewater Treatment Plant Effluents. Chem. Eng. J. 2019, 367, 304–313. [Google Scholar] [CrossRef]
- Takeda, K.; Fujisawa, K.; Nojima, H.; Kato, R.; Ueki, R.; Sakugawa, H. Hydroxyl Radical Generation with a High Power Ultraviolet Light Emitting Diode (UV-LED) and Application for Determination of Hydroxyl Radical Reaction Rate Constants. J. Photochem. Photobiol. A Chem. 2017, 340, 8–14. [Google Scholar] [CrossRef]
- Taghavi, M.; Ghaneian, M.T.; Ehrampoush, M.H.; Tabatabaee, M.; Afsharnia, M.; Alami, A.; Mardaneh, J. Feasibility of Applying the LED-UV-Induced TiO2/ZnO-Supported H3PMo12O40 Nanoparticles in Photocatalytic Degradation of Aniline. Environ. Monit. Assess. 2018, 190, 188. [Google Scholar] [CrossRef] [PubMed]
- Khademalrasool, M.; Farbod, M.; Talebzadeh, M.D. The Improvement of Photocatalytic Processes: Design of a Photoreactor Using High-Power LEDs. J. Sci. Adv. Mater. Devices 2016, 1, 382–387. [Google Scholar] [CrossRef] [Green Version]
- Farner Budarz, J.; Turolla, A.; Piasecki, A.F.; Bottero, J.Y.; Antonelli, M.; Wiesner, M.R. Influence of Aqueous Inorganic Anions on the Reactivity of Nanoparticles in TiO2 Photocatalysis. Langmuir 2017, 33, 2770–2779. [Google Scholar] [CrossRef] [PubMed]
- Bouanimba, N.; Laid, N.; Zouaghi, R.; Sehili, T. A Comparative Study of the Activity of TiO2 Degussa P25 and Millennium PCs in the Photocatalytic Degradation of Bromothymol Blue. Int. J. Chem. React. Eng. 2018, 16, 1–19. [Google Scholar] [CrossRef]
- Serrà, A.; Philippe, L.; Perreault, F.; Garcia-Segura, S. Photocatalytic Treatment of Natural Waters. Reality or Hype? The Case of Cyanotoxins Remediation. Water Res. 2021, 188, 116543. [Google Scholar] [CrossRef]
- Tufail, A.; Price, W.E.; Mohseni, M.; Pramanik, B.K.; Hai, F.I. A Critical Review of Advanced Oxidation Processes for Emerging Trace Organic Contaminant Degradation: Mechanisms, Factors, Degradation Products, and Effluent Toxicity. J. Water Process Eng. 2021, 40, 101778. [Google Scholar] [CrossRef]
- Kudlek, E.; Dudziak, M.; Bohdziewicz, J. Influence of Inorganic Ions and Organic Substances on the Degradation of Pharmaceutical Compound in Water Matrix. Water 2016, 8, 532. [Google Scholar] [CrossRef] [Green Version]
- Guillard, C.; Puzenat, E.; Lachheb, H.; Houas, A.; Herrmann, J.M. Why Inorganic Salts Decrease the TiO2 Photocatalytic Efficiency. Int. J. Photoenergy 2005, 7, 641208. [Google Scholar] [CrossRef] [Green Version]
- Šojić Merkulov, D.V.; Lazarević, M.J.; Despotović, V.N.; Banić, N.D.; Finčur, N.L.; Maletić, S.P.; Abramović, B.F. The Effects of Inorganic Anions and Organic Matter on Mesotrione (Callisto®) Removal from Environmental Waters. J. Serbian Chem. Soc. 2017, 82, 343–355. [Google Scholar] [CrossRef]
- Lado Ribeiro, A.R.; Moreira, N.F.F.; Li Puma, G.; Silva, A.M.T. Impact of Water Matrix on the Removal of Micropollutants by Advanced Oxidation Technologies. Chem. Eng. J. 2019, 363, 155–173. [Google Scholar] [CrossRef] [Green Version]
- Hatchard, C.G.; Parker, C.A. A New Sensitive Chemical Actinometer-II. Potassium Ferrioxalate as a Standard Chemical Actinometer. Proc. R. Soc. London Ser. A Math. Phys. Sci. 1956, 235, 518–536. [Google Scholar] [CrossRef]
- Zuccheri, T.; Colonna, M.; Stefanini, I.; Santini, C.; Di Gioia, D. Bactericidal Activity of Aqueous Acrylic Paint Dispersion for Wooden Substrates Based on TiO2 Nanoparticles Activated by Fluorescent Light. Materials 2013, 6, 3270–3283. [Google Scholar] [CrossRef]
- Hassellöv, M.; Readman, J.W.; Ranville, J.F.; Tiede, K. Nanoparticle Analysis and Characterization Methodologies in Environmental Risk Assessment of Engineered Nanoparticles. Ecotoxicology 2008, 17, 344–361. [Google Scholar] [CrossRef]
- White, L.; Koo, Y.; Yun, Y.; Sankar, J. TiO2 Deposition on AZ31 Magnesium Alloy Using Plasma Electrolytic Oxidation. J. Nanomater. 2013, 2013, 11. [Google Scholar] [CrossRef] [Green Version]
- Thamaphat, K.; Limsuwan, P.; Ngotawornchai, B. Phase Characterization of TiO2 Powder by XRD and TEM. Agric. Nat. Resour. 2008, 42, 357–361. [Google Scholar]
- Arefi, M.R.; Rezaei-Zarchi, S. Synthesis of Zinc Oxide Nanoparticles and Their Effect on the Compressive Strength and Setting Time of Self-Compacted Concrete Paste as Cementitious Composites. Int. J. Mol. Sci. 2012, 13, 4340–4350. [Google Scholar] [CrossRef]
- Yi, Z.; Wang, J.; Jiang, T.; Tang, Q.; Cheng, Y. Photocatalytic Degradation of Sulfamethazine in Aqueous Solution Using Zno with Different Morphologies. R. Soc. Open Sci. 2018, 5, 171457. [Google Scholar] [CrossRef] [Green Version]
- Zhu, G.; Sun, Q.; Wang, C.; Yang, Z.; Xue, Q. Removal of Sulfamethoxazole, Sulfathiazole and Sulfamethazine in Their Mixed Solution by UV/H2O2 Process. Int. J. Environ. Res. Public Health 2019, 16, 1797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, S.; Hao, C. Quantum Chemical Investigation on Photodegradation Mechanisms of Sulfamethoxypyridazine with Dissolved Inorganic Matter and Hydroxyl Radical. J. Environ. Sci. 2017, 57, 85–92. [Google Scholar] [CrossRef]
- Guo, C.; Xu, J.; Wang, S.; Zhang, Y.; He, Y.; Li, X. Photodegradation of Sulfamethazine in an Aqueous Solution by a Bismuth Molybdate Photocatalyst. Catal. Sci. Technol. 2013, 3, 1603–1611. [Google Scholar] [CrossRef]
- Yang, L.; Shi, Y.; Li, J.; Fang, L.; Luan, T. Transformation of Aqueous Sulfonamides under Horseradish Peroxidase and Characterization of Sulfur Dioxide Extrusion Products from Sulfadiazine. Chemosphere 2018, 200, 164–172. [Google Scholar] [CrossRef]
- Boreen, A.L.; Arnold, W.A.; McNeill, K. Triplet-Sensitized Photodegradation of Sulfa Drugs Containing Six-Membered Heterocyclic Groups: Identification of an SO2 Extrusion Photoproduct. Environ. Sci. Technol. 2005, 39, 3630–3638. [Google Scholar] [CrossRef]
- Ge, L.; Zhang, P.; Halsall, C.; Li, Y.; Chen, C.E.; Li, J.; Sun, H.; Yao, Z. The Importance of Reactive Oxygen Species on the Aqueous Phototransformation of Sulfonamide Antibiotics: Kinetics, Pathways, and Comparisons with Direct Photolysis. Water Res. 2019, 149, 243–250. [Google Scholar] [CrossRef]
- Park, S.; Kim, H.J.; Kim, J.S.; Yoo, K.; Lee, J.C.; Anderson, W.A.; Lee, J.H. Photocatalytic Reduction of Nitrate in Wastewater Using ZnO Nanopowder Synthesized by Solution Combustion Method. J. Nanosci. Nanotechnol. 2007, 7, 4069–4072. [Google Scholar] [CrossRef] [PubMed]
- Løgager, T.; Sehested, K. Formation and Decay of Peroxynitric Acid: A Pulse Radiolysis Study. J. Phys. Chem. 1993, 97, 10047–10052. [Google Scholar] [CrossRef]
- Náfrádi, M.; Farkas, L.; Alapi, T.; Hernádi, K.; Kovács, K.; Wojnárovits, L.; Takács, E. Application of Coumarin and Coumarin-3-Carboxylic Acid for the Determination of Hydroxyl Radicals during Different Advanced Oxidation Processes. Radiat. Phys. Chem. 2020, 170, 108610. [Google Scholar] [CrossRef]
- Ishibashi, K.I.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Detection of Active Oxidative Species in TiO2 Photocatalysis Using the Fluorescence Technique. Electrochem. Commun. 2000, 2, 207–210. [Google Scholar] [CrossRef]
- Zhang, J.; Nosaka, Y. Quantitative Detection of OH Radicals for Investigating the Reaction Mechanism of Various Visible-Light TiO2 Photocatalysts in Aqueous Suspension. J. Phys. Chem. C 2013, 117, 1383–1391. [Google Scholar] [CrossRef]
- Zhang, J.; Nosaka, Y. Generation of OH Radicals and Oxidation Mechanism in Photocatalysis of WO3 and BiVO4 Powders. J. Photochem. Photobiol. A Chem. 2015, 303–304, 53–58. [Google Scholar] [CrossRef]
- Černigoj, U.; Štangar, U.L.; Trebše, P.; Sarakha, M. Determination of Catalytic Properties of TiO2 Coatings Using Aqueous Solution of Coumarin: Standardization Efforts. J. Photochem. Photobiol. A Chem. 2009, 201, 142–150. [Google Scholar] [CrossRef]
- Baxter, J.B.; Schmuttenmaer, C.A. Conductivity of ZnO Nanowires, Nanoparticles, and Thin Films Using Time-Resolved Terahertz Spectroscopy. J. Phys. Chem. B 2006, 110, 25229–25239. [Google Scholar] [CrossRef] [PubMed]
- Meulenkamp, E.A. Electron Transport in Nanoparticulate ZnO Films. J. Phys. Chem. B 1999, 103, 7831–7838. [Google Scholar] [CrossRef]
- Sachs, M.; Pastor, E.; Kafizas, A.; Durrant, J.R. Evaluation of Surface State Mediated Charge Recombination in Anatase and Rutile TiO2. J. Phys. Chem. Lett. 2016, 7, 3742–3746. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Durrant, J.R.; Klug, D.R. Mechanism of Photocatalytic Water Splitting in TiO2. Reaction of Water with Photoholes, Importance of Charge Carrier Dynamics, and Evidence for Four-Hole Chemistry. J. Am. Chem. Soc. 2008, 130, 13885–13891. [Google Scholar] [CrossRef] [PubMed]
- Fónagy, O.; Szabó-Bárdos, E.; Horváth, O. 1,4-Benzoquinone and 1,4-Hydroquinone Based Determination of Electron and Superoxide Radical Formed in Heterogeneous Photocatalytic Systems. J. Photochem. Photobiol. A Chem. 2021, 407, 113057. [Google Scholar] [CrossRef]
- Henderson, M.A.; Shen, M. Electron-Scavenging Chemistry of Benzoquinone on TiO2(110). Top. Catal. 2017, 60, 440–445. [Google Scholar] [CrossRef]
- Adams, G.E.; Michael, B.D. Pulse Radiolysis of Benzoquinone and Hydroquinone. Semiquinone Formation by Water Elimination from Trihydroxycyclohexadienyl Radicals. Trans. Faraday Soc. 1967, 63, 1171–1180. [Google Scholar] [CrossRef]
- Al-Suhybani, A.A.; Hughes, G. Pulse Radiolysis of Hydroquinone Solutions in the Presence of N2O and O2. Isot. Isot. Environ. Heal. Stud. 1985, 21, 208–210. [Google Scholar] [CrossRef]
- Mezyk, S.P.; Neubauer, T.J.; Cooper, W.J.; Peller, J.R. Free-Radical-Induced Oxidative and Reductive Degradation of Sulfa Drugs in Water: Absolute Kinetics and Efficiencies of Hydroxyl Radical and Hydrated Electron Reactions. J. Phys. Chem. A 2007, 111, 9019–9024. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of Rate Constants for Reactions of Hydrated Electrons, Hydrogen Atoms and Hydroxyl Radicals (OH/O−in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.N.H.; Amal, R.; Beydoun, D. Effect of Formate and Methanol on Photoreduction/Removal of Toxic Cadmium Ions Using TiO2 Semiconductor as Photocatalyst. Chem. Eng. Sci. 2003, 58, 4429–4439. [Google Scholar] [CrossRef]
- Zhang, J.; Nosaka, Y. Photocatalytic Oxidation Mechanism of Methanol and the Other Reactants in Irradiated TiO2 Aqueous Suspension Investigated by OH Radical Detection. Appl. Catal. B Environ. 2015, 166–167, 32–36. [Google Scholar] [CrossRef]
- Baltrusaitis, J.; Jayaweera, P.M.; Grassian, V.H. Sulfur Dioxide Adsorption on TiO2 Nanoparticles: Influence of Particle Size, Coadsorbates, Sample Pretreatment, and Light on Surface Speciation and Surface Coverage. J. Phys. Chem. C 2011, 115, 492–500. [Google Scholar] [CrossRef]
- Langhammer, D.; Kullgren, J.; Österlund, L. Photoinduced Adsorption and Oxidation of SO2on Anatase TiO2(101). J. Am. Chem. Soc. 2020, 142, 21767–21774. [Google Scholar] [CrossRef]
- Mi, Y.; Weng, Y. Band Alignment and Controllable Electron Migration between Rutile and Anatase TiO2. Sci. Rep. 2015, 5, 11482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nosaka, Y.; Nosaka, A.Y. Reconsideration of Intrinsic Band Alignments within Anatase and Rutile TiO2. J. Phys. Chem. Lett. 2016, 7, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Kaabeche, O.N.E.H.; Zouaghi, R.; Boukhedoua, S.; Bendjabeur, S.; Sehili, T. A Comparative Study on Photocatalytic Degradation of Pyridinium-Based Ionic Liquid by TiO2 and ZnO in Aqueous Solution. Int. J. Chem. React. Eng. 2019, 17, 11482. [Google Scholar] [CrossRef]
- Wojnárovits, L.; Takács, E. Rate Constants of Sulfate Radical Anion Reactions with Organic Molecules: A Review. Chemosphere 2019, 220, 1014–1032. [Google Scholar] [CrossRef]
- Jayson, G.G.; Parsons, B.J.; Swallow, A.J. Some Simple, Highly Reactive, Inorganic Chlorne Derivatives in Aqueous Solution. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1973, 69, 1597–1607. [Google Scholar]
- Maji, T.K.; Bagchi, D.; Kar, P.; Karmakar, D.; Pal, S.K. Enhanced Charge Separation through Modulation of Defect-State in Wide Band-Gap Semiconductor for Potential Photocatalysis Application: Ultrafast Spectroscopy and Computational Studies. J. Photochem. Photobiol. A Chem. 2017, 332, 391–398. [Google Scholar] [CrossRef]

| TiO2 | ZnO | |||||||
| SMT | SMP | SMT | SMP | |||||
| r0SMT (mol dm−3 s−1) | ΦSMT | r0SMP (mol dm−3 s−1) | ΦSMP | r0 SMT (mol dm−3 s−1) | ΦSMT | r0SMP (mol dm−3 s−1) | ΦSMP | |
| LED365nm | 1.93 × 10−7 | 7.0 × 10−3 | 1.68 × 10−7 | 6.1 × 10−3 | 2.08 × 10−7 | 7.6 × 10−3 | 2.35 × 10−7 | 8.5 × 10−3 |
| LED398nm | 4.53 × 10−8 | 9.7 × 10−4 | 7.38 × 10−7 | 1.6 × 10−2 | 6.43 × 10−8 | 1.4 × 10−3 | 4.12 × 10−8 | 8.8 × 10−4 |
| TiO2 | ZnO | |||||||
| COU → 7-HC | ||||||||
| r0COU (mol dm−3 s−1) | ΦCOU | r07-HC (mol dm−3 s−1) | Φ7-HC | r0COU (mol dm−3 s−1) | ΦCOU | r07-HC (mol dm−3 s−1) | Φ7-HC | |
| LED365nm | 1.53 × 10−7 | 5.5 × 10−3 | 4.1 × 10−9 | 1.5 × 10−4 | 1.54 × 10−7 | 3.2 × 10−3 | 2.88 × 10−9 | 0.6 × 10−4 |
| LED398nm | 3.80 × 10−8 | 0.8 × 10−3 | 1.5 × 10−9 | 0.3 × 10−4 | 5.18 × 10−8 | 1.1 × 10−3 | 1.29 × 10−9 | 0.3 × 10−4 |
| 1,4-BQ → 1,4-H2Q | ||||||||
| r0BQ (mol dm−3 s−1) | Φ1,4-BQ | r01,4-H2Q (mol dm−3 s−1) | Φ1,4-H2Q | r0BQ (mol dm−3 s−1) | Φ1,4-BQ | r01,4-H2Q (mol dm−3 s−1) | Φ1,4-H2Q | |
| LED365nm | 2.78 × 10−6 | 1.0 × 10−1 | 2.61 × 10−6 | 9.5 × 10−2 | 3.12 × 10−6 | 1.1 × 10−1 | 2.47 × 10−6 | 8.9 × 10−2 |
| LED398nm | 8.88 × 10−7 | 1.9 × 10−2 | 7.61 × 10−7 | 1.5 × 10−2 | 1.19 × 10−6 | 2.6 × 10−2 | 8.07 × 10−7 | 1.7 × 10−2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Náfrádi, M.; Alapi, T.; Farkas, L.; Bencsik, G.; Kozma, G.; Hernádi, K. Wavelength Dependence of the Transformation Mechanism of Sulfonamides Using Different LED Light Sources and TiO2 and ZnO Photocatalysts. Materials 2022, 15, 49. https://doi.org/10.3390/ma15010049
Náfrádi M, Alapi T, Farkas L, Bencsik G, Kozma G, Hernádi K. Wavelength Dependence of the Transformation Mechanism of Sulfonamides Using Different LED Light Sources and TiO2 and ZnO Photocatalysts. Materials. 2022; 15(1):49. https://doi.org/10.3390/ma15010049
Chicago/Turabian StyleNáfrádi, Máté, Tünde Alapi, Luca Farkas, Gábor Bencsik, Gábor Kozma, and Klára Hernádi. 2022. "Wavelength Dependence of the Transformation Mechanism of Sulfonamides Using Different LED Light Sources and TiO2 and ZnO Photocatalysts" Materials 15, no. 1: 49. https://doi.org/10.3390/ma15010049
APA StyleNáfrádi, M., Alapi, T., Farkas, L., Bencsik, G., Kozma, G., & Hernádi, K. (2022). Wavelength Dependence of the Transformation Mechanism of Sulfonamides Using Different LED Light Sources and TiO2 and ZnO Photocatalysts. Materials, 15(1), 49. https://doi.org/10.3390/ma15010049