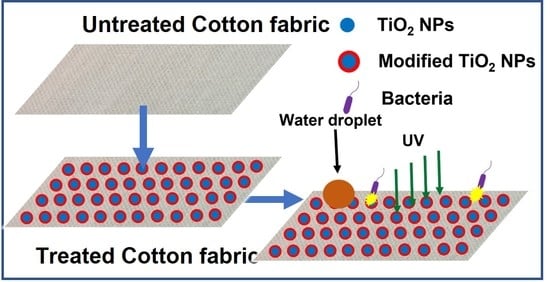
Single Step Synthesis and Functionalization of Nano Titania for Development of Multifunctional Cotton Fabrics
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Modified Nano Titania
2.3. Preparation of Multifaceted Cotton Fabric
3. Testing and Characterization
4. Results and Discussion
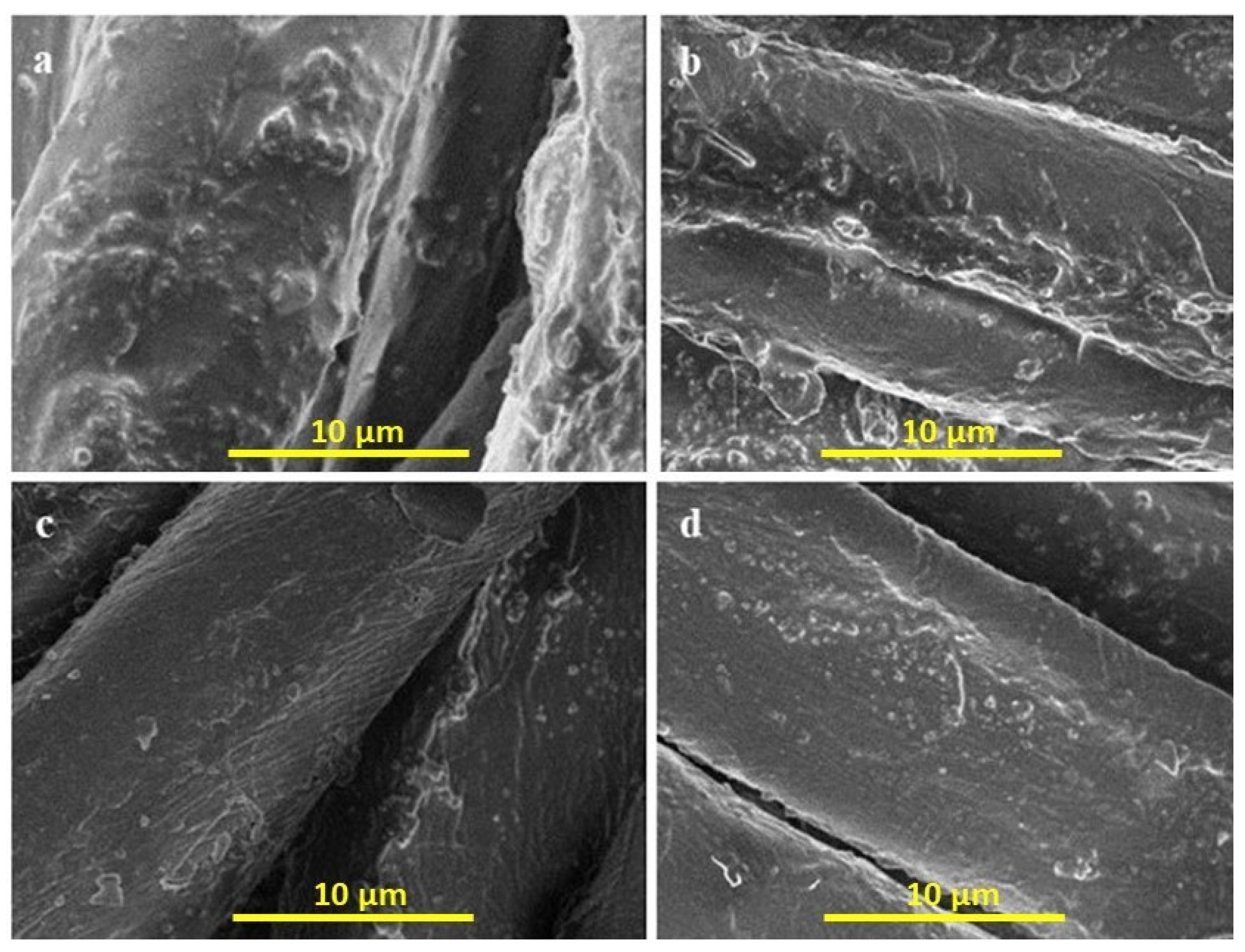
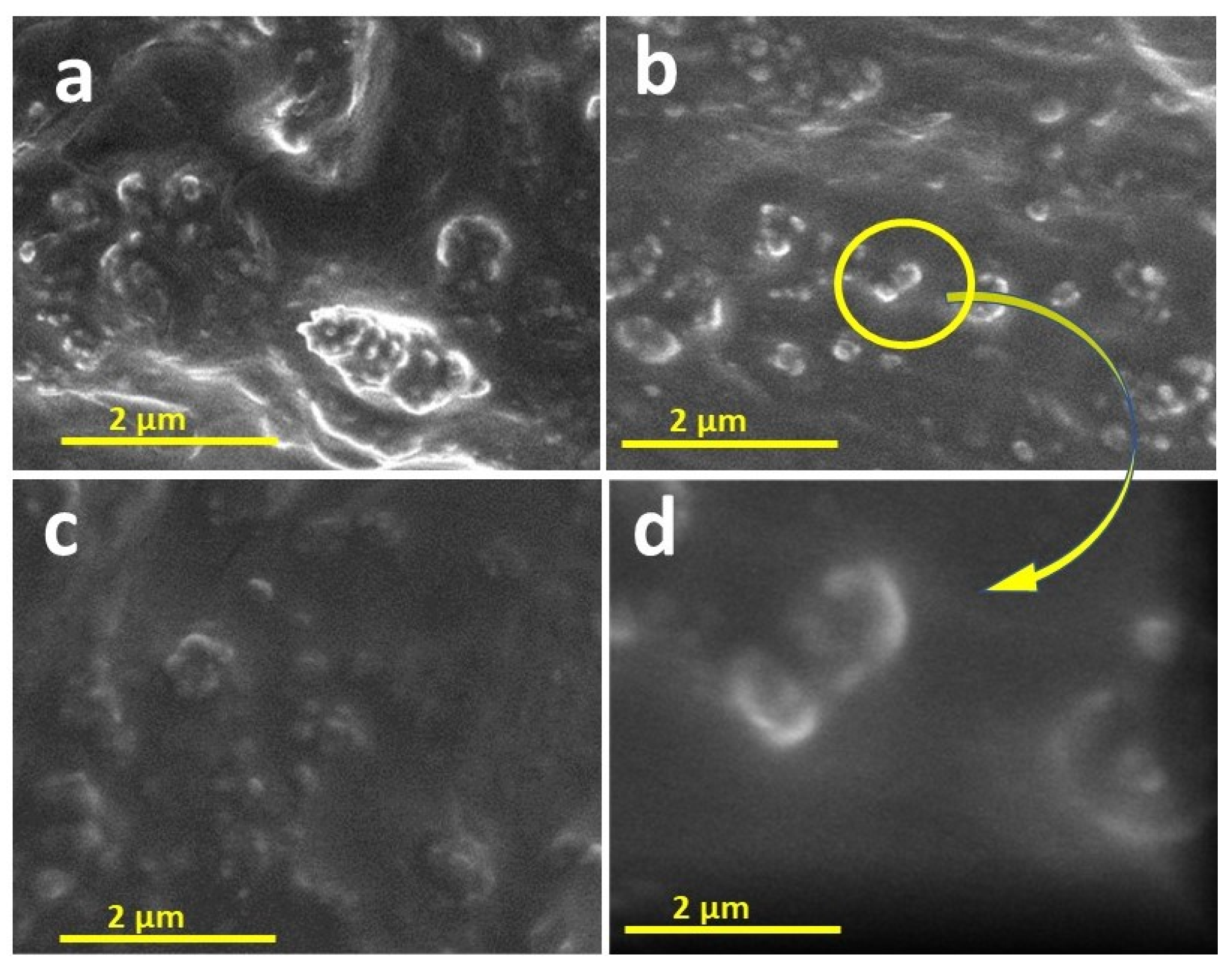
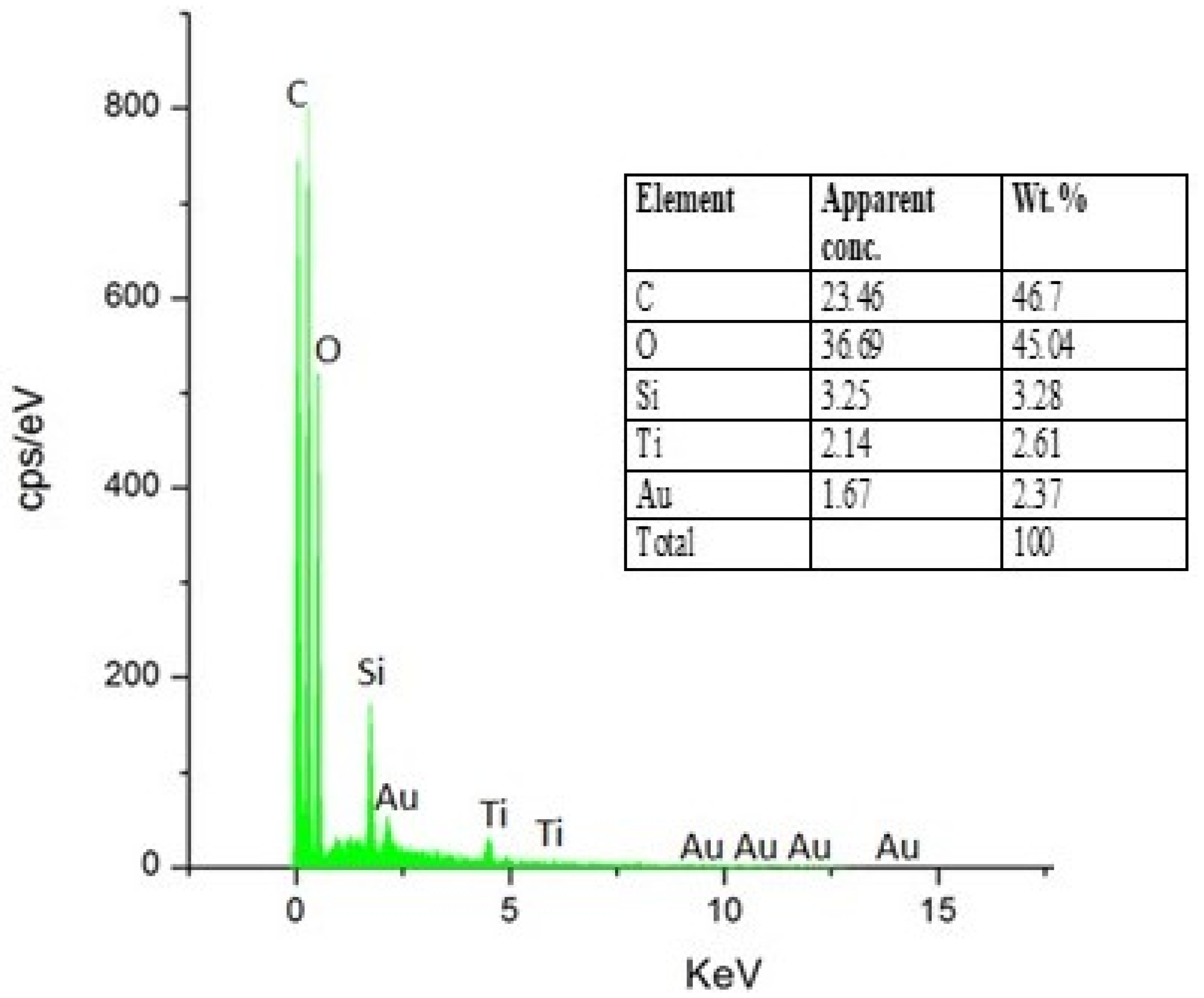
4.1. Scanning Electron Microscopy
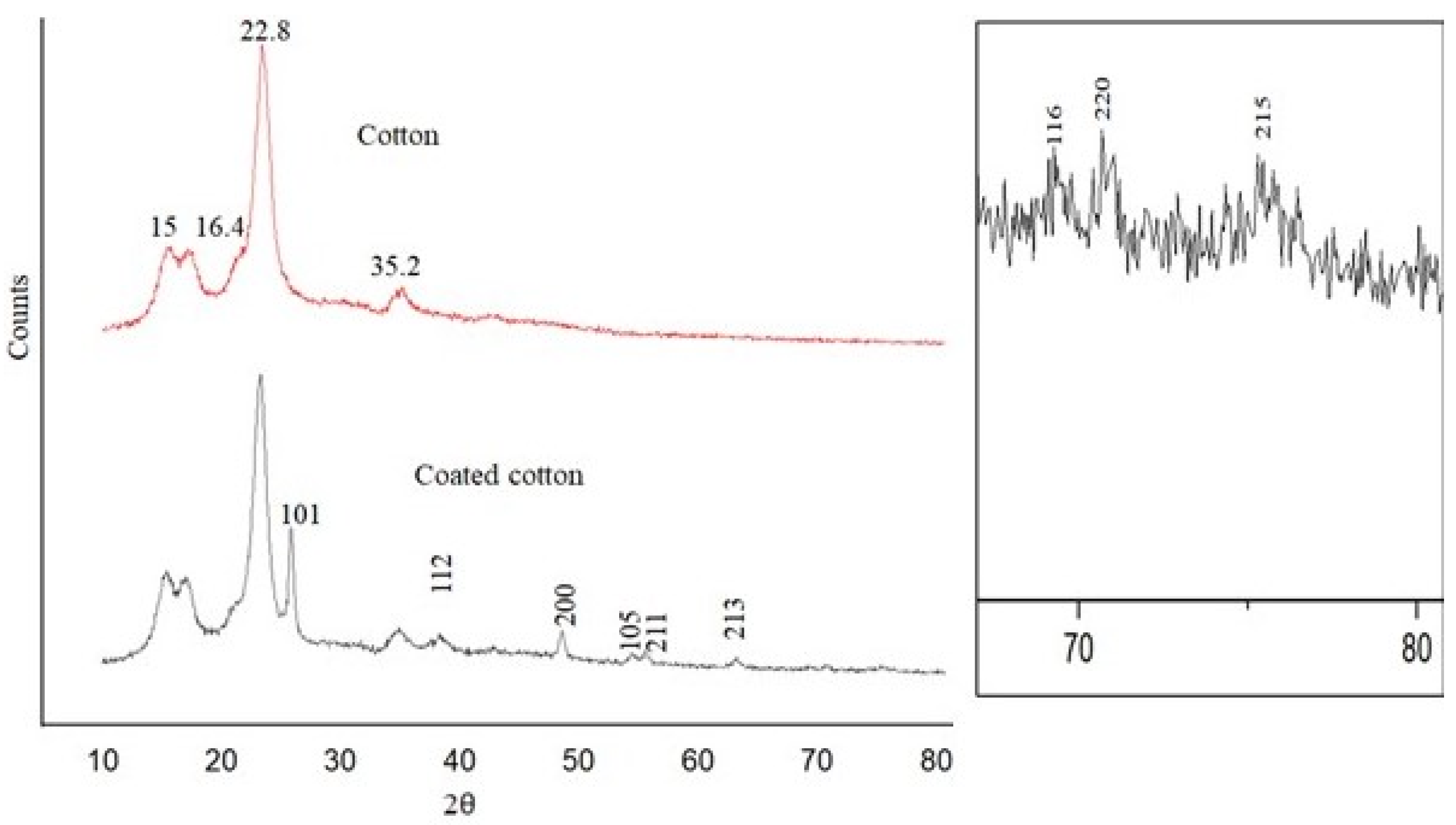
4.2. Crystallography
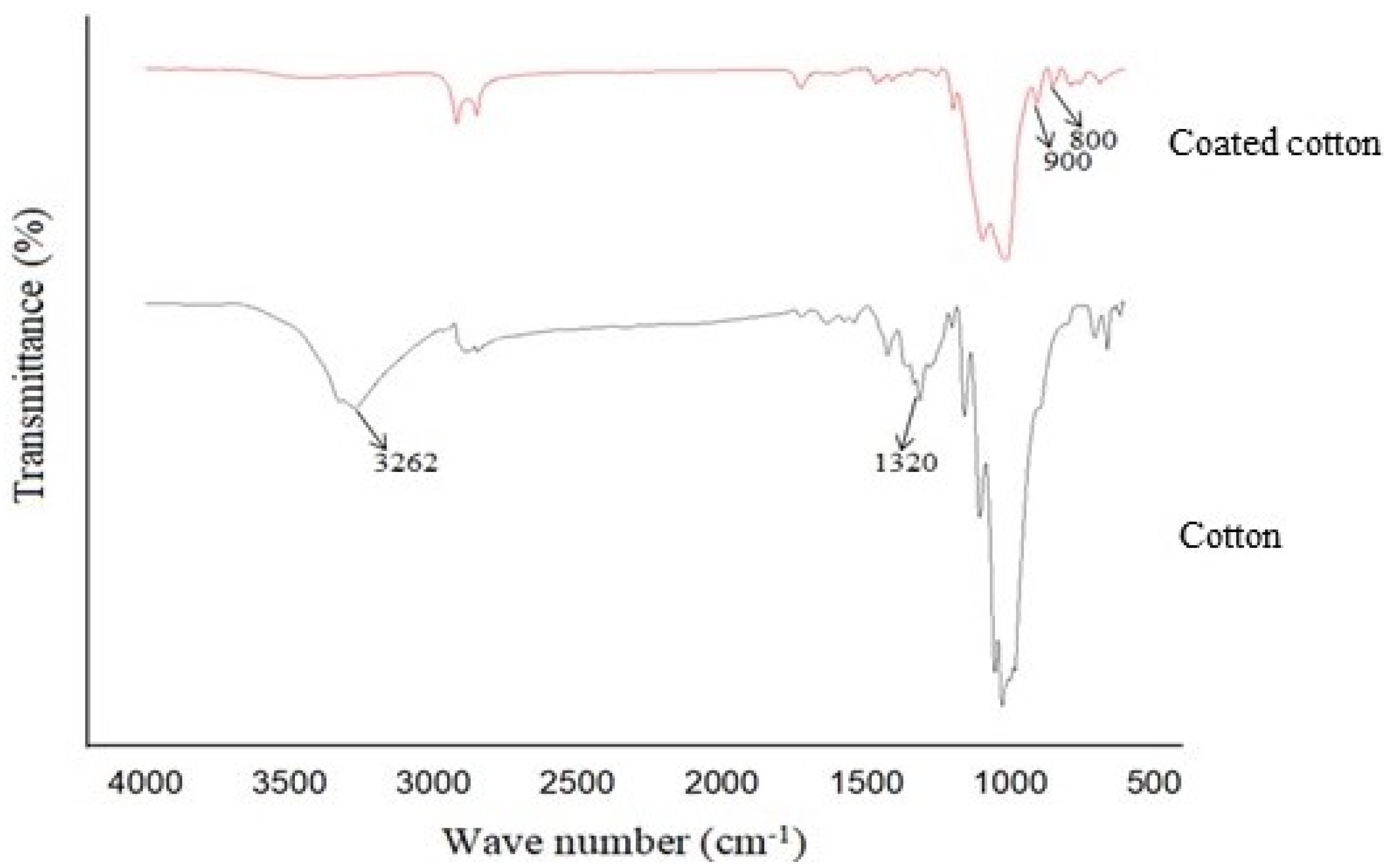
4.3. FTIR Analysis
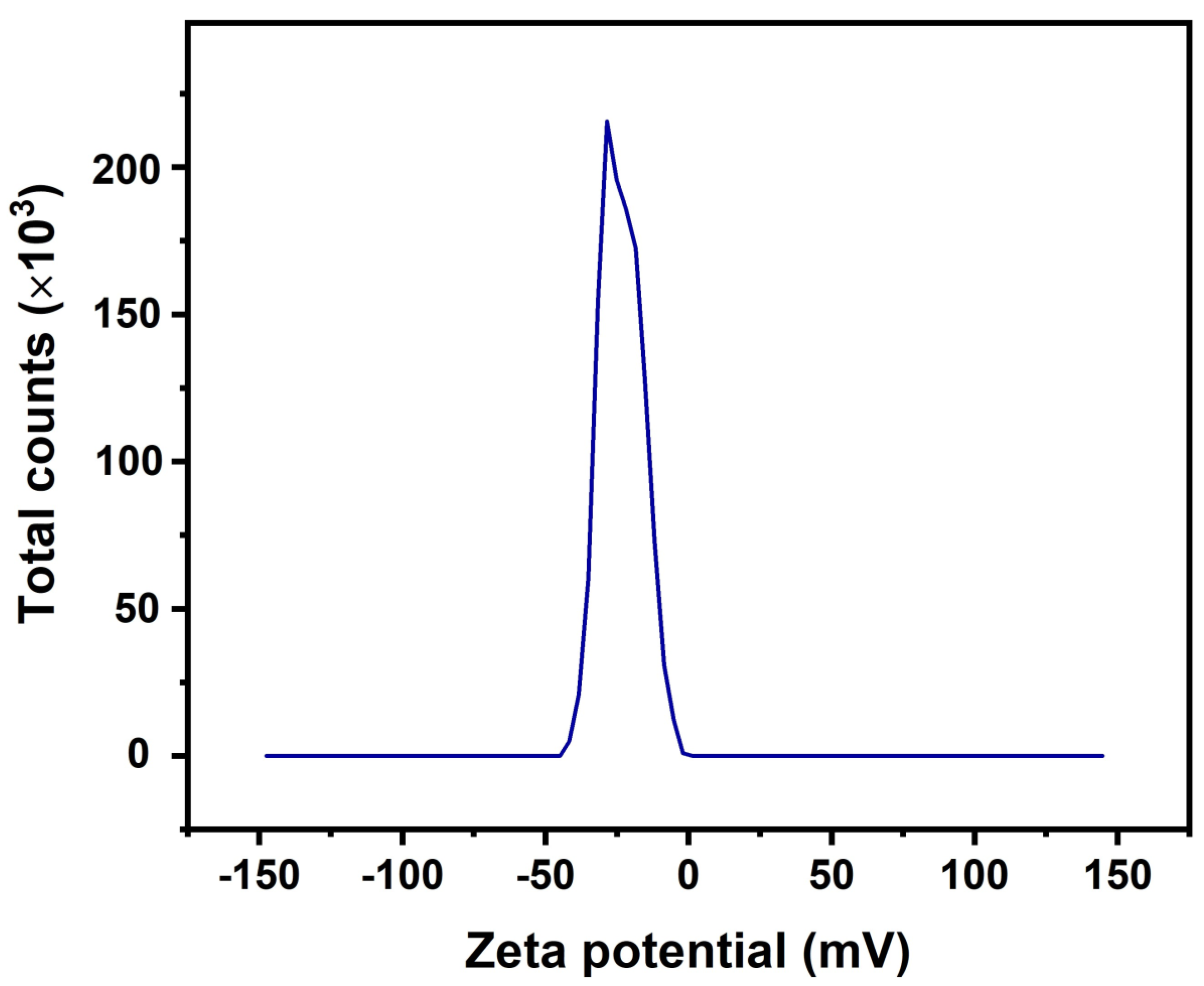
4.4. Zeta Potential of Modified Nano Titania
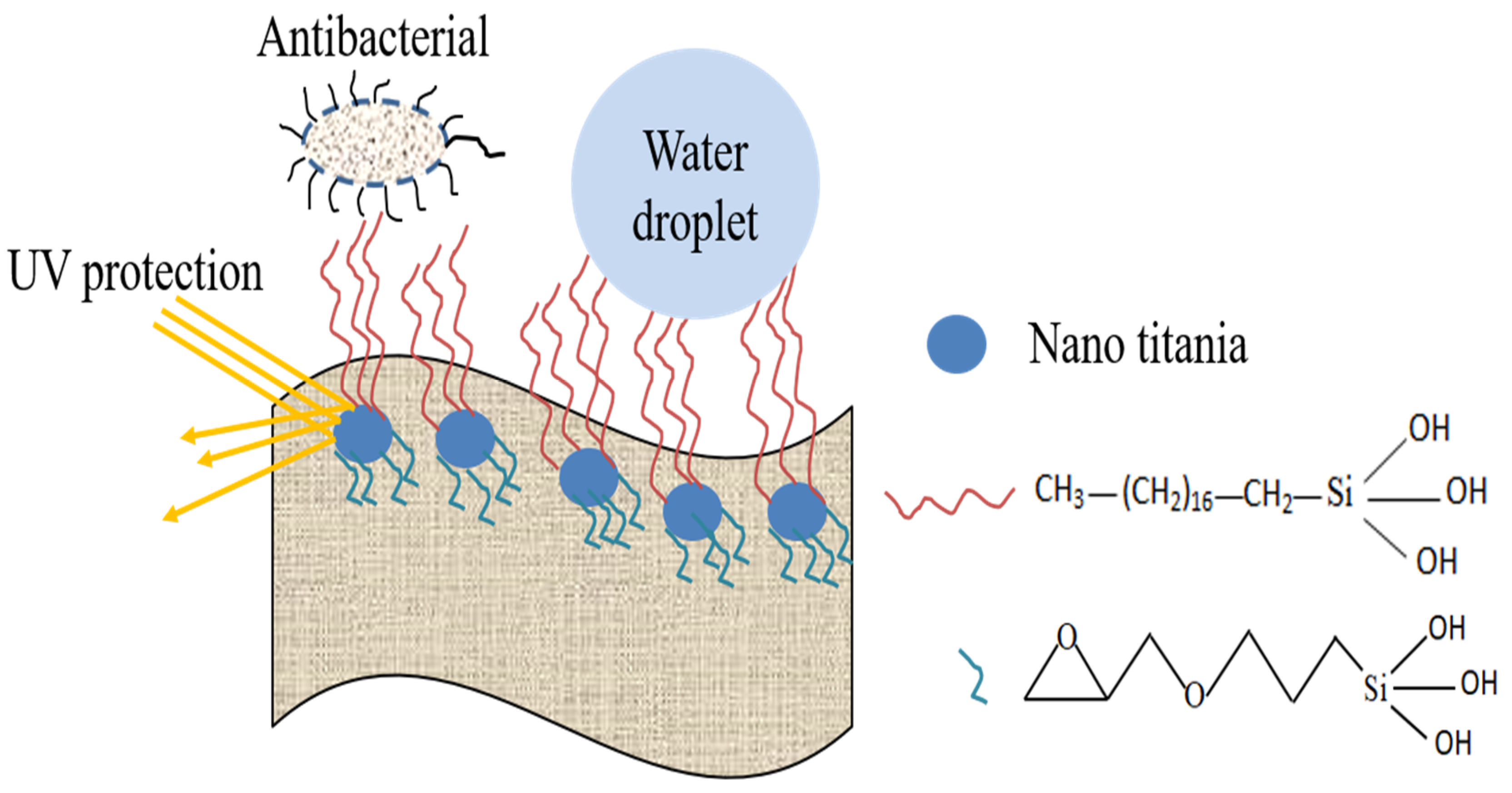
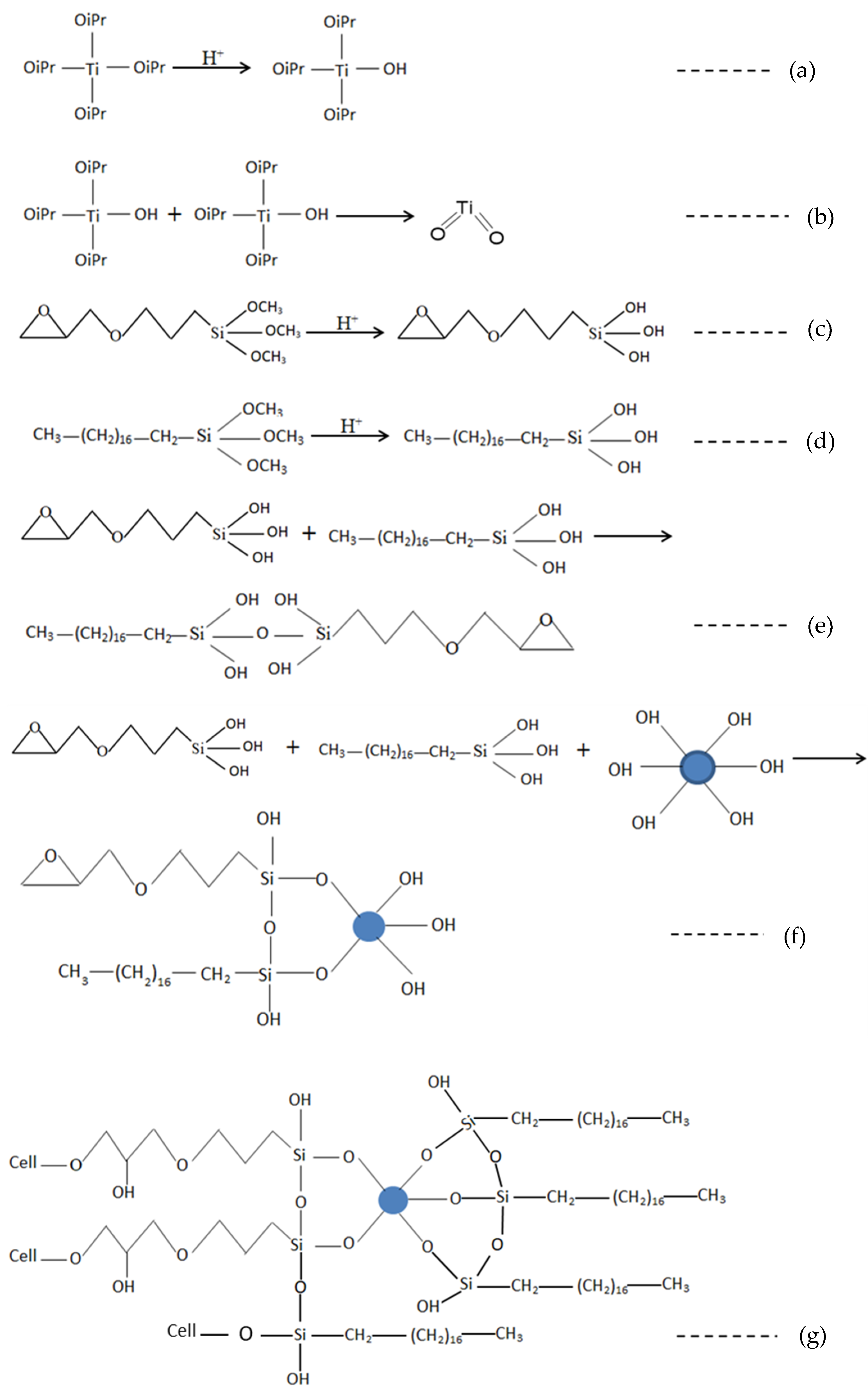
4.5. Reaction Schematics
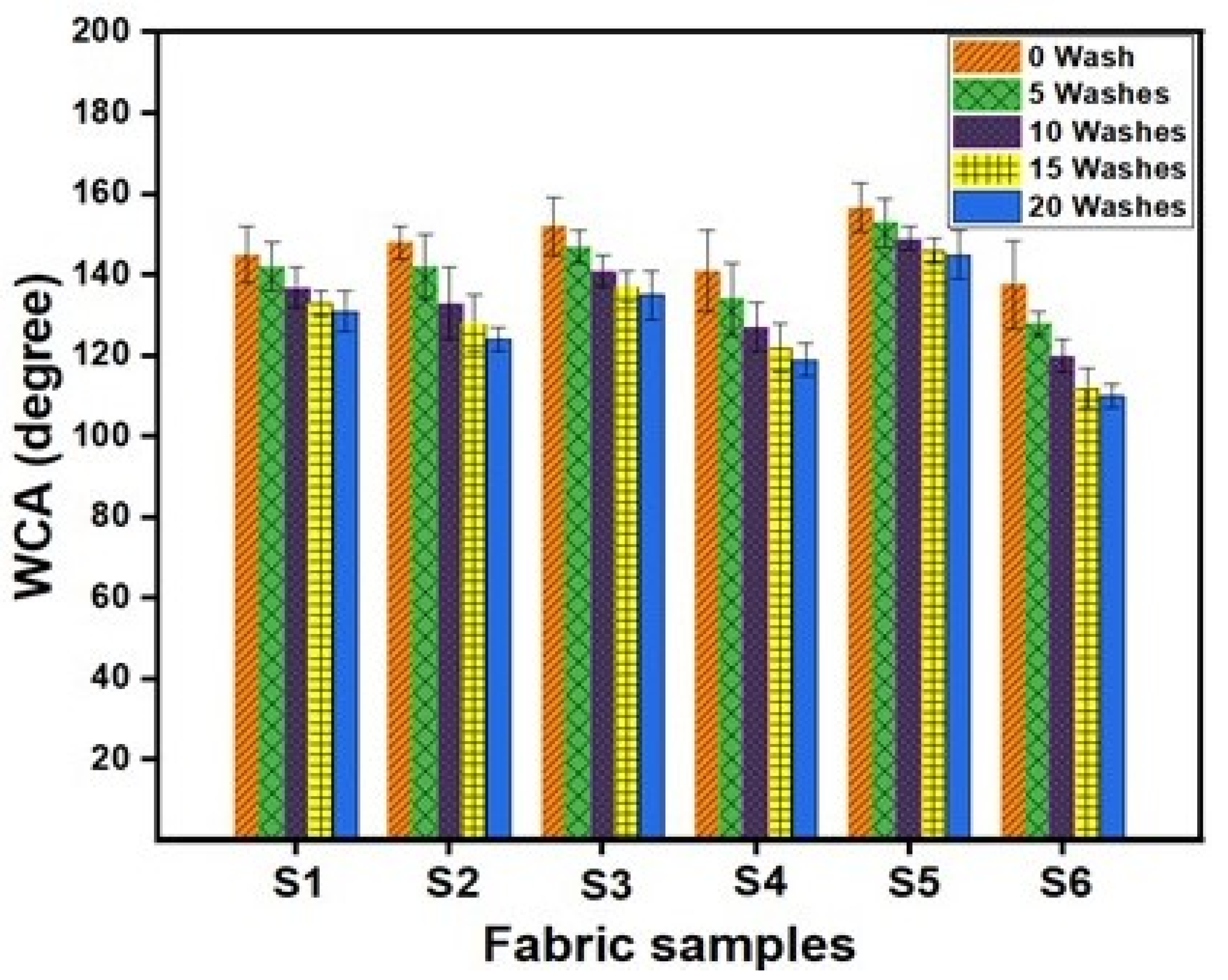
4.6. Hydrophobic Properties
4.7. Water Floating Test
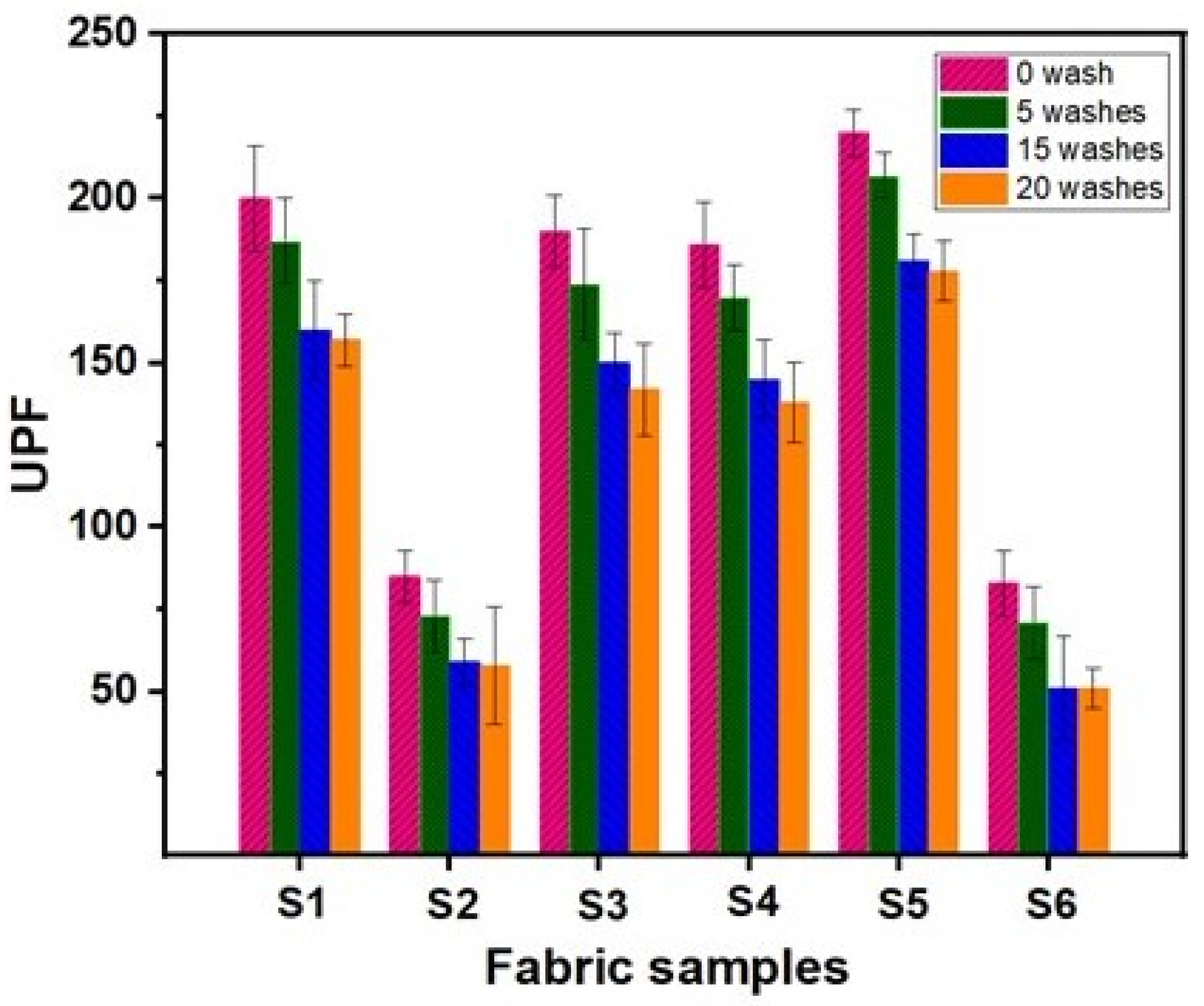
4.8. UV Protection Properties
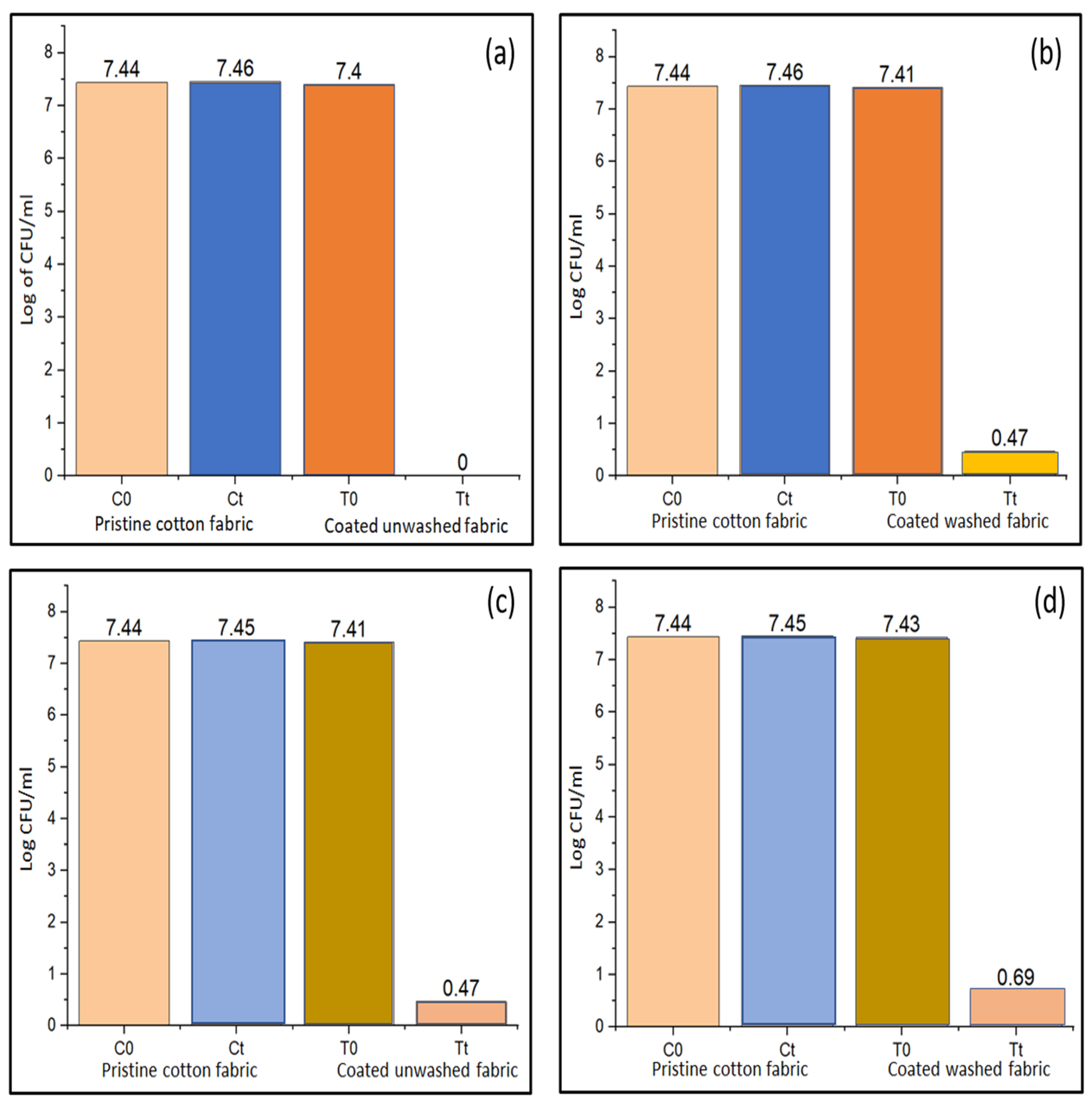
4.9. Antibacterial Properties
4.10. Surface Roughness
4.11. Air Permeability
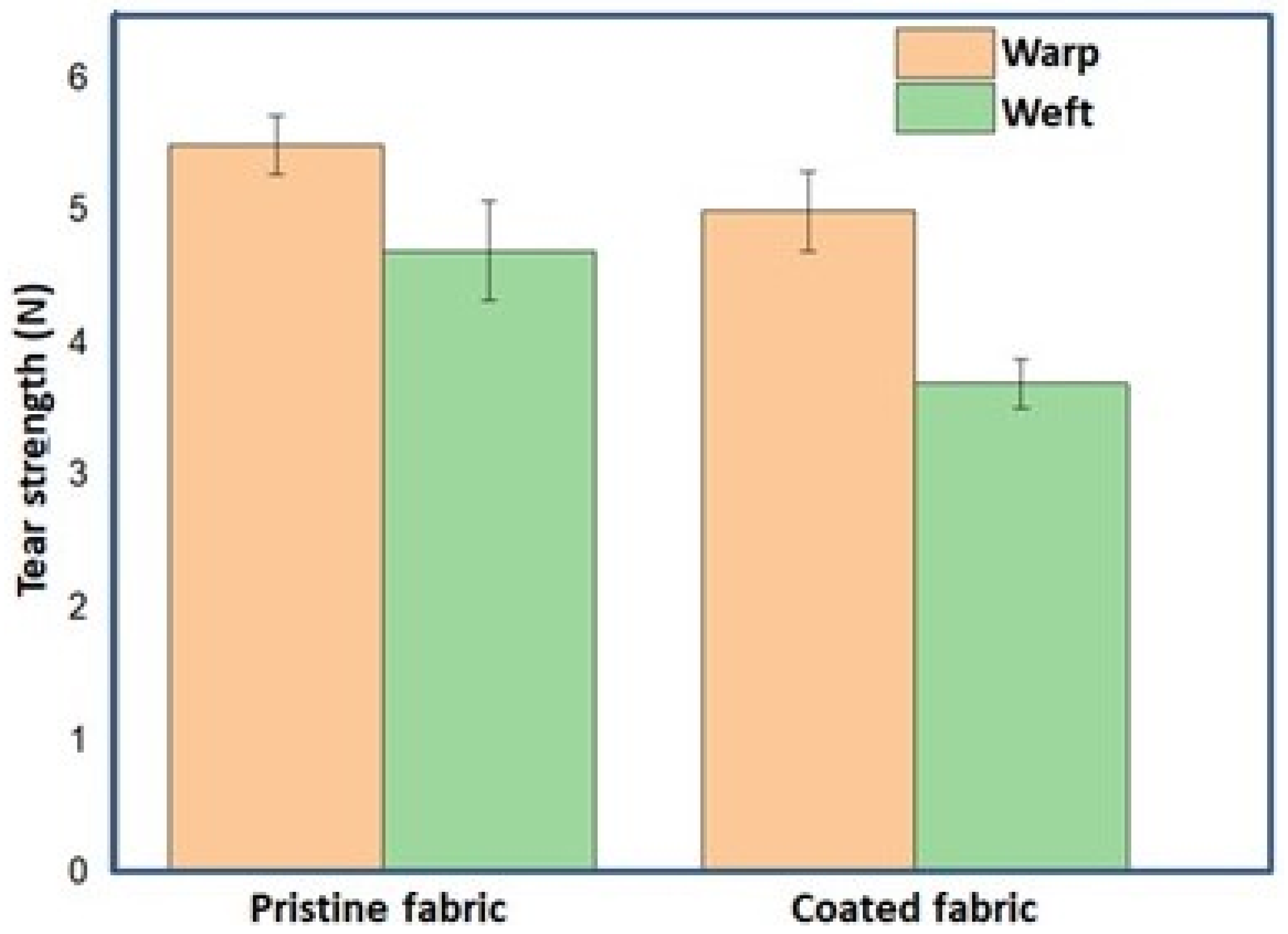
4.12. Tear Strength
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sami, S.M.; Barakat, O. Use of nanotechnology to achieve the best functional characteristics of the fabrics sheets used in hospitals. Egypt. J. Chem. 2018, 61, 705–715. [Google Scholar] [CrossRef]
- Li, S.; Huang, J.; Chen, Z.; Chen, G.; Lai, Y. A review on special wettability textiles: Theoretical models, fabrication technologies and multifunctional applications. J. Mater. Chem. A 2017, 5, 31–55. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Tang, Y.; Wang, X.; Lin, T. Superhydrophobic cotton fabric fabricated by electrostatic assembly of silica nanoparticles and its remarkable buoyancy. Appl. Surf. Sci. 2010, 256, 6736–6742. [Google Scholar] [CrossRef]
- Chen, D.; Mai, Z.; Liu, X.; Ye, D.; Zhang, H.; Yin, X.; Zhou, Y.; Liu, M.; Xu, W. UV-blocking, superhydrophobic and robust cotton fabrics fabricated using polyvinylsilsesquioxane and nano-TiO2. Cellulose 2018, 25, 3635–3647. [Google Scholar] [CrossRef]
- Gao, D.; Li, Y.; Lyu, B.; Jin, D.; Ma, J. Silicone quaternary ammonium salt based nanocomposite: A long-acting antibacterial cotton fabric finishing agent with good softness and air permeability. Cellulose 2020, 27, 1055–1069. [Google Scholar] [CrossRef]
- Guo, F.; Wen, Q.; Peng, Y.; Guo, Z. Multifunctional hollow superhydrophobic SiO2 microspheres with robust and self-cleaning and separation of oil/water emulsions properties. J. Colloid Interface Sci. 2017, 494, 54–63. [Google Scholar] [CrossRef]
- Yang, M.; Liu, W.; Jiang, C.; Xie, Y.; Shi, H.; Zhang, F. Facile fabrication of robust fluorine-free superhydrophobic cellulosic fabric for self-cleaning, photocatalysis and UV shielding. Cellulose 2019, 26, 8153–8164. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, J.; He, H.; Wang, Y.; Zhao, Y.; Lu, Q.; Qin, Y.; Ke, Y.; Peng, Y. Green synthesis of silver nanoparticles with black rice (Oryza sativa L.) extract endowing carboxymethyl chitosan modified cotton with high anti-microbial and durable properties. Cellulose 2021, 28, 1827–1842. [Google Scholar] [CrossRef]
- Hebeish, A.; Abdelhady, M.; Youssef, A. TiO2 nanowire and TiO2 nanowire doped Ag-PVP nanocomposite for antimicrobial and self-cleaning cotton textile. Carbohydr. Polym. 2013, 91, 549–559. [Google Scholar] [CrossRef]
- Hashemikia, S.; Montazer, M. Sodium hypophosphite and nano TiO2 inorganic catalysts along with citric acid on textile producing multi-functional properties. Appl. Catal. A Gen. 2012, 417, 200–208. [Google Scholar] [CrossRef]
- Kim, C.-S.; Kwon, I.-M.; Moon, B.K.; Jeong, J.H.; Choi, B.-C.; Kim, J.H.; Choi, H.; Yi, S.S.; Yoo, D.-H.; Hong, K.-S. Synthesis and particle size effect on the phase transformation of nanocrystalline TiO2. Mater. Sci. Eng. C 2007, 27, 1343–1346. [Google Scholar] [CrossRef]
- Stötzel, J.; Lötzenkirchen-Hecht, D.; Frahm, R.; Santilli, C.V.; Pulcinelli, S.H.; Kaminski, R.; Fonda, E.; Villain, F.; Briois, V. QEXAFS and UV/Vis simultaneous monitoring of the TiO2-nanoparticles formation by hydrolytic sol-gel route. J. Phys. Chem. C 2010, 114, 6228–6236. [Google Scholar] [CrossRef]
- Wang, Y.X.; Xu, M.; Sun, J. Preparation of TiO2 Nanopowders by Non-Hydrolytic Sol–Gel and Solvothermal Synthesis. Appl. Mech. Mater. 2012, 1934–1939. [Google Scholar] [CrossRef]
- An’Amt, M.; Radiman, S.; Huang, N.; Yarmo, M.; Ariyanto, N.; Lim, H.; Muhamad, M. Sol–gel hydrothermal synthesis of bismuth–TiO2 nanocubes for dye-sensitized solar cell. Ceram. Int. 2010, 36, 2215–2220. [Google Scholar] [CrossRef]
- Li, G.; Chen, L.; Dimitrijevic, N.M.; Gray, K.A. Visible light photocatalytic properties of anion-doped TiO2 materials prepared from a molecular titanium precursor. Chem. Phys. Lett. 2008, 451, 75–79. [Google Scholar] [CrossRef]
- Hosseinnia, A.; Keyanpour-Rad, M.; Kazemzad, M.; Pazouki, M. A novel approach for preparation of highly crystalline anatase TiO2 nanopowder from the agglomerates. Powder Technol. 2009, 190, 390–392. [Google Scholar] [CrossRef]
- Chen, G.; Luo, G.; Yang, X.; Sun, Y.; Wang, J. Anatase-TiO2 nano-particle preparation with a micro-mixing technique and its photocatalytic performance. Mater. Sci. Eng. A 2004, 380, 320–325. [Google Scholar] [CrossRef]
- Klein, S.M.; Choi, J.H.; Pine, D.J.; Lange, F.F. Synthesis of rutile titania powders: Agglomeration, dissolution, and reprecipitation phenomena. J. Mater. Res. 2003, 18, 1457–1464. [Google Scholar] [CrossRef] [Green Version]
- Aruna, S.; Tirosh, S.; Zaban, A. Nanosize rutile titania particle synthesis viaa hydrothermal method without mineralizers. J. Mater. Chem. 2000, 10, 2388–2391. [Google Scholar] [CrossRef]
- Zhou, W.; Cao, Q.; Tang, S. Effects on the size of nano-TiO2 powders prepared with sol–emulsion–gel method. Powder Technol. 2006, 168, 32–36. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, G.; Song, B.; Han, G. Solvent-controlled synthesis of three-dimensional TiO2 nanostructures via a one-step solvothermal route. CrystEngComm 2011, 13, 2294–2302. [Google Scholar] [CrossRef]
- Pottier, A.; Cassaignon, S.; Chanéac, C.; Villain, F.; Tronc, E.; Jolivet, J.-P. Size tailoring of TiO2 anatase nanoparticles in aqueous medium and synthesis of nanocomposites. Characterization by Raman spectroscopy. J. Mater. Chem. 2003, 13, 877–882. [Google Scholar] [CrossRef]
- Stefik, M.; Heiligtag, F.J.; Niederberger, M.; Gratzel, M. Improved nonaqueous synthesis of TiO2 for dye-sensitized solar cells. ACS Nano 2013, 7, 8981–8989. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Deng, Z.-X.; Li, Y. The synthesis of nanocrystalline anatase and rutile titania in mixed organic media. Inorg. Chem. 2001, 40, 5210–5214. [Google Scholar] [CrossRef]
- Niederberger, M. Nonaqueous sol–gel routes to metal oxide nanoparticles. Acc. Chem. Res. 2007, 40, 793–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinh, C.-T.; Nguyen, T.-D.; Kleitz, F.; Do, T.-O. Shape-controlled synthesis of highly crystalline titania nanocrystals. ACS Nano 2009, 3, 3737–3743. [Google Scholar] [CrossRef]
- Carlucci, C.; Xu, H.; Scremin, B.F.; Giannini, C.; Altamura, D.; Carlino, E.; Videtta, V.; Conciauro, F.; Gigli, G.; Ciccarella, G. Selective synthesis of TiO2 nanocrystals with morphology control with the microwave-solvothermal method. CrystEngComm 2014, 16, 1817–1824. [Google Scholar] [CrossRef]
- Galkina, O.; Vinogradov, V.; Vinogradov, A.; Agafonov, A. Development of the low-temperature sol-gel synthesis of TiO2 to provide self-cleaning effect on the textile materials. Nanotechnologies 2012, 7, 604–614. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Y.; Wang, A. Superhydrophobic kapok fiber oil-absorbent: Preparation and high oil absorbency. Chem. Eng. J. 2012, 213, 1–7. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, C.; Wang, S.; Li, J. Fabrication of superhydrophobic cotton textiles for water-oil separation based on drop-coating route. Carbohydr. Polym. 2013, 97, 59–64. [Google Scholar] [CrossRef]
- Abd El-Hady, M.; Sharaf, S.; Farouk, A. Highly hydrophobic and UV protective properties of cotton fabric using layer by layer self-assembly technique. Cellulose 2020, 27, 1099–1110. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Z.; Xu, X.; Guo, F.; Zhu, X.; Men, X.; Ge, B. Robust and durable superhydrophobic cotton fabrics for oil/water separation. ACS Appl. Mater. Interfaces 2013, 5, 7208–7214. [Google Scholar] [CrossRef]
- Wu, J.; Wang, N.; Wang, L.; Dong, H.; Zhao, Y.; Jiang, L. Electrospun porous structure fibrous film with high oil adsorption capacity. ACS Appl. Mater. Interfaces 2012, 4, 3207–3212. [Google Scholar] [CrossRef]
- Cho, E.-C.; Chang-Jian, C.-W.; Chen, H.-C.; Chuang, K.-S.; Zheng, J.-H.; Hsiao, Y.-S.; Lee, K.-C.; Huang, J.-H. Robust multifunctional superhydrophobic coatings with enhanced water/oil separation, self-cleaning, anti-corrosion, and anti-biological adhesion. Chem. Eng. J. 2017, 314, 347–357. [Google Scholar] [CrossRef]
- Cho, E.-C.; Chang-Jian, C.-W.; Hsiao, Y.-S.; Lee, K.-C.; Huang, J.-H. Interfacial engineering of melamine sponges using hydrophobic TiO2 nanoparticles for effective oil/water separation. J. Taiwan Inst. Chem. Eng. 2016, 67, 476–483. [Google Scholar] [CrossRef]
- Jiang, C.; Liu, W.; Yang, M.; Liu, C.; He, S.; Xie, Y.; Wang, Z. Facile fabrication of robust fluorine-free self-cleaning cotton textiles with superhydrophobicity, photocatalytic activity, and UV durability. Colloids Surf. A Physicochem. Eng. Asp. 2018, 559, 235–242. [Google Scholar] [CrossRef]
- Khan, M.Z.; Baheti, V.; Militky, J.; Wiener, J.; Ali, A. Self-cleaning properties of polyester fabrics coated with flower-like TiO2 particles and trimethoxy (octadecyl) silane. J. Ind. Text. 2020, 50, 543–565. [Google Scholar] [CrossRef]
- Huang, J.; Li, S.; Ge, M.; Wang, L.; Xing, T.; Chen, G.; Liu, X.; Al-Deyab, S.S.; Zhang, K.; Chen, T. Robust superhydrophobic TiO2@ fabrics for UV shielding, self-cleaning and oil–water separation. J. Mater. Chem. A 2015, 3, 2825–2832. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Lu, Z.; Chen, L.; Huang, L.; Fan, M. A robust superhydrophobic TiO2 NPs coated cellulose sponge for highly efficient oil-water separation. Sci. Rep. 2017, 7, 9428. [Google Scholar] [CrossRef] [PubMed]
- Ponyrko, S.; Kobera, L.; Brus, J.; Matějka, L. Epoxy-silica hybrids by nonaqueous sol-gel process. Polymer 2013, 54, 6271–6282. [Google Scholar] [CrossRef]
- Keshk, S.M.A.S.; Hamdy, M.S. Preparation and physicochemical characterization of zinc oxide/sodium cellulose composite for food packaging. Turk. J. Chem. 2019, 43, 94–105. [Google Scholar] [CrossRef]
- Ju, X.; Bowden, M.; Brown, E.E.; Zhang, X. An improved X-ray diffraction method for cellulose crystallinity measurement. Carbohydr. Polym. 2015, 123, 476–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivasu, P.; Singh, S.P.; Islam, A.; Han, L. Novel Approach for the Synthesis of Nanocrystalline Anatase Titania and Their Photovoltaic Application. Adv. OptoElectron. 2011, 2011, 539382. [Google Scholar] [CrossRef] [Green Version]
- Acevedo Cortez, J.S.; Kharisov, B.I.; Serrano, T.; González, L.T.; Kharissova, O.V. Hydrophobization and evaluation of absorption capacity of Aloe vera, Opuntia ficus-indica and Gelidium for oil spill cleanup. J. Dispers. Sci. Technol. 2019, 40, 884–891. [Google Scholar] [CrossRef]
- Manoharan, C.; Rajendran, V.; Sivaraj, R. Synthesis, characterization and applications of ZnO/TiO2/SiO2 nanocomposite. Orient. J. Chem. 2018, 34, 1333. [Google Scholar] [CrossRef]


| Sample | GPTMS (%) | ODS (%) |
|---|---|---|
| Control | 0 | 2 |
| S1 | 6 | 2 |
| S2 | 2 | 4 |
| S3 | 4 | 4 |
| S4 | 4 | 2 |
| S5 | 6 | 4 |
| S6 | 2 | 2 |
| MIU | MMD | SMD | |
|---|---|---|---|
| Pristine fabric | 0.182 | 0.0137 | 3.85 |
| Coated fabric | 0.374 | 0.02 | 5.206 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safdar, F.; Javid, A.; Ashraf, M. Single Step Synthesis and Functionalization of Nano Titania for Development of Multifunctional Cotton Fabrics. Materials 2022, 15, 38. https://doi.org/10.3390/ma15010038
Safdar F, Javid A, Ashraf M. Single Step Synthesis and Functionalization of Nano Titania for Development of Multifunctional Cotton Fabrics. Materials. 2022; 15(1):38. https://doi.org/10.3390/ma15010038
Chicago/Turabian StyleSafdar, Faiza, Amjed Javid, and Munir Ashraf. 2022. "Single Step Synthesis and Functionalization of Nano Titania for Development of Multifunctional Cotton Fabrics" Materials 15, no. 1: 38. https://doi.org/10.3390/ma15010038
APA StyleSafdar, F., Javid, A., & Ashraf, M. (2022). Single Step Synthesis and Functionalization of Nano Titania for Development of Multifunctional Cotton Fabrics. Materials, 15(1), 38. https://doi.org/10.3390/ma15010038