Relationship between the Crystal Structure and Tuberculostatic Activity of Some 2-Amidinothiosemicarbazone Derivatives of Pyridine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.1.1. 4-Phenylthiopicolinonitrile
2.1.2. 4-Phenylthiopicolinohydrazonamide
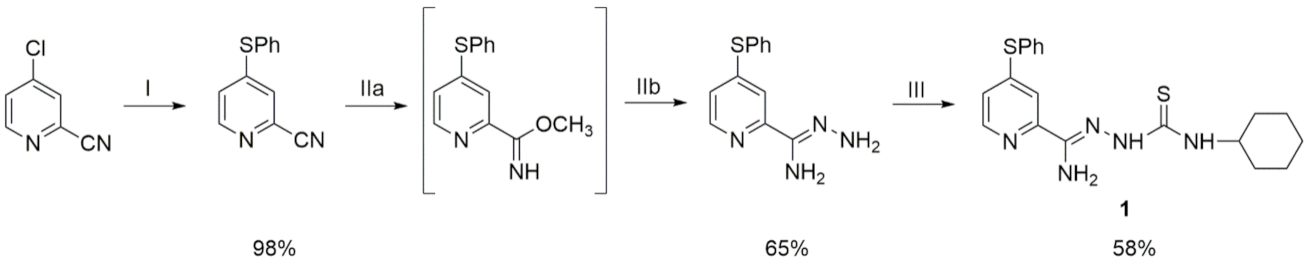
2.1.3. 2-[Amino-(4-phenylthiopyridin-2-yl)methylene]-N-cyclohexylhydrazinecarbothioamide (1)
2.2. Tuberculostatic Activity
2.3. X-ray
2.4. Solution NMR
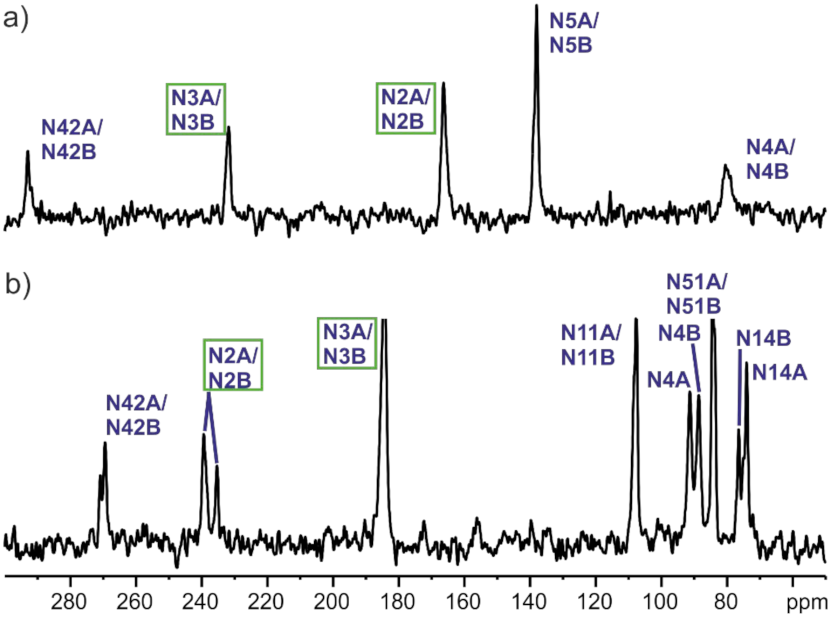
2.5. Solid-State NMR
2.6. QM Calculations
3. Results and Discussion
3.1. Synthesis
3.2. Tuberculostatic Activity
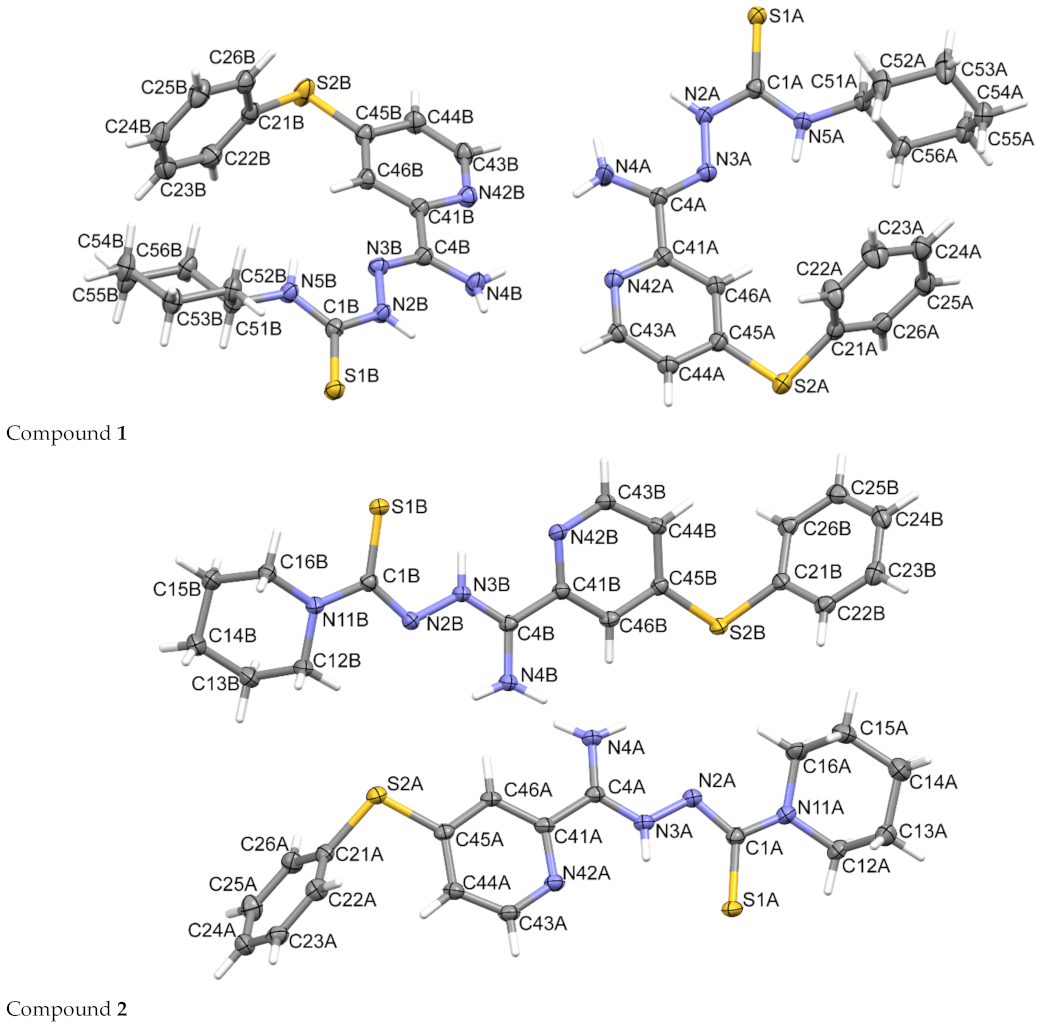
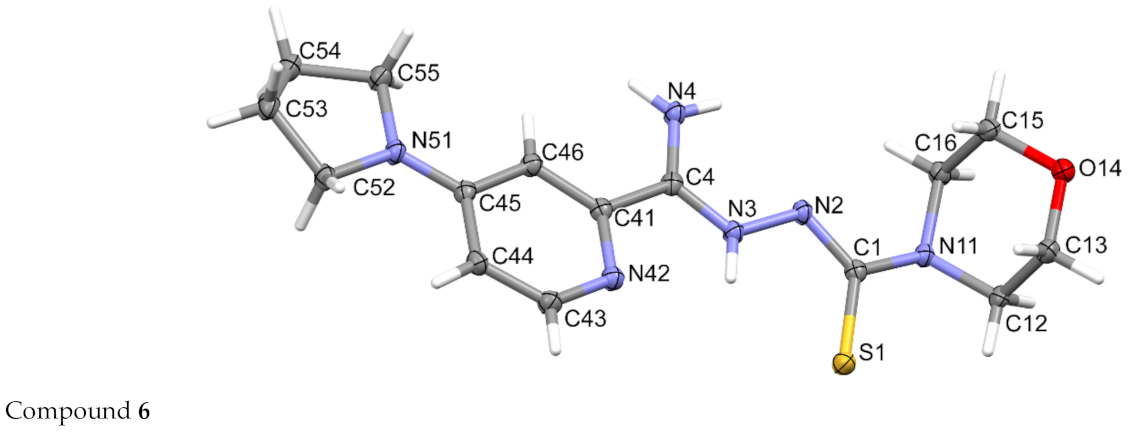
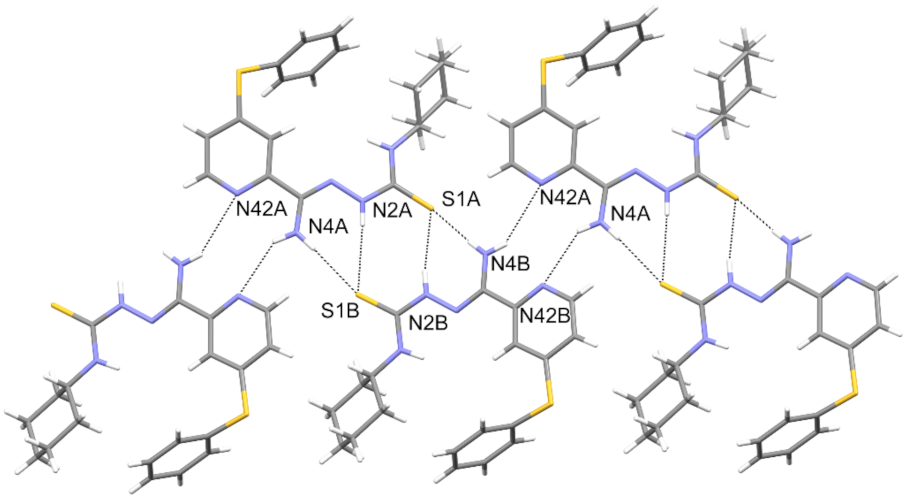
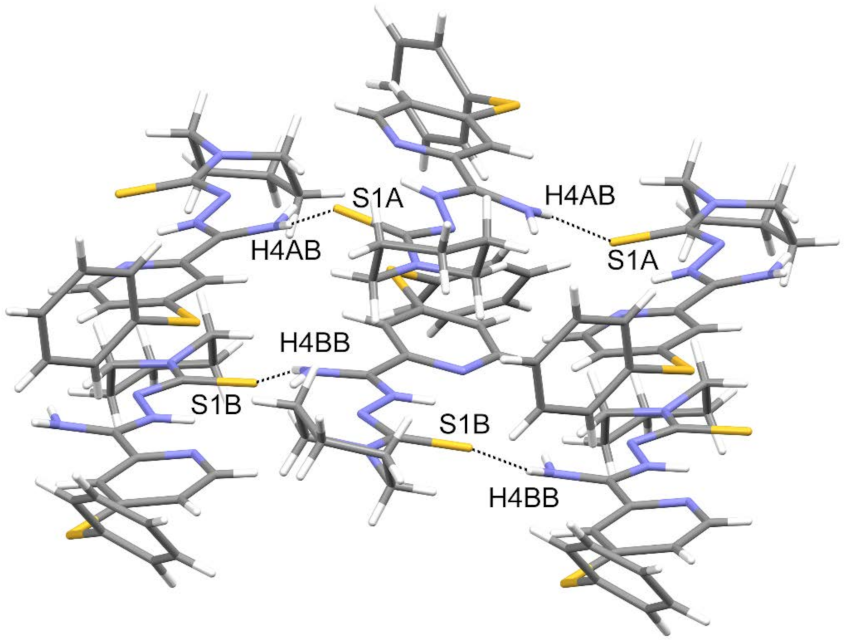
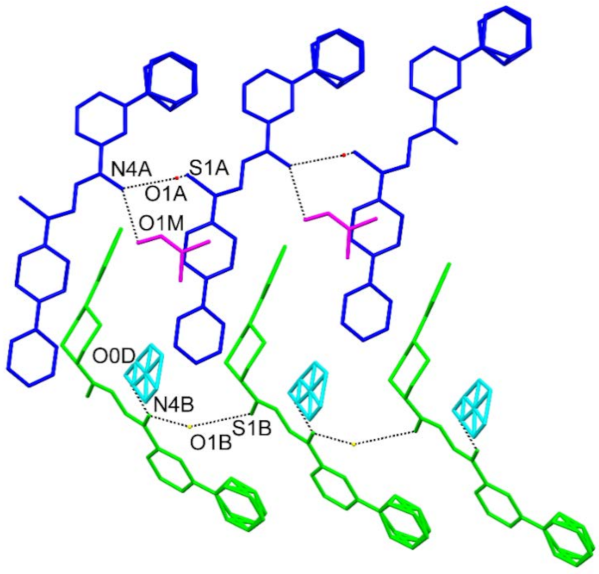
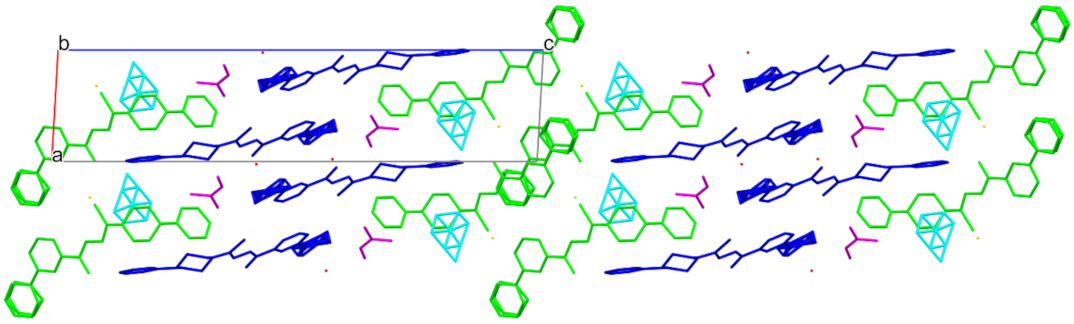
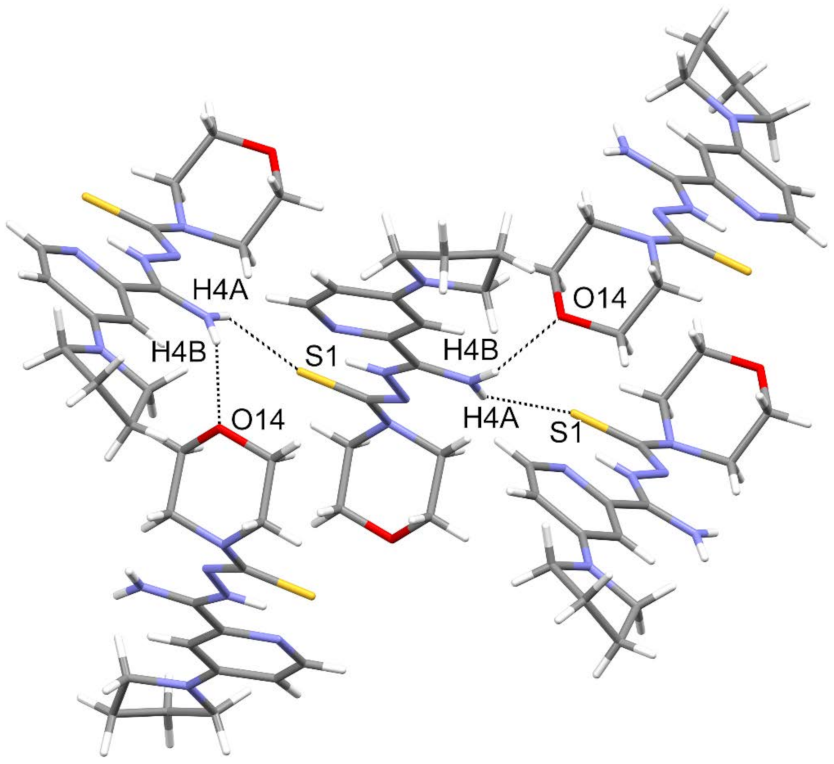
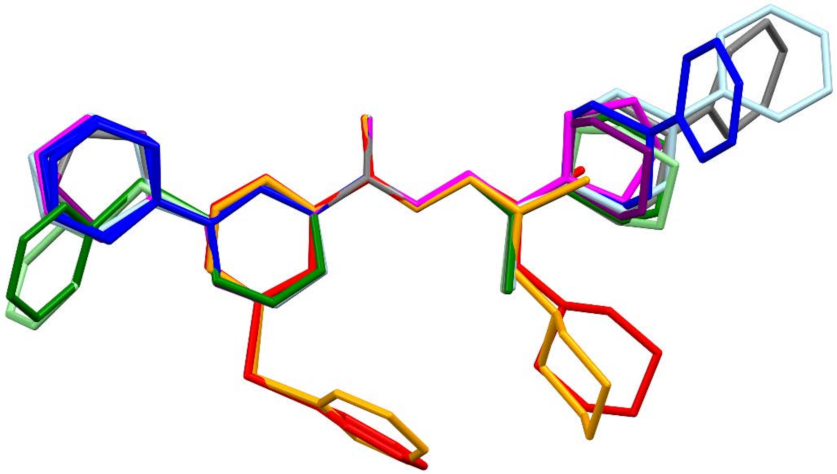
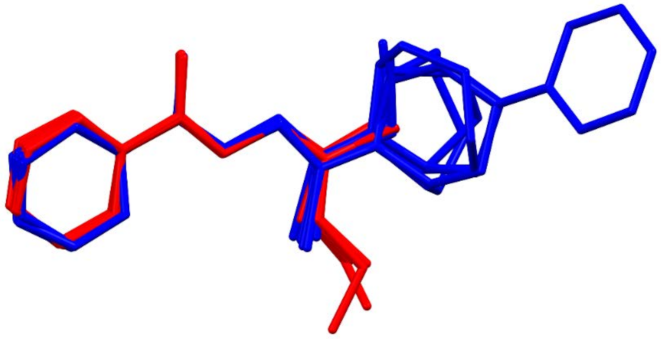
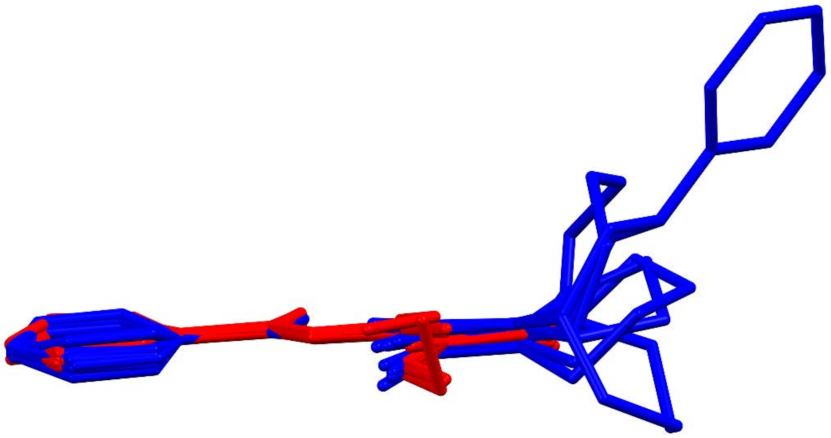
3.3. Crystallography
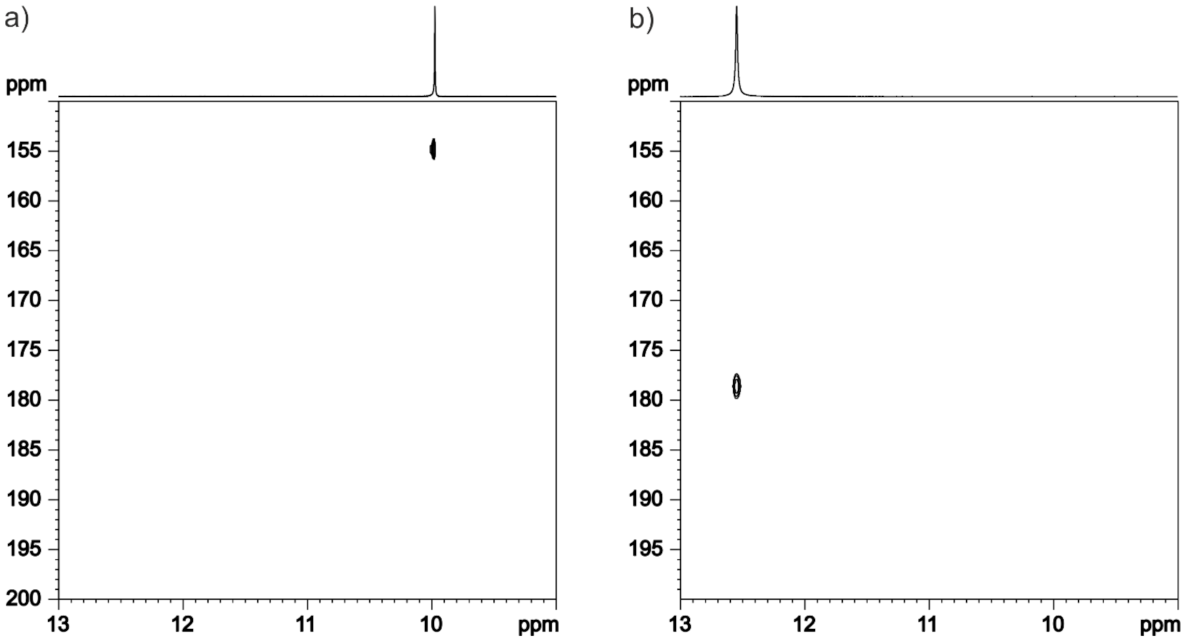
3.4. NMR
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fair, R.J.; Tor, Y. Antibiotics and Bacterial Resistance in the 21st Century. Perspect. Med. Chem. 2014, 6, 25–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koul, A.; Arnoult, E.; Lounis, N.; Guillemont, J.; Andries, K. The challenge of new drug discovery for tuberculosis. Nature 2011, 469, 483–490. [Google Scholar] [CrossRef]
- Global Tuberculosis Report. Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2021 (accessed on 14 October 2021).
- Bhat, Z.S.; Rather, M.A.; Maqbool, M.; Ahmad, Z. Drug targets exploited in Mycobacterium tuberculosis: Pitfalls and promises on the horizon. Biomed. Pharmacother. 2018, 103, 1733–1747. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Matsumoto, M.; Ishida, H.; Ohguro, K.; Yoshitake, M.; Gupta, R.; Geiter, L.; Hafkin, J. Delamanid: From discovery to its use for pulmonary multidrug-resistant tuberculosis (MDR-TB). Tuberculosis 2018, 111, 20–30. [Google Scholar] [CrossRef]
- Rather, M.A.; Maqbool, M. Cell wall: A versatile fountain of drug targets in Mycobacterium tuberculosis. Biomed. Pharmacother. 2017, 95, 1520–1534. [Google Scholar]
- Szczesio, M.; Gołka, J.; Korona-Głowniak, I.; Orlewska, C.; Gobis, K.; Olczak, A. Planarity of heteroaryldithiocarbazic acid derivatives showing tuberculostatic activity: Structure–activity relationships. Acta Cryst. 2018, C74, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.; Foks, H.; Augustynowicz-Kopeć, E.; Napiórkowska, A.; Szczesio, M.; Gobis, K. Synthesis and Tuberculostatic Activity Evaluation of Novel Benzazoles with Alkyl, Cycloalkyl or Pyridine Moiety. Molecules 2018, 23, 985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szczesio, M.; Korona-Głowniak, I.; Gobis, K. Planarity of benzoyldithiocarbazate tuberculostatics. V antibacterial activities of diesters of benzoyldithiocarbazic acid. J. Mol. Struct. 2018, 1167, 127–133. [Google Scholar] [CrossRef]
- Olczak, A.; Szczesio, M.; Gołka, J.; Orlewska, C.; Gobis, K.; Foks, H.; Główka, M.L. Planarity of heteroaryldithiocarbazic acid derivatives showing tuberculostatic activity. II Crystal structures of 3-[amino(pyrazin-2-yl)methylidene]-2-methylcarbazic acid esters. Acta Cryst. 2011, C67, o37–o42. [Google Scholar] [CrossRef] [PubMed]
- Olczak, A.; Główka, M.L.; Gołka, J.; Szczesio, M.; Bojarska, J.; Kozłowska, K.; Foks, H.; Orlewska, C. Is planarity of pyridin-2-yl- and pyrazin-2-yl-formamide thiosemicarbazones related to their tuberculostatic activity? X-ray structures of two pyrazine-2-carboxamide-N’-carbonothioyl- hydrazones. J. Mol. Struct. 2007, 830, 171–175. [Google Scholar] [CrossRef]
- Główka, M.L.; Martynowski, D.; Olczak, A.; Orlewska, C.; Foks, H.; Bojarska, J.; Szczesio, M.; Gołka, J. Planarity of N’-(amino-2-pyridylmethylene)-hydrazide carbodithioic acid frame and crystal structure of its methyl ester dihydrate. J. Chem. Crystallogr. 2005, 35, 477–480. [Google Scholar] [CrossRef]
- Szczesio, M.; Gobis, K.; Korona-Głowniak, I.; Ziembicka, D.; Olczak, A. Structure and antibacterial activity of selected hydrazide derivatives. Z. Kristall. Suppl. 2020, 40, abs. P60, 85. [Google Scholar]
- Krause, M.; Foks, H.; Ziembicka, D.; Augustynowicz-Kopeć, E.; Głogowska, A.; Korona-Głowniak, I.; Bojanowski, K.; Siluk, D.; Gobis, K. 4-Substituted picolinohydrazonamides as a new class of potential antitubercular agents. Eur. J. Med. Chem. 2020, 190, 112106. [Google Scholar] [CrossRef] [PubMed]
- Youmans, G.P.; Youmans, A.S. A method for the determination of the rate of growth of tubercle bacilli by the use of small inocula. J. Bacteriol. 1949, 58, 247–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youmans, G.P. Test tube evaluation of tuberculostatic agents. Am. Rev. Tuberc. 1947, 56, 376. [Google Scholar]
- Atlas, R.M.; Snyder, J.W. Handbook of Media for Clinical Microbiology, 2nd ed.; CRC Press: Boca Raton, MA, USA, 2006. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Cryst. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westrip, S.P. publCIF: Software for editing, validating and formatting crystallographic information files. J. Appl. Cryst. 2010, 43, 920–925. [Google Scholar] [CrossRef] [Green Version]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Bodenhausen, G.; Ruben, D.J. Natural abundance nitrogen-15 NMR by enhanced heteronuclear spectroscopy. Chem. Phys. Lett. 1980, 69, 185–189. [Google Scholar] [CrossRef] [Green Version]
- Fung, B.M.; Khitrin, A.K.; Ermolaev, K. An Improved Broadband Decoupling Sequence for Liquid Crystals and Solids. J. Magn. Reson. 2000, 142, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Topspin; Version 3.6; Bruker Biospin Gmbh: Karlsruhe, Germany, 2020.
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.J.; Refson, K.; Payne, M.C. First Principles Methods Using CASTEP. Z. Krist. 2005, 220, 567–570. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, K.M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- McNellis, E.R.; Meyer, J.; Reuter, K. Azobenzene at Coinage Metal Surfaces: Role of Dispersive van Der Waals Interactions. Phys. Rev. B 2009, 80, 205414. [Google Scholar] [CrossRef] [Green Version]
- Tkatchenko, A.; Scheffler, M. Accurate Molecular Van Der Waals Interactions from Ground-State Electron Density and Free-Atom Reference Data. Phys. Rev. Lett. 2009, 102, 073005. [Google Scholar] [CrossRef] [Green Version]
- Vanderbilt, D. Soft Self-Consistent Pseudopotentials in a Generalized Eigenvalue Formalism. Phys. Rev. B 1990, 41, 7892–7895. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. On Special Points for Brillouin Zone Integrations. Phys. Rev. B 1976, 13, 5188–5912. [Google Scholar] [CrossRef]
- Pickard, C.J.; Mauri, F. All-Electron Magnetic Response with Pseudopotentials: NMR Chemical Shifts. Phys. Rev. B 2001, 63, 245101. [Google Scholar] [CrossRef] [Green Version]
- Yates, J.R.; Pickard, C.J.; Mauri, F. Calculation of NMR Chemical Shifts for Extended Systems Using Ultrasoft Pseudopotentials. Phys. Rev. B 2007, 76, 024401. [Google Scholar] [CrossRef]
- Nocedal, J.; Wright, S.J. Numerical Optimization, 2nd ed.; Springer: Berlin, Germany; New York, NY, USA, 2006. [Google Scholar]
- Brown, K.K.; Hampton, M.B. Bological targets of isothiocyanates. Biochim. Biophys. Acta 2011, 1810, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, B72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Bruno, I.J.; Cole, J.C.; Edgington, P.R.; Kessler, M.; Macrae, C.F.; McCabe, P.; Pearson, J.; Taylor, R. New software for searching the Cambridge Structural Database and visualising crystal structures. Acta Cryst. 2002, B58, 389–397. [Google Scholar] [CrossRef]
- Almeida, J.C.L.; Amin, R.S.; Pessoa, C.; Laurenco, M.C.S.; Mendes, L.C.; Lessa, J.A. Bismuth(III) complexes with pyrazineformamide thiosemicarbazones: Investigation on the antimicrobial and cytotoxic effects. Polyhedron 2020, 189, 114709–114715. [Google Scholar] [CrossRef]
- Bermejo, E.; Castineiras, A.; Garcıa-Santos, I.; West, D.X. Structural and Coordinative Variability in Zinc(II), Cadmium(II), and Mercury(II) Complexes of 2-Pyridineformamide 3-Hexamethyleneiminylthiosemicarbazone. Z. Anorg. Allg. Chem. 2004, 630, 1096–1109. [Google Scholar] [CrossRef]
- Orlewska, C.; Foks, H.; Sowinski, P.; Martynowski, D.; Olczak, A.; Glowka, M.L. Synthesis, structure and tuberculostatic activity of N’-(amino-pyridal-methylene)-hydrazinecarbodithioic acid methyl esters. Polish J. Chem. 2001, 75, 1237–1245. [Google Scholar]
- Ketcham, K.A.; Swearingen, J.K.; Castineiras, A.; Garcia, I.; Bermejo, E.; West, D.X. Iron(III), cobalt(II,III), copper(II) and zinc(II) complexes of 2-pyridineformamide 3-piperidylthiosemicarbazone. Polyhedron 2001, 20, 3265–3273. [Google Scholar] [CrossRef]
- Bermejo, E.; Castiñeiras, A.; Fostiak, L.M.; García, I.; Llamas-Saiz, A.L.; Swearingen, J.K.; West, D.X. Synthesis, Characterization and Molecular Structure of 2-Pyridyl-formamide N(4)-Dimethylthiosemicarbazone and Some Five-Coordinated Zinc(II) and Cadmium(II) Complexes. Z. Naturforsch. 2001, 56, 1297–1305. [Google Scholar] [CrossRef]
- Bermejo, E.; Castineiras, A.; Garcia-Santos, I.; Fostiak, L.M.; Swearingen, J.K.; West, D.X. Spectral and Structural Studies of Transition Metal Complexes of 2-Pyridineformamide N(4)-ethylthiosemicarbazone. Z. Anorg. Allg. Chem. 2005, 631, 728–738. [Google Scholar] [CrossRef]
- Castineiras, A.; Garcia-Santos, I.; Nogueiras, S.; Rodriguez-Gonzalez, I.; Rodriguez-Riobo, R. Supramolecular interactions in biologically relevant compounds. 2-Pyrazineformamide thiosemicarbazones and some products of their cyclization. J. Mol. Struct. 2014, 1074, 1–18. [Google Scholar] [CrossRef]
- Amim, R.S.; Firmino, G.S.S.; Rego, A.C.P.D.; Nery, A.L.; Da-Silva, S.A.G.; de Souza, M.V.N.; Pessoa, C.; Resende, J.A.L.C.; Lessa, J.D.F.-V.J.A. Cytotoxicity and Leishmanicidal Activity of Isoniazid-Derived Hydrazones and 2-Pyrazineformamide Thiosemicarbazones. J. Braz. Chem. Soc. 2016, 27, 769–777. [Google Scholar] [CrossRef]
- Castineiras, A.; Gacia, I.; Bermejo, E.; West, D.X. Structural and Spectral Studies of 2-Pyridineformamide Thiosemicarbazone and its Complexes Prepared with Zinc Halides. Z. Naturforsch. B Chem. Sci. 2000, 55, 511. [Google Scholar] [CrossRef]
- Francuski, B.M.; Novakovic, S.B.; Francuski, D.D.; Bogdanovic, G.A. Charge Density Analysis of 2-Pyridineformamide N(4)-Methylthiosemicarbazone (Z′ = 4): Role of an Enhanced N–H···S Thioureido Dimer. Cryst. Growth Des. 2017, 17, 2993–3004. [Google Scholar] [CrossRef]
- Favre-Nicolin, V.; Černý, R. FOX, ‘free Objects for Crystallography’: A Modular Approach to Ab Initio Structure Determination from Powder Diffraction. J. Appl. Cryst. 2002, 35, 734–743. [Google Scholar] [CrossRef] [Green Version]
- Toby, B.H.; Von Dreele, R.B. GSAS-II: The Genesis of a Modern Open-Source All Purpose Crystallography Software Package. J. Appl. Cryst. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Bruno, I.J.; Cole, J.C.; Kessler, M.; Luo, J.; Motherwell, W.D.S.; Purkis, L.H.; Smith, B.R.; Taylor, R.; Cooper, R.I.; Harris, S.E.; et al. Retrieval of Crystallographically-Derived Molecular Geometry Information. J. Chem. Inf. Comput. Sci. 2004, 44, 2133–2144. [Google Scholar] [CrossRef]

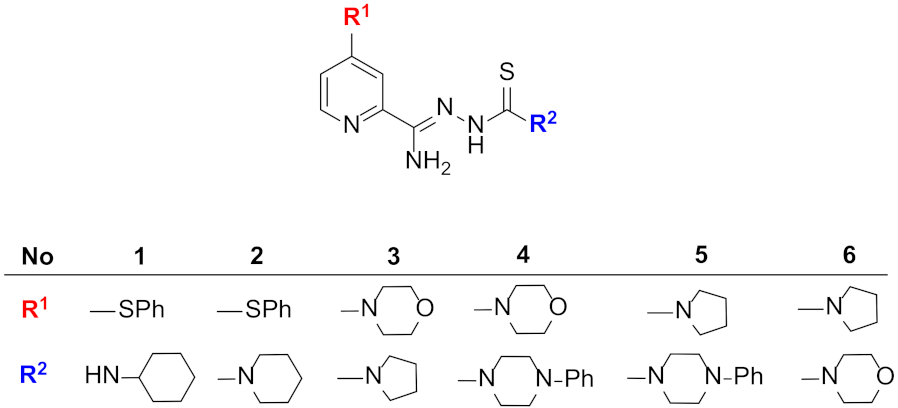



| Compound | MIC [µg/mL] * | |
|---|---|---|
| H37Rv | Spec.210 | |
| 1 | 25 | 50 |
| 2 | 12.5 | 12.5 |
| 3 | 6.25 | 6.25 |
| 4 | 6.26 | 6.25 |
| 5 | 6.25 | 6.25 |
| 6 | 0.4 | 0.4 |
| INH | 3.1 | 12.5 |
| PZA | 25 | >400 |
| Compound | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Chemical formula | C19H23N5S2 | C18H21N5S2 | C15H22N6OS | C21H27N7OSC3H7NO·H2O | C21H27N7S | C15H22N6OS |
| Mr | 385.54 | 371.52 | 334.45 | 516.67 | 409.56 | 334.45 |
| Crystal system, space group | Triclinic, P-1 | Monoclinic, P21/n | Monoclinic, P21/n | Triclinic, P-1 | Triclinic, P-1 | Monoclinic, P21/c |
| Temperature (K) | 100 | 100 | 110 | 120 | 100 | 100 |
| a, b, c (Å) | 9.9607 (1), 11.4800 (2), 17.9470 (2) | 9.63579 (7), 11.57077 (6), 32.5353 (2) | 9.6759 (2), 15.3925 (4), 11.4019 (3) | 9.1277 (5), 9.2367 (5), 35.3349 (19) | 6.5854 (3), 7.8371 (3), 19.5806 (6) | 9.7946 (1), 12.8549 (2), 12.6723 (2) |
| α, β, γ (°) | 79.552 (1), 75.589 (1), 85.158 (1) | 90, 97.4227 (7), 90 | 90, 97.977 (1), 90 | 90.778 (2), 92.528 (2), 116.791 (3) | 95.660 (3), 95.484 (3), 98.811 (3) | 90, 91.200 (1), 90 |
| V (Å3) | 1953.03 (5) | 3597.08 (4) | 1681.73 (7) | 2654.8 (3) | 987.46 (7) | 1595.20 (4) |
| Z (Z’) | 4 (2) | 8 (2) | 4 (1) | 4 (2) | 2 (1) | 4 (1) |
| μ (mm−1) | 2.57 | 2.77 | 1.83 | 1.42 | 1.64 | 1.93 |
| Crystal size (mm) | 0.81 × 0.42 × 0.20 | 0.81 × 0.38 × 0.07 | 0.85 × 0.40 × 0.25 | 1.06 × 0.50 × 0.21 | 0.17 × 0.05 × 0.03 | 0.55 × 0.4 × 0.3 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 39,531, 8158, 7475 | 174,670, 7668, 7186 | 17,901, 3252, 3216 | 42,785, 10,708, 9883 | 9850, 3829, 3243 | 16,851, 3188, 3010 |
| Rint | 0.054 | 0.082 | 0.022 | 0.037 | 0.041 | 0.030 |
| (sin θ/λ)max (Å−1) | 0.637 | 0.637 | 0.618 | 0.626 | 0.635 | 0.637 |
| R[F2 > 2σ(F2)], wR(F2), S | 0.040, 0.110, 1.10 | 0.055, 0.153, 1.19 | 0.031, 0.082, 1.05 | 0.065, 0.155, 1.25 | 0.040, 0.108, 1.06 | 0.030, 0.080, 1.05 |
| No. of parameters | 494 | 471 | 266 | 772 | 274 | 220 |
| Δmax, Δmin (e Å−3) | 0.39, −0.36 | 0.77, −0.46 | 0.21, −0.32 | 0.54, −0.33 | 0.41, −0.33 | 0.24, −0.25 |
| D—H···A | D—H | H···A | D···A | D—H···A |
|---|---|---|---|---|
| N4A—H4AA···S1B i | 0.85 (3) | 2.47 (3) | 3.2938 (16) | 166 (3) |
| N4A—H4AB···N42A | 0.86 (3) | 2.30 (3) | 2.676 (2) | 107 (2) |
| N4A—H4AB···N42B | 0.86 (3) | 2.53 (3) | 3.256 (2) | 142 (3) |
| N2A—H2A···S1B i | 0.85 (2) | 2.65 (2) | 3.4953 (14) | 176.1 (18) |
| N2B—H2B···S1A ii | 0.84 (2) | 2.67 (2) | 3.4657 (14) | 158.1 (18) |
| N4B—H4BA···N42B | 0.83 (2) | 2.26 (2) | 2.648 (2) | 108.8 (19) |
| N4B—H4BB···S1A ii | 0.86 (2) | 2.43 (2) | 3.2872 (16) | 173 (2) |
| D—H···A | D—H | H···A | D···A | D—H···A |
|---|---|---|---|---|
| N4B—H4BA···N2B | 0.84 (5) | 2.30 (5) | 2.702 (3) | 109 (4) |
| N4B—H4BB···S1 B i | 0.90 (5) | 2.53 (5) | 3.423 (3) | 171 (4) |
| N4A—H4AA···N2A | 0.85 (4) | 2.31 (4) | 2.691 (4) | 108 (4) |
| N3A—H3A···S1A | 0.84 (5) | 2.35 (4) | 2.827 (2) | 116 (3) |
| N3A—H3A···N42A | 0.84 (5) | 2.27 (4) | 2.636 (3) | 106 (3) |
| N3B—H3B···S1B | 0.90 (5) | 2.30 (4) | 2.839 (2) | 119 (3) |
| N3B—H3B···N42B | 0.90 (5) | 2.32 (4) | 2.660 (3) | 103 (3) |
| N4A—H4AB···S1A ii | 0.87 (4) | 2.49 (4) | 3.348 (3) | 170 (4) |
| D—H···A | D—H | H···A | D···A | D—H···A |
|---|---|---|---|---|
| N4—HbB···S1 i | 0.911 (18) | 2.350 (18) | 3.2508 (11) | 169.7 (15) |
| N4—H4A···N2 | 0.876 (17) | 2.213 (17) | 2.6222 (15) | 108.2 (12) |
| D—H···A | D—H | H···A | D···A | D—H···A |
|---|---|---|---|---|
| N4B—H4BA···O0D | 0.88 | 2.20 | 3.003 (4) | 152 |
| O1A—H1A···O54X | 0.82 (5) | 2.04 (6) | 2.85 (2) | 170 (5) |
| O1A—H1A···O54A | 0.82 (5) | 2.02 (6) | 2.84 (3) | 178 (7) |
| O1B—H1B···S1B i | 0.92 (5) | 2.34 (5) | 3.247 (3) | 171 (4) |
| N4B—H4BB···O1B | 0.88 | 1.99 | 2.827 (4) | 159 |
| O1A—H2A···S1A ii | 0.94 (6) | 2.30 (6) | 3.232 (4) | 172 (4) |
| O1B—H2B···O54Q iii | 0.84 (6) | 2.21 (6) | 3.025 (19) | 164 (5) |
| O1B—H2B···O54B iii | 0.84 (6) | 1.90 (6) | 2.70 (3) | 161 (5) |
| N4A—H4AA···O1M | 0.88 | 2.17 | 2.969 (4) | 150 |
| N4A—H4AB···O1A iv | 0.88 | 1.99 | 2.831 (4) | 160 |
| D—H···A | D—H | H···A | D···A | D—H···A |
|---|---|---|---|---|
| N3—H3···S1 | 0.94 (3) | 2.29 (2) | 2.8351 (14) | 116.5 (18) |
| N4—H4B···S1 i | 0.87 (3) | 2.70 (3) | 3.4792 (16) | 150 (2) |
| D—H···A | D—H | H···A | D···A | D—H···A |
|---|---|---|---|---|
| N3—H3···S1 | 0.871 (18) | 2.395 (17) | 2.8596 (10) | 113.8 (13) |
| N3—H3···N42 | 0.871 (18) | 2.237 (17) | 2.6219 (14) | 106.6 (13) |
| N4—H4A···N2 | 0.828 (19) | 2.332 (18) | 2.6827 (15) | 106.1 (14) |
| N4—H4A···S1 i | 0.828 (19) | 2.679 (19) | 3.3937 (11) | 145.5 (16) |
| N4—H4B···O14 ii | 0.855 (19) | 2.176 (18) | 2.9458 (14) | 149.8 (17) |
| Structure (Position) | δExp | δGIPAW (Hydrogen on N2) | δGIPAW (Hydrogen on N3) |
|---|---|---|---|
| N2A | 166.3 | 173.1 | 256.6 |
| N2B | 166.3 | 177.9 | 247.4 |
| N3A | 231.8 | 230.4 | 176.1 |
| N3B | 231.8 | 230.2 | 165.1 |
| N4A | 80.0 | 78.8 | 86.8 |
| N4B | 80.0 | 79.6 | 95.0 |
| N5A | 138.2 | 136.2 | 114.3 |
| N5B | 138.2 | 135.1 | 119.0 |
| N42A | 292.9 | 288.2 | 276.2 |
| N42B | 292.9 | 282.7 | 286.0 |
| Structure (Position) | δExp | δGIPAW (Hydrogen on N2) | δGIPAW (Hydrogen on N3) |
|---|---|---|---|
| N2A | 235.3 | 174.4 | 237.9 |
| N2B | 239.33 | 171.9 | 237.6 |
| N3A | 184.3 | 248.2 | 183.6 |
| N3B | 184.3 | 246.0 | 181.5 |
| N4A | 88.6 | 78.3 | 92.2 |
| N4B | 91.3 | 81.5 | 94.1 |
| N11A | 107.7 | 111.8 | 115.0 |
| N11B | 107.7 | 115.0 | 110.8 |
| N14A | 74.9 | 73.4 | 78.9 |
| N14B | 76.5 | 76.3 | 76.0 |
| N42A | 269.4 | 282.9 | 262.2 |
| N42B | 270.8 | 281.6 | 263.6 |
| N51A | 84.5 | 76.6 | 82.4 |
| N51B | 84.5 | 77.9 | 82.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gobis, K.; Szczesio, M.; Olczak, A.; Pawlak, T.; Augustynowicz-Kopeć, E.; Krause, M.; Główka, M.L. Relationship between the Crystal Structure and Tuberculostatic Activity of Some 2-Amidinothiosemicarbazone Derivatives of Pyridine. Materials 2022, 15, 349. https://doi.org/10.3390/ma15010349
Gobis K, Szczesio M, Olczak A, Pawlak T, Augustynowicz-Kopeć E, Krause M, Główka ML. Relationship between the Crystal Structure and Tuberculostatic Activity of Some 2-Amidinothiosemicarbazone Derivatives of Pyridine. Materials. 2022; 15(1):349. https://doi.org/10.3390/ma15010349
Chicago/Turabian StyleGobis, Katarzyna, Małgorzata Szczesio, Andrzej Olczak, Tomasz Pawlak, Ewa Augustynowicz-Kopeć, Malwina Krause, and Marek L. Główka. 2022. "Relationship between the Crystal Structure and Tuberculostatic Activity of Some 2-Amidinothiosemicarbazone Derivatives of Pyridine" Materials 15, no. 1: 349. https://doi.org/10.3390/ma15010349
APA StyleGobis, K., Szczesio, M., Olczak, A., Pawlak, T., Augustynowicz-Kopeć, E., Krause, M., & Główka, M. L. (2022). Relationship between the Crystal Structure and Tuberculostatic Activity of Some 2-Amidinothiosemicarbazone Derivatives of Pyridine. Materials, 15(1), 349. https://doi.org/10.3390/ma15010349








