Green Production of Zero-Valent Iron (ZVI) Using Tea-Leaf Extracts for Fenton Degradation of Mixed Rhodamine B and Methyl Orange Dyes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Tea Extract Preparation
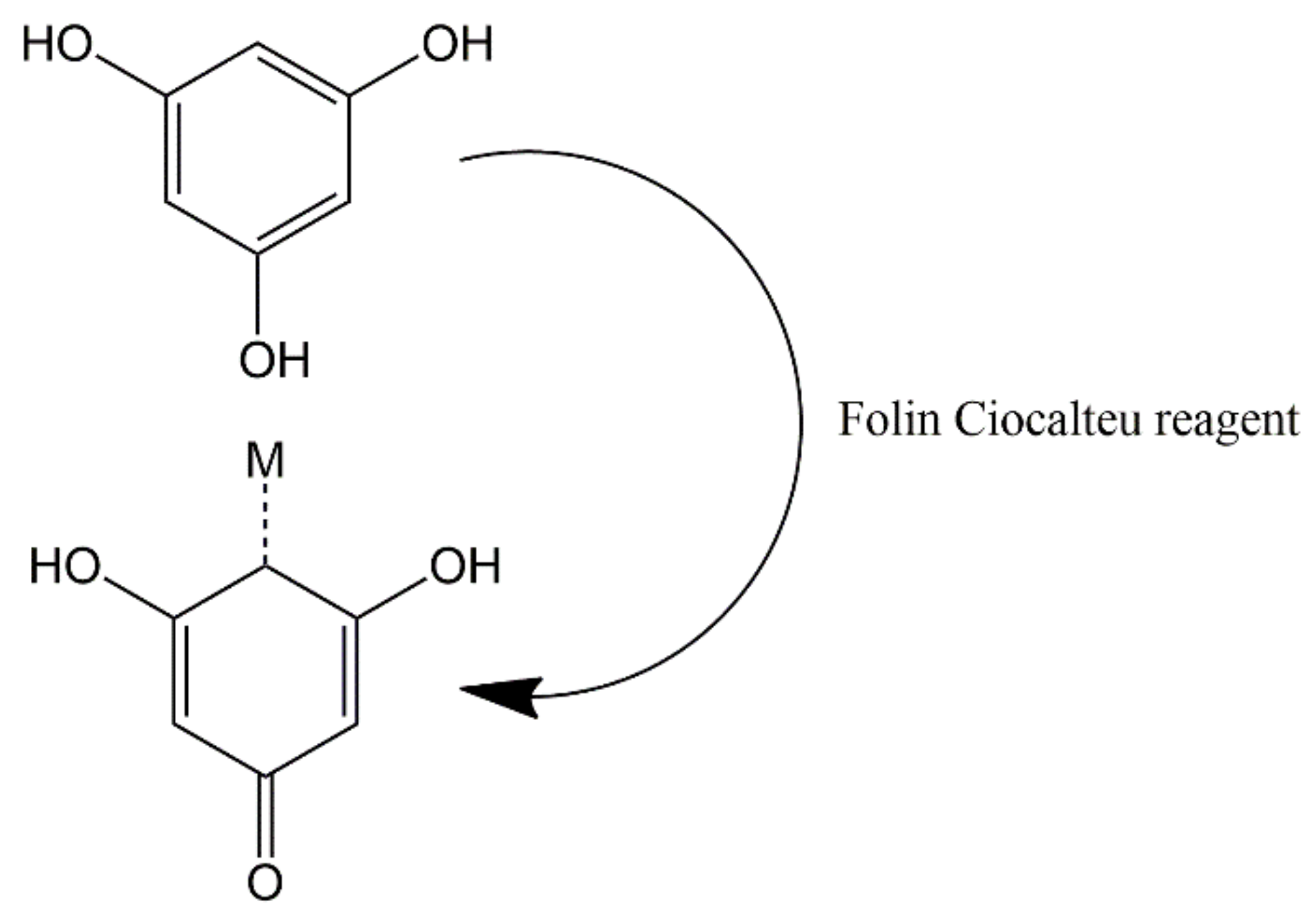
2.3. Determination of Polyphenol Content in Tea Extract
2.4. Synthesis and Characterization of Zero-Valent Iron (ZVI)
2.5. ZVI Catalytic Test for Dyes
2.6. Chemical Oxygen Demand (COD) Test
3. Results and Discussions
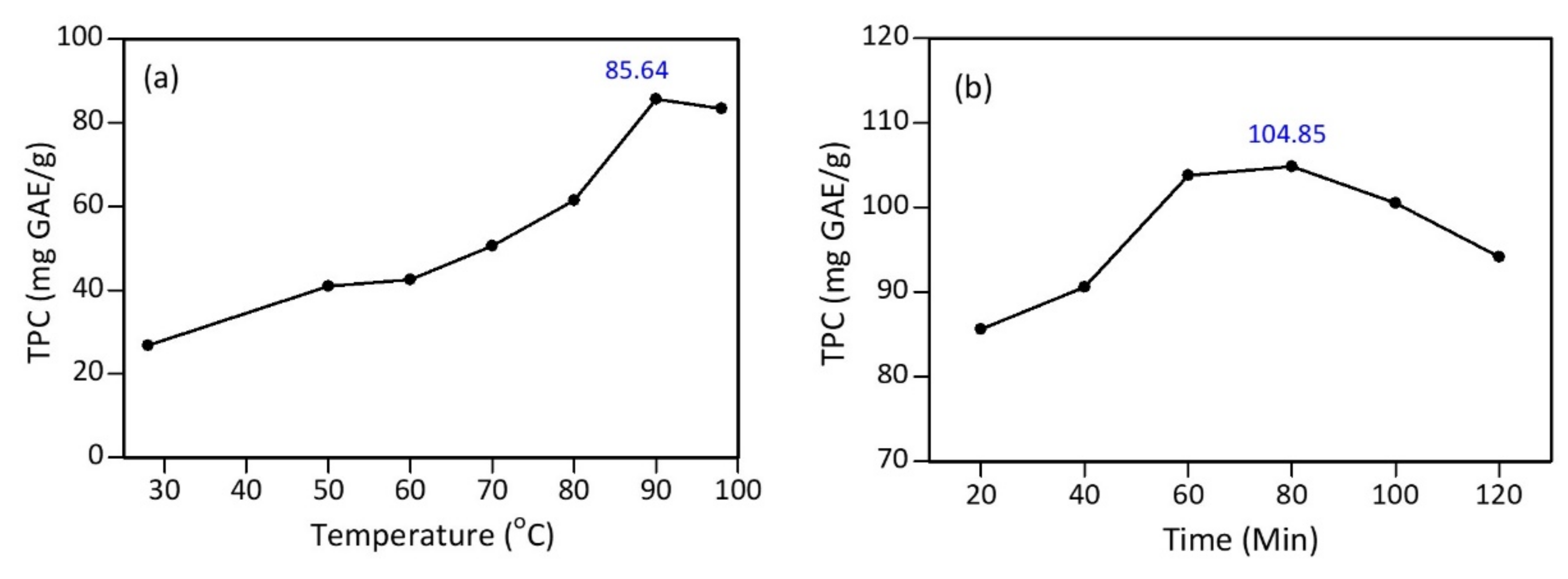
3.1. Preparation and Polyphenol Content of Tea Extract
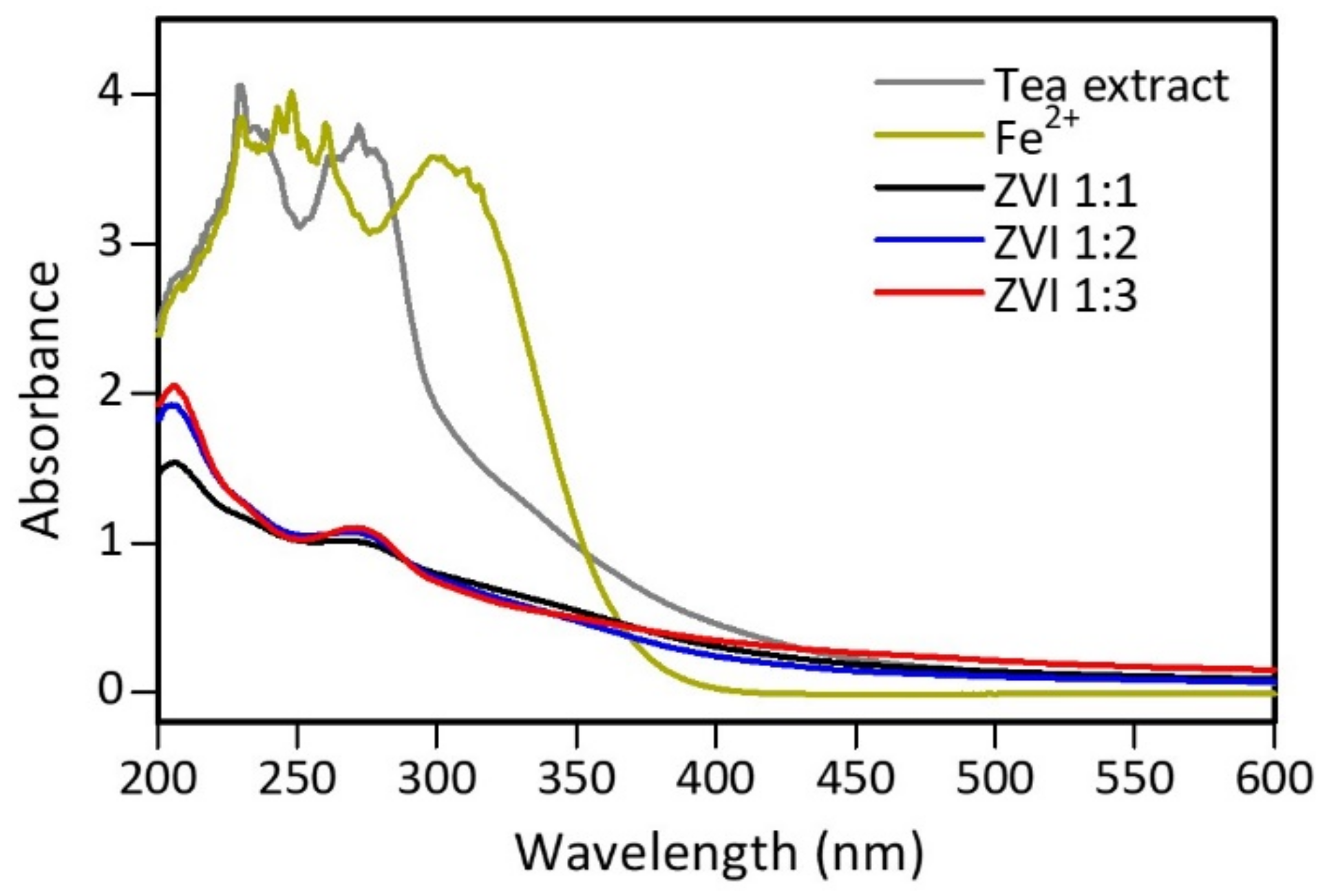
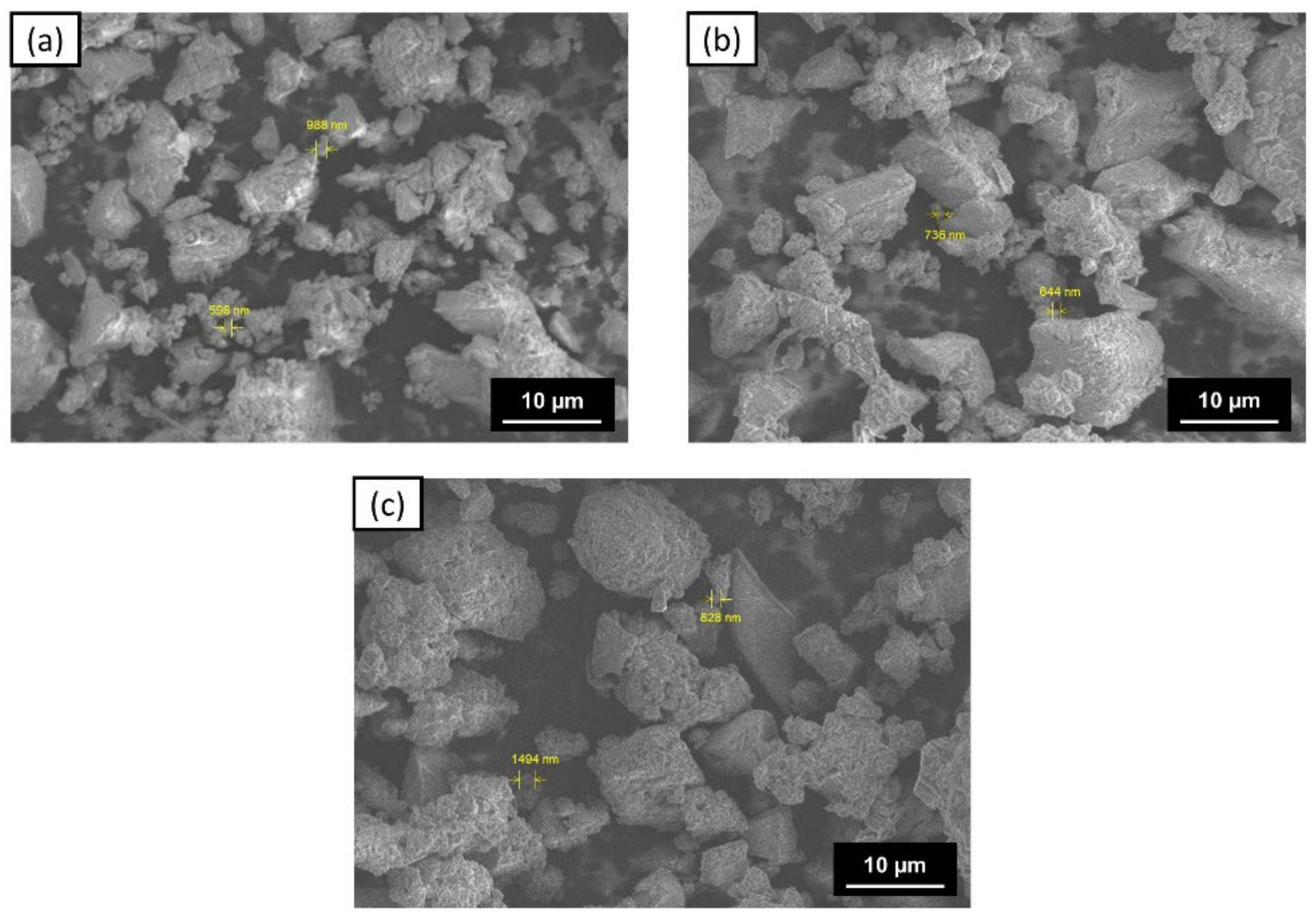
3.2. Characteristics of ZVI
3.3. Color Intensity Test
3.4. Degradation Pathway and Identification of the Intermediates
3.5. COD Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pratiknyo, P. Proyeksi Ketersediaan dan Kebutuhan Air Industri di Kab. Tangerang. In Proceedings of the Nasional Kebumian XI-FTM UPN Veteran Yogyakarta, Yogyakarta, Indonesia, 3–4 November 2016. [Google Scholar]
- Singh, K.; Kumar, P.; Srivastava, R. An overview of textile dyes and their removal techniques: Indian perspective. Pollut. Res. 2017, 36, 790–797. [Google Scholar]
- Haley, T.J. Benzidine revisited: A review of the literature and problems associated with the use of benzidine and its congeners. Clin. Toxicol. 1975, 8, 13–42. [Google Scholar] [CrossRef] [PubMed]
- Hassani, A.; Khataee, A.; Karaca, S.; Karaca, M.; Kiranşan, M. Adsorption of two cationic textile dyes from water with modified nanoclay: A comparative study by using central composite design. J. Environ. Chem. Eng. 2015, 3, 2738–2749. [Google Scholar] [CrossRef]
- Agarwal, S.; Tyagi, I.; Gupta, V.K.; Dastkhoon, M.; Ghaedi, M.; Yousefi, F.; Asfaram, A. Ultrasound-assisted adsorption of Sunset Yellow CFC dye onto Cu doped ZnS nanoparticles loaded on activated carbon using response surface methodology based on central composite design. J. Mol. Liq. 2016, 219, 332–340. [Google Scholar] [CrossRef]
- Kasperchik, V.P.; Yaskevich, A.L.; Bil’Dyukevich, A.V. Wastewater treatment for removal of dyes by coagulation and membrane processes. Pet. Chem. 2012, 52, 545–556. [Google Scholar] [CrossRef]
- Rodrigues, C.S.; Madeira, L.M.; Boaventura, R.A. Treatment of textile dye wastewaters using ferrous sulphate in a chemical coagulation/flocculation process. Environ. Technol. 2013, 34, 719–729. [Google Scholar] [CrossRef]
- Cheikh S’Id, E.; Kheribech, A.; Degu, M.; Hatim, Z.; Chourak, R.; M’Bareck, C. Removal of Methylene Blue from Water by Polyacrylonitrile Co Sodium Methallylsulfonate Copolymer (AN69) and Polysulfone (PSf) Synthetic Membranes. Prog. Color Color. Coat. 2021, 14, 89–100. [Google Scholar]
- Etezad, S.M.; Sadeghi-Kiakhani, M. Decolorization of Malachite Green Dye Solution by Bacterial Biodegradation. Prog. Color Color. Coat. 2021, 14, 79–87. [Google Scholar]
- Klavarioti, M.; Mantzavinos, D.; Kassinos, D. Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ. Int. 2009, 35, 402–417. [Google Scholar] [CrossRef]
- Seabra, A.B.; Haddad, P.; Duran, N. Biogenic synthesis of nanostructured iron compounds: Applications and perspectives. IET Nanobiotechnol. 2013, 7, 90–99. [Google Scholar] [CrossRef]
- Krishnan, S.; Rawindran, H.; Sinnathambi, C.M.; Lim, J.W. Comparison of various advanced oxidation processes used in remediation of industrial wastewater laden with recalcitrant pollutants. IOP Conf. Ser. Mater. Sci. Eng. 2017, 206, 012089. [Google Scholar] [CrossRef]
- Blowes, D.W.; Ptacek, C.J.; Benner, S.G.; McRae, C.W.; Bennett, T.A.; Puls, R.W. Treatment of inorganic contaminants using permeable reactive barriers. J. Contam. Hydrol. 2000, 45, 123–137. [Google Scholar] [CrossRef]
- Gavaskar, A.; Tatar, L. Cost and Performance Report Nanoscale Zero-Valent Iron Technologies for Source Remediation; Naval Facilities Engineering Service Center Port Hueneme: Port Hueneme, CA, USA, 2005. [Google Scholar]
- Rashmi, S.H.; Madhu, G.M.; Kittur, A.; Suresh, R. Synthesis, characterization and application of zero valent iron nanoparticles for the removal of toxic metal hexavalent chromium [Cr (VI)] from aqueous solution. Int. J. Curr. Eng. Technol. 2013, 1, 37–42. [Google Scholar]
- Nursyamsiah, D.; Solihudin; Eddy, D.R. Green Synthesis of Zero Valent Iron (ZVI) using Tea Leaves Extract and its Application as Fenton like Catalyst for Textile Dyes Removal. Asian J. Chem. 2021, 33, 963–968. [Google Scholar] [CrossRef]
- Wardiyati, S.; Fisli, A.; Yusuf, S. Sintesis dan Karakterisasi Nano Zero Valent Iron (NZVI) dengan Metode Presipitasi. J. Kimia Kemasan 2013, 35, 37–44. [Google Scholar] [CrossRef]
- Kanel, S.R.; Manning, B.; Charlet, L.; Choi, H. Removal of arsenic (III) from groundwater by nanoscale zero-valent iron. Environ. Sci. Technol. 2005, 39, 1291–1298. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Castle, A.B.; Murdock, R.C.; Hussain, S.M.; Varma, R.S. In vitro biocompatibility of nanoscale zerovalent iron particles (NZVI) synthesized using tea polyphenols. Green Chem. 2010, 12, 114–122. [Google Scholar] [CrossRef]
- Machado, S.; Pinto, S.L.; Grosso, J.P.; Nouws, H.P.A.; Albergaria, J.T.; Delerue-Matos, C. Application of green zero-valent iron nanoparticles to the remediation of soils contaminated with ibuprofen. Sci. Total Environ. 2013, 445, 323–329. [Google Scholar] [CrossRef] [Green Version]
- Hoag, G.E.; Collins, J.B.; Holcomb, J.L.; Hoag, J.R.; Nadagouda, M.N.; Varma, R.S. Degradation of bromothymol blue by ‘greener’nano-scale zero-valent iron synthesized using tea polyphenols. J. Mater. Chem. 2009, 19, 8671–8677. [Google Scholar] [CrossRef]
- Dhuper, S.; Panda, D.; Nayak, P.L. Green synthesis and characterization of zero valent iron nanoparticles from the leaf extract of Mangifera indica. J. Nanotechnol. Its Appl. 2012, 13, 16–22. [Google Scholar]
- Huang, L.; Weng, X.; Chen, Z.; Megharaj, M.; Naidu, R. Synthesis of iron-based nanoparticles using oolong tea extract for the degradation of malachite green. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 117, 801–804. [Google Scholar] [CrossRef]
- Murgueitio, E.; Debut, A.; Landivar, J.; Cumbal, L. Synthesis of iron nanoparticles through extracts of native fruits of Ecuador, as capuli (Prunus serotina) and mortiño (Vaccinium floribundum). Biol. Med. 2016, 8, 1. [Google Scholar] [CrossRef]
- Kecić, V.; Kerkez, Đ.; Prica, M.; Lužanin, O.; Bečelić-Tomin, M.; Pilipović, D.T.; Dalmacija, B. Optimization of azo printing dye removal with oak leaves-nZVI/H2O2 system using statistically designed experiment. J. Clean Prod. 2018, 202, 65–80. [Google Scholar] [CrossRef]
- Desalegn, B.; Megharaj, M.; Chen, Z.; Naidu, R. Green synthesis of zero valent iron nanoparticle using mango peel extract and surface characterization using XPS and GC-MS. Heliyon 2019, 5, e01750. [Google Scholar] [CrossRef] [Green Version]
- Sumpio, B.E.; Cordova, A.C.; Berke-Schlessel, D.W.; Qin, F.; Chen, Q.H. Green tea, the “Asian paradox,” and cardiovascular disease. Chen. J. Am. Coll. Surg. 2006, 202, 813–825. [Google Scholar] [CrossRef]
- Anjarsari, I.R.D. Indonesia tea catechin: Prospect and benefits. J. Kultivasi 2016, 15, 99–106. [Google Scholar]
- Chang, K. World Tea Production and Trade Current and Future Development. 2015. Available online: http://www.fao.org/3/i4480e/i4480e.pdf (accessed on 20 May 2020).
- Neyens, E.; Baeyens, J.A. A review of classic Fenton’s peroxidation as an advanced oxidation technique. J. Hazard Mater. 2003, 98, 33–50. [Google Scholar] [CrossRef]
- Haddad, M.E.; Regti, A.; Laamari, M.R.; Mamouni, R.; Saffaj, N. Use of Fenton reagent as advanced oxidative process for removing textile dyes from aqueous solutions. J. Mater. Environ. Sci. 2014, 5, 667–674. [Google Scholar]
- Walling, C. Fenton’s reagent revisited. Acc. Chem. Res. 1975, 8, 125–131. [Google Scholar] [CrossRef]
- Goi, A. Advanced Oxidation Processes for Water Purification and Soil Remediation. Ph.D. Thesis, Tallinn University of Technology, Tallinn, Estonia, 2005. [Google Scholar]
- Shahwan, T.; Sirriah, S.A.; Nairat, M.; Boyacı, E.; Eroğlu, A.E.; Scott, T.B.; Hallam, K.R. Green synthesis of iron nanoparticles and their application as a Fenton-like catalyst for the degradation of aqueous cationic and anionic dyes. Chem. Eng. J. 2011, 172, 258–266. [Google Scholar] [CrossRef]
- Truskewycz, A.; Shukla, R.; Ball, A.S. Iron nanoparticles synthesized using green tea extracts for the fenton-like degradation of concentrated dye mixtures at elevated temperatures. J. Environ. Chem. Eng. 2016, 4, 4409–4417. [Google Scholar] [CrossRef]
- Ghanim, D.; Al-Kindi, G.Y.; Hassan, A.K. Green synthesis of iron nanoparticles using black tea leaves extract as adsorbent for removing eriochrome blue-black B dye. Eng. Technol. J. 2020, 38, 1558–1569. [Google Scholar] [CrossRef]
- Xiao, C.; Li, H.; Zhao, Y.; Zhang, X.; Wang, X. Green synthesis of iron nanoparticle by tea extract (polyphenols) and its selective removal of cationic dyes. J. Environ. Manag. 2020, 275, 111262. [Google Scholar] [CrossRef] [PubMed]
- Sibarani, J.; Purba, D.L.; Suprihatin, I.E.; Manurung, M. Fotodegradasi Rhodamin B menggunakan ZnO/UV/Reagen Fenton. Cakra Kimia 2016, 4, 84–93. [Google Scholar]
- Bafana, A.; Devi, S.S.; Chakrabarti, T. Azo dyes: Past, present and the future. Environ. Rev. 2011, 19, 350–370. [Google Scholar] [CrossRef]
- Hou, M.F.; Liao, L.; Zhang, W.D.; Tang, X.Y.; Wan, H.F.; Yin, G.C. Degradation of rhodamine B by Fe (0)-based Fenton process with H2O2. Chemosphere 2011, 83, 1279–1283. [Google Scholar] [CrossRef]
- Youssef, N.A.; Shaban, S.A.; Ibrahim, F.A.; Mahmoud, A.S. Degradation of methyl orange using Fenton catalytic reaction. Egypt J. Pet. 2016, 25, 317–321. [Google Scholar] [CrossRef] [Green Version]
- Yoon, S.; Bae, S. Novel synthesis of nanoscale zerovalent iron from coal fly ash and its application in oxidative degradation of methyl orange by Fenton reaction. J. Hazard. Mater. 2019, 365, 751–758. [Google Scholar] [CrossRef]
- Liang, L.; Cheng, L.; Zhang, Y.; Wang, Q.; Wu, Q.; Xue, Y.; Meng, X. Efficiency and mechanisms of rhodamine B degradation in Fenton-like systems based on zero-valent iron. RSC Adv. 2020, 10, 28509–28515. [Google Scholar] [CrossRef]
- Devi, L.G.; Kumar, S.G.; Reddy, K.M.; Munikrishnappa, C. Photo degradation of Methyl Orange an azo dye by Advanced Fenton Process using zero valent metallic iron: Influence of various reaction parameters and its degradation mechanism. J. Hazard. Mater. 2009, 164, 459–467. [Google Scholar] [CrossRef]
- Effendi, H.; Romanto; Wardiatno, Y. Water quality status of Ciambulawung River, Banten Province, based on pollution index and NSF-WQI. Procedia Environ. Sci. 2015, 24, 228–237. [Google Scholar] [CrossRef] [Green Version]
- Alara, O.R.; Abdurahman, N.H.; Olalere, O.A. Ethanolic extraction of bioactive compounds from Vernonia amygdalina leaf using response surface methodology as an optimization tool. J. Food Meas. Charact. 2018, 12, 1107–1122. [Google Scholar] [CrossRef]
- Barchan, A.; Bakkali, M.; Arakrak, A.; Pagán, R.; Laglaoui, A. The effects of solvents polarity on the phenolic contents and antioxidant activity of three Mentha species extracts. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 399–412. [Google Scholar]
- Unachukwu, U.J.; Ahmed, S.; Kavalier, A.; Lyles, J.T.; Kennelly, E.J. White and green teas (Camellia sinensis var. sinensis): Variation in phenolic, methylxanthine, and antioxidant profiles. J. Food Sci. 2010, 75, C541–C548. [Google Scholar] [CrossRef] [Green Version]
- Peng, L.; Song, X.; Shi, X.; Li, J.; Ye, C. An improved HPLC method for simultaneous determination of phenolic compounds, purine alkaloids and theanine in Camellia species. J. Food Compos. Anal. 2008, 21, 559–563. [Google Scholar] [CrossRef]
- Khadijah, K.; Jayali, A.M.; Umar, S.; Sasmita, I. Penentuan total fenolik dan aktivitas antioksidan ekstrak etanolik daun samama (Anthocephalus macrophylus) asal Ternate, Maluku Utara. J. Kimia Mulawarman 2017, 15, 11–18. [Google Scholar]
- Ford, L.; Theodoridou, K.; Sheldrake, G.N.; Walsh, P.J. A critical review of analytical methods used for the chemical characterisation and quantification of phlorotannin compounds in brown seaweeds. Phytochem. Anal. 2019, 30, 587–599. [Google Scholar] [CrossRef]
- Puspitasari, L.; Arief, S.; Zulhadjri, Z. Ekstrak Daun Andalas sebagai Capping Agent dalam Green Hydrothermal Synthesis Nanopartikel Mangan Ferrit dan Aplikasinya sebagai Antibakteri. Chim. Nat. Acta 2019, 7, 20–26. [Google Scholar] [CrossRef] [Green Version]
- Phan, C.M.; Nguyen, H.M. Role of capping agent in wet synthesis of nanoparticles. J. Phys. Chem. A 2017, 121, 3213–3219. [Google Scholar] [CrossRef]
- Yunita, Y.; Nurlina, N.; Syahbanu, I. Sintesis Nanopartikel Zink Oksida (ZnO) dengan Penambahan Ekstrak Klorofil sebagai Capping Agent. Positron 2020, 10, 123–130. [Google Scholar] [CrossRef]
- Gulati, S.; Sachdeva, M.; Bhasin, K.K. Capping agents in nanoparticle synthesis: Surfactant and solvent system. AIP Conf. Proc. 2018, 1953, 030214. [Google Scholar]
- Yuvakkumar, R.; Elango, V.; Rajendran, V.; Kannan, N. Preparation and characterization of zero valent iron nanoparticles. Dig. J. Nanomater. Biostruct. 2011, 6, 1771–1776. [Google Scholar]
- Buckingham, M.A.; Marken, F.; Aldous, L. The thermoelectrochemistry of the aqueous iron (ii)/iron (iii) redox couple: Significance of the anion and pH in thermogalvanic thermal-to-electrical energy conversion. Sustain. Energy Fuels 2018, 2, 2717–2726. [Google Scholar] [CrossRef] [Green Version]
- Joseph, J.; Nagashri, K. Novel copper-based therapeutic agent for anti-inflammatory: Synthesis, characterization, and biochemical activities of copper (II) complexes of hydroxyflavone Schiff bases. Appl. Biochem. Biotechnol. 2012, 167, 1446–1458. [Google Scholar] [CrossRef]
- Kumar, K.M.; Mandal, B.K.; Kumar, K.S.; Reddy, P.S.; Sreedhar, B. Biobased green method to synthesise palladium and iron nanoparticles using Terminalia chebula aqueous extract. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 102, 128–133. [Google Scholar] [CrossRef]
- Wang, T.; Lin, J.; Chen, Z.; Megharaj, M.; Naidu, R. Green synthesized iron nanoparticles by green tea and eucalyptus leaves extracts used for removal of nitrate in aqueous solution. J. Clean Prod. 2014, 83, 413–419. [Google Scholar] [CrossRef]
- Geng, S.; Shan, S.; Ma, H.; Liu, B. Antioxidant activity and α-glucosidase inhibitory activities of the polycondensate of catechin with glyoxylic acid. PLoS ONE 2016, 11, e0150412. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Vásquez, M.J.; Valenzuela-Buitimea, E.L.; Plascencia-Jatomea, M.; Encinas-Encinas, J.C.; Rodríguez-Félix, F.; Sánchez-Valdes, S.; Rosas-Burgos, E.C.; Ocaño-Higuera, V.M.; Graciano-Verdugo, A.Z. Functionalization of chitosan by a free radical reaction: Characterization, antioxidant and antibacterial potential. Carbohydr. Polym. 2017, 155, 117–127. [Google Scholar] [CrossRef]
- Mohamed, S.E.; Khalifa, M.G.; Sayed, S.A.; Kamel, A.M.; Shalabi, M.E.H. Removal of Lignin from Pulp Waste Water’s Black Liquor via By-Pass Cement Dust. Eurasian Chem.-Technol. J. 2009, 11, 51–59. [Google Scholar]
- D’Souza, L.; Devi, P.; Divya Shridhar, M.P.; Naik, C.G. Use of Fourier Transform Infrared (FTIR) spectroscopy to study cadmium-induced changes in Padina tetrastromatica (Hauck). Anal. Chem. Insights 2008, 3, 135–143. [Google Scholar] [CrossRef]
- Dong, H.; Zhao, F.; He, Q.; Xie, Y.; Zeng, Y.; Zhang, L.; Tang, L.; Zeng, G. Physicochemical transformation of carboxymethyl cellulose-coated zero-valent iron nanoparticles (nZVI) in simulated groundwater under anaerobic conditions. Sep. Purif. Technol. 2017, 175, 376–383. [Google Scholar] [CrossRef]
- Mishra, D.; Arora, R.; Lahiri, S.; Amritphale, S.S.; Chandra, N. Synthesis and characterization of iron oxide nanoparticles by solvothermal method. Prot. Met. Phys. Chem. Surfaces 2014, 50, 628–631. [Google Scholar] [CrossRef]
- Fitria, W.; Fatriasari, E.; Hermiati, N.S. Pengaruh lokasi tempat tumbuh terhadap kandungan kimia kayu sengon (Paraserianthes falcataria) sebagai bahan baku pulp. J. Ilmu Teknol. Has. Hutan 2010, 3, 45–50. [Google Scholar]
- Muhammad, F.; Xia, M.; Li, S.; Yu, X.; Mao, Y.; Muhammad, F.; Huang, X.; Jiao, B.; Yu, L.; Li, D. The reduction of chromite ore processing residues by green tea synthesized nano zerovalent iron and its solidification/stabilization in composite geopolymer. J. Clean Prod. 2019, 234, 381–391. [Google Scholar] [CrossRef]
- Wang, W.; Jin, Z.H.; Li, T.L.; Zhang, H.; Gao, S. Preparation of spherical iron nanoclusters in ethanol–water solution for nitrate removal. Chemosphere 2006, 65, 1396–1404. [Google Scholar] [CrossRef]
- Aziztyana, A.P.; Wardhani, S.; Prananto, Y.P.; Purwonugroho, D.; Darjito. Optimisation of Methyl Orange Photodegradation Using TiO2-Zeolite Photocatalyst and H2O2 in Acid Condition. IOP Conf. Ser. Mater. Sci. Eng. 2019, 546, 042047. [Google Scholar] [CrossRef]



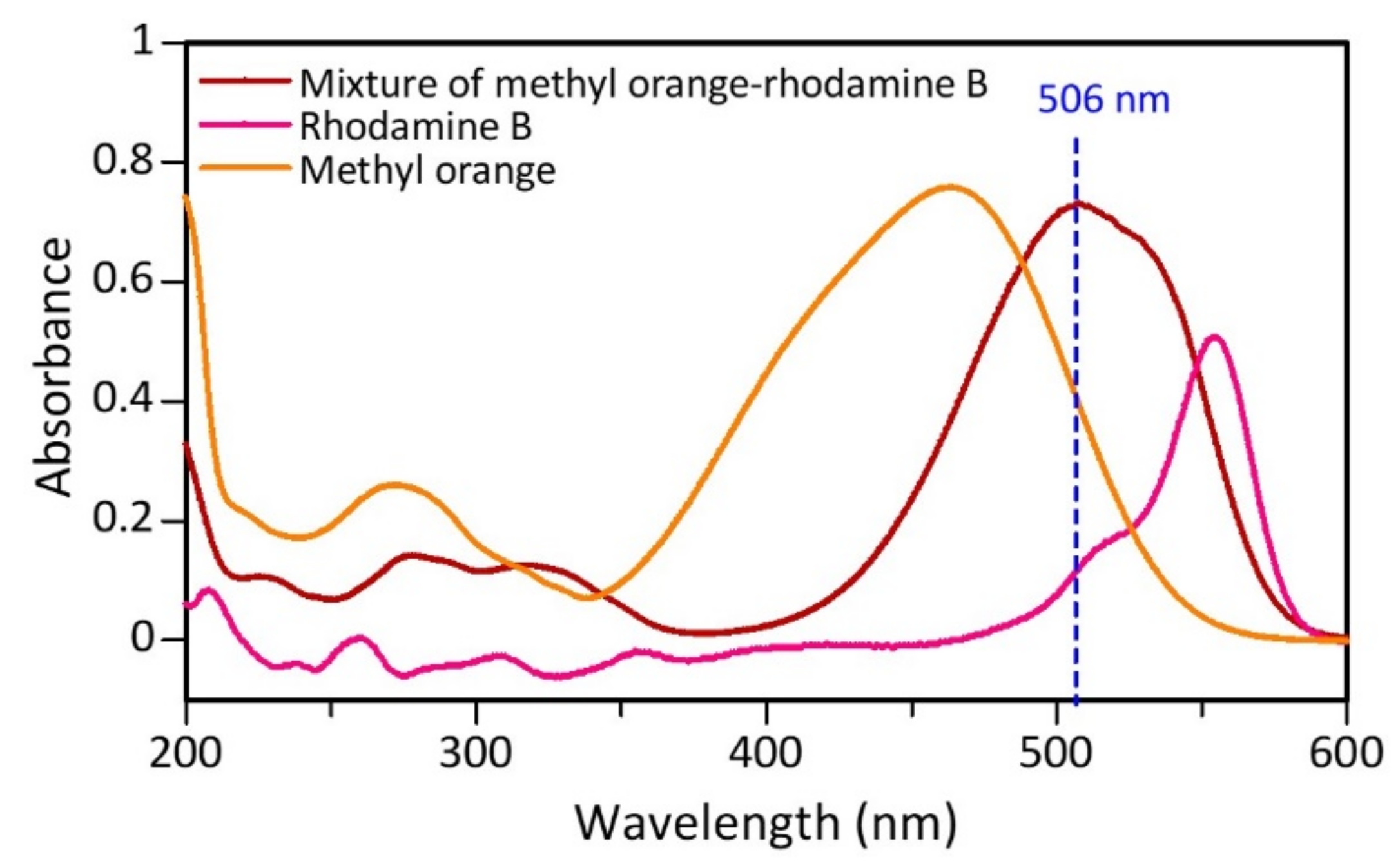

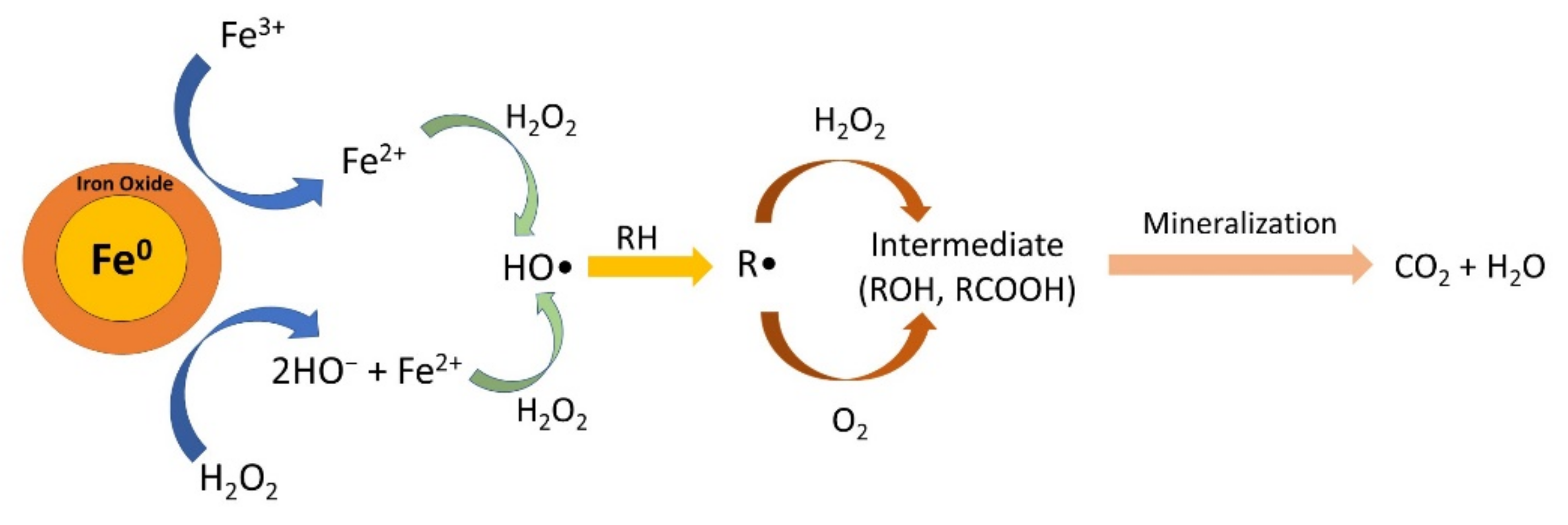
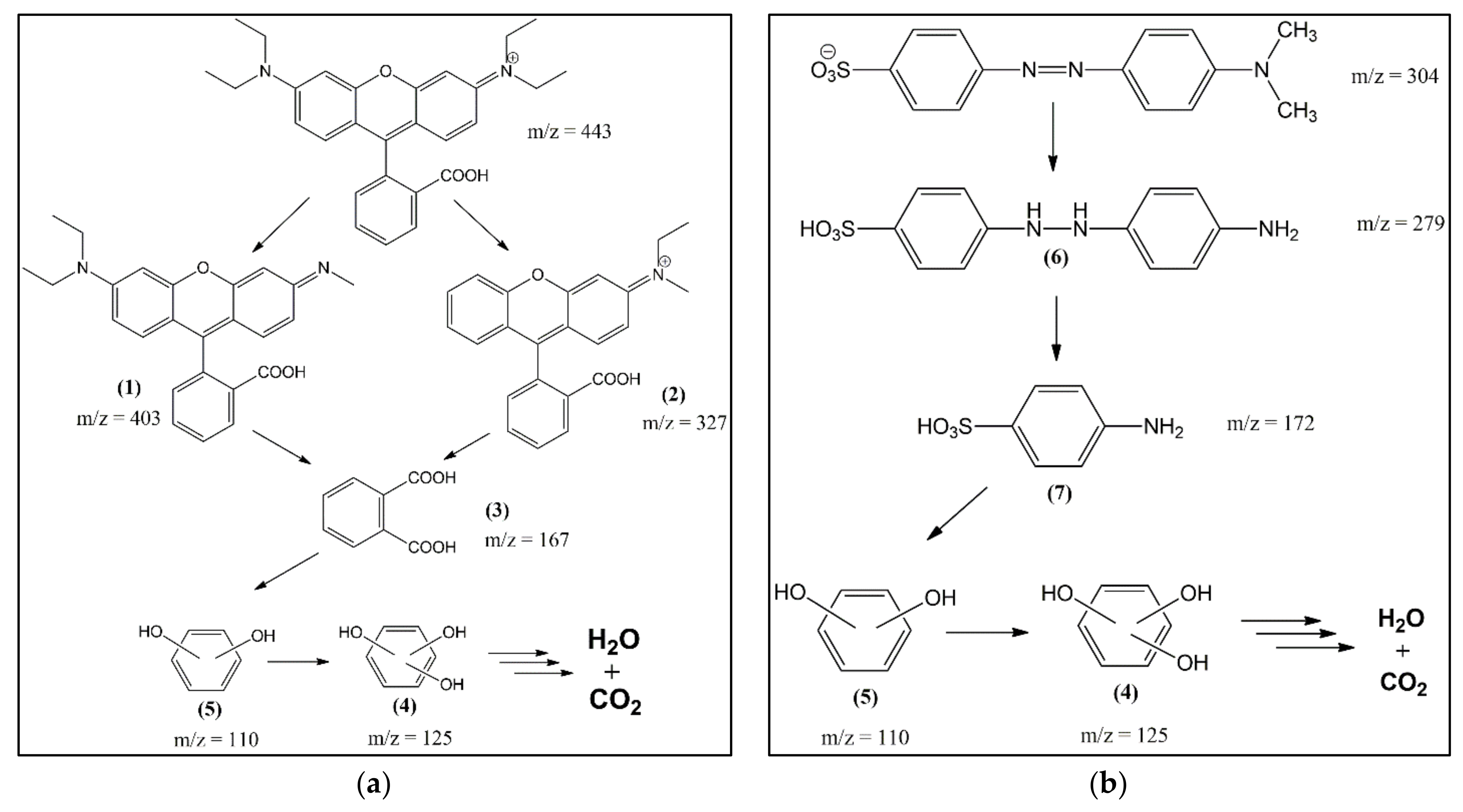
| ZVI | Size Range (nm) | Average Size (nm) | ||
|---|---|---|---|---|
| First Peak | Second Peak | First Peak | Second Peak | |
| 1:1 | 151–171 | 945–1207 | 153 | 1012 |
| 1:2 | 93–105 | 740–945 | 93 | 733 |
| 1:3 | 279–356 | - | 301 | - |
| Element | Weight Percent (%) | ||
|---|---|---|---|
| ZVI 1:1 | ZVI 1:2 | ZVI 1:3 | |
| O | 36.97 | 42.92 | 44.91 |
| C | 19.97 | 20.96 | 27.98 |
| S | 13.39 | 14.46 | 5.40 |
| Fe | 10.12 | 11.30 | 11.90 |
| Na | 3.42 | 2.69 | 3.24 |
| K | 3.32 | 4.17 | 2.25 |
| N | 2.97 | 3.50 | 4.32 |
| Compound No. | Retention Time (min) | m/z |
|---|---|---|
| (1) | 12.02 | 403 |
| (2) | 10.32 | 327 |
| (3) | 13.27 | 167 |
| (4) | 0.87 | 125 |
| (5) | 24.13 | 110 |
| (6) | 11.27 | 279 |
| (7) | 12.45 | 172 |
| Sample | COD (mg O2/g) | Decrease (%) |
|---|---|---|
| Mixture before catalytic | 92.76 | - |
| ZVI 1:1 | 7.32 | 92.11 |
| Iron(II) sulfate | 48.83 | 47.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eddy, D.R.; Nursyamsiah, D.; Permana, M.D.; Solihudin; Noviyanti, A.R.; Rahayu, I. Green Production of Zero-Valent Iron (ZVI) Using Tea-Leaf Extracts for Fenton Degradation of Mixed Rhodamine B and Methyl Orange Dyes. Materials 2022, 15, 332. https://doi.org/10.3390/ma15010332
Eddy DR, Nursyamsiah D, Permana MD, Solihudin, Noviyanti AR, Rahayu I. Green Production of Zero-Valent Iron (ZVI) Using Tea-Leaf Extracts for Fenton Degradation of Mixed Rhodamine B and Methyl Orange Dyes. Materials. 2022; 15(1):332. https://doi.org/10.3390/ma15010332
Chicago/Turabian StyleEddy, Diana Rakhmawaty, Dian Nursyamsiah, Muhamad Diki Permana, Solihudin, Atiek Rostika Noviyanti, and Iman Rahayu. 2022. "Green Production of Zero-Valent Iron (ZVI) Using Tea-Leaf Extracts for Fenton Degradation of Mixed Rhodamine B and Methyl Orange Dyes" Materials 15, no. 1: 332. https://doi.org/10.3390/ma15010332
APA StyleEddy, D. R., Nursyamsiah, D., Permana, M. D., Solihudin, Noviyanti, A. R., & Rahayu, I. (2022). Green Production of Zero-Valent Iron (ZVI) Using Tea-Leaf Extracts for Fenton Degradation of Mixed Rhodamine B and Methyl Orange Dyes. Materials, 15(1), 332. https://doi.org/10.3390/ma15010332









