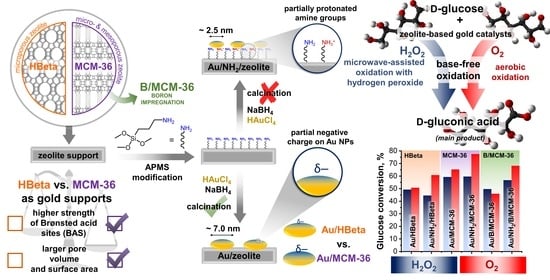
Modification of Gold Zeolitic Supports for Catalytic Oxidation of Glucose to Gluconic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials/Compounds
2.2. Synthesis of MCM-36 Zeolite
2.3. Modification of MCM-36 Zeolites with Boron
2.4. Modification with (3-Aminopropyl)trimethoxysilane (APMS)
2.5. Gold Catalysts Preparation
2.6. Characterization of the Materials
2.7. Glucose Oxidation
2.8. Analysis of Reactant and Products
3. Results and Discussion
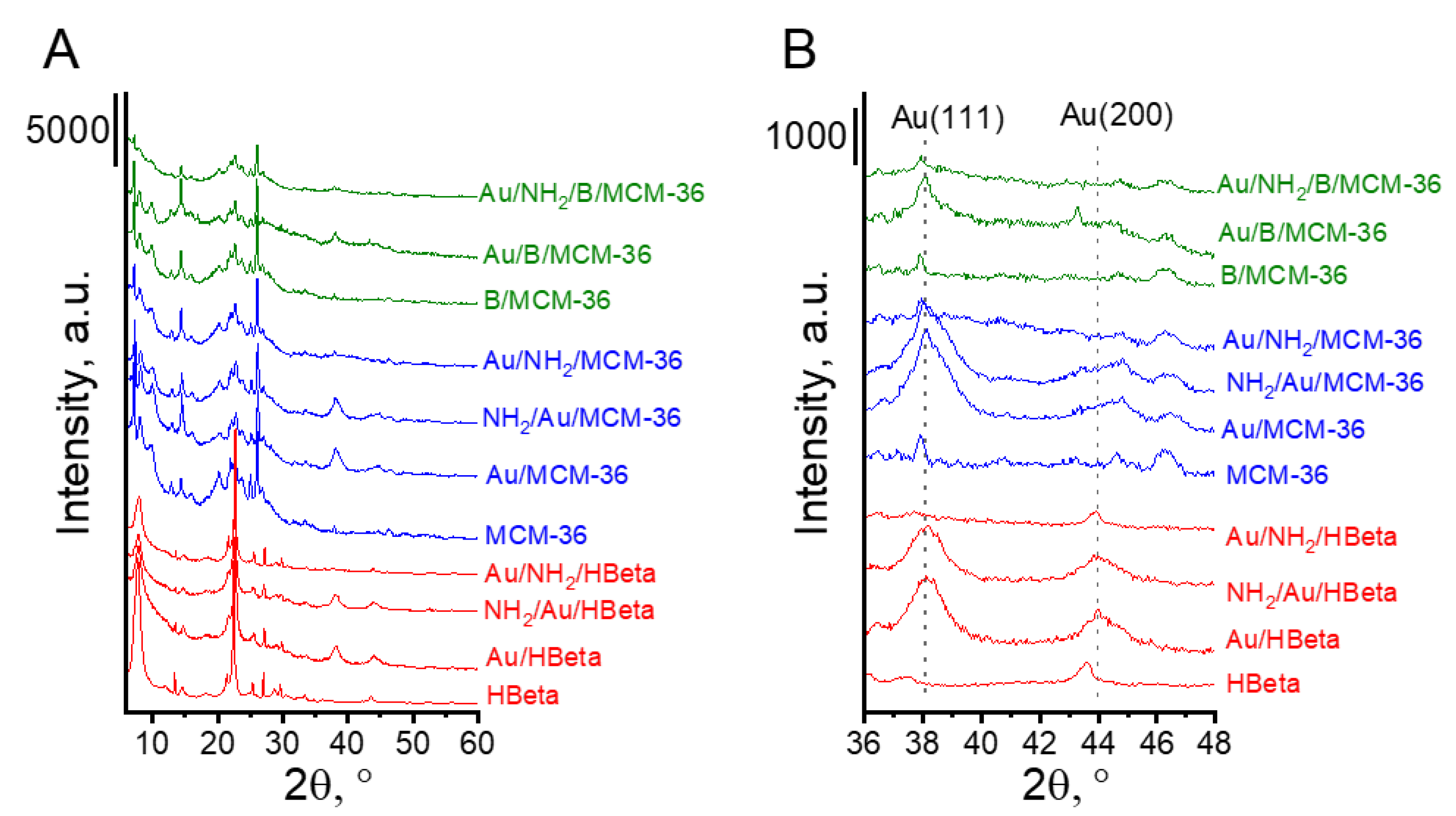
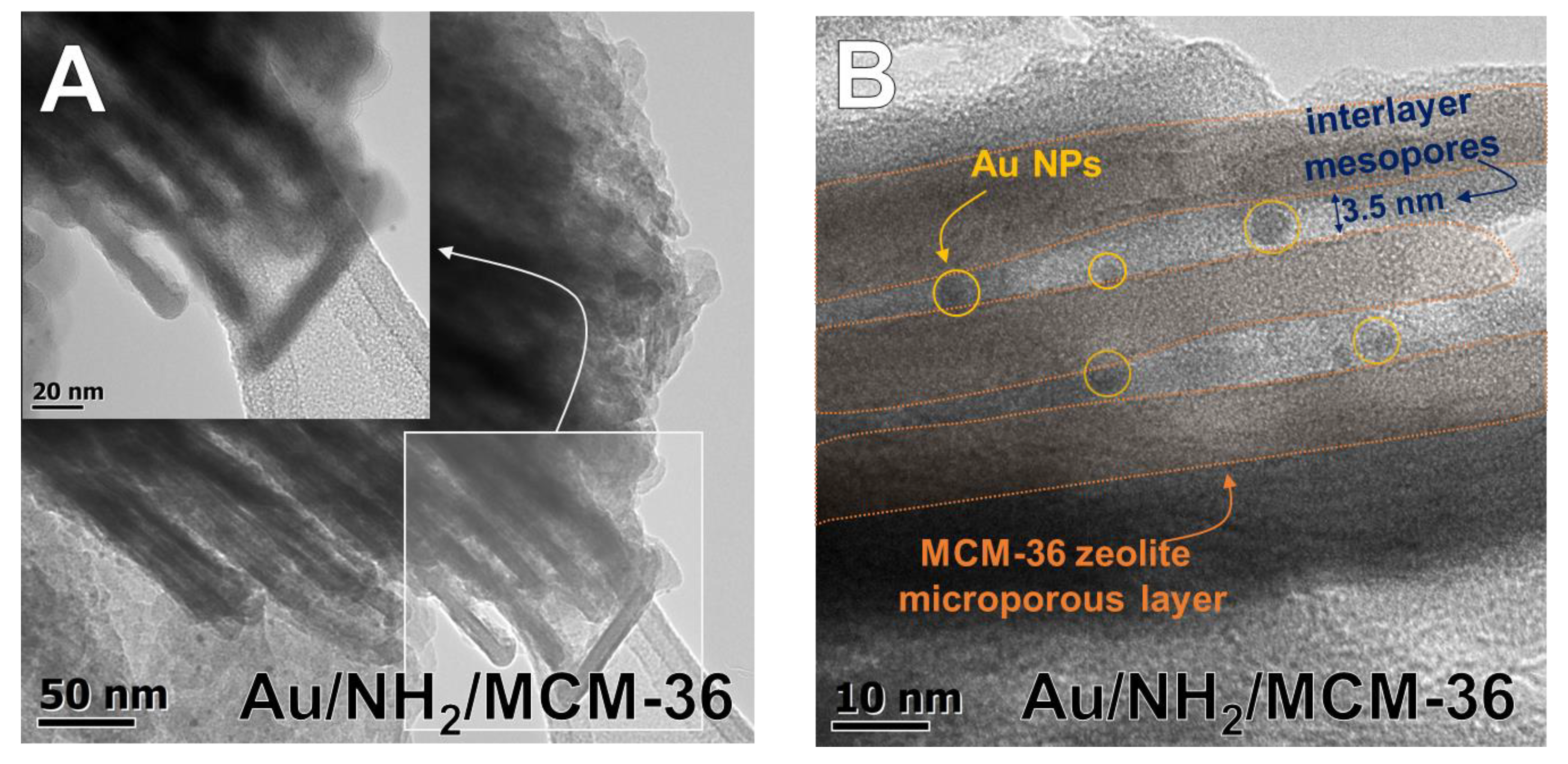
3.1. Composition of Catalysts and Their Textural/Structural Properties
3.2. Surface Properties of Catalysts
3.2.1. Acidity of Selected Catalysts
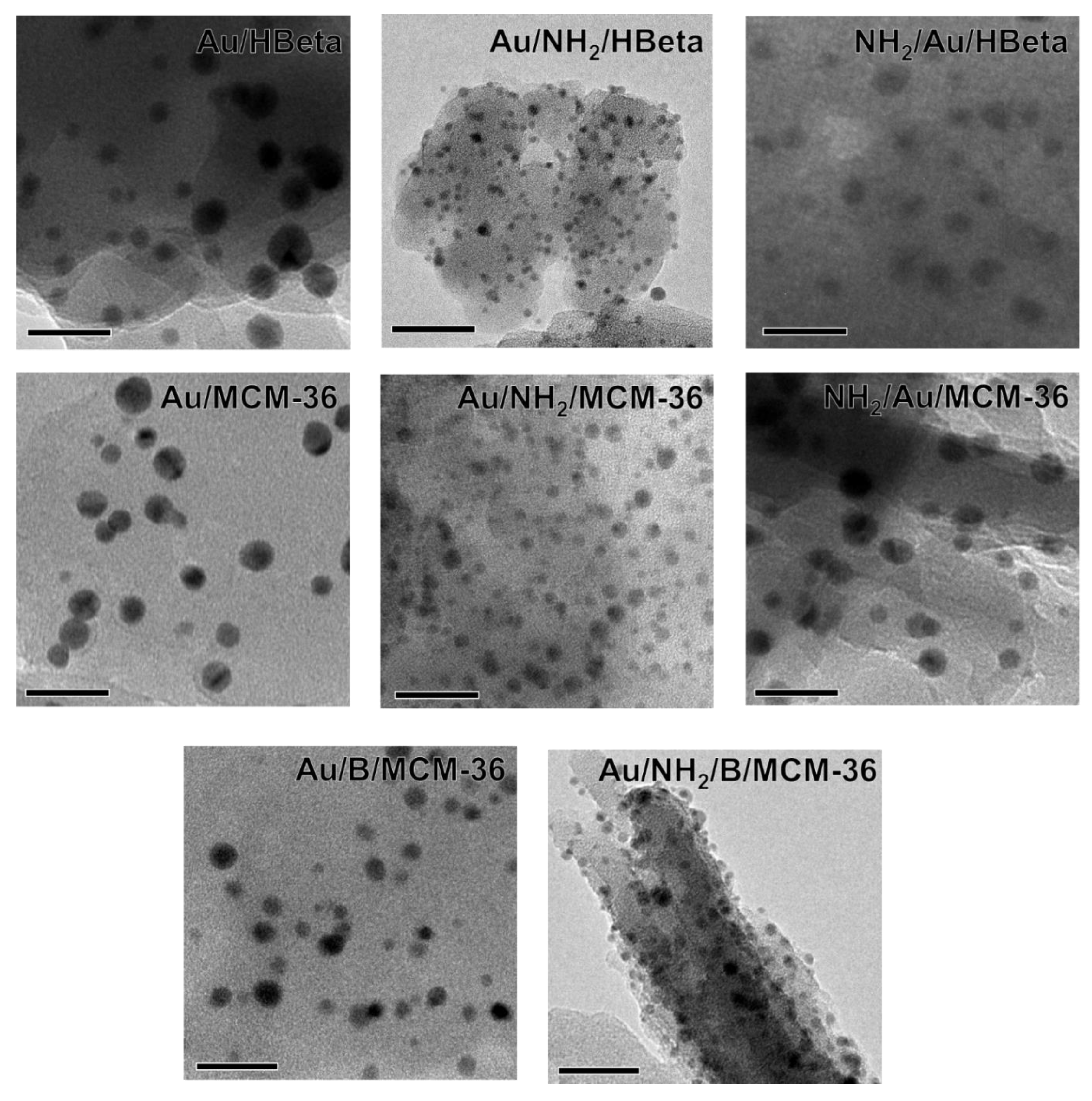
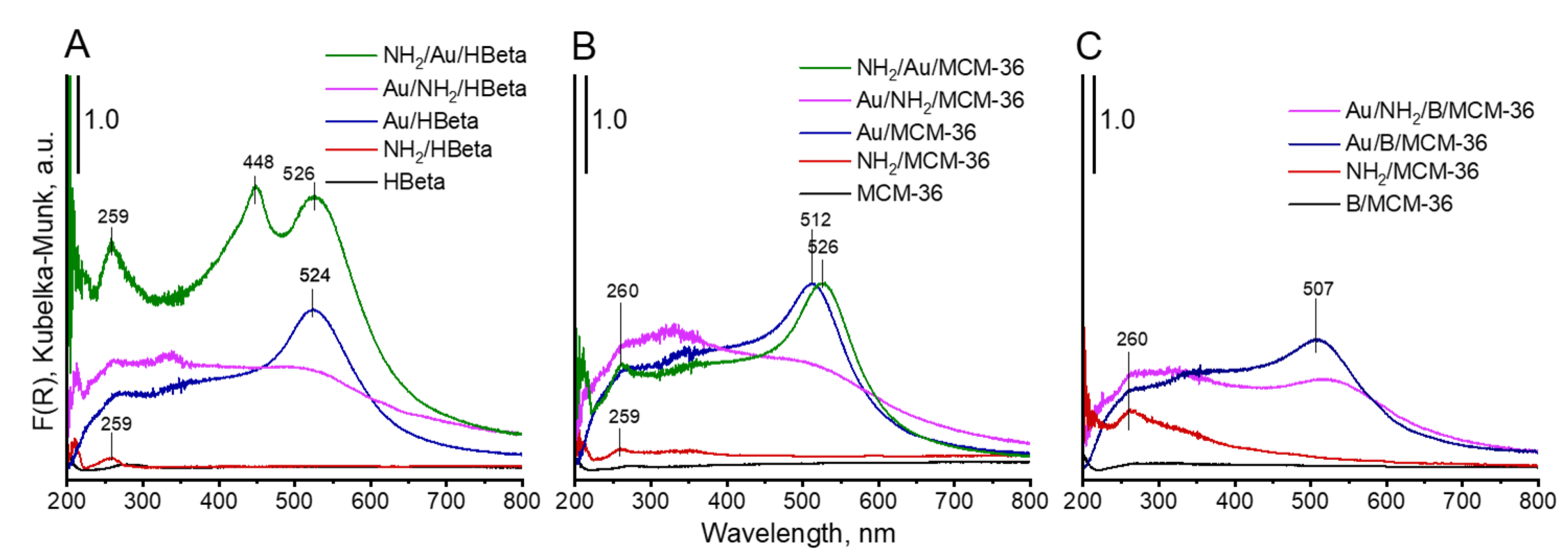
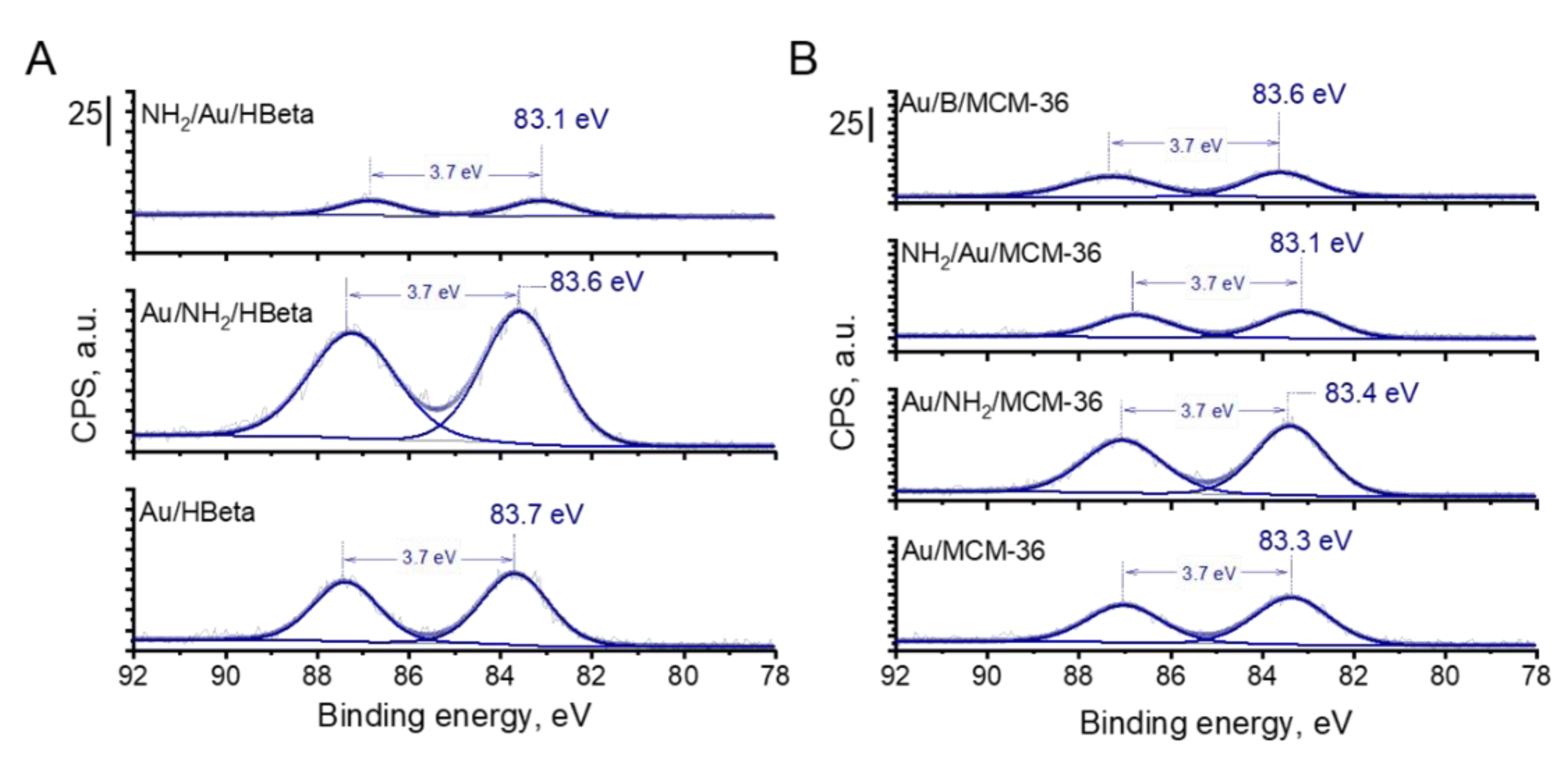
3.2.2. Particle Size and Oxidation State of Gold
3.3. Glucose Oxidation with Molecular Oxygen
3.3.1. Influence of Zeolite Support on the Activity of Gold Catalysts
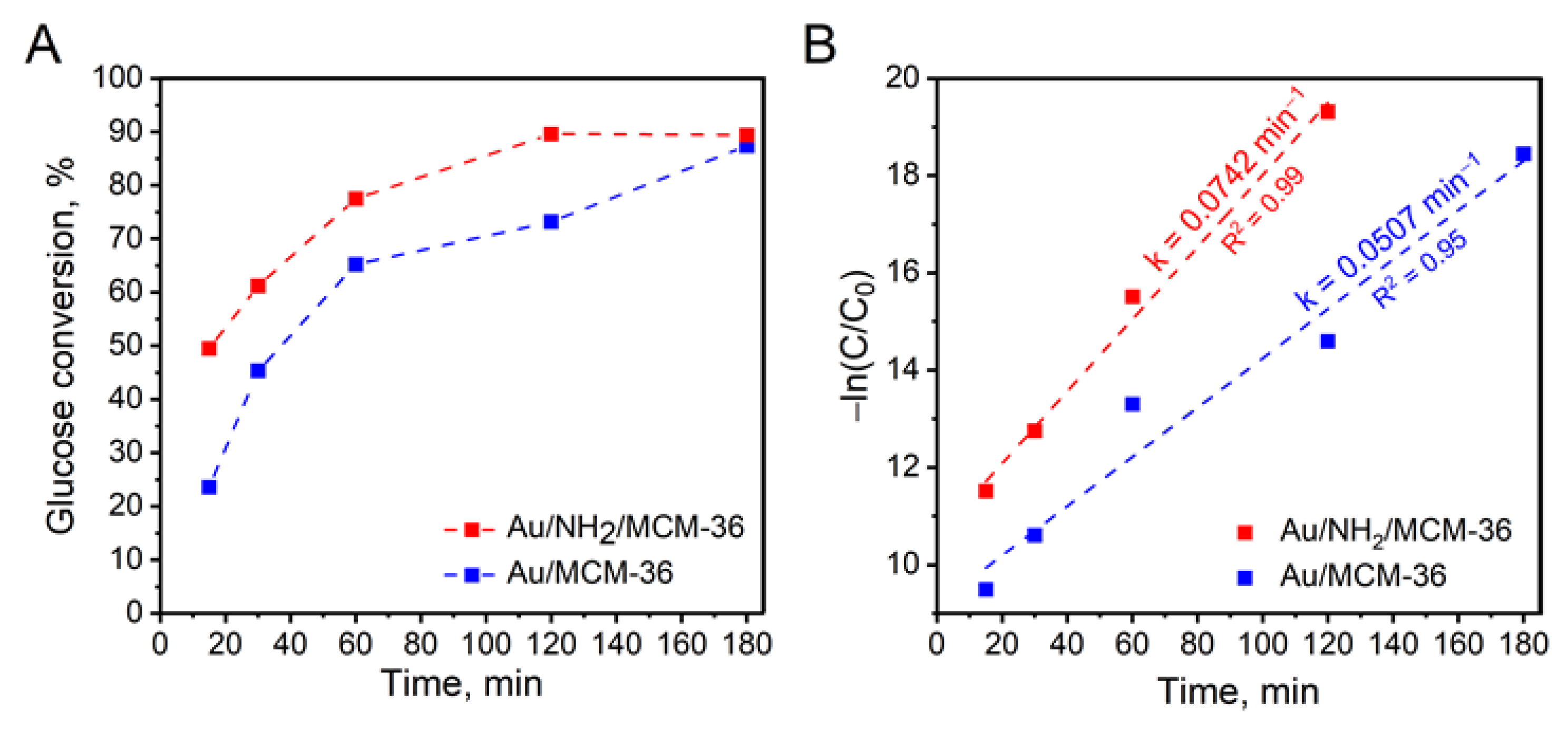
3.3.2. Time Dependence of Glucose Conversion
3.4. Glucose Oxidation with Hydrogen Peroxide
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haruta, M. Gold catalysts prepared by coprecipitation for low-temperature oxidation of hydrogen and of carbon monoxide. J. Catal. 1989, 115, 301–309. [Google Scholar] [CrossRef]
- Hutchings, G.J. Spiers Memorial Lecture: Understanding reaction mechanisms in heterogeneously catalysed reactions. Faraday Discuss. 2021, 229, 9–34. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Liebana, M.C.; Bonet-Aleta, J.; Hueso, J.L.; Santamaria, J. Gold-Based Nanoparticles on Amino-Functionalized Mesoporous Silica Supports as Nanozymes for Glucose Oxidation. Catalysts 2020, 10, 333. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Fang, Q.; Li, Z.; Zhang, J.; Zhang, J.; Li, G. Efficient base-free direct oxidation of glucose to gluconic acid over TiO2-supported gold clusters. Nanoscale 2018, 11, 1326–1334. [Google Scholar] [CrossRef]
- da Silva, A.G.; Rodrigues, T.S.; Candido, E.G.; de Freitas, I.C.; da Silva, A.H.; Fajardo, H.V.; Balzer, R.; Gomes, J.F.; Assaf, J.M.; de Oliveira, D.C.; et al. Combining active phase and support optimization in MnO2-Au nanoflowers: Enabling high activities towards green oxidations. J. Colloid Interface Sci. 2018, 530, 282–291. [Google Scholar] [CrossRef]
- Megías-Sayago, C.; Reina, T.R.; Ivanova, S.; Odriozola, J.A. Au/CeO2-ZnO/Al2O3 as Versatile Catalysts for Oxidation Reactions: Application in Gas/Liquid Environmental Processes. Front. Chem. 2019, 7, 504. [Google Scholar] [CrossRef] [Green Version]
- Franz, S.; Shcherban, N.D.; Bezverkhyy, I.; Sergiienko, S.A.; Simakova, I.L.; Salmi, T.; Murzin, D.Y. Catalytic activity of gold nanoparticles deposited on N-doped carbon-based supports in oxidation of glucose and arabinose mixtures. Res. Chem. Intermed. 2021, 1–15. [Google Scholar] [CrossRef]
- Perovic, M.; Zeininger, L.; Oschatz, M. Immobilization of Gold-on-Carbon Catalysts Onto Perfluorocarbon Emulsion Droplets to Promote Oxygen Delivery in Aqueous Phase D-Glucose Oxidation. ChemCatChem 2020, 13, 196–201. [Google Scholar] [CrossRef]
- Wojcieszak, R.; Ferraz, C.P.; Sha, J.; Houda, S.; Rossi, L.M.; Paul, S. Advances in Base-Free Oxidation of Bio-Based Compounds on Supported Gold Catalysts. Catalysts 2017, 7, 352. [Google Scholar] [CrossRef] [Green Version]
- Mihályi, M.R.; Kollár, M.; Klébert, S.; Mavrodinova, V. Transformation of ethylbenzene-m-xylene feed over MCM-22 zeolites with different acidities. Appl. Catal. A Gen. 2014, 476, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Shvets, O.; Shamzhy, M.; Yaremov, P.S.; Musilová, Z.; Procházková, D.; Čejka, J. Isomorphous Introduction of Boron in Germanosilicate Zeolites with UTL Topology. Chem. Mater. 2011, 23, 2573–2585. [Google Scholar] [CrossRef]
- Zhang, X.; Lai, E.S.M.; Martin-Aranda, R.; Yeung, K.L. An investigation of Knoevenagel condensation reaction in microreactors using a new zeolite catalyst. Appl. Catal. A Gen. 2004, 261, 109–118. [Google Scholar] [CrossRef]
- Chatti, R.; Bansiwal, A.K.; Thote, J.A.; Kumar, V.; Jadhav, P.; Lokhande, S.K.; Biniwale, R.B.; Labhsetwar, N.K.; Rayalu, S.S. Amine loaded zeolites for carbon dioxide capture: Amine loading and adsorption studies. Microporous Mesoporous Mater. 2009, 121, 84–89. [Google Scholar] [CrossRef]
- Baerlocher, C.; McCusker, L. Database of Zeolite Structures. Framework Type LTA (Material: Linde Type A, Zeolite A). 2020. Available online: http://www.iza-structure.org/databases/ (accessed on 10 July 2021).
- Ishida, T.; Murayama, T.; Taketoshi, A.; Haruta, M. Importance of Size and Contact Structure of Gold Nanoparticles for the Genesis of Unique Catalytic Processes. Chem. Rev. 2019, 120, 464–525. [Google Scholar] [CrossRef] [Green Version]
- Ouellette, R.J.; Rawn, J.D. Aldehydes and Ketones: Nucleophilic Addition Reactions. In Organic Chemistry: Structure, Mechanism, Synthesis, 2nd ed.; Elsevier: Oxford, UK, 2018; pp. 595–623. [Google Scholar] [CrossRef]
- Wolska, J.; Walkowiak, A.; Sobczak, I.; Wolski, L.; Ziolek, M. Gold-containing Beta zeolite in base-free glucose oxidation—The role of Au deposition procedure and zeolite dopants. Catal. Today 2021. [Google Scholar] [CrossRef]
- Prati, L.; Villa, A.; Lupini, A.R.; Veith, G.M. Gold on carbon: One billion catalysts under a single label. Phys. Chem. Chem. Phys. 2012, 14, 2969–2978. [Google Scholar] [CrossRef] [PubMed]
- Megías-Sayago, C.; Santos, J.L.; Ammari, F.; Chenouf, M.; Ivanova, S.; Centeno, M.; Odriozola, J. Influence of gold particle size in Au/C catalysts for base-free oxidation of glucose. Catal. Today 2018, 306, 183–190. [Google Scholar] [CrossRef]
- Khawaji, M.; Zhang, Y.; Loh, M.; Graça, I.; Ware, E.; Chadwick, D. Composition dependent selectivity of bimetallic Au-Pd NPs immobilised on titanate nanotubes in catalytic oxidation of glucose. Appl. Catal. B Environ. 2019, 256, 117799. [Google Scholar] [CrossRef]
- Roth, W.; Chlubná, P.; Kubů, M.; Vitvarová, D. Swelling of MCM-56 and MCM-22P with a new medium—surfactant–tetramethylammonium hydroxide mixtures. Catal. Today 2013, 204, 8–14. [Google Scholar] [CrossRef]
- Leonowicz, M.E.; Lawton, J.A.; Lawton, S.L.; Rubin, M.K. MCM-22: A Molecular Sieve with Two Independent Multidimensional Channel Systems. Science 1994, 264, 1910–1913. [Google Scholar] [CrossRef]
- Gil, B.; Marszałek, B.; Micek-Ilnicka, A.; Olejniczak, Z. The Influence of Si/Al Ratio on the Distribution of OH Groups in Zeolites with MWW Topology. Top. Catal. 2010, 53, 1340–1348. [Google Scholar] [CrossRef] [Green Version]
- Koranyi, T.I.; Nagy, J.B. Distribution of Aluminum and Boron in the Periodical Building Units of Boron-Containing β Zeolites. J. Phys. Chem. B 2006, 110, 14728–14735. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ernst, H.; Freude, D.; Scheffler, F.; Schwieger, W. In situ 11B MAS NMR study of the synthesis of a boron-containing MFI type zeolite. Microporous Mesoporous Mater. 2002, 54, 319–330. [Google Scholar] [CrossRef]
- Parry, E. An infrared study of pyridine adsorbed on acidic solids. Characterization of surface acidity. J. Catal. 1963, 2, 371–379. [Google Scholar] [CrossRef]
- Busch, O.M.; Brijoux, W.; Thomson, S.J.; Schüth, F. Spatially resolving infrared spectroscopy for parallelized characterization of acid sites of catalysts via pyridine sorption: Possibilities and limitations. J. Catal. 2004, 222, 174–179. [Google Scholar] [CrossRef]
- Emeis, C. Determination of Integrated Molar Extinction Coefficients for Infrared Absorption Bands of Pyridine Adsorbed on Solid Acid Catalysts. J. Catal. 1993, 141, 347–354. [Google Scholar] [CrossRef]
- Sekhar, A.S.; Sivaranjani, K.; Gopinath, C.S.; Vinod, C. A simple one pot synthesis of nano gold–mesoporous silica and its oxidation catalysis. Catal. Today 2012, 198, 92–97. [Google Scholar] [CrossRef]
- da Silva, F.P.; Fiorio, J.L.; Rossi, L.M. Tuning the Catalytic Activity and Selectivity of Pd Nanoparticles Using Ligand-Modified Supports and Surfaces. ACS Omega 2017, 2, 6014–6022. [Google Scholar] [CrossRef]
- Feldheim, D.L.; Foss, C.A. Metal Nanoparticles Synthesis: Characterization and Applications; Marcel Dekker: New York, NY, USA, 2002. [Google Scholar]
- Nakamura, T.; Herbani, Y.; Ursescu, D.; Banici, R.; Dabu, R.V.; Sato, S. Spectroscopic study of gold nanoparticle formation through high intensity laser irradiation of solution. AIP Adv. 2013, 3, 082101. [Google Scholar] [CrossRef] [Green Version]
- Abdelhalim, M.A.K.; Mady, M.M.; Ghannam, M.M. Physical Properties of Different Gold Nanoparticles: Ultraviolet-Visible and Fluorescence Measurements. J. Nanomed. Nanotechnol. 2012, 3. [Google Scholar] [CrossRef]
- Dyer, J.R. Applications of Absorption Spectroscopy of Organic Compounds; Prentice-Hall: Englewood Cliffs, NJ, USA, 1965. [Google Scholar]
- Tiwari, I.; Singh, M.; Tripathi, V.S.; Lakshminarayana, G.; Nogami, M. An Amperometric Sensor for Nanomolar Detection of Hydrogen Peroxide Based on Encapsulation of Horseradish Peroxidase in Thymol Blue-Ormosil Composite. Sens. Lett. 2011, 9, 1323–1330. [Google Scholar] [CrossRef]
- Sobczak, I.; Calvino-Casilda, V.; Wolski, L.; Siodła, T.; Martin-Aranda, R.; Ziolek, M. The role of gold dopant in AP-Nb/MCF and AP-MCF on the Knoevenagel condensation of ethyl cyanoacetate with benzaldehyde and 2,4-dichlorobenzaldehyde. Catal. Today 2018, 325, 81–88. [Google Scholar] [CrossRef]
- Dias, D.R.; Moreira, A.F.; Correia, I.J. The effect of the shape of gold core–mesoporous silica shell nanoparticles on the cellular behavior and tumor spheroid penetration. J. Mater. Chem. B 2016, 4, 7630–7640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sham, T.K. X-Ray Studies of the Structure and Electronic Behavior of Alkanethiolate-Capped Gold Nanoparticles: The Interplay of Size and Surface Effects. Phys. Rev. Lett. 2003, 90, 245502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Li, J.; Zhou, J.; Liu, Y.; Wei, Z.; Zhang, H. Layered double hydroxides supported atomically precise Aun nanoclusters for air oxidation of benzyl alcohol: Effects of size and active site structure. J. Catal. 2020, 389, 409–420. [Google Scholar] [CrossRef]
- Besson, M.; Gallezot, P. Selective oxidation of alcohols and aldehydes on metal catalysts. Catal. Today 2000, 57, 127–141. [Google Scholar] [CrossRef]
- Sharma, A.S.; Kaur, H.; Shah, D. Selective oxidation of alcohols by supported gold nanoparticles: Recent advances. RSC Adv. 2016, 6, 28688–28727. [Google Scholar] [CrossRef]
- Ibrahim, M.; Alaam, M.; Elhaes, H.; Jalbout, A.F.; De Leon, A. Analysis of the structure and vibrational spectra of glucose and fructose. Eclet. Quim. 2006, 31, 15–21. [Google Scholar] [CrossRef]
- Liu, X.; Wang, A.; Yang, X.; Zhang, T.; Mou, C.-Y.; Su, D.-S.; Li, J. Synthesis of Thermally Stable and Highly Active Bimetallic Au−Ag Nanoparticles on Inert Supports. Chem. Mater. 2008, 21, 410–418. [Google Scholar] [CrossRef]
- Wang, A.; Liu, X.Y.; Mou, C.-Y.; Zhang, T. Understanding the synergistic effects of gold bimetallic catalysts. J. Catal. 2013, 308, 258–271. [Google Scholar] [CrossRef]
- Graf, N.; Yegen, E.; Gross, T.; Lippitz, A.; Weigel, W.; Krakert, S.; Terfort, A.; Unger, W.E. XPS and NEXAFS studies of aliphatic and aromatic amine species on functionalized surfaces. Surf. Sci. 2009, 603, 2849–2860. [Google Scholar] [CrossRef]
- Qi, P.; Chen, S.; Chen, J.; Zheng, J.; Zheng, X.; Yuan, Y. Catalysis and Reactivation of Ordered Mesoporous Carbon-Supported Gold Nanoparticles for the Base-Free Oxidation of Glucose to Gluconic Acid. ACS Catal. 2015, 5, 2659–2670. [Google Scholar] [CrossRef]
- Wisniewska, J.; Sobczak, I.; Ziolek, M. Gold based on SBA-15 supports–Promising catalysts in base-free glucose oxidation. Chem. Eng. J. 2020, 413, 127548. [Google Scholar] [CrossRef]
- Zhuge, Y.; Fan, G.; Lin, Y.; Yang, L.; Li, F. A hybrid composite of hydroxyapatite and Ca–Al layered double hydroxide supported Au nanoparticles for highly efficient base-free aerobic oxidation of glucose. Dalton Trans. 2019, 48, 9161–9172. [Google Scholar] [CrossRef] [PubMed]
- Kiyonaga, T.; Jin, Q.; Kobayashi, H.; Tada, H. Size-Dependence of Catalytic Activity of Gold Nanoparticles Loaded on Titanium (IV) Dioxide for Hydrogen Peroxide Decomposition. ChemPhysChem 2009, 10, 2935–2938. [Google Scholar] [CrossRef] [PubMed]
- Rautiainen, S.; Lehtinen, P.; Vehkamäki, M.; Niemelä, K.; Kemell, M.; Heikkilä, M.; Repo, T. Microwave-assisted base-free oxidation of glucose on gold nanoparticle catalysts. Catal. Commun. 2016, 74, 115–118. [Google Scholar] [CrossRef]
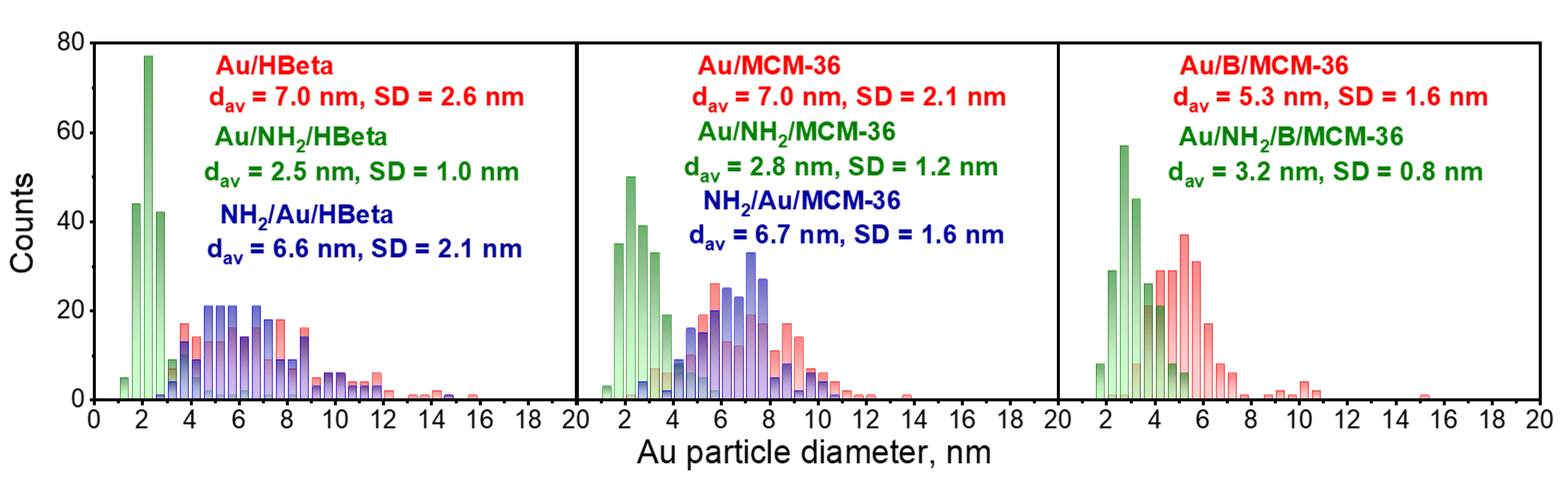
| Entry | Catalyst | %wt. Au a | %wt. N b | Au NPs Size c, nm | BET Surface Area, m2 g−1 | t-Plot External Surface Area m2 g−1 | Single Point Total Pore Volume d cm3 g−1 | t-Plot Micropore Volume, cm3 g−1 |
|---|---|---|---|---|---|---|---|---|
| 1. | HBeta | – | traces | – | 526 | 149 | 0.34 | 0.20 |
| 2. | Au/HBeta | 1.87 | traces | 7.0 | 338 | 82 | 0.24 | 0.14 |
| 3. | Au/NH2/HBeta | 2.37 | 1.56 | 2.5 | 132 | 78 | 0.13 | 0.03 |
| 4. | NH2/Au/HBeta | 1.78 | 1.74 | 6.6 | 57 | 25 | 0.04 | 0.02 |
| 5. | MCM-36 | – | traces | – | 534 | 361 | 0.54 | 0.10 |
| 6. | Au/MCM-36 | 1.84 | traces | 7.0 | 413 | 191 | 0.43 | 0.10 |
| 7. | Au/NH2/MCM-36 | 2.33 | 1.50 | 2.8 | 217 | 154 | 0.32 | 0.03 |
| 8. | NH2/Au/MCM-36 | 1.91 | 1.36 | 6.7 | 210 | 106 | 0.24 | 0.06 |
| 9. | B/MCM-36 | – | traces | – | 546 | 401 | 0.53 | 0.08 |
| 10. | Au/B/MCM-36 | 1.58 | traces | 5.3 | 401 | 241 | 0.41 | 0.09 |
| 11. | Au/NH2/B/MCM-36 | 1.55 | 1.87 | 3.2 | 108 | 38 | 0.21 | 0.02 |
| Entry | Catalyst | Evacuation Temp., °C | Number of BAS Occupied by Pyridine, after Evacuation, μmol g−1 | Number of LAS Occupied by Pyridine after Evacuation at 300 °C, μmol g−1 | Pyridine Desorbed at 300 °C from BAS, % a | BAS/LAS Ratio After Evacuation at 300 °C |
|---|---|---|---|---|---|---|
| 1. | HBeta | 150 | 434 | – | 34.3 | 1.54 |
| 200 | 452 | – | ||||
| 250 | 360 | – | ||||
| 300 | 297 | 193 | ||||
| 2. | Au/HBeta | 150 | 344 | – | 44.8 | 1.49 |
| 200 | 348 | – | ||||
| 250 | 244 | – | ||||
| 300 | 192 | 129 | ||||
| 3. | MCM-36 | 150 | 425 | – | 22.4 | 5.73 |
| 200 | 406 | – | ||||
| 250 | 373 | – | ||||
| 300 | 315 | 55 | ||||
| 4. | Au/MCM-36 | 150 | 188 | – | 24.7 | 3.77 |
| 200 | 150 | – | ||||
| 250 | 148 | – | ||||
| 300 | 113 | 30 | ||||
| 5. | Au/B/MCM-36 | 150 | 198 | – | 35.2 | 4.07 |
| 200 | 176 | – | ||||
| 250 | 150 | – | ||||
| 300 | 114 | 28 | ||||
| 6. | B/MCM-36 | 150 | 482 | – | 5.3 | 6.31 |
| 200 | 360 | – | ||||
| 250 | 328 | – | ||||
| 300 | 341 | 54 |
| Entry | Catalyst | Glucose Conv. a, % | Selectivity, % | TOF b, h−1 | |
|---|---|---|---|---|---|
| A | B | ||||
| 1. | HBeta | 1.2 | 82.0 | 18.0 | – |
| 2. | Au/HBeta | 50.6 | 98.8 | 1.2 | 24,200 |
| 3. | Au/NH2/HBeta | 60.7 | 99.2 | 0.8 | 10,400 |
| 4. | NH2/Au/HBeta | 12.0 | >99.9 | traces | 5410 |
| 5. | MCM-36 | 6.2 | 89.0 | 11.0 | – |
| 6. | Au/MCM-36 | 65.2 | 99.2 | 0.8 | 31,300 |
| 7. | Au/NH2/MCM-36 | 77.5 | 99.3 | 0.7 | 14,800 |
| 8. | NH2/Au/MCM-36 | 61.0 | 99.1 | 0.9 | 28,000 |
| 9. | B/MCM-36 | <1 | >99.9 | traces | – |
| 10. | Au/B/MCM-36 | 45.8 | 98.9 | 1.1 | 16,600 |
| 11. | Au/NH2/B/MCM-36 | 68.1 | 99.1 | 0.9 | 14,900 |
| Entry | Catalyst Symbol | %wt. Au a | Au NPs Size, nm | Reaction Conditions | TOF, h−1 | Reference |
|---|---|---|---|---|---|---|
| 1. | Au/MCM-36 | 1.84 | 7.0 | 20 mL of 0.2 M glucose solution, glucose/Au molar ratio = 1970, T = 110 °C, p(O2) = 0.5 MPa | 31,300 a | This work |
| 2. | Au/TH-150 | 0.5 | 1.2 | 10 mL of 0.1 M glucose solution, glucose/Au molar ratio = 1000, T = 110 °C, p(O2) = 1 MPa | 1920 b | [4] |
| 3. | Au/CMK-3 | 0.94 | 3.0 | 20 mL of 0.1 M glucose solution, glucose/Au molar ratio = 1000, T = 110 °C, p(O2) = 0.3 MPa | 17,700 c | [46] |
| 4. | Au-HBeta(AP) | 1.4 | 6.0 | 20 mL of 0.2 M glucose solution, glucose/Au molar ratio = 1970, T = 110 °C, p(O2) = 0.5 MPa | 26,500 d | [17] |
| 5. | Au/SBA-15 | 2.1 | 4.9 | 10 mL of 0.2 M glucose solution glucose/Au molar ratio = 1970, T = 110 °C, p(O2) = 0.5 MPa | 16,900 d | [47] |
| 6. | Au/HAP-LDH | 0.22 | 6.6 | 12 mL of 0.167 M glucose solution glucose/Au molar ratio = 1000, T = 110 °C, p(O2) = 0.5 MPa | 20,200 e | [48] |
| Entry | Catalyst | Glucose Conv. a, % | Selectivity, % | TOF b, h−1 | |
|---|---|---|---|---|---|
| A | B | ||||
| 1. | HBeta | 13.3 | 93.9 | 6.1 | |
| 2. | Au/HBeta | 49.1 | 99.0 | 1.0 | 158,000 |
| 3. | Au/NH2/HBeta | 44.4 | 98.9 | 1.1 | 50,900 |
| 4. | NH2/Au/HBeta | 12.2 | 95.2 | 4.8 | 37,100 |
| 5. | MCM-36 | 3.8 | 83.6 | 16.4 | – |
| 6. | Au/MCM-36 | 59.2 | 99.2 | 0.8 | 191,000 |
| 7. | Au/NH2/MCM-36 | 59.5 | 99.2 | 0.8 | 76,500 |
| 8. | NH2/Au/MCM-36 | 45.9 | 99.0 | 1.0 | 142,000 |
| 9. | B/MCM-36 | 7.2 | 72.2 | 27.7 | – |
| 10. | Au/B/MCM-36 | 49.5 | 99.0 | 1.0 | 121,000 |
| 11. | Au/NH2/B/MCM-36 | 56.6 | 99.1 | 0.9 | 83,800 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walkowiak, A.; Wolska, J.; Wojtaszek-Gurdak, A.; Sobczak, I.; Wolski, L.; Ziolek, M. Modification of Gold Zeolitic Supports for Catalytic Oxidation of Glucose to Gluconic Acid. Materials 2021, 14, 5250. https://doi.org/10.3390/ma14185250
Walkowiak A, Wolska J, Wojtaszek-Gurdak A, Sobczak I, Wolski L, Ziolek M. Modification of Gold Zeolitic Supports for Catalytic Oxidation of Glucose to Gluconic Acid. Materials. 2021; 14(18):5250. https://doi.org/10.3390/ma14185250
Chicago/Turabian StyleWalkowiak, Adrian, Joanna Wolska, Anna Wojtaszek-Gurdak, Izabela Sobczak, Lukasz Wolski, and Maria Ziolek. 2021. "Modification of Gold Zeolitic Supports for Catalytic Oxidation of Glucose to Gluconic Acid" Materials 14, no. 18: 5250. https://doi.org/10.3390/ma14185250
APA StyleWalkowiak, A., Wolska, J., Wojtaszek-Gurdak, A., Sobczak, I., Wolski, L., & Ziolek, M. (2021). Modification of Gold Zeolitic Supports for Catalytic Oxidation of Glucose to Gluconic Acid. Materials, 14(18), 5250. https://doi.org/10.3390/ma14185250