Engineering Commercial TiO2 Powder into Tailored Beads for Efficient Water Purification
Abstract
:1. Introduction
2. Materials and Methods
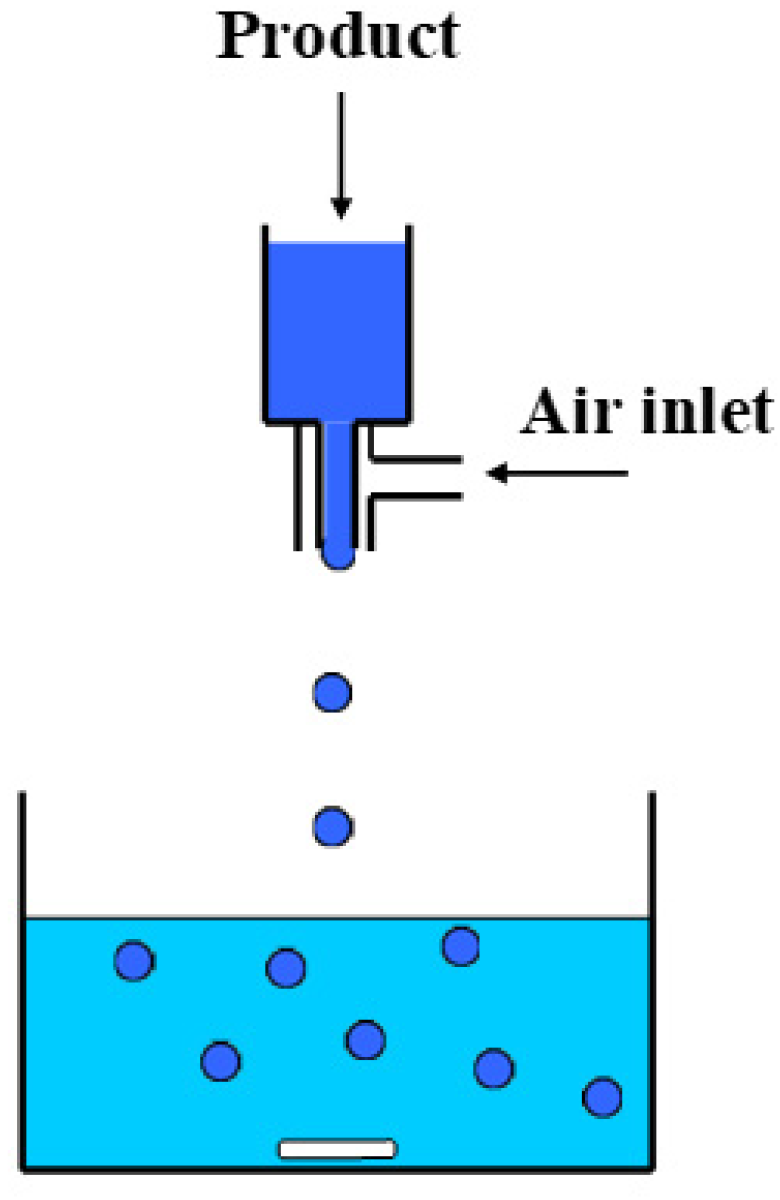
2.1. Fabrication of Beads
2.2. Characterization Techniques
2.3. Photocatalytic Experiments of the Developed Beads
3. Results and Discussion
3.1. Materials Characterization
3.1.1. Morphological Analysis of the Ceramic Beads
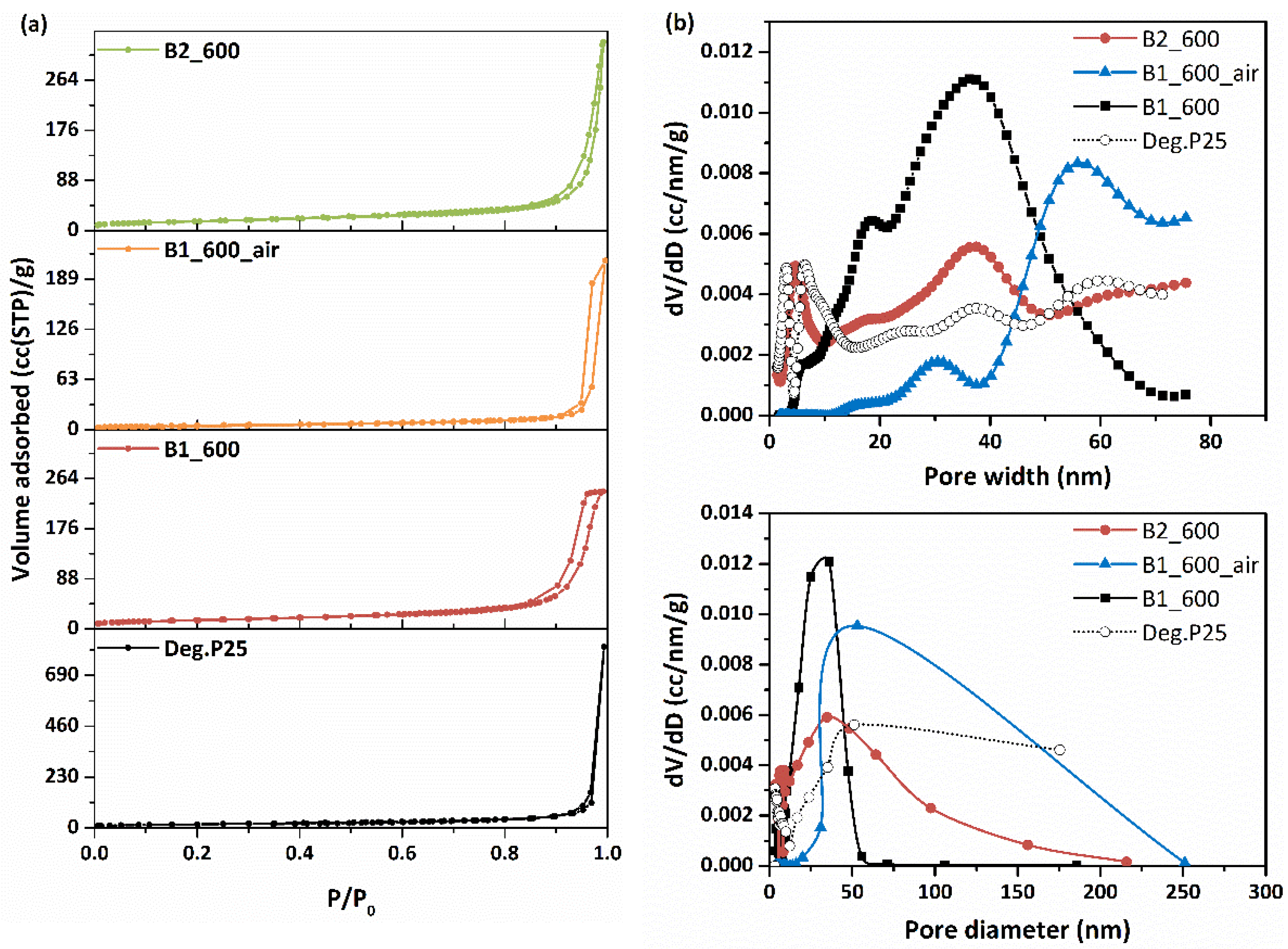
3.1.2. LN2 Porosimetry of Ceramic Beads
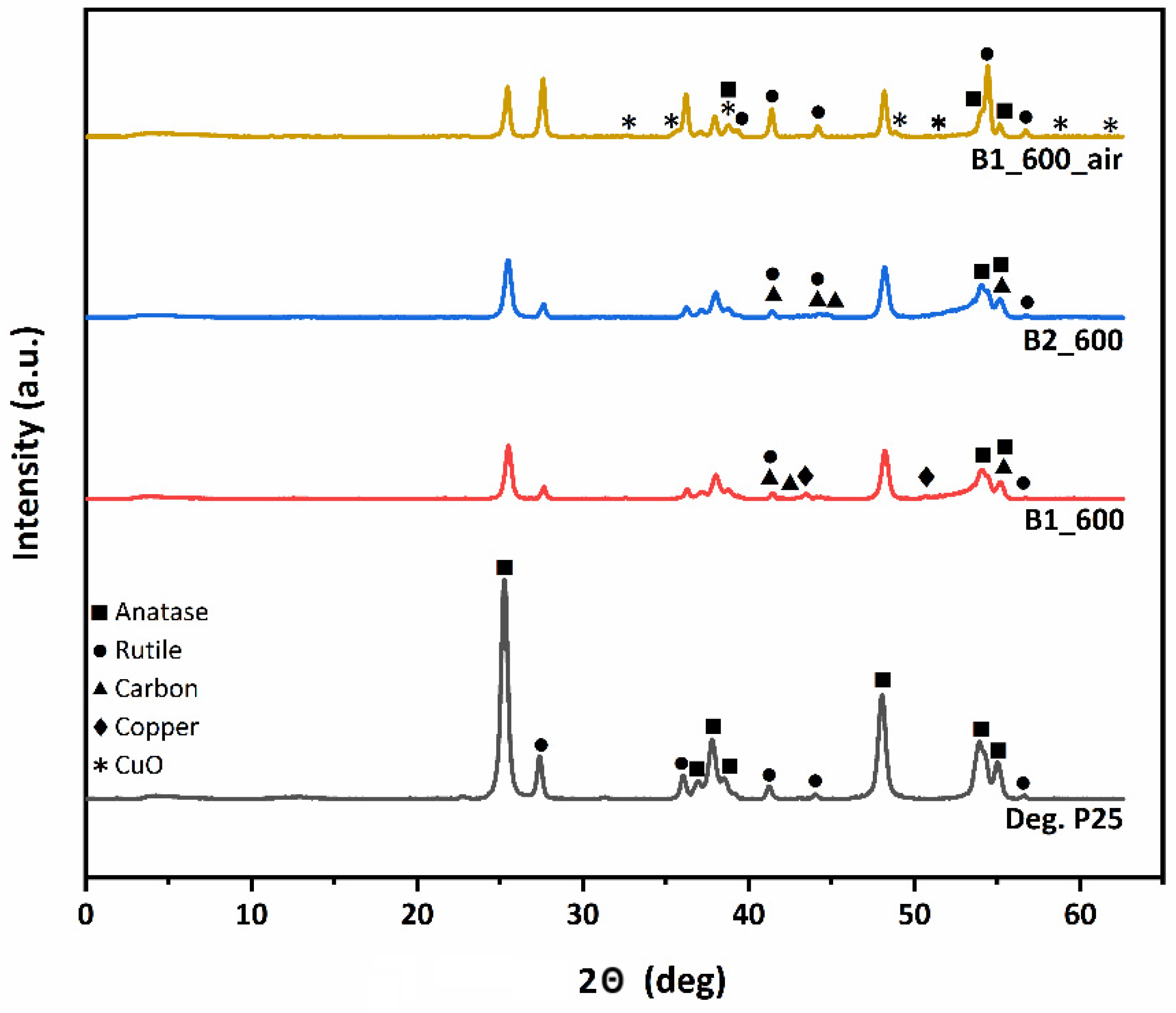
3.2. XRD Analysis
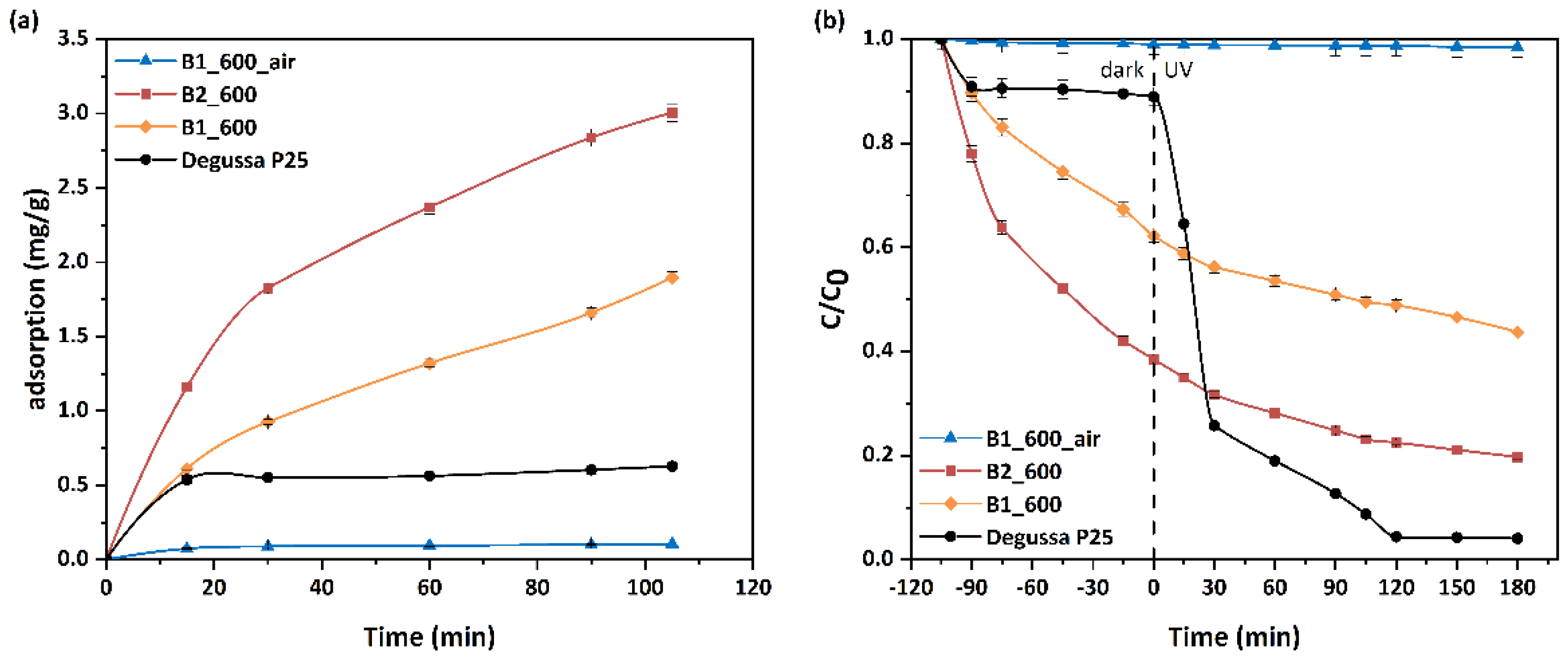
3.3. MO Adsorption and Photocatalytic Performance of Beads
3.4. Stability of the Photocatalytic Performance of Beads
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Chkirida, S.; Zari, N.; Achour, R.; Hassoune, H.; Lachehab, A.; El kacem Qaiss, A.; Bouhfid, R. Highly synergic adsorption/photocatalytic efficiency of alginate/bentonite impregnated TiO2 beads for wastewater treatment. J. Photochem. Photobiol. A Chem. 2021, 412, 113215. [Google Scholar] [CrossRef]
- Theodorakopoulos, G.; Athanasekou, C.; Romanos, G.E.; Papageorgiou, S.K. Current photocatalytic systems for intensified water purification applications. In Handbook of Smart Photocatalytic Materials: Fundamentals, Fabrications, and Water Resources Applications; Hussain, C.M., Mishra, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 231–232. [Google Scholar] [CrossRef]
- Wang, C.; Liu, H.; Qu, Y. TiO2-based photocatalytic process for purification of polluted water: Bridging fundamentals to applications. J. Nanomater. 2013, 2013, 319637. [Google Scholar] [CrossRef] [Green Version]
- Ibhadon, A.O.; Fitzpatrick, P. Heterogeneous photocatalysis: Recent advances and applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef] [Green Version]
- Ajmal, A.; Majeed, Ι.; Malik, R.N.; Idriss, H.; Nadeem, M.A. Principles and mechanisms of photocatalytic dye degradation on TiO2 based photocatalysts: A comparative overview. RSC Adv. 2014, 4, 37003–37026. [Google Scholar] [CrossRef]
- Taghizadeh, M.T.; Siyahi, V.; Ashassi-Sorkhabi, H.; Zarrini, G. ZnO, AgCl and AgCl/ZnO nanocomposites incorporated chitosan in the form of hydrogel beads for photocatalytic degradation of MB, E. coli and S. aureus. Int. J. Biol. Macromol. 2020, 147, 1018–1028. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Cui, Y.; Du, H.; Wen, L. Doped-TiO2 Photocatalysts and synthesis methods to prepare TiO2 films. J. Mat. Sci. Technol. 2008, 24, 675–689. [Google Scholar]
- Colmenares, J.C.; Luque, R.; Campelo, J.M.; Colmenares, F.; Karpiński, Z.; Romero, A.A. Nanostructured photocatalysts and their applications in the photocatalytic transformation of lignocellulosic biomass: An overview. Materials 2009, 2, 2228–2258. [Google Scholar] [CrossRef]
- Xing, Z.; Zhang, J.; Cui, J.; Yin, J.; Zhao, T.; Kuang, J.; Xiu, Z.; Wan, N.; Zhou, W. Recent advances in floating TiO2-based photocatalysts for environmental application. Appl. Catal. B Environ. 2018, 225, 452–467. [Google Scholar] [CrossRef]
- Yahya, N.; Aziz, F.; Jamaludin, N.A.; Mutalib, M.A.; Ismail, A.F.; Salleh, W.N.W.; Jaafar, J.; Yusof, N.; Ludin, N.A. A review of integrated photocatalyst adsorbents for wastewater treatment. J. Environ. Chem. Eng. 2018, 6, 7411–7425. [Google Scholar] [CrossRef]
- Molinari, R.; Lavorato, C.; Argurio, P. Visible-light photocatalysts and their perspectives for building photocatalytic membrane reactors for various liquid phase chemical conversions. Catalysts 2020, 10, 1334. [Google Scholar] [CrossRef]
- Fabiyi, M.E.; Skelton, R.L. Photocatalytic mineralisation of methylene blue using buoyant TiO2-coated polystyrene beads. J. Photochem. Photobiol. A Chem. 2000, 132, 121–128. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, X.; Wang, H.; Chen, A.; Liu, S.; Guo, Y.; He, Y.; Hu, X.; Li, J.; Liu, S.; et al. Photoreduction of Cr(VI) from acidic aqueous solution using TiO2-impregnated glutaraldehyde-crosslinked alginate beads and the effects of Fe(III) ions. Chem. Eng. J. 2013, 226, 131–138. [Google Scholar] [CrossRef]
- Verma, A.; Prakash, N.T.; Toor, A.P. An efficient TiO2 coated immobilized system for the degradation studies of herbicide isoproturon: Durability studies. Chemosphere 2014, 109, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Nouri, L.; Hemidouche, S.; Boudjemaa, A.; Kaouah, F.; Sadaoui, Z.; Bachari, K. Elaboration and characterization of photobiocomposite beads, based on titanium (IV) oxide and sodium alginate biopolymer, for basic blue 41 adsorption/photocatalytic degradation. Int. J. Biol. Macromol. 2020, 151, 66–84. [Google Scholar] [CrossRef]
- Dijkstra, M.F.J.; Michorius, A.; Buwalda, H.; Panneman, H.J.; Winkelman, J.G.M.; Beenackers, A.A.C.M. Comparison of the efficiency of immobilized and suspended systems in photocatalytic degradation. Catal. Today 2001, 66, 487–494. [Google Scholar] [CrossRef]
- Mehrotra, K.; Yablonsky, G.S.; Ray, A.K. Kinetic studies of photocatalytic degradation in a TiO2 slurry system: Distinguishing working regimes and determining rate dependences. Ind. Eng. Chem. Res. 2003, 42, 2273–2281. [Google Scholar] [CrossRef]
- Jawad, A.H.; Mubarak, N.S.A.; Ishak, M.A.M.; Ismail, K.; Nawawi, W.I. Kinetics of photocatalytic decolourization of cationic dye using porous TiO2 film. J. Taibah Univ. Sci. 2016, 10, 352–362. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, R.; Ahmad, Z.; Khan, A.U.; Mastoi, N.R.; Aslam, M.; Kim, J. Photocatalytic systems as an advanced environmental remediation: Recent developments, limitations and new avenues for applications. J. Environ. Chem. Eng. 2016, 4, 4143–4164. [Google Scholar] [CrossRef]
- Hui, J.; Pestana, C.J.; Caux, M.; Gunaratne, H.Q.N.; Edwards, C.; Robertson, P.K.J.; Lawton, L.A.; Irvine, J.T.S. Graphitic-C3N4 coated floating glass beads for photocatalytic destruction of synthetic and natural organic compounds in water under UV light. J. Photochem. Photobiol. A Chem. 2021, 405, 112935. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, H.; Yang, M. TiO2 nanoparticles supported on PMMA nanofibers for photocatalytic degradation of methyl orange. J. Colloid Interface Sci. 2017, 508, 500–507. [Google Scholar] [CrossRef]
- Altın, İ.; Sökmen, M. Buoyant photocatalyst based on ZnO immobilized on polystyrene beads for pollutants treatment. Clean Soil Air Water 2015, 43, 1025–1030. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Appunni, S.; Gopalram, K. Immobilized TiO2/chitosan beads for photocatalytic degradation of 2,4-dichlorophenoxyacetic acid. Int. J. Biol. Macromol. 2020, 161, 282–291. [Google Scholar] [CrossRef]
- Papageorgiou, S.K.; Kouvelos, E.P.; Katsaros, F.K. Calcium alginate beads from Laminaria digitata for the removal of Cu2+ and Cd2+ from dilute aqueous metal solutions. Desalination 2008, 224, 293–306. [Google Scholar] [CrossRef]
- Isik, Z.; Bilici, Z.; Adiguzel, S.K.; Yatmaz, H.C.; Dizge, N. Entrapment of TiO2 and ZnO powders in alginate beads: Photocatalytic and reuse efficiencies for dye solutions and toxicity effect for DNA damage. Environ. Technol. Innov. 2019, 14, 100358. [Google Scholar] [CrossRef]
- Dalponte, I.; de Sousa, B.C.; Mathias, A.L.; Jorge, R.M.M. Formulation and optimization of a novel TiO2/calcium alginate floating photocatalyst. Int. J. Biol. Macromol. 2019, 137, 992–1001. [Google Scholar] [CrossRef]
- Choi, B.Y.; Park, H.J.; Hwang, S.J.; Park, J.B. Preparation of alginate beads for floating drug delivery system: Effects of CO2 gas-forming agents. Int. J. Pharm. 2002, 239, 81–91. [Google Scholar] [CrossRef]
- Yousif, E.; Haddad, R. Photodegradation and photostabilization of polymers, especially polystyrene: Review. SpringerPlus 2013, 2, 398–429. [Google Scholar] [CrossRef] [Green Version]
- Siew, C.K.; Williams, P.A.; Young, N.W.G. New insights into the mechanism of gelation of alginate and pectin: Charge annihilation and reversal mechanism. Biomacromolecules 2005, 6, 963–969. [Google Scholar] [CrossRef]
- Liu, S.; Li, H.; Tang, B.; Bi, S.; Li, L. Scaling law and microstructure of alginate hydrogel. Carbohydr. Polym. 2016, 135, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Tahtat, D.; Bouaicha, M.N.; Benamer, S.; Nacer-Khodja, A.; Mahlous, M. Development of alginate gel beads with a potential use in the treatment against acute lead poisoning. Int. J. Biol. Macromol. 2017, 105, 1010–1016. [Google Scholar] [CrossRef]
- Soares, J.P.; Santos, J.E.; Chierice, G.O.; Cavalheiro, E.T.G. Thermal behavior of alginic acid and its sodium salt. Eclética Química 2004, 29, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Papageorgiou, S.K.; Katsaros, F.K.; Kouvelos, E.P.; Nolan, J.W.; Deit, H.L.; Kanellopoulos, N.K. Heavy metal sorption by calcium alginate beads from Laminaria digitata. J. Hazard. Mater. 2006, 137, 1765–1772. [Google Scholar] [CrossRef]
- Theodorakopoulos, G.V.; Romanos, G.E.; Katsaros, F.K.; Papageorgiou, S.K.; Kontos, A.G.; Spyrou, K.; Beazi-Katsioti, M.; Falaras, P. Structuring efficient photocatalysts into bespoke fiber shaped systems for applied water treatment. Chemosphere 2021, 277, 130253. [Google Scholar] [CrossRef]
- Lowell, S.; Shields, J.E. Pore analysis by adsorption. In Powder Surface Area and Porosity, 3rd ed.; Lowell, S., Shields, J.E., Eds.; Chapman & Hall Ltd.: London, UK, 1991; pp. 52–71. [Google Scholar]
- Gregg, S.J.; Sing, K.S.W. Introduction. In Adsorption, Surface Area and Porosity, 2nd ed.; Gregg, S.J., Sing, K.S.W., Eds.; Academic Press Inc. (London) Ltd.: London, UK, 1982; pp. 1–40. [Google Scholar]
- Papageorgiou, S.K.; Favvas, E.P.; Sapalidis, A.A.; Romanos, G.E.; Katsaros, F.K. Facile synthesis of carbon supported copper nanoparticles from alginate precursor with controlled metal content and catalytic NO reduction properties. J. Hazard. Mater. 2011, 189, 384–390. [Google Scholar] [CrossRef]
- Woelki, S.; Kohler, H.-H. Orientation of chain molecules in ionotropic gels: A Brownian dynamics model. Chem. Phys. 2003, 293, 323–340. [Google Scholar] [CrossRef]
- Quong, D.; Neufeld, R.J.; Skjåk-Bræk, G.; Poncelet, D. External versus internal source of calcium during the gelation of alginate beads for DNA encapsulation. Biotechnol. Bioeng. 1998, 57, 438–446. [Google Scholar] [CrossRef]
- Zhao, Y.; Carvajal, M.T.; Won, Y.-Y.; Harris, M.T. Preparation of calcium alginate microgel beads in an electrodispersion reactor using an internal source of calcium carbonate nanoparticles. Langmuir 2007, 23, 12489–12496. [Google Scholar] [CrossRef]
- Wang, S.; Vincent, T.; Faur, C.; Guibal, E. Alginate and algal-based beads for the sorption of metal cations: Cu(II) and Pb(II). Int. J. Mol. Sci. 2016, 17, 1453. [Google Scholar] [CrossRef] [Green Version]
- Jeong, C.; Kim, S.; Lee, C.; Cho, S.; Kim, S.-B. Changes in the physical properties of calcium alginate gel beads under a wide range of gelation temperature conditions. Foods 2020, 9, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, A.W.; Whitney, R.A.; Neufeld, R.J. Semisynthesis of a controlled stimuli-responsive alginate hydrogel. Biomacromolecules 2009, 10, 609–616. [Google Scholar] [CrossRef]
- Spurr, R.A.; Myers, H. Quantitative analysis of anatase-rutile mixtures with an X-ray diffractometer. Anal. Chem. 1957, 29, 760–762. [Google Scholar] [CrossRef]
- Fang, Y.; Al-Assaf, S.; Phillips, G.O.; Nishinari, K.; Funami, T.; Williams, P.A.; Li, L. Multiple steps and critical behaviors of the binding of calcium to alginate. J. Phys. Chem. B 2007, 111, 2456–2462. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-F.; Wu, N.-Z.; Xie, Y.-C.; Tang, Y.-Q. A monolayer dispersion study of titania-supported copper oxide. J. Mater. Chem. 2000, 10, 1629–1634. [Google Scholar] [CrossRef]
- Gong, R.; Ye, J.; Dai, W.; Yan, X.; Hu, J.; Hu, X.; Li, S.; Huang, H. Adsorptive removal of methyl orange and methylene blue from aqueous solution with finger-citron-residue-based activated carbon. Ind. Eng. Chem. Res. 2013, 52, 14297–14303. [Google Scholar] [CrossRef]
- Ying, Y.; He, P.; Ding, G.; Peng, X. Ultrafast adsorption and selective desorption of aqueous aromatic dyes by graphene sheets modified by graphene quantum dots. Nanotechnology 2016, 27, 245703. [Google Scholar] [CrossRef]
- Bernal, V.; Giraldo, L.; Moreno-Piraján, J.C.; Balsamo, M.; Erto, A. Mechanisms of methylparaben adsorption onto activated carbons: Removal tests supported by a calorimetric study of the adsorbent-adsorbate interactions. Molecules 2019, 24, 413. [Google Scholar] [CrossRef] [Green Version]
- Manassero, A.; Satuf, M.L.; Alfano, O.M. Photocatalytic reactors with suspended and immobilized TiO2: Comparative efficiency evaluation. Chem. Eng. J. 2017, 326, 29–36. [Google Scholar] [CrossRef]
- Mukherjee, D.; Barghi, S.; Ray, A.K. Degradation of methyl orange by TiO2/polymeric film photocatalyst. Can. J. Chem. Eng. 2014, 92, 1661–1666. [Google Scholar] [CrossRef]
- Hadi, H.M.; Wahab, H.S. Visible light photocatalytic decolourization of methyl orange using n-doped TiO2 nanoparticles. J. Al-Nahrain Univ. Sci. 2015, 18, 1–9. [Google Scholar] [CrossRef]
- Guettaï, N.; Amar, H.A. Photocatalytic oxidation of methyl orange in presence of titanium dioxide in aqueous suspension. Part II: Kinetics study. Desalination 2005, 185, 439–448. [Google Scholar] [CrossRef]
- Andronic, L.; Duta, A. TiO2 thin films for dyes photodegradation. Thin Solid Film. 2007, 515, 6294–6297. [Google Scholar] [CrossRef]
- Devi, L.G.; Reddy, K.M. Enhanced photocatalytic activity of silver metallized TiO2 particles in the degradation of an azo dye methyl orange: Characterization and activity at different pH values. Appl. Surf. Sci. 2010, 256, 3116–3121. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.-T.; Ji, H.-B.; Huang, X.-J. Photocatalytic degradation of methyl orange over metalloporphyrins supported on TiO2 degussa P25. Molecules 2012, 17, 1149–1158. [Google Scholar] [CrossRef] [Green Version]
- Barka, N.; Qourzal, S.; Assabbane, A.; Ait-Ichou, Y. Kinetic modeling of the photocatalytic degradation of methyl orange by supported TiO2. J. Environ. Sci. Eng. 2010, 4, 1–5. [Google Scholar] [CrossRef]
- Bouarioua, A.; Zerdaoui, M. Photocatalytic activities of TiO2 layers immobilized on glass substrates by dip-coating technique toward the decolorization of methyl orange as a model organic pollutant. J. Environ. Chem. Eng. 2017, 5, 1565–1574. [Google Scholar] [CrossRef]

| Code Sample | Cross-Linker | Process | Τ | Isothermal Step | |
|---|---|---|---|---|---|
| Glutaraldehyde | Cu2+ | (°C) | (h) | ||
| B1_600 | − | + | pyrolysis | 600 | 6 |
| B1_600_air | − | + | calcination | 600 | 6 |
| B2_600 | + | + | pyrolysis | 600 | 6 |
| Sample | TPV 1 | SBET | Porosity ε | dmean 2 | dBJH 3 | Dparticle 4 |
|---|---|---|---|---|---|---|
| (mL/g) | (m2/g) | (%) | (nm) | (nm) | (nm) | |
| B1_600 | 0.374 | 53.8 | 59.3 | 27.8 | 36.1 | 29.5 |
| B1_600_air | 0.228 | 19.4 | 47.1 | 46.9 | 53.1 | 79.1 |
| B2_600 | 0.513 | 57.2 | 66.7 | 35.9 | 34.8 | 26.9 |
| Deg. P25 | 0.269 | 62.0 | 51.2 | 17.3 | 51.2 | 24.8 |
| Sample | Anatase (nm) | Rutile (nm) | wa (%) | wr (%) |
|---|---|---|---|---|
| B1_600 | 17.8 | 23.9 | 78.6 | 21.4 |
| B1_600_air | 24.1 | 25.2 | 39.9 | 60.1 |
| B2_600 | 17.4 | 23.8 | 80.0 | 20.0 |
| Deg. P25 | 17.3 | 23.6 | 81.0 | 19.0 |
| Form | Catalyst Amount (g/L) | Light Intensity (mW/cm2) | Results | Reference |
|---|---|---|---|---|
| powder | 0.8 | 9.4 | R ≈ 65% (15 ppm, 3 h) | [55] |
| powder | 1 | 11.1 | R ≈ 60% (15.6 ppm, 3 h) | [56] |
| powder | 0.16 | 7.75 | R ≈ 50% (10 ppm, 1 h) | [57] |
| powder | 0.3 | n/a | R ≈ 58% (20 ppm, 1.5 h) | [58] |
| coating * | 1 | 25.3 | R ≈ 55% (12 ppm, 3 h) | [59] |
| coating | 0.2 | 1.73 | R ≈ 29% (9.8 ppm, 3 h) | [60] |
| powder | 2.5 | 0.5 | R = 95.4% (12 ppm, 3 h) | This work |
| beads | 2.5 | 0.5 | R = 48.8% (12 ppm, 3 h), R = 80.3% for the overall process at the same conditions | This work |
| Sample | qe | R |
|---|---|---|
| (mg/g) | (%) | |
| B2_600 | 2.24 (3.00) | 43.2 (48.8) |
| B1_600 | 1.85 (1.90) | 25.3 (29.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theodorakopoulos, G.V.; Katsaros, F.K.; Papageorgiou, S.K.; Beazi-Katsioti, M.; Romanos, G.E. Engineering Commercial TiO2 Powder into Tailored Beads for Efficient Water Purification. Materials 2022, 15, 326. https://doi.org/10.3390/ma15010326
Theodorakopoulos GV, Katsaros FK, Papageorgiou SK, Beazi-Katsioti M, Romanos GE. Engineering Commercial TiO2 Powder into Tailored Beads for Efficient Water Purification. Materials. 2022; 15(1):326. https://doi.org/10.3390/ma15010326
Chicago/Turabian StyleTheodorakopoulos, George V., Fotios K. Katsaros, Sergios K. Papageorgiou, Margarita Beazi-Katsioti, and George Em. Romanos. 2022. "Engineering Commercial TiO2 Powder into Tailored Beads for Efficient Water Purification" Materials 15, no. 1: 326. https://doi.org/10.3390/ma15010326
APA StyleTheodorakopoulos, G. V., Katsaros, F. K., Papageorgiou, S. K., Beazi-Katsioti, M., & Romanos, G. E. (2022). Engineering Commercial TiO2 Powder into Tailored Beads for Efficient Water Purification. Materials, 15(1), 326. https://doi.org/10.3390/ma15010326







