Effect of Conditioning on PU Foam Matrix Materials Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
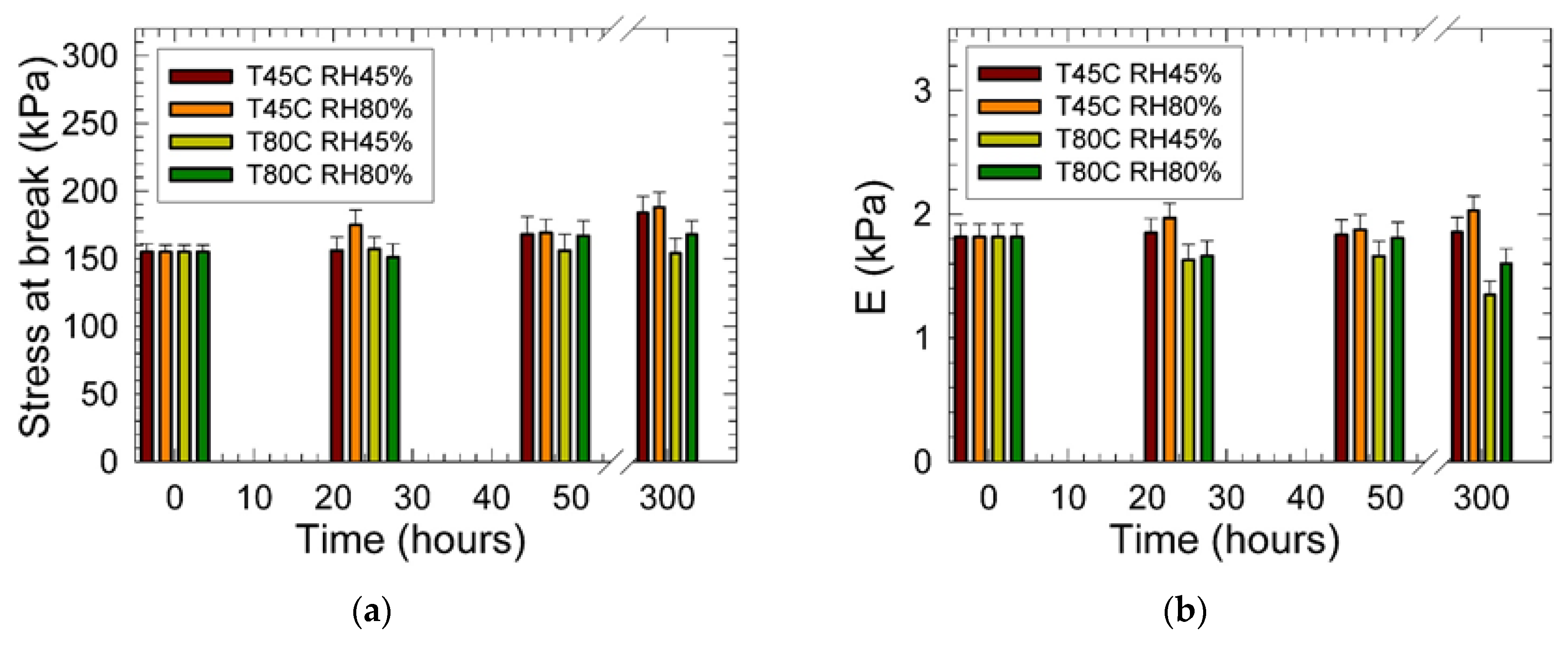
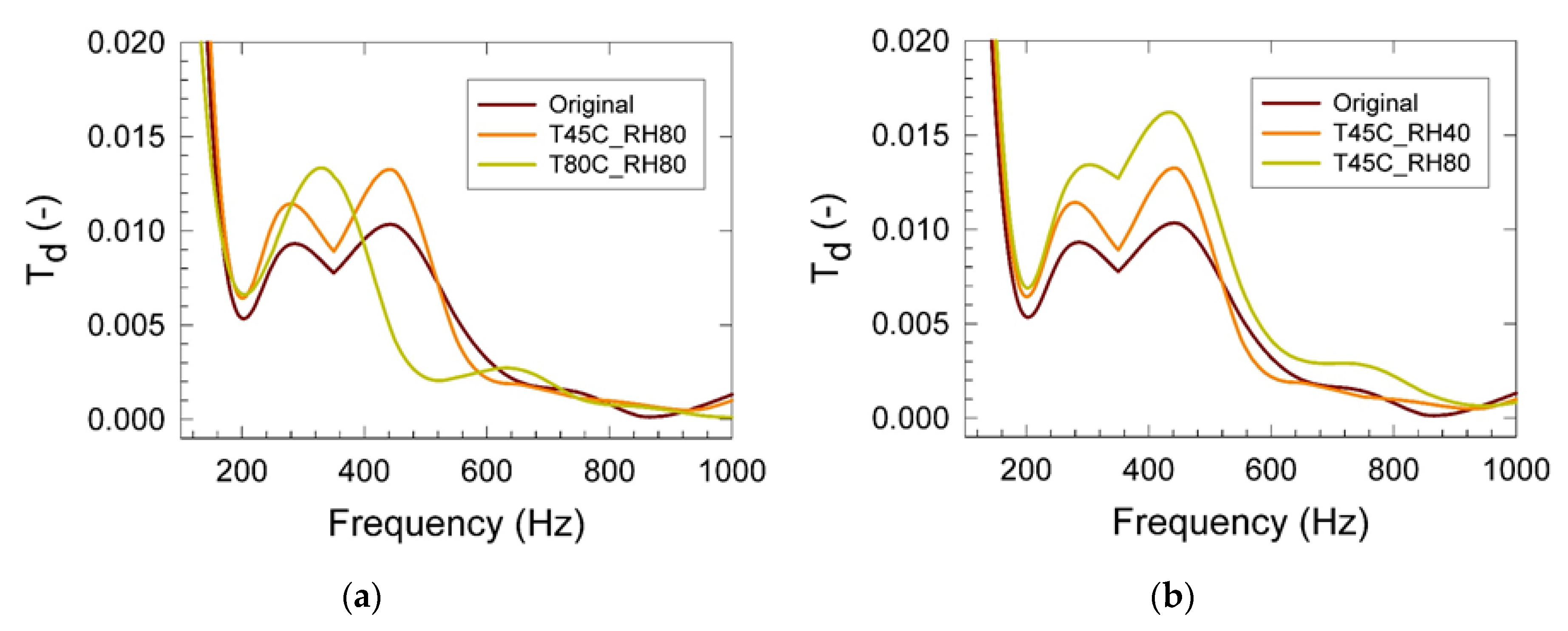
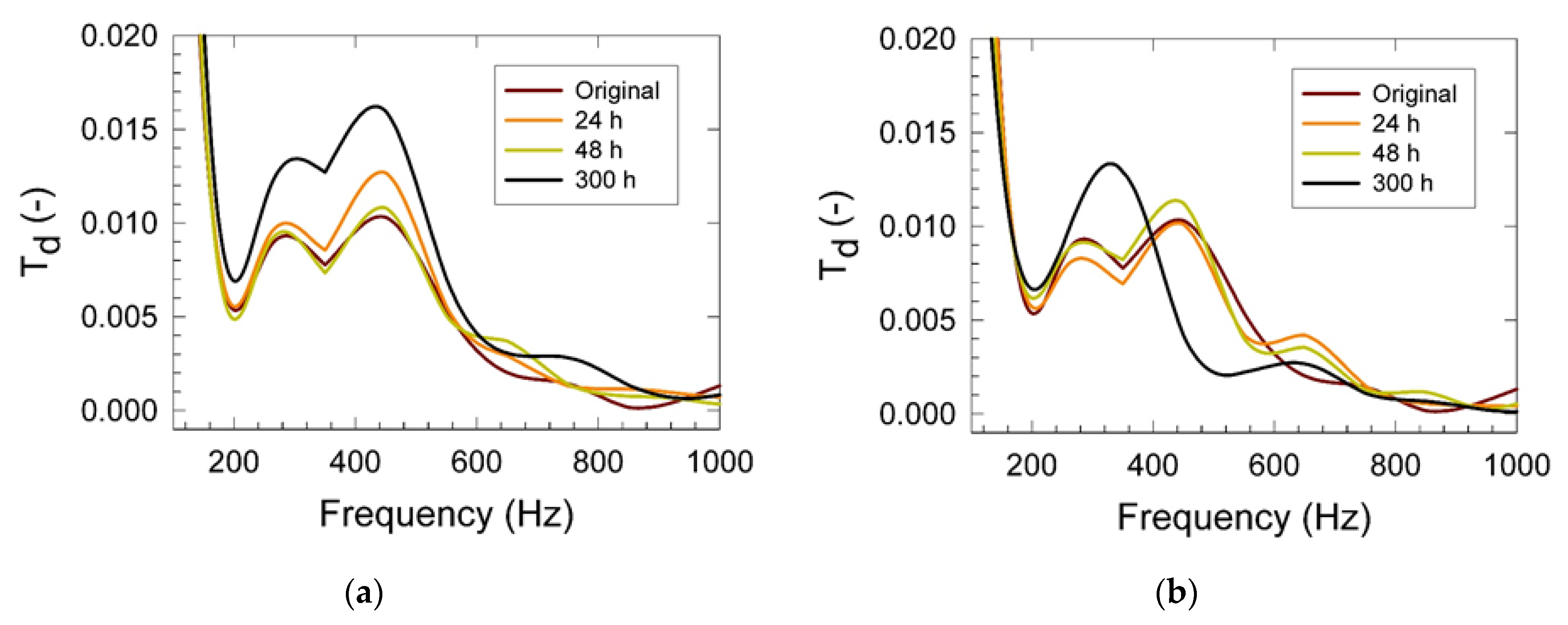
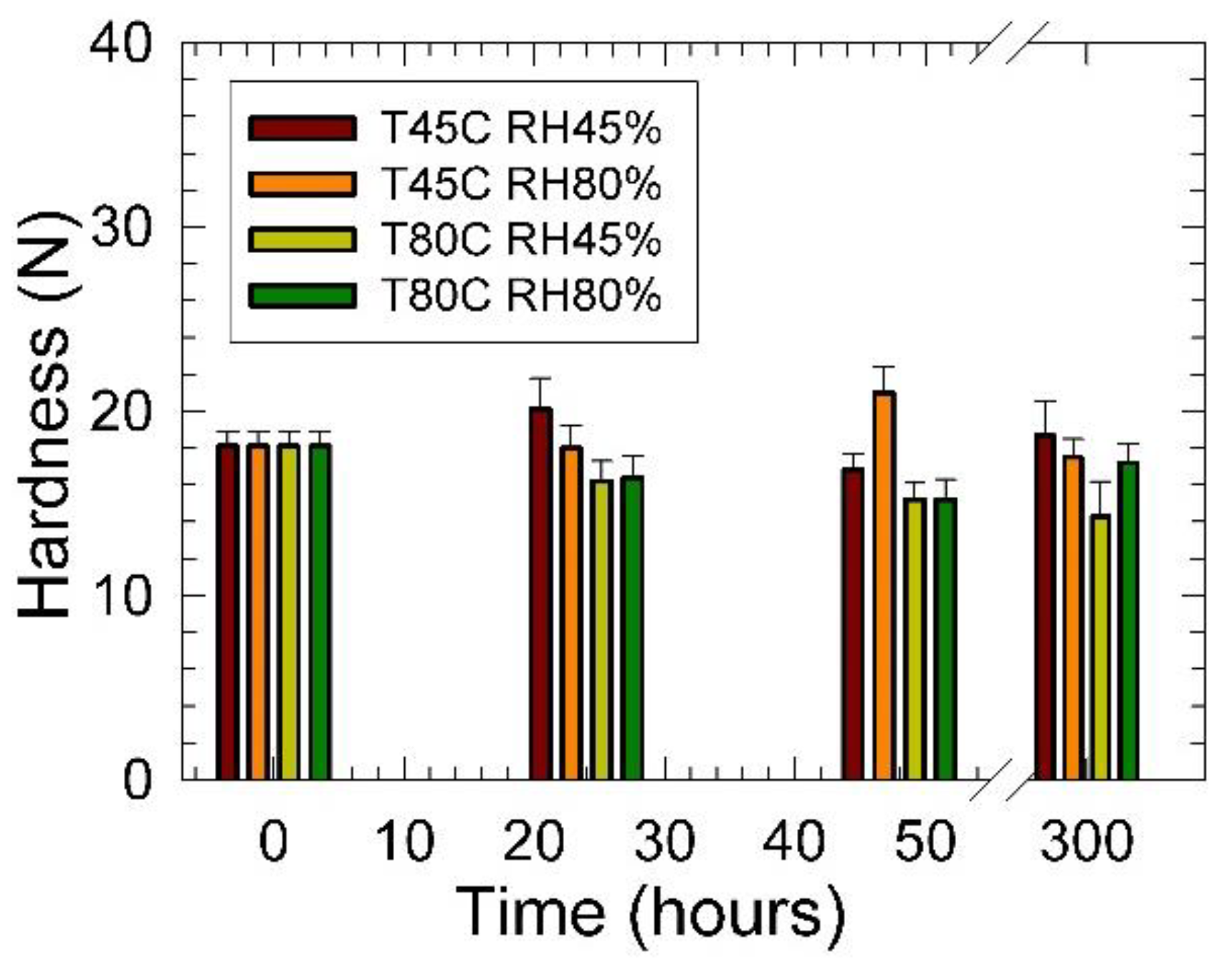
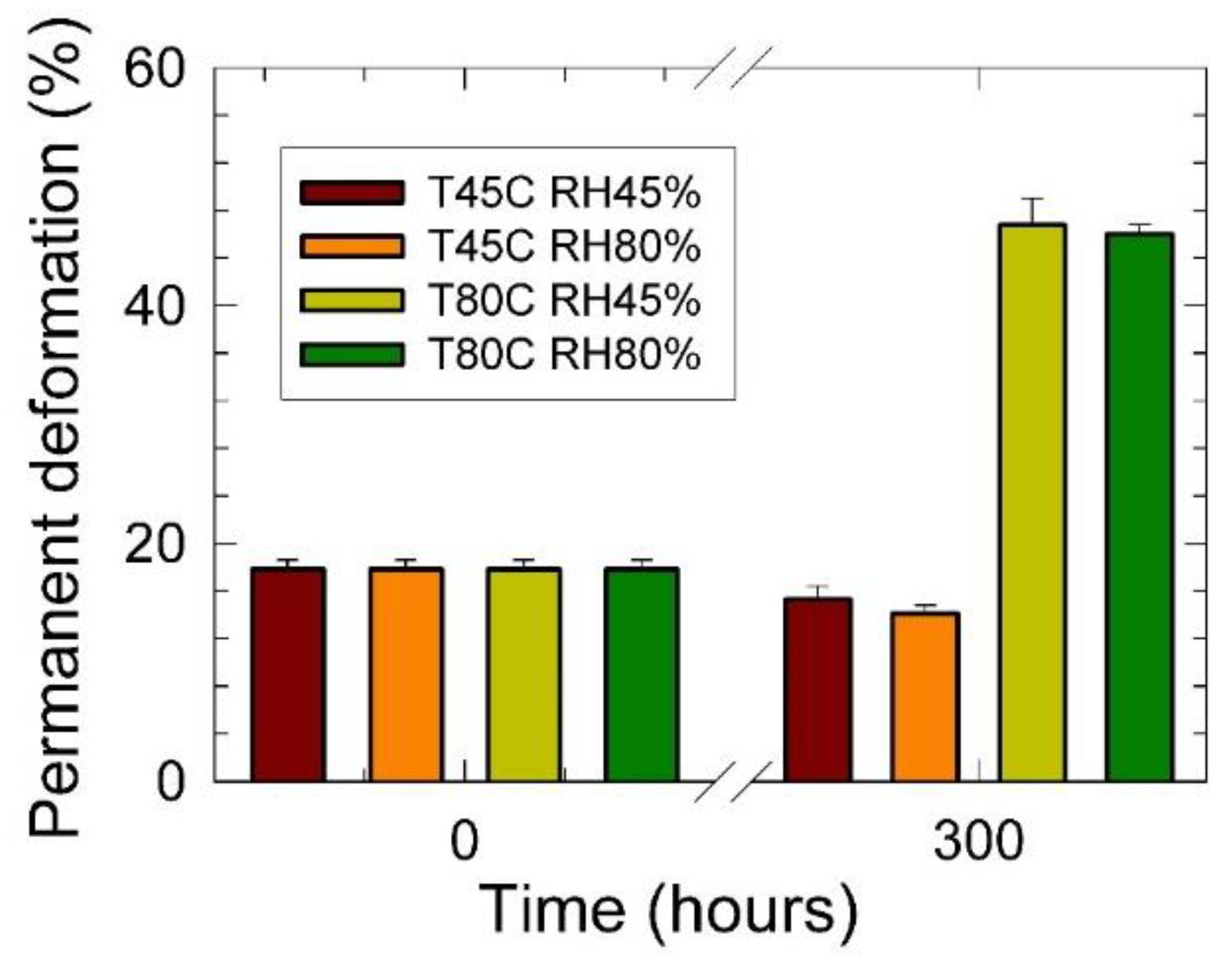
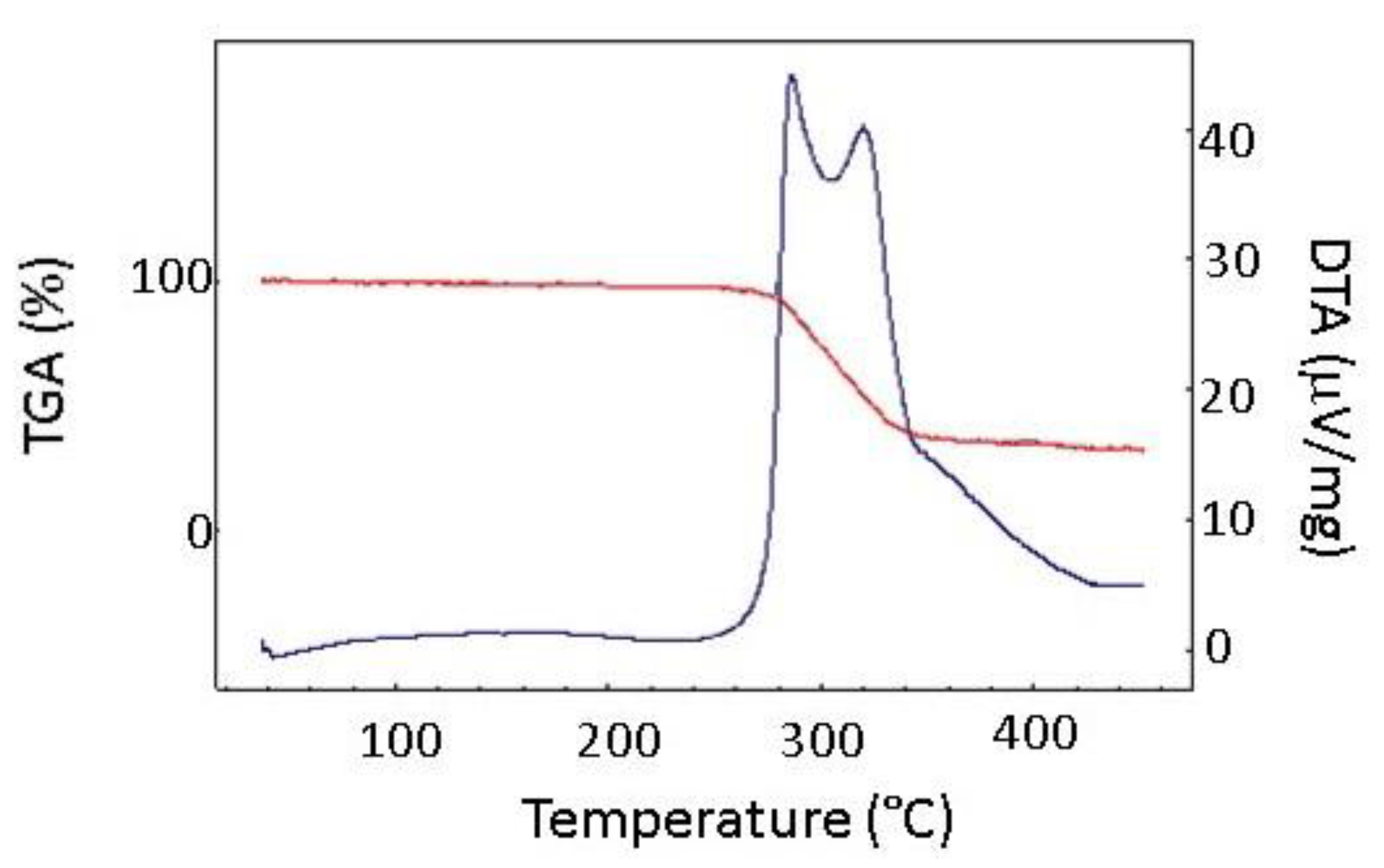
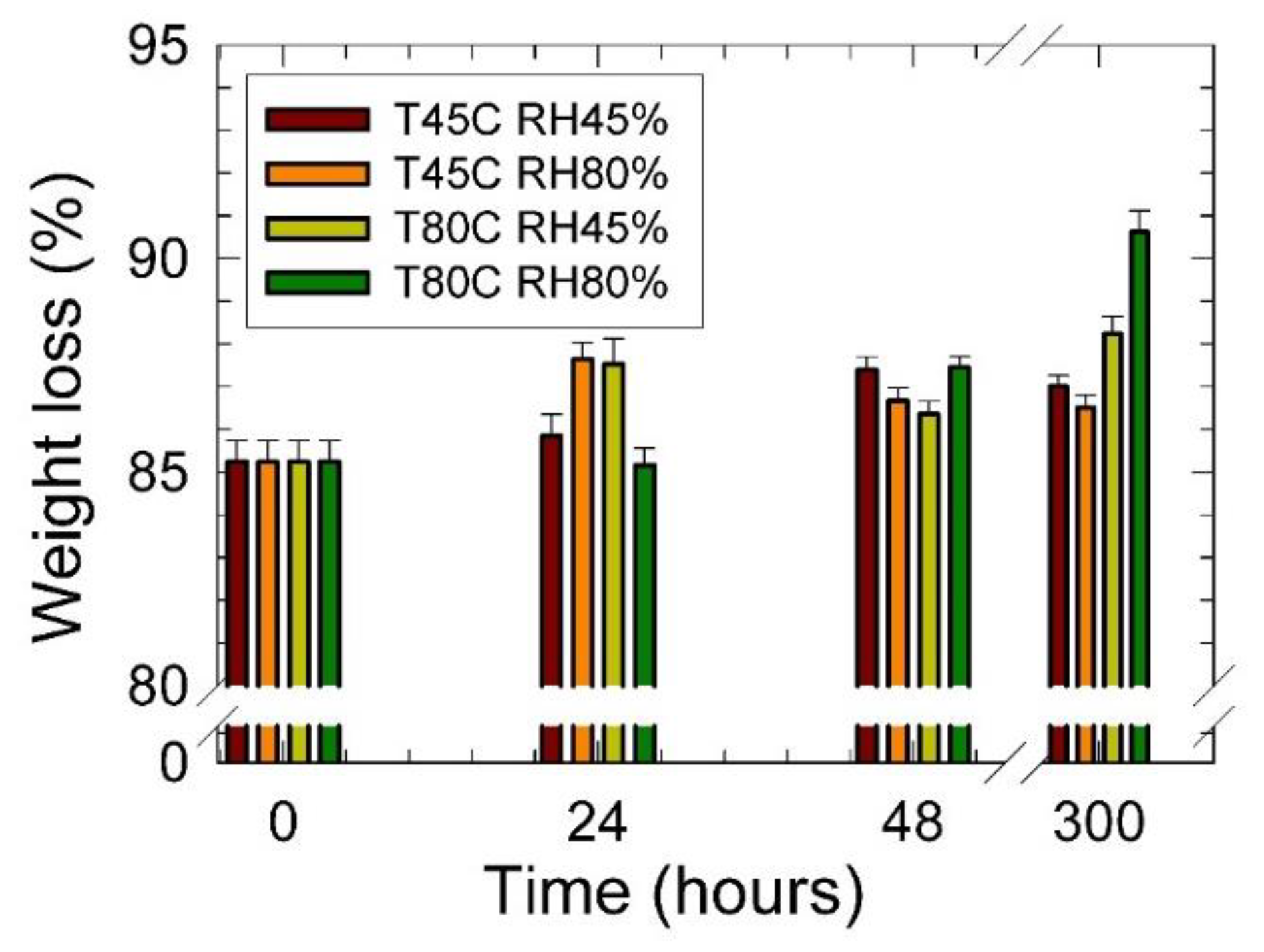
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krol, P. Polyurethanes—A Review of 60 Years of their Syntheses and Applications. Polimery 2009, 54, 489–500. [Google Scholar] [CrossRef] [Green Version]
- Stachak, P.; Lukaszewska, I.; Hebda, E.; Pielichowski, K. Recent Advances in Fabrication of Non-Isocyanate Polyurethane-Based Composite Materials. Materials 2021, 14, 3497. [Google Scholar] [CrossRef] [PubMed]
- Lapcik, L.; Cetkovsky, V.; Lapcikova, B.; Vasut, S. Materials for noise and vibration attenuation. Chem. Listy 2000, 94, 117–122. [Google Scholar]
- Shaw, S.D.; Harris, J.H.; Berger, M.L.; Subedi, B.; Kannan, K. Brominated Flame Retardants and Their Replacements in Food Packaging and Household Products: Uses, Human Exposure, and Health Effects. In Toxicants in Food Packaging and Household Plastics: Exposure and Health Risks to Consumers; Springer: London, UK, 2014; pp. 61–93. [Google Scholar] [CrossRef]
- Song, H.Y.; Cheng, X.X.; Chu, L. Effect of Density and Ambient Temperature on Coefficient of Thermal Conductivity of Heat-Insulated EPS and PU Materials for Food Packaging. Res. Food Packag. Technol. 2014, 469, 152–155. [Google Scholar] [CrossRef]
- Volcik, V.; Lapcikova, B.; Lapcik, L.; Asuquo, R. Uses of polyurethane matrixes in the environmental field. Plasty Kauc. 2002, 39, 164–169. [Google Scholar]
- Tomin, M.; Kmetty, A. Polymer foams as advanced energy absorbing materials for sports applications-A review. J. Appl. Polym. Sci. 2022, 139, 51714. [Google Scholar] [CrossRef]
- Lapcikova, B.; Lapcik, L., Jr. TG and DTG Study of Decomposition of Commercial PUR Cellular Materials. J. Polym. Mater. 2011, 28, 353–366. [Google Scholar]
- Scholz, P.; Wachtendorf, V.; Panne, U.; Weidner, S.M. Degradation of MDI-based polyether and polyester-polyurethanes in various environments—Effects on molecular mass and crosslinking. Polym. Test. 2019, 77, 105881. [Google Scholar] [CrossRef]
- Oprea, S.; Oprea, V. Mechanical behavior during different weathering tests of the polyurethane elastomers films. Eur. Polym. J. 2002, 38, 1205–1210. [Google Scholar] [CrossRef]
- Scholz, P.; Wachtendorf, V.; Elert, A.-M.; Falkenhagen, J.; Becker, R.; Hoffmann, K.; Resch-Genger, U.; Tschiche, H.; Reinsch, S.; Weidner, S. Analytical toolset to characterize polyurethanes after exposure to artificial weathering under systematically varied moisture conditions. Polym. Test. 2019, 78, 105996. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85069503515&doi=10.1016%2fj.polymertesting.2019.105996&partnerID=40&md5=5d3e6a62259aeed6cdeb3912a2ec95d6 (accessed on 14 December 2021). [CrossRef]
- Kuranska, M.; Pinto, J.A.; Salach, K.; Barreiro, M.F.; Prociak, A. Synthesis of thermal insulating polyurethane foams from lignin and rapeseed based polyols: A comparative study. Ind. Crops Prod. 2020, 143, 111882. [Google Scholar] [CrossRef]
- Mort, R.; Vorst, K.; Curtzwiler, G.; Jiang, S. Biobased foams for thermal insulation: Material selection, processing, modelling, and performance. RSC Adv. 2021, 11, 4375–4394. [Google Scholar] [CrossRef]
- Jonjaroen, V.; Ummartyotin, S.; Chittapun, S. Algal cellulose as a reinforcement in rigid polyurethane foam. Algal Res. Biomass Biofuels Bioprod. 2020, 51, 102057. [Google Scholar] [CrossRef]
- Cornille, A.; Auvergne, R.; Figovsky, O.; Boutevin, B.; Caillol, S. A perspective approach to sustainable routes for non-isocyanate polyurethanes. Eur. Polym. J. 2017, 87, 535–552. [Google Scholar] [CrossRef]
- Rodrigues, J.D.O.; Andrade, C.K.Z.; Quirino, R.L.; Sales, M.J.A. Non-isocyanate poly(acyl-urethane) obtained from urea and castor (Ricinus communis L.) oil. Prog. Org. Coat. 2022, 162, 106557. [Google Scholar] [CrossRef]
- Wilhelm, C.; Rivaton, A.; Gardette, J.-L. Infrared analysis of the photochemical behaviour of segmented polyurethanes: 3. Aromatic diisocyanate based polymers. Polymer 1998, 39, 1223–1232. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-0032028577&doi=10.1016%2fS0032-3861%2897%2900353-4&partnerID=40&md5=4ffb65e5dd9477d91e7672f4dff2de01 (accessed on 14 December 2021). [CrossRef]
- Wilhelm, C.; Gardette, J.-L. Infrared analysis of the photochemical behaviour of segmented polyurethanes: Aliphatic poly(ether-urethane)s. Polymer 1998, 39, 5973–5980. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-0032210353&doi=10.1016%2fS0032-3861%2897%2910065-9&partnerID=40&md5=71595238af20bfe5abd9a439287d94e8 (accessed on 14 December 2021). [CrossRef]
- Weise, N.K.; Bertocchi, M.J.; Wynne, J.H.; Long, I.; Mera, A.E. High performance vibrational damping poly(urethane) coatings: Blending ‘soft’ macrodiols for improved mechanical stability under weathering. Prog. Org. Coat. 2019, 136, 105240. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85071912699&doi=10.1016%2fj.porgcoat.2019.105240&partnerID=40&md5=aeb9c93665d568497d6faf5157645215 (accessed on 14 December 2021). [CrossRef]
- Lapcik, L.; Manas, D.; Lapcikova, B.; Vasina, M.; Stanek, M.; Cepe, K.; Vlcek, J.; Waters, K.E.; Greenwood, R.W.; Rowson, N.A. Effect of filler particle shape on plastic-elastic mechanical behavior of high density poly(ethylene)/mica and poly(ethylene)/wollastonite composites. Compos. Part B Eng. 2018, 141, 92–99. [Google Scholar] [CrossRef]
- Rao, S.S. Mechanical Vibrations, 5th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2010; p. 1105. [Google Scholar]
- Liu, K.; Liu, J. The damped dynamic vibration absorbers: Revisited and new result. J. Sound Vib. 2005, 284, 1181–1189. [Google Scholar] [CrossRef]
- Hadas, Z.; Ondrusek, C. Nonlinear spring-less electromagnetic vibration energy harvesting system. Eur. Phys. J.-Spec. Top. 2015, 224, 2881–2896. [Google Scholar] [CrossRef]
- Carrella, A.; Brennan, M.J.; Waters, T.P.; Lopes, V., Jr. Force and displacement transmissibility of a nonlinear isolator with high-static-low-dynamic-stiffness. Int. J. Mech. Sci. 2012, 55, 22–29. [Google Scholar] [CrossRef]
- Dupuis, R.; Duboeuf, O.; Kirtz, B.; Aubry, E. Characterization of Vibrational Mechanical Properties of Polyurethane Foam. Dyn. Behav. Mater. 2016, 1, 123–128. [Google Scholar] [CrossRef]
- Lapcik, L.; Vasina, M.; Lapcikova, B.; Stanek, M.; Ovsik, M.; Murtaja, Y. Study of the material engineering properties of high-density poly(ethylene)/perlite nanocomposite materials. Nanotechnol. Rev. 2020, 9, 1491–1499. [Google Scholar] [CrossRef]
- Platonova, E.; Chechenov, I.; Pavlov, A.; Solodilov, V.; Afanasyev, E.; Shapagin, A.; Polezhaev, A. Thermally Remendable Polyurethane Network Cross-Linked via Reversible Diels-Alder Reaction. Polymers 2021, 13, 1935. [Google Scholar] [CrossRef] [PubMed]
- Nemade, A.M.; Mishra, S.; Zope, V.S. Kinetics and Thermodynamics of Neutral Hydrolytic Depolymerization of Polyurethane Foam Waste Using Different Catalysts at Higher Temperature and Autogenious Pressures. Polym. Plast. Technol. Eng. 2010, 49, 83–89. [Google Scholar] [CrossRef]
- Casati, F.; Herrington, R.; Broos, R.; Miyazaki, Y. Tailoring the performance of molded flexible polyurethane foams for car seats (Reprinted from Polyurethanes World Congress ′97, 29 September–1 October 1997). J. Cell. Plast. 1998, 34, 430–466. [Google Scholar] [CrossRef]
- Suh, K.; Park, C.; Maurer, M.; Tusim, M.; De Genova, R.; Broos, R.; Sophiea, D. Lightweight cellular plastics. Adv. Mater. 2000, 12, 1779–1789. [Google Scholar] [CrossRef]
- Vakil, A.U.; Petryk, N.M.; Shepherd, E.; Beaman, H.T.; Ganesh, P.S.; Dong, K.S.; Monroe, M.B.B. Shape Memory Polymer Foams with Tunable Degradation Profiles. ACS Appl. Bio. Mater. 2021, 4, 6769–6779. [Google Scholar] [CrossRef]
- Zahedifar, P.; Pazdur, L.; Vande Velde, C.M.L.; Billen, P. Multistage Chemical Recycling of Polyurethanes and Dicarbamates: A Glycolysis-Hydrolysis Demonstration. Sustainability 2021, 13, 3583. [Google Scholar] [CrossRef]
- Gaboriaud, F.; Vantelon, J.P. Thermal-Degradation of Polyurethane Based on Mdi and Propoxylated Trimethylol Propane. J. Polym. Sci. Part A Polym. Chem. 1981, 19, 139–150. [Google Scholar] [CrossRef]
- Ballistreri, A.; Foti, S.; Maravigna, P.; Montaudo, G.; Scamporrino, E. Mechanism of Thermal-Degradation of Polyurethanes Investigated by Direct Pyrolysis in the Mass-Spectrometer. J. Polym. Sci. Part A Polym. Chem. 1980, 18, 1923–1931. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lapčík, L.; Vašina, M.; Lapčíková, B.; Murtaja, Y. Effect of Conditioning on PU Foam Matrix Materials Properties. Materials 2022, 15, 195. https://doi.org/10.3390/ma15010195
Lapčík L, Vašina M, Lapčíková B, Murtaja Y. Effect of Conditioning on PU Foam Matrix Materials Properties. Materials. 2022; 15(1):195. https://doi.org/10.3390/ma15010195
Chicago/Turabian StyleLapčík, Lubomír, Martin Vašina, Barbora Lapčíková, and Yousef Murtaja. 2022. "Effect of Conditioning on PU Foam Matrix Materials Properties" Materials 15, no. 1: 195. https://doi.org/10.3390/ma15010195
APA StyleLapčík, L., Vašina, M., Lapčíková, B., & Murtaja, Y. (2022). Effect of Conditioning on PU Foam Matrix Materials Properties. Materials, 15(1), 195. https://doi.org/10.3390/ma15010195






