Process Phenomena and Material Properties in Selective Laser Sintering of Polymers: A Review
Abstract
1. Introduction
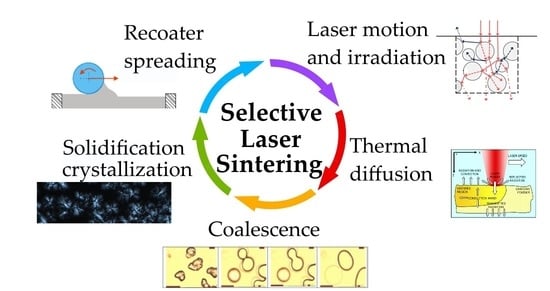
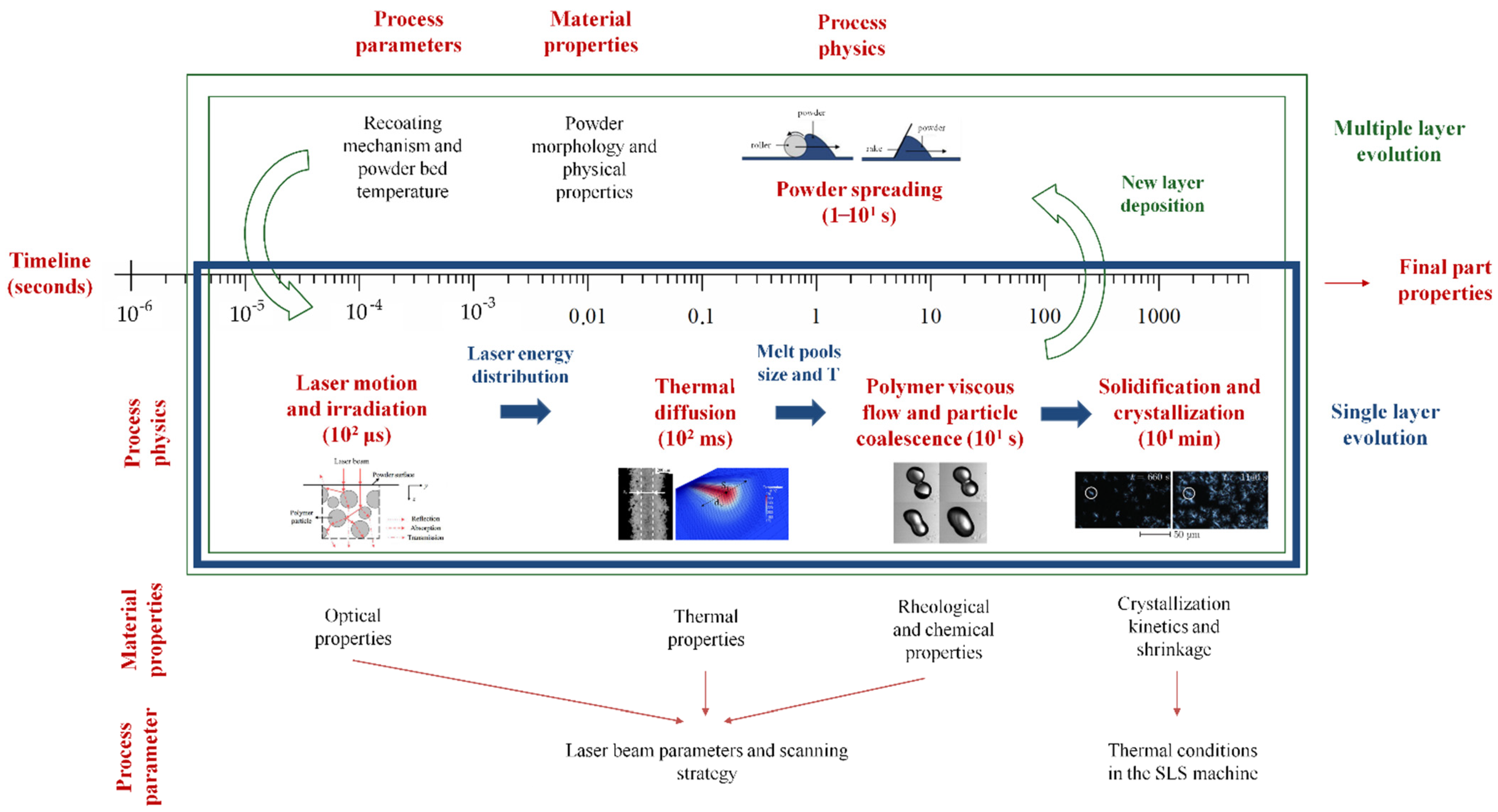
2. Selective Laser Sintering Process
- Laser motion and irradiation (102 μs);
- Thermal diffusion (102 ms);
- Polymer viscous flow and particle coalescence (101 s);
- Powder spreading (1–101 s);
- Solidification / crystallization (101 min).
2.1. Powder Spreading (1–101 s)
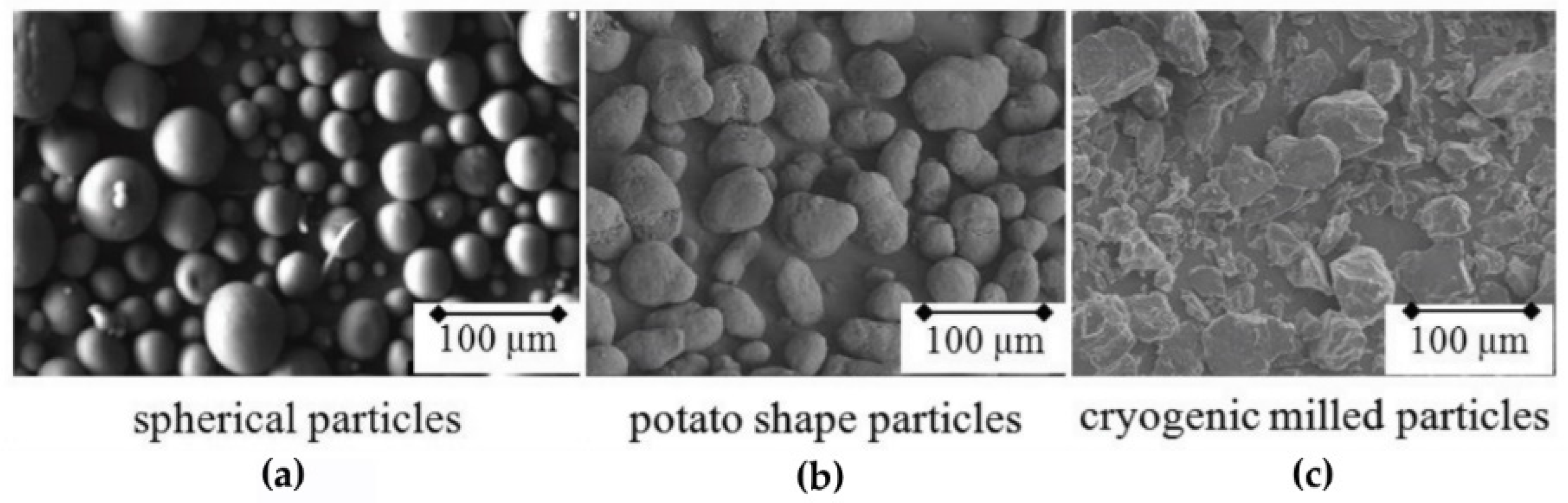
2.1.1. Particle Size Distribution and Shape
2.1.2. Interparticle Forces
2.1.3. Temperature and Humidity
2.2. Laser Motion and Irradiation (102 μs)
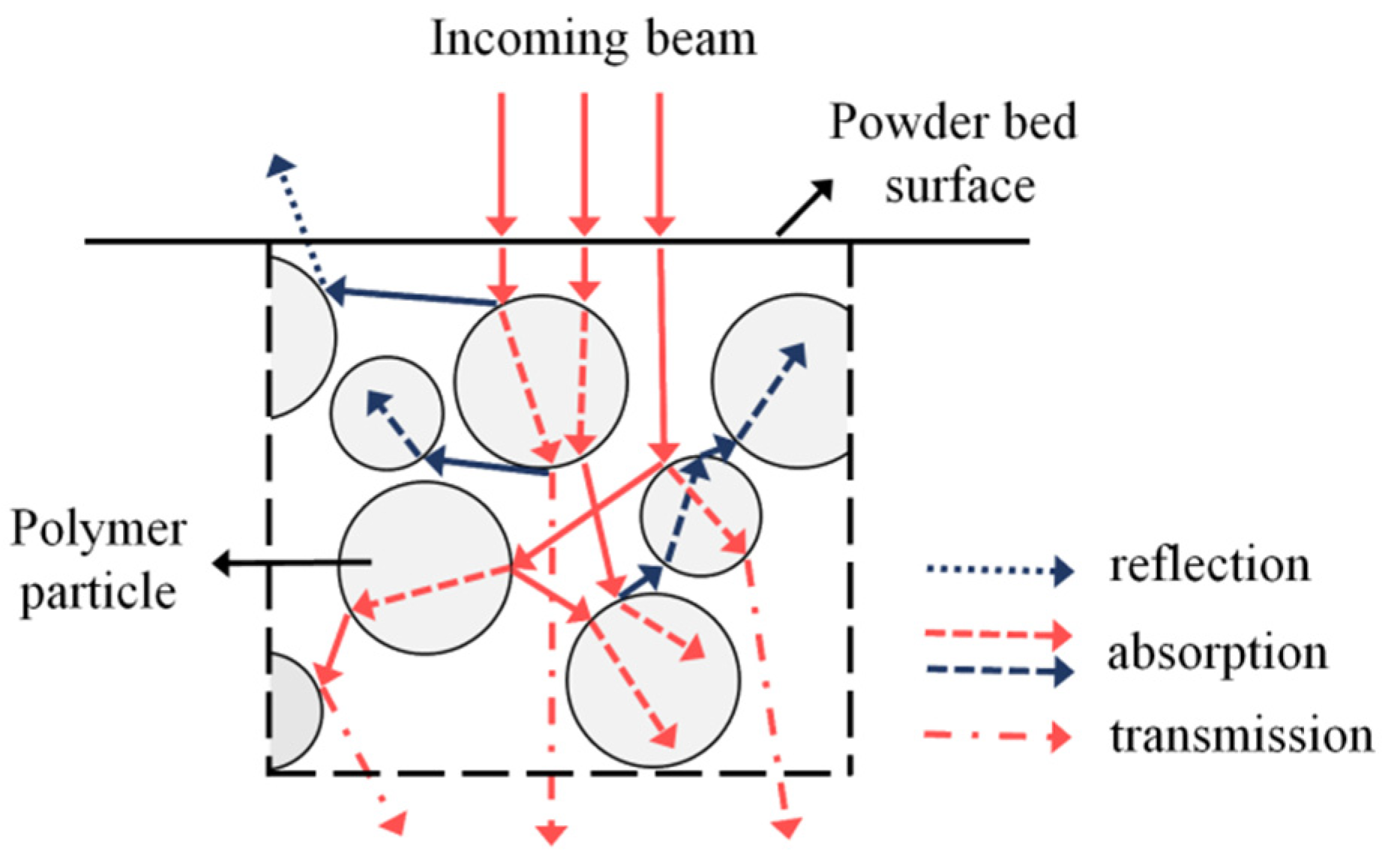
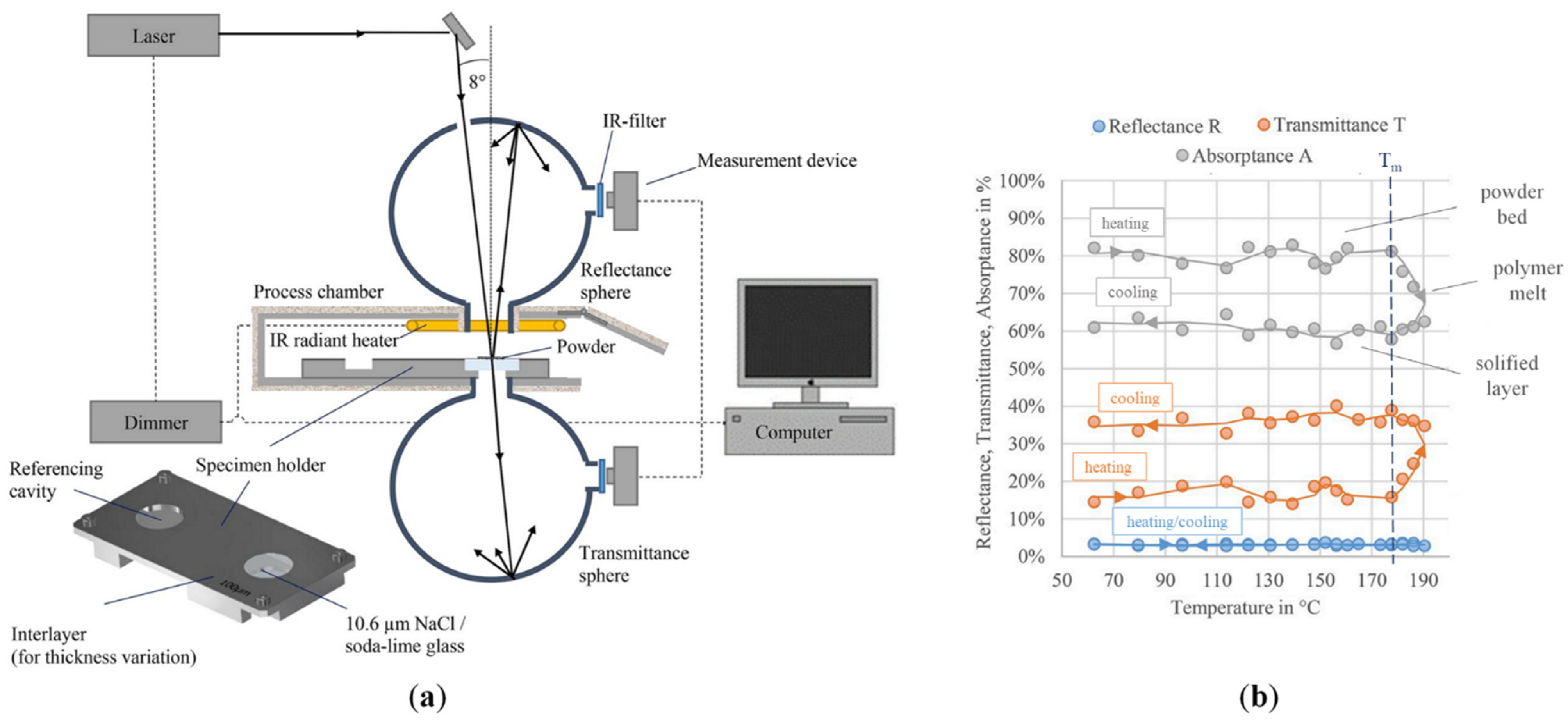
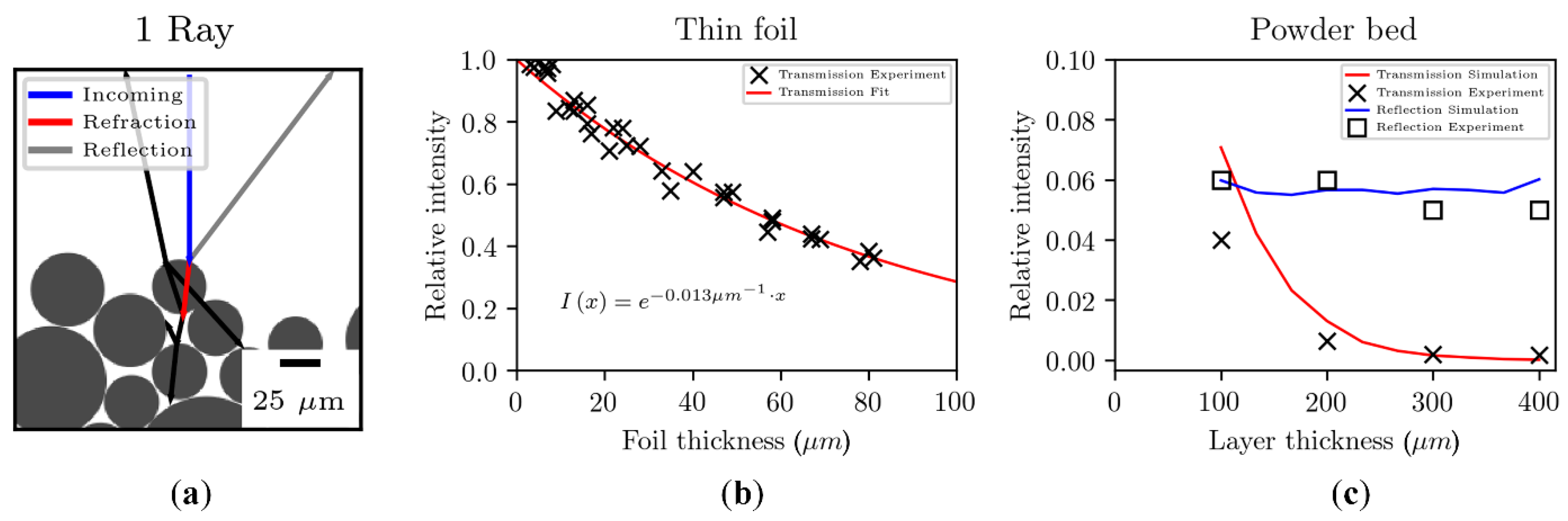
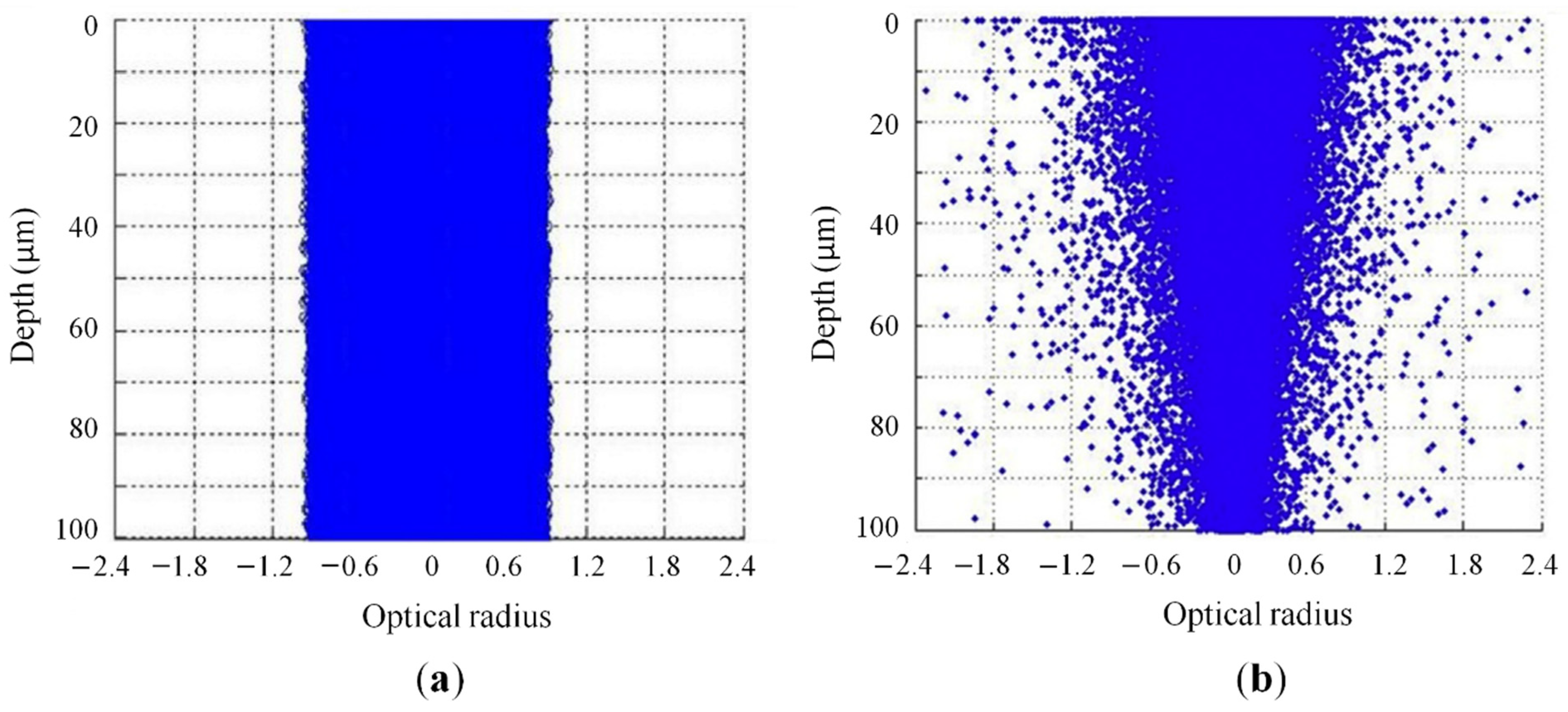
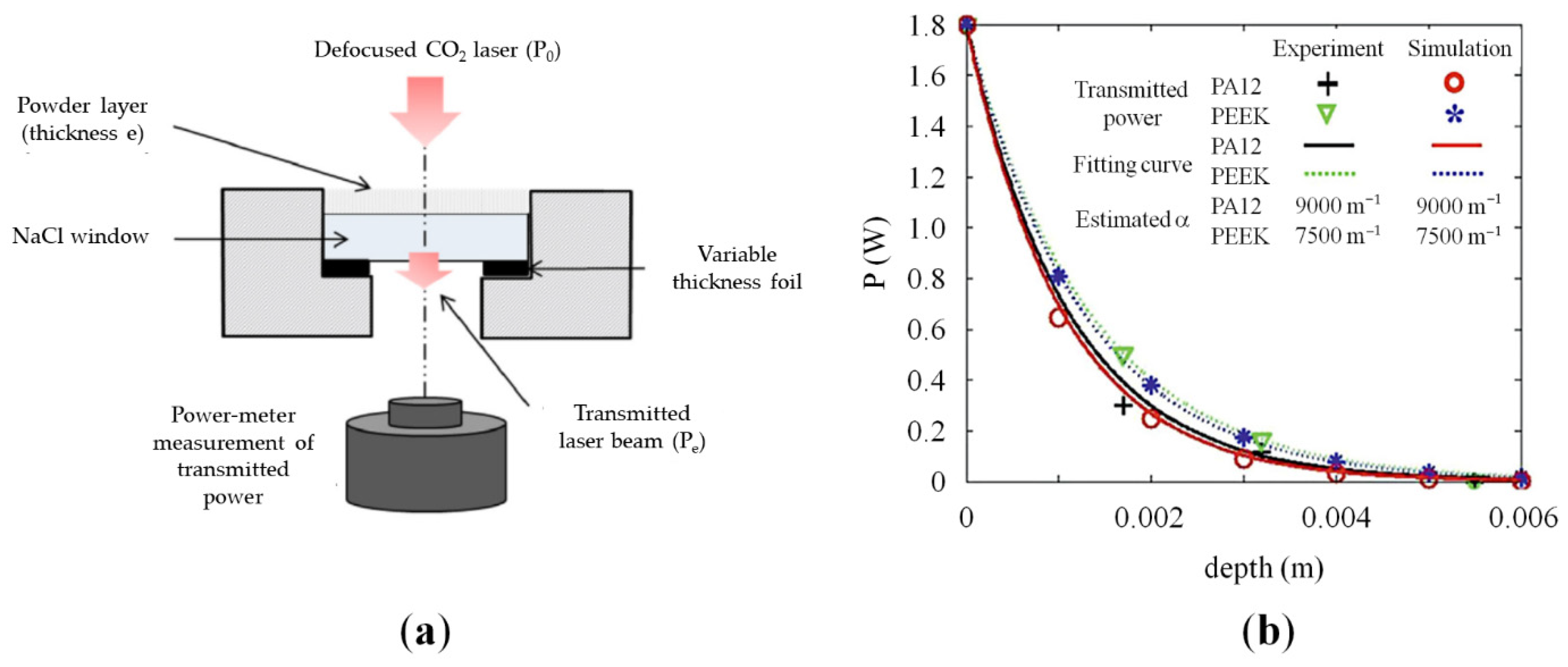
2.2.1. Optical Properties of Polymeric Powders
- The calorimetry method, which directly measures the absorptance from the temperature variations developed across a thin sample irradiated on the top surface;
- The radiometric method, which indirectly determines absorptance through the analysis of optical radiative properties such as reflectance and emissivity.
2.2.2. Effect of Filler or Absorbing Additives
2.3. Thermal Diffusion (102 ms)
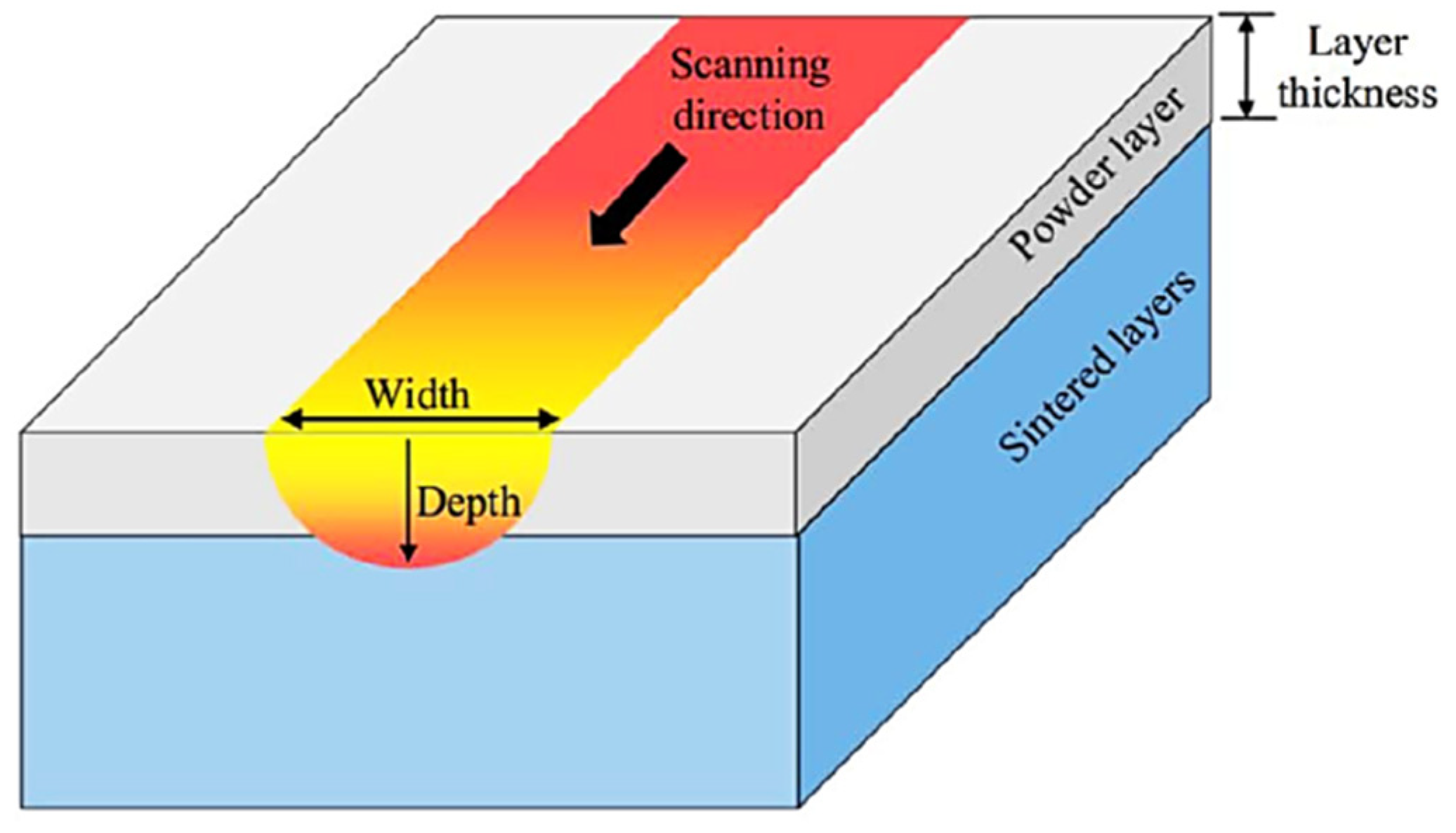
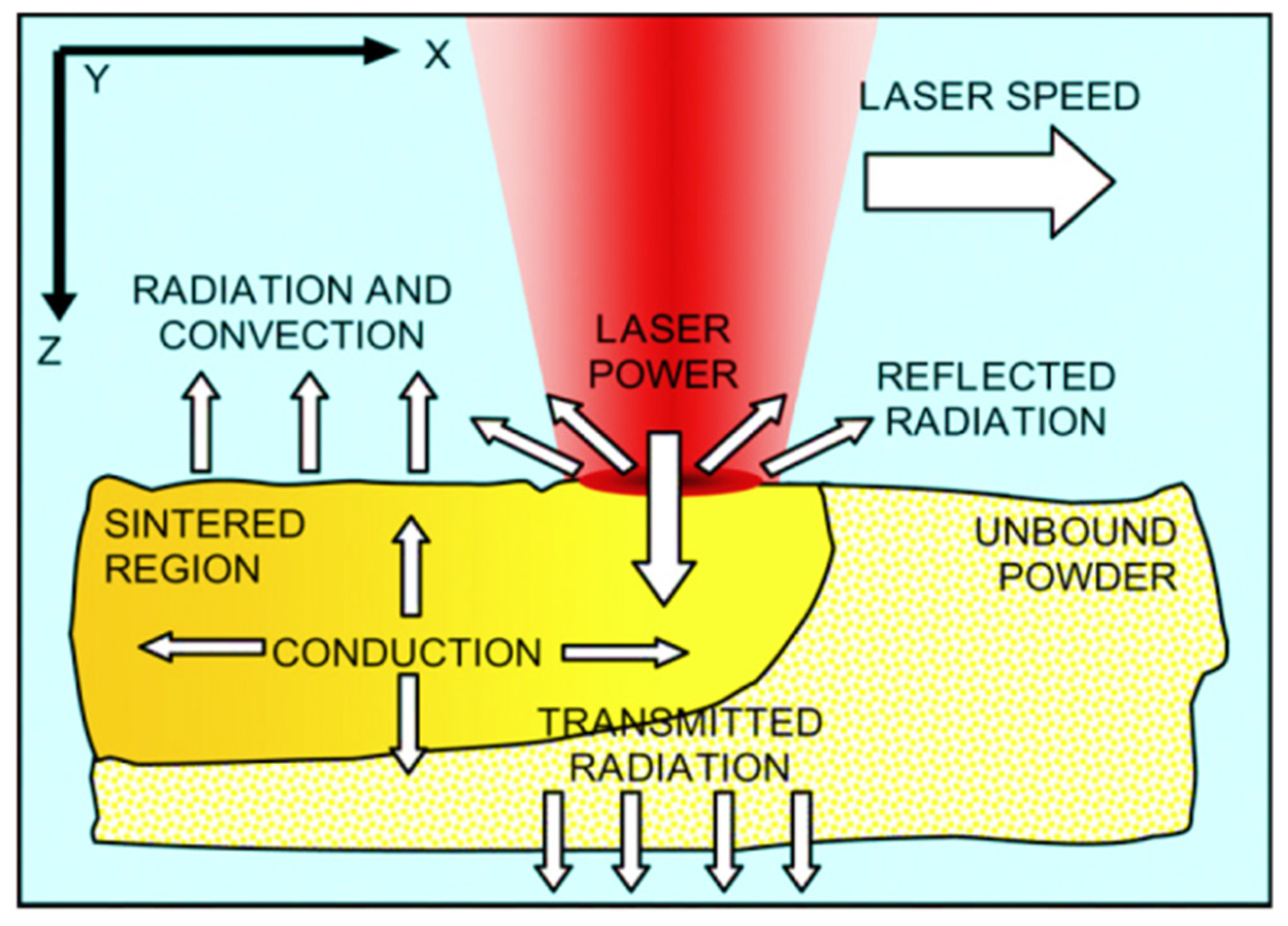
2.3.1. The “Melt Pool”
2.3.2. Main Process Parameters Involved in the Formation of the Melt Pool
2.3.3. Powder Properties Affecting the Melt Pool
2.3.4. Evaluation of the Melt Pool: Modeling and Experimental Approaches
2.4. Polymer Viscous Flow and Particle Coalescence (101 s)
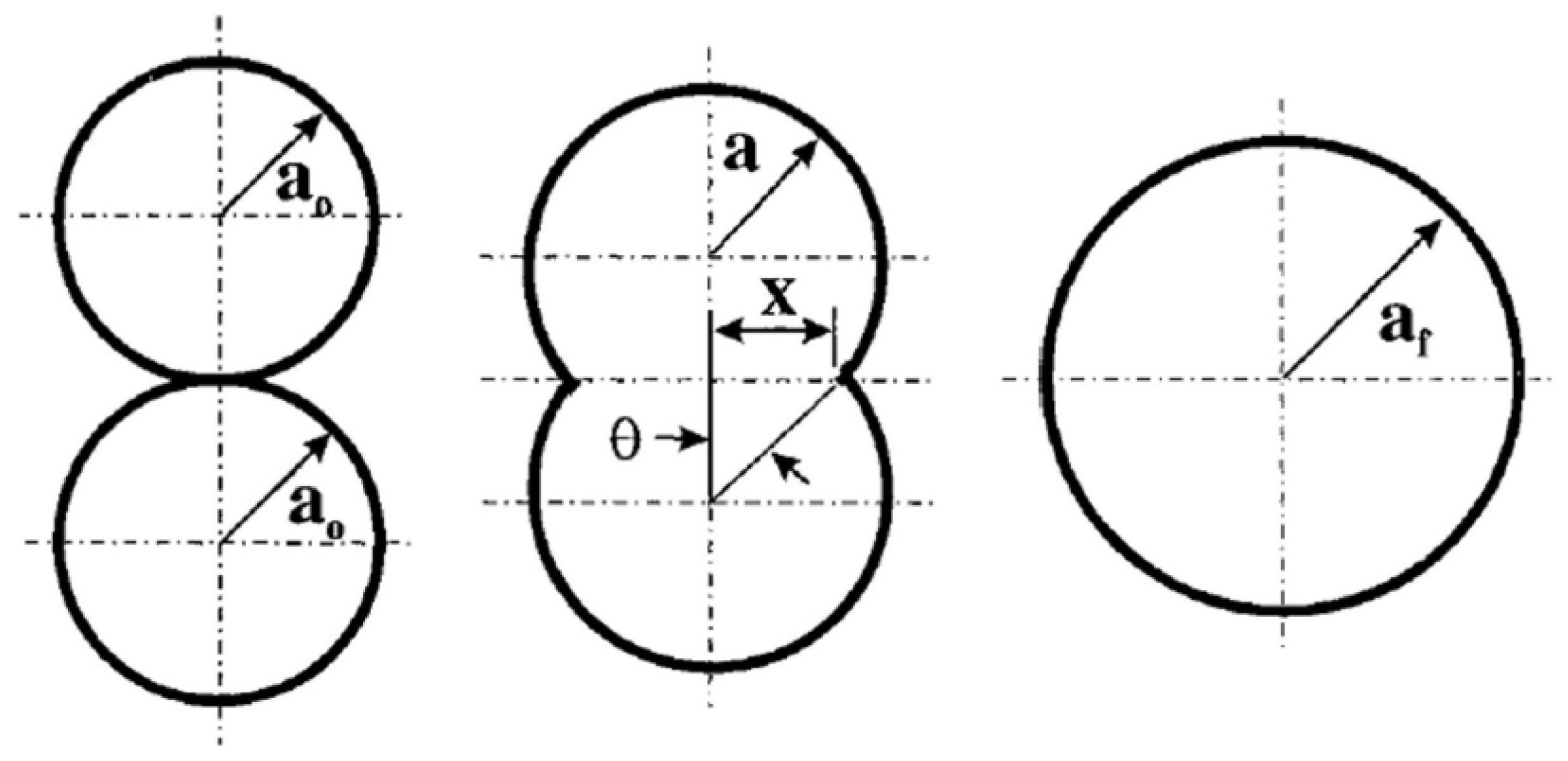
2.4.1. Binding Mechanisms in SLS
2.4.2. Models of Viscous Sintering
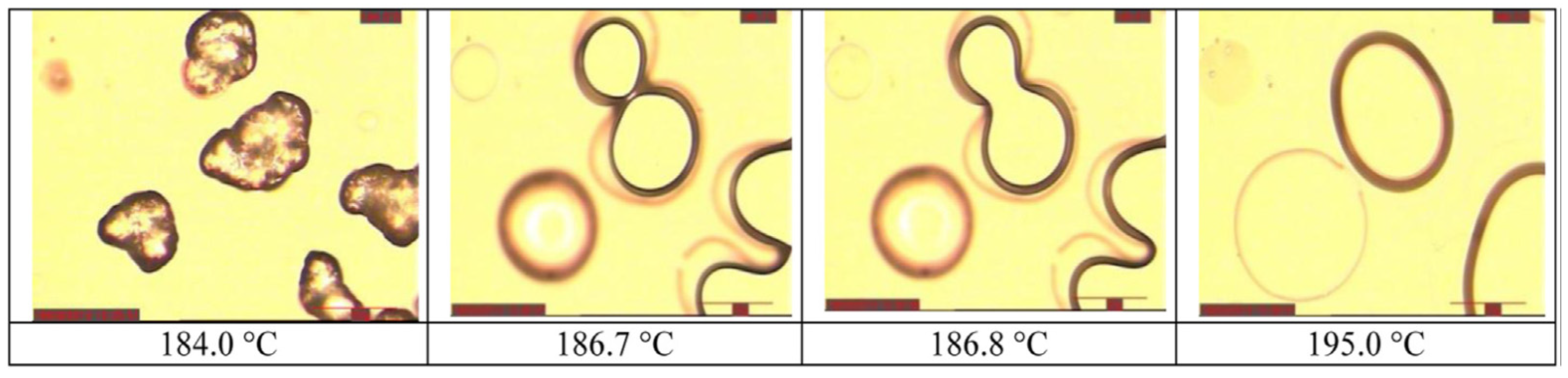
2.4.3. Experimental Evaluation of Coalescence: Hot-Stage Microscopy
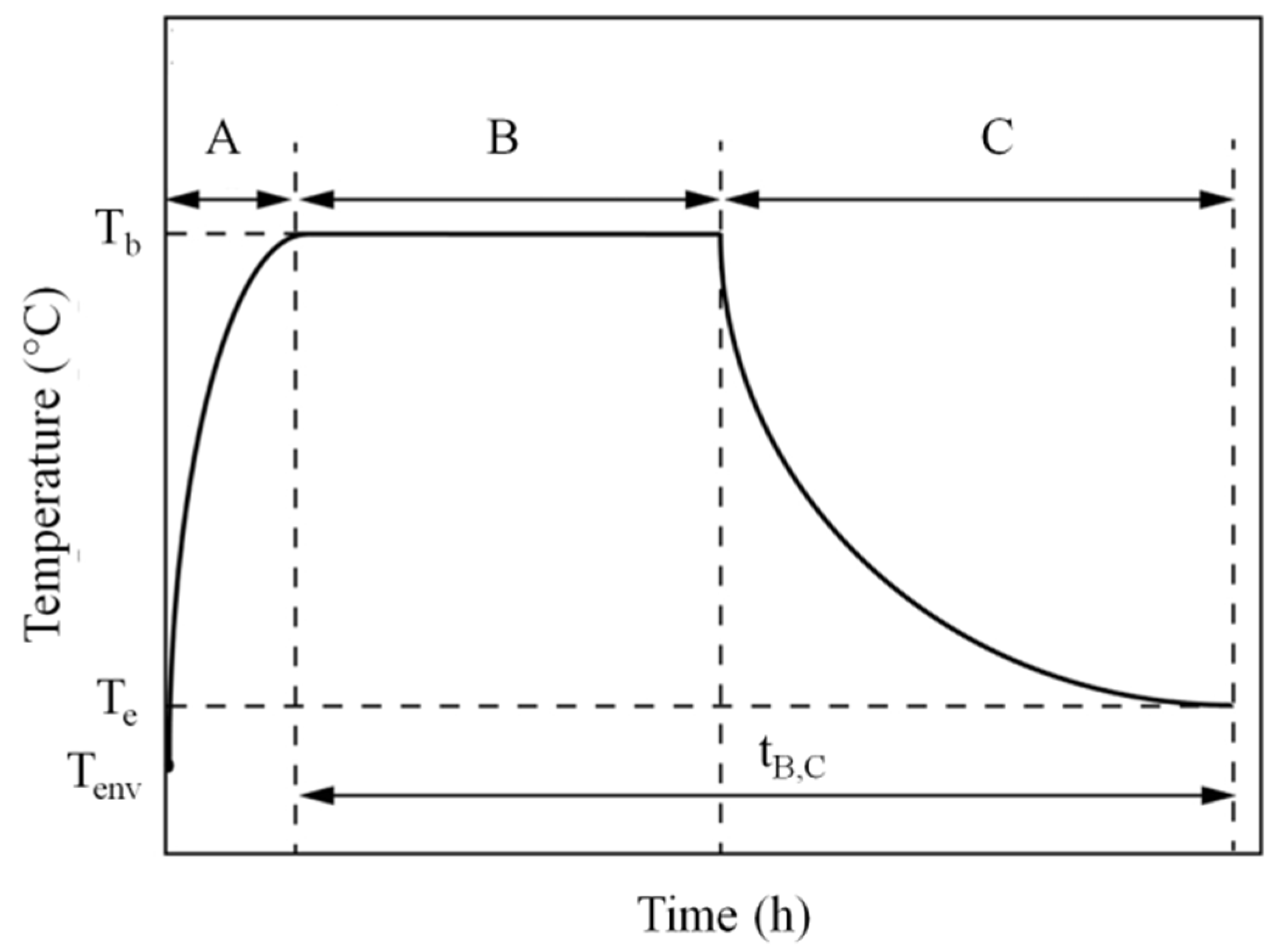
2.5. Solidification/Crystallization (101 min)
Influence of Crystallization on Part Shrinkage and Deformation
3. Conclusions and Future Perspectives
3.1. Powder Spreading
3.2. Laser Motion and Irradiation
3.3. Thermal Diffusion
3.4. Polymer Viscous Flow and Particle Coalescence
3.5. Solidification/Crystallization
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goodridge, R.D.; Tuck, C.J.; Hague, R.J.M. Laser sintering of polyamides and other polymers. Prog. Mater. Sci. 2012, 57, 229–267. [Google Scholar] [CrossRef]
- Özer, G.; Özbay, B.; Öter, Z.Ç.; Tarakçi, G.; Yılmaz, M.S.; Bulduk, M.E.; Koç, E.; Acar, S.; Güler, K.A. Investigation of the surface quality and dimensional accuracy of polymer patterns produced by selective laser sintering (SLS) method for investment casting (IC). Int. J. Cast Met. Res. 2020, 33, 146–152. [Google Scholar] [CrossRef]
- Yang, J.; Shi, Y.; Shen, Q.; Yan, C. Selective laser sintering of HIPS and investment casting technology. J. Mater. Process. Technol. 2009, 209, 1901–1908. [Google Scholar] [CrossRef]
- Yuan, S.; Shen, F.; Chua, C.K.; Zhou, K. Polymeric composites for powder-based additive manufacturing: Materials and applications. Prog. Polym. Sci. 2019, 91, 141–168. [Google Scholar] [CrossRef]
- Özbay, B.; Serhatlı, E. Processing and Characterization of Hollow Glass-Filled Polyamide 12 Composites by Selective Laser Sintering Method. Mater. Technol. 2020, 1–11. [Google Scholar] [CrossRef]
- Drummer, D.; Greiner, S.; Zhao, M.; Wudy, K. A novel approach for understanding laser sintering of polymers. Addit. Manuf. 2019, 27, 379–388. [Google Scholar] [CrossRef]
- Stichel, T.; Laumer, T.; Baumüller, T.; Amend, P.; Roth, S. Powder layer preparation using vibration-controlled capillary steel nozzles for additive manufacturing. Phys. Procedia 2014, 56, 157–166. [Google Scholar] [CrossRef]
- Stichel, T.; Laumer, T.; Linnenweber, T.; Amend, P.; Roth, S. Mass flow characterization of selective deposition of polymer powders with vibrating nozzles for laser beam melting of multi-material components. Phys. Procedia 2016, 83, 947–953. [Google Scholar] [CrossRef]
- Aerosint SA. How Multi-Powder Deposition Will Transform Industrial 3d Printing. Available online: https://aerosint.com/powder-deposition-multimaterial-printing/ (accessed on 25 September 2021).
- Amado Becker, A.F. Characterization and Prediction of SLS Processability of Polymer Powders with Respect to Powder Flow and Part Warpage; ETH Zurich: Zurich, Switzerland, 2016. [Google Scholar]
- Chatham, C.A.; Long, T.E.; Williams, C.B. A review of the process physics and material screening methods for polymer powder bed fusion additive manufacturing. Prog. Polym. Sci. 2019, 93, 68–95. [Google Scholar] [CrossRef]
- Brighenti, R.; Cosma, M.P.; Marsavina, L.; Spagnoli, A.; Terzano, M. Laser-based additively manufactured polymers: A review on processes and mechanical models. J. Mater. Sci. 2021, 56, 961–998. [Google Scholar] [CrossRef]
- Campbell, I.; Bourell, D.; Gibson, I. Additive manufacturing: Rapid prototyping comes of age. Rapid Prototyp. J. 2012, 18, 255–258. [Google Scholar] [CrossRef]
- Bourell, D.L.; Watt, T.J.; Leigh, D.K.; Fulcher, B. Performance limitations in polymer laser sintering. Phys. Procedia 2014, 56, 147–156. [Google Scholar] [CrossRef]
- Mokrane, A.; Boutaous, M.; Xin, S. Process of selective laser sintering of polymer powders: Modeling, simulation, and validation. Comptes Rendus Mec. 2018, 346, 1087–1103. [Google Scholar] [CrossRef]
- Dupin, S.; Lame, O.; Barrès, C.; Charmeau, J.Y. Microstructural origin of physical and mechanical properties of polyamide 12 processed by laser sintering. Eur. Polym. J. 2012, 48, 1611–1621. [Google Scholar] [CrossRef]
- Soe, S.P. Quantitative analysis on SLS part curling using EOS P700 machine. J. Mater. Process. Technol. 2012, 212, 2433–2442. [Google Scholar] [CrossRef]
- Bierwisch, C.; Mohseni-Mofidi, S.; Dietemann, B.; Grünewald, M.; Rudloff, J.; Lang, M. Universal process diagrams for laser sintering of polymers. Mater. Des. 2021, 199, 109432. [Google Scholar] [CrossRef]
- Schmid, M.; Amado, A.; Wegener, K. Materials perspective of polymers for additive manufacturing with selective laser sintering. J. Mater. Res. 2014, 29, 1824–1832. [Google Scholar] [CrossRef]
- Schmid, M.; Wegener, K. Additive Manufacturing: Polymers applicable for laser sintering (LS). In Proceedings of the Procedia Engineering; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; Volume 149, pp. 457–464. [Google Scholar]
- Badini, C.; Padovano, E.; Lambertini, V.G. Preferred orientation of chopped fibers in polymer-based composites processed by selective laser sintering and fused deposition modeling: Effects on mechanical properties. J. Appl. Polym. Sci. 2020, 137, 1–12. [Google Scholar] [CrossRef]
- Amado, A.; Schmid, M.; Levy, G.; Wegener, K. Advances in SLS powder characterization. In Proceedings of the 22th Solid Freeform Fabrication Symposium, Austin, TX, USA, 17 August 2011; pp. 438–452. [Google Scholar]
- Clayton, J.; Millington-Smith, D.; Armstrong, B. The Application of Powder Rheology in Additive Manufacturing. JOM 2015, 67, 544–548. [Google Scholar] [CrossRef]
- Schmid, M.; Amado, A.; Wegener, K. Polymer powders for selective laser sintering (SLS). In Proceedings of the AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2015; Volume 1664. [Google Scholar]
- Beltrán, M. Measurement Science Needs for Real-time Control of Additive Manufacturing Powder Bed Fusion Processes; NIST Interagency/Internal Report (NISTIR); National Institute of Standards and Technology: Gaithersburg, MD, USA, 2015. [CrossRef]
- Gibson, I.; Shi, D. Material properties and fabrication parameters in selective laser sintering process. Rapid Prototyp. J. 1997, 3, 129–136. [Google Scholar] [CrossRef]
- Wang, L.; Yu, A.; Li, E.; Shen, H.; Zhou, Z. Effects of spreader geometry on powder spreading process in powder bed additive manufacturing. Powder Technol. 2021, 384, 211–222. [Google Scholar] [CrossRef]
- Haeri, S.; Wang, Y.; Ghita, O.; Sun, J. Discrete element simulation and experimental study of powder spreading process in additive manufacturing. Powder Technol. 2017, 306, 45–54. [Google Scholar] [CrossRef]
- Haeri, S. Optimisation of blade type spreaders for powder bed preparation in Additive Manufacturing using DEM simulations. Powder Technol. 2017, 321, 94–104. [Google Scholar] [CrossRef]
- Wang, L.; Li, E.L.; Shen, H.; Zou, R.P.; Yu, A.B.; Zhou, Z.Y. Adhesion effects on spreading of metal powders in selective laser melting. Powder Technol. 2020, 363, 602–610. [Google Scholar] [CrossRef]
- Parteli, E.J.R. DEM simulation of particles of complex shapes using the multisphere method: Application for additive manufacturing. AIP Conf. Proc. 2013, 1542, 185–188. [Google Scholar] [CrossRef]
- Lee, Y.S.; Nandwana, P.; Zhang, W. Dynamic simulation of powder packing structure for powder bed additive manufacturing. Int. J. Adv. Manuf. Technol. 2018, 96, 1507–1520. [Google Scholar] [CrossRef]
- Prescott, J.K.; Barnum, R.A. On powder flowability. Pharm. Technol. 2000, 24, 60–84. [Google Scholar]
- Ruggi, D.; Lupo, M.; Sofia, D.; Barrès, C.; Barletta, D.; Poletto, M. Flow properties of polymeric powders for selective laser sintering. Powder Technol. 2020, 370, 288–297. [Google Scholar] [CrossRef]
- Ahmed, M.; Pasha, M.; Nan, W.; Ghadiri, M. A simple method for assessing powder spreadability for additive manufacturing. Powder Technol. 2020, 367, 671–679. [Google Scholar] [CrossRef]
- Mellin, P.; Lyckfeldt, O.; Harlin, P.; Brodin, H.; Blom, H.; Strondl, A. Evaluating flowability of additive manufacturing powders, using the Gustavsson flow meter. Met. Powder Rep. 2017, 72, 322–326. [Google Scholar] [CrossRef]
- Xu, G.; Lu, P.; Li, M.; Liang, C.; Xu, P.; Liu, D.; Chen, X. Investigation on characterization of powder flowability using different testing methods. Exp. Therm. Fluid Sci. 2018, 92, 390–401. [Google Scholar] [CrossRef]
- Escano, L.I.; Parab, N.D.; Xiong, L.; Guo, Q.; Zhao, C.; Fezzaa, K.; Everhart, W.; Sun, T.; Chen, L. Revealing particle-scale powder spreading dynamics in powder-bed-based additive manufacturing process by high-speed x-ray imaging. Sci. Rep. 2018, 8, 15079. [Google Scholar] [CrossRef]
- Chen, H.; Wei, Q.; Wen, S.; Li, Z.; Shi, Y. Flow behavior of powder particles in layering process of selective laser melting: Numerical modeling and experimental verification based on discrete element method. Int. J. Mach. Tools Manuf. 2017, 123, 146–159. [Google Scholar] [CrossRef]
- Nan, W.; Pasha, M.; Bonakdar, T.; Lopez, A.; Zafar, U.; Nadimi, S.; Ghadiri, M. Jamming during particle spreading in additive manufacturing. Powder Technol. 2018, 338, 253–262. [Google Scholar] [CrossRef]
- Meier, C.; Weissbach, R.; Weinberg, J.; Wall, W.A.; Hart, A.J. Critical influences of particle size and adhesion on the powder layer uniformity in metal additive manufacturing. J. Mater. Process. Technol. 2019, 266, 484–501. [Google Scholar] [CrossRef]
- Chen, H.; Wei, Q.; Zhang, Y.; Chen, F.; Shi, Y.; Yan, W. Powder-spreading mechanisms in powder-bed-based additive manufacturing: Experiments and computational modeling. Acta Mater. 2019, 179, 158–171. [Google Scholar] [CrossRef]
- Fouda, Y.M.; Bayly, A.E. A DEM study of powder spreading in additive layer manufacturing. Granul. Matter 2020, 22, 10. [Google Scholar] [CrossRef]
- He, Y.; Hassanpour, A.; Bayly, A.E. Linking particle properties to layer characteristics: Discrete element modelling of cohesive fine powder spreading in additive manufacturing. Addit. Manuf. 2020, 36, 101685. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Y.; Liu, Y.; Wei, Q.; Shi, Y.; Yan, W. Packing quality of powder layer during counter-rolling-type powder spreading process in additive manufacturing. Int. J. Mach. Tools Manuf. 2020, 153, 103553. [Google Scholar] [CrossRef]
- Chen, H.; Zhu, W.; Tang, H.; Yan, W. Oriented structure of short fiber reinforced polymer composites processed by selective laser sintering: The role of powder-spreading process. Int. J. Mach. Tools Manuf. 2021, 163, 103703. [Google Scholar] [CrossRef]
- Yuan, S.; Bai, J.; Chua, C.K.; Wei, J.; Zhou, K. Material evaluation and process optimization of CNT-coated polymer powders for selective laser sintering. Polymers 2016, 8, 370. [Google Scholar] [CrossRef]
- Yuan, S.; Zheng, Y.; Chua, C.K.; Yan, Q.; Zhou, K. Electrical and thermal conductivities of MWCNT/polymer composites fabricated by selective laser sintering. Compos. A Appl. Sci. Manuf. 2018, 105, 203–213. [Google Scholar] [CrossRef]
- Van den Eynde, M.; Verbelen, L.; Van Puyvelde, P. Assessing polymer powder flow for the application of laser sintering. Powder Technol. 2015, 286, 151–155. [Google Scholar] [CrossRef]
- Berretta, S.; Ghita, O.; Evans, K.E.; Anderson, A.; Newman, C. Size, shape and flow of powders for use in Selective Laser Sintering (SLS). In High Value Manufacturing: Advanced Research in Virtual and Rapid Prototyping—Proceedings of the 6th International Conference on Advanced Research and Rapid Prototyping, VR@P, Leiria, Portugal, 1–5 October 2013; CRC Press: Boca Raton, FL, USA, 2014; pp. 49–54. [Google Scholar]
- Kumar, S. Selective laser sintering/melting. In Comprehensive Materials Processing; Elsevier: Amsterdam, The Netherlands, 2014; pp. 93–104. ISBN 9780080965321. [Google Scholar]
- Berretta, S.; Ghita, O.; Evans, K.E. Morphology of polymeric powders in Laser Sintering (LS): From Polyamide to new PEEK powders. Eur. Polym. J. 2014, 59, 218–229. [Google Scholar] [CrossRef]
- Wang, G.; Wang, P.; Zhen, Z.; Zhang, W.; Ji, J. Preparation of PA12 microspheres with tunable morphology and size for use in SLS processing. Mater. Des. 2015, 87, 656–662. [Google Scholar] [CrossRef]
- Chung, H.; Das, S. Processing and properties of glass bead particulate-filled functionally graded Nylon-11 composites produced by selective laser sintering. Mater. Sci. Eng. A 2006, 437, 226–234. [Google Scholar] [CrossRef]
- Shi, Y.; Li, Z.; Sun, H.; Huang, S.; Zeng, F. Effect of the properties of the polymer materials on the quality of selective laser sintering parts. Proc. Inst. Mech. Eng. L J. Mater. Des. Appl. 2004, 218, 247–252. [Google Scholar] [CrossRef]
- Yuksel, A.; Cullinan, M. Modeling of nanoparticle agglomeration and powder bed formation in microscale selective laser sintering systems. Addit. Manuf. 2016, 12, 204–215. [Google Scholar] [CrossRef]
- Tayeb, R.; Dou, X.; Mao, Y.; Zhang, Y. Analysis of cohesive microsized particle packing structure using history-dependent contact models. J. Manuf. Sci. Eng. Trans. ASME 2016, 138, 041005. [Google Scholar] [CrossRef]
- Huang, Q.; Mesbah-Nejad, A.; Tadayyon, S.M.; Norton, P.; Zhang, H.; Zhu, J. Measurement of inter-particle forces by an interfacial force microscope. Particuology 2010, 8, 400–406. [Google Scholar] [CrossRef]
- Katainen, J.; Paajanen, M.; Ahtola, E.; Pore, V.; Lahtinen, J. Adhesion as an interplay between particle size and surface roughness. J. Colloid Interface Sci. 2006, 304, 524–529. [Google Scholar] [CrossRef]
- Amado, A.; Schmid, M.; Wegener, K. Flowability of SLS powders at elevated temperature. Rapid. Tech. 2014, 2, 1–7. [Google Scholar]
- Chirone, R.; Barletta, D.; Poletto, M.; Lettieri, P. Detection and estimation of capillary interparticle forces in the material of a fluidized bed reactor at high temperature by powder flow characterization. Powder Technol. 2018, 330, 371–385. [Google Scholar] [CrossRef]
- Macrì, D.; Barletta, D.; Lettieri, P.; Poletto, M. Experimental and theoretical analysis of TiO2 powders flow properties at ambient and high temperatures. Chem. Eng. Sci. 2017, 167, 172–190. [Google Scholar] [CrossRef]
- Kruth, J.P.; Wang, X.; Laoui, T.; Froyen, L. Lasers and materials in selective laser sintering. Assem. Autom. 2003, 23, 357–371. [Google Scholar] [CrossRef]
- Gusarov, A.V.; Yadroitsev, I.; Bertrand, P.; Smurov, I. Model of radiation and heat transfer in laser-powder interaction zone at selective laser melting. J. Heat Transf. 2009, 131, 072101. [Google Scholar] [CrossRef]
- Xin, L.; Boutaous, M.; Xin, S.; Siginer, D.A. Numerical modeling of the heating phase of the selective laser sintering process. Int. J. Therm. Sci. 2017, 120, 50–62. [Google Scholar] [CrossRef]
- Foroozmehr, A.; Badrossamay, M.; Foroozmehr, E.; Golabi, S. Finite Element Simulation of Selective Laser Melting process considering Optical Penetration Depth of laser in powder bed. Mater. Des. 2016, 89, 255–263. [Google Scholar] [CrossRef]
- Peyre, P.; Rouchausse, Y.; Defauchy, D.; Régnier, G. Experimental and numerical analysis of the selective laser sintering (SLS) of PA12 and PEKK semi-crystalline polymers. J. Mater. Process. Technol. 2015, 225, 326–336. [Google Scholar] [CrossRef]
- Greiner, S.; Wudy, K.; Lanzl, L.; Drummer, D. Selective laser sintering of polymer blends: Bulk properties and process behavior. Polym. Test. 2017, 64, 136–144. [Google Scholar] [CrossRef]
- Salmoria, G.V.; Paggi, R.A.; Lago, A.; Beal, V.E. Microstructural and mechanical characterization of PA12/MWCNTs nanocomposite manufactured by selective laser sintering. Polym. Test. 2011, 30, 611–615. [Google Scholar] [CrossRef]
- Wang, Y.; Rouholamin, D.; Davies, R.; Ghita, O.R. Powder characteristics, microstructure and properties of graphite platelet reinforced Poly Ether Ether Ketone composites in High Temperature Laser Sintering (HT-LS). Mater. Des. 2015, 88, 1310–1320. [Google Scholar] [CrossRef]
- Xi, S.; Zhang, P.; Huang, Y.; Kong, M.; Yang, Q.; Li, G. Laser sintering of cryogenically ground polymer powders into high-performance parts: The role of dry particle coating with a conductive flow agent. Polymer 2020, 186, 122044. [Google Scholar] [CrossRef]
- Indhu, R.; Vivek, V.; Loganathan, S.; Bharatish, A.; Soundarapandian, S. Overview of Laser Absorptivity Measurement Techniques for Material Processing. Lasers Manuf. Mater. Process. 2018, 5, 458–481. [Google Scholar] [CrossRef]
- Tolochko, N.K.; Laoui, T.; Khlopkov, Y.V.; Mozzharov, S.E.; Titov, V.I.; Ignatiev, M.B. Absorptance of powder materials suitable for laser sintering. Rapid Prototyp. J. 2000, 6, 155–160. [Google Scholar] [CrossRef]
- Laumer, T.; Stichel, T.; Nagulin, K.; Schmidt, M. Optical analysis of polymer powder materials for Selective Laser Sintering. Polym. Test. 2016, 56, 207–213. [Google Scholar] [CrossRef]
- Shen, F.; Yuan, S.; Chua, C.K.; Zhou, K. Development of process efficiency maps for selective laser sintering of polymeric composite powders: Modeling and experimental testing. J. Mater. Process. Technol. 2018, 254, 52–59. [Google Scholar] [CrossRef]
- Laumer, T.; Stichel, T.; Sachs, M.; Amend, P.; Schmidt, M. Qualification and modification of new polymer powders for laser beam melting using Ulbricht spheres. In High Value Manufacturing: Advanced Research in Virtual and Rapid Prototyping—Proceedings of the 6th International Conference on Advanced Research in Virtual and Rapid Prototyping, Leiria, Portugal, 1–5 October 2013; CRC Press: Boca Raton, FL, USA, 2014; pp. 255–260. [Google Scholar] [CrossRef]
- Heinl, M.; Laumer, T.; Bayer, F.; Hausotte, T. Temperature-dependent optical material properties of polymer powders regarding in-situ measurement techniques in additive manufacturing. Polym. Test. 2018, 71, 378–383. [Google Scholar] [CrossRef]
- Schuffenhauer, T.; Stichel, T.; Schmidt, M. Experimental determination of scattering processes in the interaction of laser radiation with polyamide 12 powder. Procedia CIRP 2020, 94, 85–88. [Google Scholar] [CrossRef]
- Osmanlic, F.; Wudy, K.; Laumer, T.; Schmidt, M.; Drummer, D.; Körner, C. Modeling of laser beam absorption in a polymer powder bed. Polymers 2018, 10, 784. [Google Scholar] [CrossRef]
- Defauchy, D. Simulation du Procédé de Fabrication Directe de Pièces Thermoplastiques par Fusion Laser de Poudre. Ph.D. Thesis, L’École Nationale Supérieure d’Arts et Métiers, Paris, France, 2013; p. 219. [Google Scholar]
- Xin, L.; Boutaous, M.; Xin, S.; Siginer, D.A. Multiphysical modeling of the heating phase in the polymer powder bed fusion process. Addit. Manuf. 2017, 18, 121–135. [Google Scholar] [CrossRef]
- Fan, K.M.; Wong, K.W.; Cheung, W.L.; Gibson, I. Reflectance and transmittance of TrueFormTM powder and its composites to CO2 laser. Rapid Prototyp. J. 2007, 13, 175–181. [Google Scholar] [CrossRef]
- Ho, H.C.H.; Cheung, W.L.; Gibson, I. Effects of graphite powder on the laser sintering behaviour of polycarbonate. Rapid Prototyp. J. 2002, 8, 233–242. [Google Scholar] [CrossRef]
- Athreya, S.R.; Kalaitzidou, K.; Das, S. Processing and characterization of a carbon black-filled electrically conductive Nylon-12 nanocomposite produced by selective laser sintering. Mater. Sci. Eng. A 2010, 527, 2637–2642. [Google Scholar] [CrossRef]
- Woicke, N.; Wagner, T.; Eyerer, P. Carbon assisted laser sintering of thermoplastic polymers. Annu. Tech. Conf. ANTEC Conf. Proc. 2005, 7, 36–40. [Google Scholar]
- Lanzl, L.; Wudy, K.; Drummer, D. The effect of short glass fibers on the process behavior of polyamide 12 during selective laser beam melting. Polym. Test. 2020, 83, 106313. [Google Scholar] [CrossRef]
- Tian, X.; Peng, G.; Yan, M.; He, S.; Yao, R. Process prediction of selective laser sintering based on heat transfer analysis for polyamide composite powders. Int. J. Heat Mass Transf. 2018, 120, 379–386. [Google Scholar] [CrossRef]
- Erdal, M.; Dag, S.; Jande, Y.; Tekin, C.M. Manufacturing of functionally graded porous products by selective laser sintering. Mater. Sci. Forum 2010, 631–632, 253–258. [Google Scholar] [CrossRef]
- Du, Y.; Liu, H.; Shuang, J.; Wang, J.; Ma, J.; Zhang, S. Microsphere-based selective laser sintering for building macroporous bone scaffolds with controlled microstructure and excellent biocompatibility. Colloids Surf. B Biointerfaces 2015, 135, 81–89. [Google Scholar] [CrossRef]
- Vasquez, M.; Haworth, B.; Hopkinson, N. Optimum sintering region for laser sintered Nylon-12. In Proceedings of the Proceedings of the Institution of Mechanical Engineers, Part B: Journal of Engineering Manufacture; Sage Publications: London, UK, 2011; Volume 225, pp. 2240–2248. [Google Scholar]
- Lupone, F.; Padovano, E.; Pietroluongo, M.; Giudice, S.; Ostrovskaya, O.; Badini, C. Optimization of selective laser sintering process conditions using stable sintering region approach. Express Polym. Lett. 2021, 15, 177–192. [Google Scholar] [CrossRef]
- Drexler, M.; Lexow, M.; Drummer, D. Selective Laser Melting of Polymer Powder—Part Mechanics as Function of Exposure Speed. Phys. Procedia 2015, 78, 328–336. [Google Scholar] [CrossRef]
- Starr, T.L.; Gornet, T.J.; Usher, J.S. The effect of process conditions on mechanical properties of laser-sintered nylon. Rapid Prototyp. J. 2011, 17, 418–423. [Google Scholar] [CrossRef]
- Berretta, S.; Evans, K.E.; Ghita, O.R. Predicting processing parameters in high temperature laser sintering (HT-LS) from powder properties. Mater. Des. 2016, 105, 301–314. [Google Scholar] [CrossRef]
- Caulfield, B.; McHugh, P.E.; Lohfeld, S. Dependence of mechanical properties of polyamide components on build parameters in the SLS process. J. Mater. Process. Technol. 2007, 182, 477–488. [Google Scholar] [CrossRef]
- Dong, L.; Makradi, A.; Ahzi, S.; Remond, Y. Three-dimensional transient finite element analysis of the selective laser sintering process. J. Mater. Process. Technol. 2009, 209, 700–706. [Google Scholar] [CrossRef]
- Vasquez, G.M.; Majewski, C.E.; Haworth, B.; Hopkinson, N. A targeted material selection process for polymers in laser sintering. Addit. Manuf. 2014, 1, 127–138. [Google Scholar] [CrossRef]
- Franco, A.; Lanzetta, M.; Romoli, L. Experimental analysis of selective laser sintering of polyamide powders: An energy perspective. J. Clean. Prod. 2010, 18, 1722–1730. [Google Scholar] [CrossRef]
- Riedlbauer, D.; Drexler, M.; Drummer, D.; Steinmann, P.; Mergheim, J. Modelling, simulation and experimental validation of heat transfer in selective laser melting of the polymeric material PA12. Comput. Mater. Sci. 2014, 93, 239–248. [Google Scholar] [CrossRef]
- Yuan, S.; Li, J.; Yao, X.; Zhu, J.; Gu, X.; Gao, T.; Xu, Y.; Zhang, W. Intelligent optimization system for powder bed fusion of processable thermoplastics. Addit. Manuf. 2020, 34, 101182. [Google Scholar] [CrossRef]
- Drummer, D.; Drexler, M.; Wudy, K. Impact of heating rate during exposure of laser molten parts on the processing window of PA12 powder. Phys. Procedia 2014, 56, 184–192. [Google Scholar] [CrossRef]
- Wudy, K.; Drummer, D.; Drexler, M. Characterization of polymer materials and powders for selective laser melting. AIP Conf. Proc. 2014, 1593, 702–707. [Google Scholar] [CrossRef]
- Kruth, J.P.; Levy, G.; Klocke, F.; Childs, T.H.C. Consolidation phenomena in laser and powder-bed based layered manufacturing. CIRP Ann. Manuf. Technol. 2007, 56, 730–759. [Google Scholar] [CrossRef]
- Kruth, J.P.; Mercelis, P.; Van Vaerenbergh, J.; Froyen, L.; Rombouts, M. Binding mechanisms in selective laser sintering and selective laser melting. Rapid Prototyp. J. 2005, 11, 26–36. [Google Scholar] [CrossRef]
- Zarringhalam, H.; Hopkinson, N.; Kamperman, N.F.; de Vlieger, J.J. Effects of processing on microstructure and properties of SLS Nylon 12. Mater. Sci. Eng. A 2006, 435–436, 172–180. [Google Scholar] [CrossRef]
- Dadbakhsh, S.; Verbelen, L.; Verkinderen, O.; Strobbe, D.; Van Puyvelde, P.; Kruth, J.P. Effect of PA12 powder reuse on coalescence behaviour and microstructure of SLS parts. Eur. Polym. J. 2017, 92, 250–262. [Google Scholar] [CrossRef]
- Zhao, M.; Drummer, D.; Wudy, K.; Drexler, M. Sintering Study of Polyamide 12 Particles for Selective Laser Melting. Int. J. Recent Contrib. Eng. Sci. IT 2015, 3, 28. [Google Scholar] [CrossRef]
- Benedetti, L.; Brulé, B.; Decraemer, N.; Evans, K.E.; Ghita, O. Evaluation of particle coalescence and its implications in laser sintering. Powder Technol. 2019, 342, 917–928. [Google Scholar] [CrossRef]
- Sun, M.M.; Nelson, J.C.; Beaman, J. A Model for Partial Viscous Sintering; The University of Texas at Austin: Austin, TX, USA, 1991; pp. 46–55. [Google Scholar]
- Pokluda, O.; Bellehumeur, C.T.; Vlachopoulos, J. Modification of Frenkel’s Model for Sintering. AIChE J. 1997, 43, 3253–3256. [Google Scholar] [CrossRef]
- Bellehumeur, C.T.; Kontopoulou, M.; Vlachopoulos, J. The role of viscoelasticity in polymer sintering. Rheol. Acta 1998, 37, 270–278. [Google Scholar] [CrossRef]
- Verbelen, L.; Dadbakhsh, S.; Van Den Eynde, M.; Kruth, J.P.; Goderis, B.; Van Puyvelde, P. Characterization of polyamide powders for determination of laser sintering processability. Eur. Polym. J. 2016, 75, 163–174. [Google Scholar] [CrossRef]
- Sauer, B.B.; Dipaolo, N.V. Surface tension and dynamic wetting on polymers using the Wihelmy method: Applications to high molecular weights and elevated temperatures. J. Colloid Interface Sci. 1991, 144, 527–537. [Google Scholar] [CrossRef]
- Yan, M.; Tian, X.; Peng, G.; Li, D.; Zhang, X. High temperature rheological behavior and sintering kinetics of CF/PEEK composites during selective laser sintering. Compos. Sci. Technol. 2018, 165, 140–147. [Google Scholar] [CrossRef]
- Kim, J.; Creasy, T.S. Selective laser sintering characteristics of nylon 6/clay-reinforced nanocomposite. Polym. Test. 2004, 23, 629–636. [Google Scholar] [CrossRef]
- Berretta, S.; Wang, Y.; Davies, R.; Ghita, O.R. Polymer viscosity, particle coalescence and mechanical performance in high-temperature laser sintering. J. Mater. Sci. 2016, 51, 4778–4794. [Google Scholar] [CrossRef]
- Haworth, B.; Hopkinson, N.; Hitt, D.; Zhong, X. Shear viscosity measurements on Polyamide-12 polymers for laser sintering. Rapid Prototyp. J. 2013, 19, 28–36. [Google Scholar] [CrossRef]
- Bai, J.; Goodridge, R.D.; Hague, R.J.M.; Song, M.; Okamoto, M. Influence of carbon nanotubes on the rheology and dynamic mechanical properties of polyamide-12 for laser sintering. Polym. Test. 2014, 36, 95–100. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, P.; Nanda, A. Hot stage microscopy and its applications in pharmaceutical characterization. Appl. Microsc. 2020, 50, 12. [Google Scholar] [CrossRef]
- Laumer, T.; Wudy, K.; Drexler, M.; Amend, P.; Roth, S.; Drummer, D.; Schmidt, M. Fundamental investigation of laser beam melting of polymers for additive manufacture. J. Laser Appl. 2014, 26, 042003. [Google Scholar] [CrossRef]
- Verbelen, L.; Dadbakhsh, S.; Van den Eynde, M.; Strobbe, D.; Kruth, J.P.; Goderis, B.; Van Puyvelde, P. Analysis of the material properties involved in laser sintering of thermoplastic polyurethane. Addit. Manuf. 2017, 15, 12–19. [Google Scholar] [CrossRef]
- Schmachtenberg, E.; Seul, T. Model of isothermic lasersintering. In Proceedings of the 60th Anual Technical Conference of the Society of Plastic Engineers (ANTEC), San Francisco, CA, USA, 5–9 May 2002. [Google Scholar]
- Drummer, D.; Rietzel, D.; Kühnlein, F. Development of a characterization approach for the sintering behavior of new thermoplastics for selective laser sintering. Phys. Procedia 2010, 5, 533–542. [Google Scholar] [CrossRef]
- Wendel, B.; Rietzel, D.; Kühnlein, F.; Feulner, R.; Hülder, G.; Schmachtenberg, E. Additive processing of polymers. Macromol. Mater. Eng. 2008, 293, 799–809. [Google Scholar] [CrossRef]
- Amado, A.; Wegener, K.; Schmid, M.; Levy, G. Characterization and modeling of non-isothermal crystallization of Polyamide 12 and co-Polypropylene during the SLS process. In Proceedings of the 5th International Polymers & Moulds Innovations Conference, Ghent, Belgium, 12–14 September 2012; Volume 60. [Google Scholar]
- Greiner, S.; Jaksch, A.; Cholewa, S.; Drummer, D. Development of material-adapted processing strategies for laser sintering of polyamide 12. Adv. Ind. Eng. Polym. Res. 2021, 4, 251–263. [Google Scholar] [CrossRef]
- Zhao, M.; Wudy, K.; Drummer, D. Crystallization kinetics of polyamide 12 during Selective laser sintering. Polymers 2018, 10, 168. [Google Scholar] [CrossRef]
- Tan, L.J.; Zhu, W.; Sagar, K.; Zhou, K. Comparative study on the selective laser sintering of polypropylene homopolymer and copolymer: Processability, crystallization kinetics, crystal phases and mechanical properties. Addit. Manuf. 2021, 37, 101610. [Google Scholar] [CrossRef]
- Amado, A.; Schmid, M.; Wegener, K. Simulation of warpage induced by non-isothermal crystallization of co-polypropylene during the SLS proceb. AIP Conf. Proc. 2015, 1664, 2–6. [Google Scholar] [CrossRef]
- Chen, P.; Cai, H.; Li, Z.; Li, M.; Wu, H.; Su, J.; Wen, S.; Zhou, Y.; Liu, J.; Wang, C.; et al. Crystallization kinetics of polyetheretherketone during high temperature-selective laser sintering. Addit. Manuf. 2020, 36, 101615. [Google Scholar] [CrossRef]
- Negi, S.; Sharma, R.K. Study on shrinkage behaviour of laser sintered PA 3200GF specimens using RSM and ANN. Rapid Prototyp. J. 2016, 22, 645–659. [Google Scholar] [CrossRef]
- Wang, Y.; Beard, J.D.; Evans, K.E.; Ghita, O. Unusual crystalline morphology of Poly Aryl Ether Ketones (PAEKs). RSC Adv. 2016, 6, 3198–3209. [Google Scholar] [CrossRef]
- Garcia-Leiner, M.; Dennies, D.P.; Yardimci, A. High Performance Polymers in Additive Manufacturing Processes: Understanding Process, Structure and Property. Microsc. Microanal. 2015, 21, 127–128. [Google Scholar] [CrossRef][Green Version]
- Van den Eynde, M.; Strobbe, D.; Verkinderen, O.; Verbelen, L.; Goderis, B.; Kruth, J.P.; Van Puyvelde, P. Effect of thermal treatments on the laser sinterability of cryogenically milled polybutene-1. Mater. Des. 2018, 153, 15–23. [Google Scholar] [CrossRef]
- Benedetti, L.; Brulé, B.; Decreamer, N.; Evans, K.E.; Ghita, O. Shrinkage behaviour of semi-crystalline polymers in laser sintering: PEKK and PA12. Mater. Des. 2019, 181, 107906. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lupone, F.; Padovano, E.; Casamento, F.; Badini, C. Process Phenomena and Material Properties in Selective Laser Sintering of Polymers: A Review. Materials 2022, 15, 183. https://doi.org/10.3390/ma15010183
Lupone F, Padovano E, Casamento F, Badini C. Process Phenomena and Material Properties in Selective Laser Sintering of Polymers: A Review. Materials. 2022; 15(1):183. https://doi.org/10.3390/ma15010183
Chicago/Turabian StyleLupone, Federico, Elisa Padovano, Francesco Casamento, and Claudio Badini. 2022. "Process Phenomena and Material Properties in Selective Laser Sintering of Polymers: A Review" Materials 15, no. 1: 183. https://doi.org/10.3390/ma15010183
APA StyleLupone, F., Padovano, E., Casamento, F., & Badini, C. (2022). Process Phenomena and Material Properties in Selective Laser Sintering of Polymers: A Review. Materials, 15(1), 183. https://doi.org/10.3390/ma15010183