Abstract
Doping plays a vital role in the application of transition-metal dichalcogenides (TMDCs) because it can increase the functionality of TMDCs by tuning their native characteristics. In this study, the influence of Mn, Fe, Co, and Cu doping on the photoelectric properties of HfS2 was investigated. Pristine, Mn-, Fe-, Co-, and Cu-doped HfS2 crystals were grown using the chemical vapor transport method. Scanning electron microscopy images showed that the crystals were layered and transmission electron microscopy, X-ray diffraction, and Raman spectroscopy measurements confirmed that the crystals were in the 1T-phase with a CdI2-like structure. The bandgap of pristine HfS2 obtained from the absorption and photoconductivity spectra was approximately 1.99 eV. As the dopant changed from Mn, Fe, and Co, to Cu, the bandgap gradually increased. The activation energies of the samples were determined using temperature-dependent current-voltage curves. After doping, the activation energy decreased, and the Co-doped HfS2 exhibited the smallest activation energy. Time-resolved photoresponse measurements showed that doping improved the response of HfS2 to light; the Co-doped HfS2 exhibited the best response. The photoresponsivity of HfS2 as a function of the laser power and bias voltage was measured. After doping, the photoresponsivity increased markedly; the Co-doped HfS2 exhibited the highest photoresponsivity. All the experimental results indicated that doping with Mn, Fe, Co, and Cu significantly improved the photoresponsive performance of HfS2, of which Co-doped HfS2 had the best performance.
1. Introduction
Transition metal dichalcogenides (TMDCs) [1,2,3,4,5,6,7,8,9] refer to the compounds of transition metal (TM) elements from group IVB to group VIIB in the periodic table and chalcogen elements. They have the general chemical formula TX2, where T represents a TM atom, and X represents a chalcogen atom such as S, Se, or Te. TMDCs are layered materials in which one unit layer is composed of three atomic planes. One TM atomic plane and two chalcogen atomic planes form an X-T-X sandwich structure via strong covalent bonds, and the X-T-X monolayers are gathered together by van der Waals forces. Owing to the weak van der Waals forces, foreign atoms or molecules can be easily inserted between the X-T-X monolayers [2], and TMDCs can be exfoliated into structures with few monolayers [10] or graphene-like two-dimensional (2D) freestanding monolayers [11]. Such 2D TMDCs with atomic-scale thicknesses exhibit a direct bandgap and strong spin-orbit coupling. Thus, 2D TMDCs have unique mechanical, chemical, optical, and electrical properties and have promising applications as catalysts [8,12], energy storage devices, electronic devices, biosensors, piezoelectric devices, photonic devices, and gas sensors [6].
The most noticeable TMDCs are those of the group VIB TM elements Mo and W, such as MoS2, WS2, MoSe2, WSe2, and MoTe2. They have an appropriate bandgap value between 1 and 2 eV, and by changing the dimensionality, this bandgap can be tuned from indirect in bulk materials to direct in monolayers. Their unique properties are favorable for applications in optoelectronics, sensing, photovoltaics, and nanoelectronics [3,7,13]. For example, Radisavljevic et al. fabricated the first monolayer MoS2 field-effect transistor [14], and Splendiani et al. and Mak et al. discovered strong photoluminescence in MoS2 monolayers [15,16].
Furthermore, TMDCs of the group IVB TM elements Zr and Hf, such as ZrS2, HfS2, ZrSe2, and HfSe2, also show semiconductor characteristics, and related research has been conducted [17]. According to previous reports, HfS2 and ZrS2 bulk materials have indirect bandgaps; the bandgap of HfS2 is approximately 1.80–2.13 eV [18,19,20,21,22,23], and the bandgap of ZrS2 is approximately 1.68–1.78 eV [18,19,20,21,24,25]. The corresponding light wavelengths fall within the range of the visible and infrared regimes. Therefore, they may be suitable for various applications in optoelectronics, such as photodetectors and photovoltaic devices [17,25].
2D layered HfS2 crystals have recently attracted significant attention owing to their prospective properties [26]. Kaur et al. employed a solvent-assisted ultrasonification method to chemically exfoliate HfS2 crystals into single or multiple monolayers. The HfS2 nanosheets exhibited an indirect bandgap of 1.3 eV and held a high potential for applications in field-effect transistors [27]. Wang et al. grew an HfS2 monolayer on hexagonal boron nitride (h-BN) via chemical vapor deposition; photodetectors based on HfS2/h-BN heterostructures exhibited excellent sensing performance [28]. Li et al. used exfoliated HfS2 nanosheets to manufacture memory devices, which exhibited typical bipolar resistive switching behavior with a high switching voltage and a small ratio of high and low resistive states [29]. Yin et al. showed that HfS2 nanosheets exhibit excellent nonlinear optical absorption in broadband and have the potential for applications in ultrafast photonics [30]. Hoat et al. utilized density functional theory to theoretically investigate the electronic structure and optical properties of HfS2 monolayers under vertical strain. The application of vertical strains can remarkably adjust the bandgap, and an indirect-direct gap transition may occur with compressive strains [31].
In optoelectronic devices and field-effect transistors, the most widely studied TMDC is MoS2; however, several experiments have shown that the performance of HfS2 is not inferior to that of MoS2 [32,33,34,35,36]. In addition, theoretical calculations predicted that the room-temperature mobility of 2D HfS2 is 1833 cm2 V−1 s−1, which is much higher than the 340 cm2 V−1 s−1 value of 2D MoS2 [37]. Xu et al. studied the electronic and optoelectronic properties of a few-layered HfS2 phototransistor. This ultrathin HfS2 phototransistor had a very high on/off ratio ≈107 and an ultrahigh photoresponsivity of over 890 AW−1 [34]. These results indicate that HfS2 has a high application potential and is worthy of further in-depth research.
Doping is the intentional introduction of foreign impurities into the host material. It plays an essential role in the research on TMDCs because it can increase the functionality of TMDCs by providing routes to tune their native characteristics. For example, Suh et al. established stable p-type conduction in MoS2, which had intrinsic n-type conduction, by substitutional Nb doping [38]. Zhang et al. doped Mn into a MoS2 monolayer on a graphene substrate and altered its band structure [39]. Gong et al. demonstrated that changing the Se concentration can fine-tune the optical bandgap of Se-doped MoS2 [40]. Mouri et al. demonstrated the tunability of the photoluminescence properties of MoS2 monolayers via chemical doping [41]. Li et al. reported that field-effect transistors based on an Fe-doped SnS2 monolayer exhibited high optoelectronic performance [42].
Theoretically, many calculations have been conducted to investigate the structural, electronic, thermoelectric, optical, and magnetic properties of doped HfS2. In these studies, the dopants were of group IIIA, VA, VIIA [43,44,45], TM [46,47,48,49], or lanthanide atoms [50]. All these studies showed that doping is an effective method of modulating the properties of HfS2, and the doped HfS2 may have significant potential applications in photocatalysts, and tunable electronic, optoelectronic, thermoelectric, magneto-optic, and spintronic devices.
Based on theoretical studies, it is interesting and essential to experimentally investigate the properties of doped HfS2. However, the influence of doping with TM atoms on the photoelectric properties of HfS2 crystals is not completely understood. Thus, in this study, pristine, Mn-, Fe-, Co-, and Cu-doped HfS2 crystals were grown using the chemical vapor transport (CVT) method, and their photoelectric properties were explored. All the experimental results indicated that doping with Mn, Fe, Co, and Cu could significantly improve the photoresponsive performance of HfS2 crystals. The Co-doped HfS2 crystal exhibited the best performance.
2. Materials and Methods
Pristine, Mn-, Fe-, Co- and Cu-doped HfS2 crystals were grown using the CVT method. First, Hf and S were weighed using an electronic balance to generate a Hf to S molar ratio of 1:2. Then, these quantities of Hf and S as well as 0.5 g of I2 used as a transport agent were placed into a quartz ampoule along with the doping elements Mn, Fe, Co, and Cu. The designed doping concentration was 2%. The quartz ampoule was evacuated to 1 × 10−3 Pa, sealed, and then placed in a three-zone furnace for 300 h. To obtain the best diffusion gradient for crystal growth, the temperatures at both ends of the quartz ampoule were set to 880 °C and 730 °C, respectively. The temperature gradient was approximately 5 °C/cm.
After the growth of the pristine, Mn-, Fe-, Co-, and Cu-doped HfS2 crystals, a JEOL JXA-8530F (Tokyo, Japan) field-emission electron probe micro-analyzer (FE-EPMA) was used to identify the chemical composition. The morphology of the crystals was characterized by a HITACHI S-4800 (Hitachi High-Tech-, Tokyo, Japan) field-emission scanning electron microscope (SEM). A JEOL JEM-3010 (Jeol, Tokyo, Japan) transmission electron microscope (TEM) was used to obtain crystal images. The crystal structures of the samples were examined using a Bruker D8 SSS (Bruker, Billerica, MA, USA) high-resolution X-ray diffractometer (XRD) with Cu Kα radiation (λ = 1.5418 Å). A Dongwoo Ramboss (DongWoo Optron, Gwangju-Si, Korea) micro Raman system equipped with a solid-state laser source was used for the Raman analysis. The wavelength of the incident laser beam was 532 nm. The photoluminescence (PL) spectra of the samples were collected using a HORIBA iHR550 (Horiba, Kyoto, Japan) spectrometer. The wavelength of the excitation laser beam was 532 nm.
For absorption and photoconductivity (PC) measurements, a 1/4 m monochromator (MKS, Irvine, CA, USA) equipped with a 130 W halogen lamp was used to produce monochromatic light with a wide photon energy range. A mechanical chopper was used to modulate continuous light from the monochromator into alternating light. For the absorption measurements, the frequency of the alternating incident light was 200 Hz. To detect the intensity of the transmitted light, a silicon photodetector (Thorlabs, Newton, NJ, USA) with an amplifier was placed on the back of the measured sample. The output signals of the photodetector were recorded by a dual-phase lock-in amplifier (Ametek, Berwyn, PA, USA) to suppress the noise signals. For the PC measurements, the frequency of the incident alternating light was 20 Hz. A stable bias voltage of 20 V applied to the measured sample was supplied by a Keithley 2400 SourceMeter (Tektronix, Beaverton, OR, USA). To obtain the photoresponsivity of the measured sample, the photocurrent was recorded using a dual-phase lock-in amplifier and then divided by the power of the incident light at each wavelength.
A solar simulator, which provided a stable irradiation intensity of 100 mW/cm2, was used as the light source to record the current-voltage (I-V) characteristics of the measured sample under dark and illumination conditions. In addition, a Keithley 2400 SourceMeter was used to apply a bias voltage to the measured sample and record the current. To construct the temperature-dependent I-V curves, a Janis Research CCS-250 system (Lake Shore Cryotronics, Westerville, OH, USA) was utilized; this system was equipped with a Model 32 B cryogenic thermometer controller (Cryogenic Control Systems, Rancho Santa Fe, CA, USA) to adjust the temperature of the measured sample within the range of 300 to 380 K.
For time-resolved photoresponse measurements, a 532 nm wavelength laser was controlled by an AFG-2225 function generator (GW Instek, New Taipei City, Taiwan) to apply on/off light modulation to the measured sample. First, a stable bias voltage of 100 V was applied to the measured sample using a Keithley 2410 SourceMeter (Tektronix, Beaverton, OR, USA). Then, the photocurrent signals were collected using a data acquisition device with a sampling frequency of 1 MHz.
A laser with a wavelength of 532 nm was used as the excitation source to measure the photoresponsivity of the measured sample as a function of the laser power and bias voltage. The laser light was modulated into alternating light with a frequency of 1 Hz using an AFG-2225 function generator. For the laser power-dependent photoresponsivity measurements, the laser power was adjusted using neutral-density filters. A stable bias voltage of 100 V was applied to the measured sample using a Keithley 2400 SourceMeter. The photocurrent was recorded using a dual-phase lock-in amplifier and divided by the laser power to obtain the photoresponsivity of the measured sample. For the measurements of the bias-dependent photoresponsivity, the laser power was set to 1.3 mW, and a Keithley 2400 SourceMeter was used to apply a bias voltage to the measured sample and record the induced current. The difference between the average currents under dark and illumination conditions was divided by the incident laser power to obtain the photoresponsivity of the measured sample.
3. Results and Discussion
An FE-EPMA was used to determine the chemical composition of the grown samples, and the results are listed in Table 1. Each value in Table 1 is an average value after multiple measurements; therefore, the sum of the atomic percentages of Hf, S and the dopant for each sample was not exactly equal to 100%. The atomic ratio of Hf to S in the pristine HfS2 crystal was approximately 1:2. The Mn-, Co-, and Cu-doped HfS2 crystals were S-rich, whereas the Fe-doped HfS2 crystal was Hf-rich. In the Mn-, Co-, and Cu-doped HfS2 crystals, the atomic percentages of the dopants were much higher than that in the Fe-doped HfS2 crystal. Theoretical calculations [46] showed that under S-rich conditions, it is energetically favorable and relatively easier to incorporate TM atoms in HfS2. The FE-EPMA results were consistent with this prediction.

Table 1.
Atomic percentages of Hf, S, and dopants in the pristine, Mn-, Fe-, Co-, and Cu-doped HfS2 crystals.
Figure 1a shows the schematic structure of a 1T-HfS2 crystal, and the top view and side view of layered forms are presented. In a HfS2 single layer, the Hf atomic plane is sandwiched between two S atomic planes. The Hf atom is octahedrally coordinated with the S atoms. 1T-HfS2 adopts a CdI2-like layered structure belonging to the space group 3m1. Figure 1b shows an SEM image of the Cu-doped HfS2 sample. The grown HfS2 crystal was composed of multiple layers, and an angle of 120° characterized the edge of each layer. SEM images of the other samples are similar to that shown in Figure 1b.
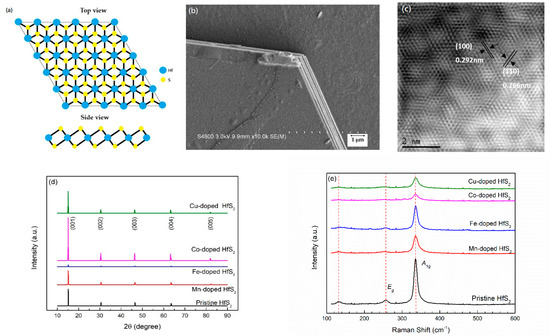
Figure 1.
(a) Schematic structure of a 1T-HfS2 crystal; (b) Scanning electron microscopy image of the Cu-doped HfS2 crystal; (c) transmission electron microscopy image of the Co-doped HfS2 crystal; (d) X-ray diffraction patterns of the pristine, Mn-, Fe-, Co-, and Cu-doped HfS2 crystals; (e) Raman spectra of the pristine, Mn-, Fe-, Co-, and Cu-doped HfS2 crystals.
Figure 1c shows a TEM image of the Co-doped HfS2 sample. This image confirms that the grown HfS2 crystal has a hexagonal 1T structure, and TEM images of the other samples are similar to that shown in Figure 1c. First, the TEM image of each HfS2 crystal was used to estimate the lattice plane spacings, d100 and d110. Then, the lattice constant a was calculated using the formula
The calculated results are listed in Table 2. It can be seen that as the dopant changed from Mn, Fe, and Co to Cu, the lattice spacings d100, d110 and the lattice constant a gradually decreased. This reduction may be due to the substitution of doping atoms for Hf atoms. As the radius of the doping atoms decreased, d100, d110, and a also decreased.

Table 2.
Lattice parameters of the pristine, Mn-, Fe-, Co-, and Cu-doped HfS2 crystals.
XRD and Raman spectroscopy were used to confirm the crystal structures of the samples. Figure 1d shows the XRD patterns of the pristine, Mn-, Fe-, Co-, and Cu-doped HfS2 crystals. Only the (00l) diffraction peaks of the HfS2 crystals are observed in Figure 1d. The peaks at approximately 2θ = 15.16°, 30.52°, 46.54°, 63.48°, and 82.3° correspond to the (001), (002), (003), (004), and (005) planes of the HfS2 crystals, respectively. The diffraction patterns of the HfS2 crystals matched well with JCPDS card No. 28-0444, confirming that the crystals had a CdI2-like layered structure belonging to the space group 3m1 [52]. As shown in Figure 1d, as the dopant of the HfS2 crystals changed from Mn, Fe, and Co to Cu, the position of the (001) peak shifted slightly to a smaller angle. Using the characteristic wavelength of the Kα radiation of copper λ = 1.5418 Å and Bragg’s formula
The lattice constant c of each HfS2 crystal was calculated and is listed in Table 2. The estimated c value of the pristine HfS2 crystal matched well with the value reported by Lucovsky et al. [51]. As shown in Table 2, as the dopant of the HfS2 crystals changed from Mn, Fe, and Co to Cu, the lattice constant c increased slightly; this small effect may be due to the doping of TM atoms, which reduces the lattice constant a, deforms the lattice, and then slightly increases the lattice constant c.
According to group theory, 1T-HfS2 has vibrational modes with symmetries of A1g + Eg + A2u + Eu at the Γ point. The A1g + Eg modes are Raman-active, while the A2u + Eu modes are infrared-active [21,53,54]. The Raman spectra of the pristine, Mn-, Fe-, Co-, and Cu-doped HfS2 crystals recorded at room temperature are shown in Figure 1e. There are weak peaks observed at approximately 131.7 cm−1. Neal et al. considered these peaks were due to the Eu(TO) mode, which resulted from the in-plane out-of-phase vibrations of the atomic planes of S and the atomic planes of Hf [53]. However, the same Eu mode was found in the infrared spectra at around 166 cm−1 [52] and 155 cm−1 [53]. Therefore, the origin of these weak peaks observed at approximately 131.7 cm−1 should still be further investigated. The weak peaks observed at approximately 256.2 cm−1 were due to the Eg mode, which resulted from the in-plane out-of-phase vibrations of the atomic planes of S. The intense peaks observed at approximately 335.3 cm−1 were due to the A1g mode, which resulted from the out-of-plane out-of-phase vibrations of the atomic planes of S [21,52,53,54,55]. The Eg and A1g peaks are the two most commonly observed signals in 1T HfS2 crystals [21,27,53,55,56,57,58,59,60]. Figure 1e indicates that the grown HfS2 crystals were in the 1T phase. Furthermore, Figure 1e shows that the doping of Mn, Fe, Co, and Cu in the HfS2 crystals had no significant effect on the positions of the Eg, and A1g peaks.
Figure 2 shows the absorption spectra of the pristine, Mn-, Fe-, Co-, and Cu-doped HfS2 crystals recorded at room temperature. From these spectra, the bandgap of each sample was determined. The bandgap of pristine HfS2 was approximately 1.99 eV, which is similar to the values reported by other researchers [18,19,20,21,22,23]. The bandgaps of the Mn-, Fe-, Co-, and Cu-doped HfS2 crystals were approximately 2.05, 2.08, 2.11, and 2.22 eV, respectively. As the dopant of the HfS2 crystals changed from Mn, Fe, and Co to Cu, the bandgap gradually increased. This increase may be a result of reducing the lattice spacings d100 and d110 and the lattice constant a.

Figure 2.
Absorption spectra of the pristine, Mn-, Fe-, Co-, and Cu-doped HfS2 crystals.
The bandgaps of the HfS2 crystals could also be determined from their PC spectra. Figure 3 shows the PC spectra of the HfS2 crystals. The bandgaps of the pristine, Mn-, Fe-, Co-, and Cu-doped HfS2 crystals were approximately 1.99, 2.08, 2.10, 2.11, and 2.15 eV, respectively. These values were close to those specified by the absorption spectra.
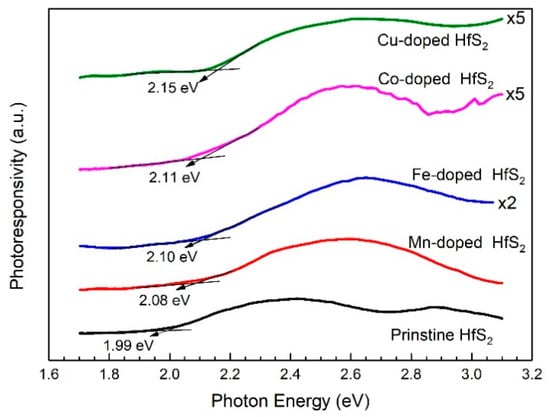
Figure 3.
Photoconductivity spectra of the pristine, Mn-, Fe-, Co-, and Cu-doped HfS2 crystals.
Figure 4 shows the PL spectra of the HfS2 samples recorded at room temperature. The peaks of the spectra were located at 1.40~1.45 eV. Fu et al. recorded the PL spectra of HfS2 nanoflakes with a thickness of <1~5 nm [32]. The peaks of their spectra were located at approximately 1.45 eV. Fu et al. attributed the PL to the near-indirect bandgap emission of the HfS2 nanoflakes. Therefore, the PL from the samples in the present study may have resulted from the near-bandgap emission of the HfS2 nanoflakes in these samples. As the dopant changed from Mn, Fe, and Co to Cu, the PL peak blue-shifted; this shift may be due to an increase in the bandgap of the HfS2 nanoflakes in the samples.
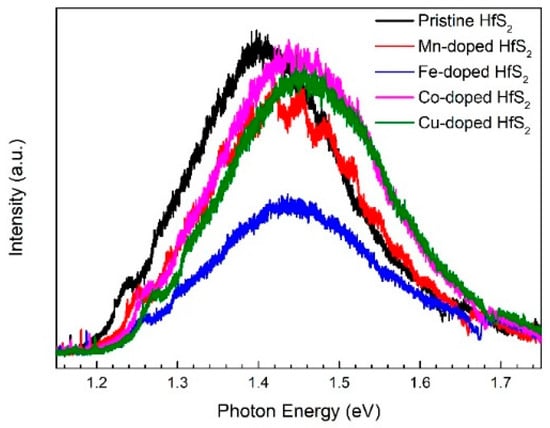
Figure 4.
Photoluminescence spectra of the pristine, Mn-, Fe-, Co-, and Cu-doped HfS2 crystals.
Figure 5 shows the I-V characteristic curves of the pristine and Co-doped HfS2 crystals with and without light illumination; the I-V curves of the other samples revealed similar behaviors. The resistivity of each sample was determined from the I–V curves and is listed in Table 3. As shown, doping with Mn, Fe, Co, and Cu can reduce the resistivity of HfS2 crystals. Notably, the Co-doped crystal exhibited the lowest resistivity, and when the HfS2 crystals were illuminated, their resistivity was considerably reduced.
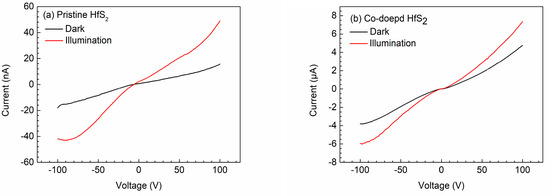
Figure 5.
Current-voltage curves of (a) the pristine and (b) the Co-doped HfS2 crystals.

Table 3.
The resistivity of the pristine, Mn-, Fe-, Co-, and Cu-doped HfS2 crystals.
To understand the relationship between the conductivity and temperature, I-V curves of each sample at various temperatures were constructed. Figure 6a shows the results for Cu-doped HfS2. Under a given bias voltage, the current of the Cu-doped HfS2 crystal increased as the temperature increased, and the temperature-dependent I-V curves of the other samples exhibited similar behaviors. This phenomenon resulted from the increase in carrier concentration with the increase in temperature, leading to a decrease in the resistivity of the samples.
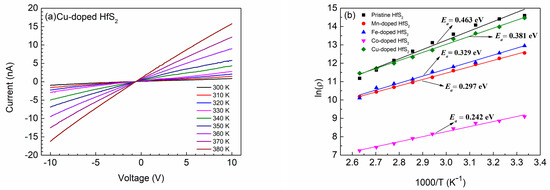
Figure 6.
(a) Temperature-dependent I-V curves of the Cu-doped HfS2 crystal. (b) Plots of ln(ρ) versus 1000/T of the pristine, Mn-, Fe-, Co-, and Cu-doped HfS2 crystals.
For n-type semiconductors, the conduction process is mainly contributed by electrons that transition from the donor level to the conduction band. The relationship between resistivity ρ and temperature T can be expressed as [29,61,62,63]
where ρ0 is a constant, kB is the Boltzmann constant, and Ea is the activation energy, which approximately measures the energy difference between the bottom of the conduction band and the donor level. The lower the activation energy, the easier it is for the donor electrons to jump to the conduction band.
The resistivity ρ of each sample at various temperatures was determined from the temperature-dependent I-V curves. The curves of ln[ρ (T)] versus 1000/T for the samples are plotted in Figure 6b. As shown, the curve of each sample was approximately a straight line, and the Co-doped HfS2 crystal had the smallest ρ at any given temperature T. The activation energy of each sample was determined from the slope of each line in Figure 6b; the activation energies of the Mn-, Fe-, Co-, and Cu-doped HfS2 crystals were 0.297, 0.329, 0.242, and 0.381 eV, respectively. These values were all less than the activation energy of pristine HfS2, which was 0.463 eV. The Co-doped HfS2 crystal had the smallest activation energy; hence, its donor electrons were the easiest to transition to the conduction band.
Figure 7 shows the time-resolved photoresponse measurements of the pristine and Co-doped HfS2 samples; this reveals how the photocurrent of each sample changed with time under an illumination frequency of 500 Hz. The photocurrents of the other samples exhibited similar behaviors under different illumination frequencies. Table 4 lists the rise time trise (from 10% to 90% of the maximum photocurrent) and the fall time tfall (from 90% to 10% of the maximum photocurrent) of each sample under different illumination frequencies. As shown in Table 4, under any illumination frequency, the rise and fall times of the Mn-, Fe-, Co-, and Cu-doped HfS2 crystals were shorter than those of the pristine HfS2 crystal. The rise and fall times of the Co-doped HfS2 crystal were the shortest.
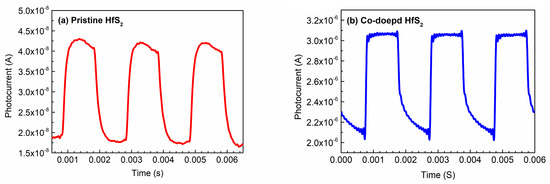
Figure 7.
Photocurrents of (a) the pristine and (b) the Co-doped HfS2 crystals as a function of time under an illumination frequency of 500 Hz.

Table 4.
Rise time trise and fall time tfall of the pristine, Mn-, Fe-, Co-, and Cu-doped HfS2 crystals under different illumination frequencies.
The current amplitude, which is defined as the difference between the maximum and minimum photocurrents in a rising-falling period, of each sample under different illumination frequencies, is listed in Table 5. Under any illumination frequency, the current amplitude of each TM-doped HfS2 crystal was greater than that of the pristine HfS2 crystal, and the Co-doped HfS2 crystal had the largest current amplitude. According to the data listed in Table 4 and Table 5, Mn, Fe, Co, and Cu doping significantly improved the response of HfS2 crystals to light. In particular, the Co-doped HfS2 crystal exhibited the best response to light.

Table 5.
Current amplitudes of the pristine, Mn-, Fe-, Co-, and Cu-doped HfS2 crystals under different illumination frequencies.
Figure 8 shows how the photoresponsivity of each sample varied with the incident laser power. As the laser power gradually decreased from the order of 10−3 W to the order of 10−7 W, the photoresponsivity of each sample gradually increased; this increase reached two orders of magnitude. For a given incident laser power, the photoresponsivity of each TM-doped HfS2 crystal was greater than that of the pristine HfS2 crystal, and the Co-doped HfS2 crystal exhibited the highest photoresponsivity. Its maximum value reached 49.96 μA/W at a laser power of 10−7 W, which is greater than the maximum photoresponsivity of the Mn-doped HfS2 crystal, 30.93 μA/W, and much greater than those of the pristine, Fe-, and Cu-doped HfS2 crystals.
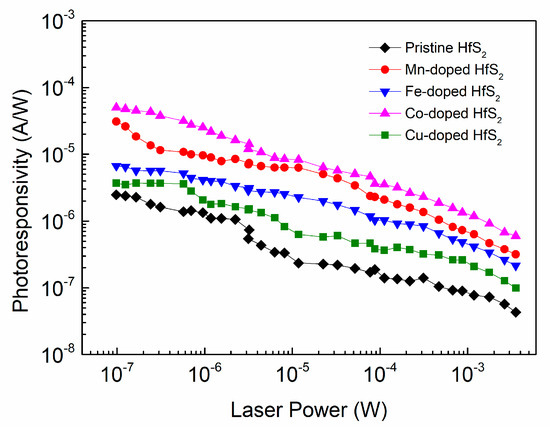
Figure 8.
Photoresponsivity of the pristine, Mn-, Fe-, Co-, and Cu-doped HfS2 crystals as a function of laser power.
Figure 9 shows how the photoresponsivity of each sample varied with the bias voltage. As the applied bias voltage increased, the photoresponsivity of each sample also increased. For a given bias voltage, the photoresponsivity of each TM-doped HfS2 crystal was greater than that of the pristine HfS2 crystal, and the Co-doped HfS2 crystal exhibited the highest photoresponsivity. Its maximum value reached 55.70 μA/W at 100 V, which is greater than the maximum photoresponsivity of the Mn-doped HfS2 crystal, 29.16 μA/W, and much greater than those of the pristine, Fe-, and Cu-doped HfS2 crystals.
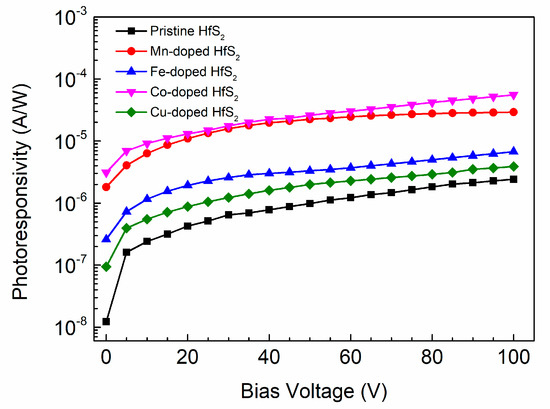
Figure 9.
Photoresponsivity of the pristine, Mn-, Fe-, Co-, and Cu-doped HfS2 crystals as a function of bias voltage.
4. Conclusions
In conclusion, pristine, Mn-, Fe-, Co-, and Cu-doped HfS2 crystals were grown using the CVT method to study their structural, optical, and photoelectric properties. SEM images showed that the HfS2 crystals were layered materials, with an angle of 120° characterizing the edge of each layer. The TEM and XRD results showed that the HfS2 crystals had a CdI2-like 1T structure belonging to the space group 3m1. As the dopant changed from Mn, Fe, and Co to Cu, the lattice constant a gradually decreased and the lattice constant c slightly increased. The signals of the Eg and A1g vibration modes in the Raman spectra also confirmed that the HfS2 crystals were in the 1T phase. Moreover, the bandgap of the pristine HfS2 crystal was determined as approximately 1.99 eV using the absorption and photoconductivity spectra. When the dopant was changed from Mn, Fe, and Co to Cu, the bandgap gradually increased. The PL peak from the HfS2 nanoflakes in the pristine sample was located at approximately 1.40 eV and blue-shifted as the dopant changed from Mn, Fe, and Co to Cu. The I-V curves revealed that Mn, Fe, Co, and Cu doping significantly increased the conductivity of HfS2; the Co-doped HfS2 crystal exhibited the highest conductivity. Light illumination also improved the conductivity of the samples. Furthermore, the activation energy of each HfS2 crystal was determined from the temperature-dependent I-V curves. After doping with Mn, Fe, Co, and Cu, the activation energy of HfS2 decreased, and the Co-doped HfS2 crystal had the lowest activation energy. The time-resolved photoresponse measurements revealed that Mn, Fe, Co, and Cu doping significantly improved the response of HfS2 to light. The Co-doped HfS2 crystal, which had the shortest rise and fall times and the largest current amplitude, exhibited the best response to light. Additionally, experiments on laser power-dependent and bias voltage-dependent photoresponsivity revealed that Mn, Fe, Co, and Cu doping increased the photoresponsivity of HfS2, of which the Co-doped HfS2 crystal exhibited the maximum photoresponsivity. Overall, doping with Mn, Fe, Co, and Cu significantly improved the photoresponsive performance of HfS2. In particular, the Co-doped HfS2 crystal exhibited the best photoresponsive performance. HfS2 crystals doped with Mn, Fe, Co, and Cu have tunable and excellent photoelectric properties, making them promising for use in sensing and photoelectronic devices.
Author Contributions
Conceptualization, D.-Y.L. and Y.-T.S.; methodology, D.-Y.L. and C.-F.L.; software, D.-Y.L. and W.-C.T.; validation, D.-Y.L. and Y.-T.S.; formal analysis, D.-Y.L., Y.-T.S. and H.-Z.C.; investigation, D.-Y.L. and Y.-T.S.; resources, D.-Y.L., Y.-T.S., C.-F.L. and H.-Z.C.; data curation, Y.-T.S. and W.-C.T.; writing—original draft preparation, Y.-T.S.; writing—review and editing, D.-Y.L., Y.-T.S. and H.-Z.C.; visualization, D.-Y.L. and Y.-T.S.; supervision, D.-Y.L. and Y.-T.S.; project administration, D.-Y.L. and Y.-T.S.; funding acquisition, D.-Y.L. and Y.-T.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Technology (MOST) of the Republic of China, grant number MOST-109-2221-E-018-008, MOST-110-2221-E-018-009 and National Changhua University of Education (NCUE). The APC was funded by MOST-110-2221-E-018-009.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wilson, J.A.; Yoffe, A.D. The transition metal dichalcogenides discussion and interpretation of the observed optical, electrical and structural properties. Adv. Phys. 1969, 18, 193–335. [Google Scholar] [CrossRef]
- Friend, R.H.; Yoffe, A.D. Electronic properties of intercalation complexes of the transition metal dichalcogenides. Adv. Phys. 1987, 36, 1–94. [Google Scholar] [CrossRef]
- Zeng, H.; Cui, X. An optical spectroscopic study on two dimensional group-VI transition metal dichalcogenides. Chem. Soc. Rev. 2015, 44, 2629–2642. [Google Scholar] [CrossRef] [PubMed]
- Voiry, D.; Mohite, A.; Chhowalla, M. Phase engineering of transition metal dichalcogenides. Chem. Soc. Rev. 2015, 44, 2702–2712. [Google Scholar] [CrossRef]
- Kolobov, A.V.; Tominaga, J. Two-Dimensional Transition-Metal Dichalcogenides; Springer: Cham, Switzerland, 2016; pp. 29–77. [Google Scholar]
- Choi, W.; Choudhary, N.; Han, G.H.; Park, J.; Akinwande, D.; Lee, Y.H. Recent development of two-dimensional transition metal dichalcogenides and their applications. Mater. Today 2017, 20, 116–130. [Google Scholar] [CrossRef]
- Manzeli, S.; Ovchinnikov, D.; Pasquier, D.; Yazyev, O.V.; Kis, A. 2D transition metal dichalcogenides. Nat. Rev. Mater. 2017, 2, 17033. [Google Scholar] [CrossRef]
- Li, H.; Jia, X.; Zhang, Q.; Wang, X. Metallic Transition-Metal Dichalcogenide Nanocatalysts for Energy Conversion. Chem 2018, 4, 1510–1537. [Google Scholar] [CrossRef] [Green Version]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.J.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef]
- Frindt, R.F. Single Crystals of MoS2 Several Molecular Layers Thick. J. Appl. Phys. 1966, 37, 1928–1929. [Google Scholar] [CrossRef]
- Joensen, P.; Frindt, R.F.; Morrison, S.R. Single-layer MoS2. Mat. Res. Bull. 1986, 21, 457–461. [Google Scholar] [CrossRef]
- Liu, Q.; Li, X.; He, Q.; Khalil, A.; Liu, D.; Xiang, T.; Wu, X.; Song, L. Gram-scale aqueous synthesis of stable few-layered 1T-MoS2: Applications for visible-light-driven photocatalytic hydrogen evolution. Small 2015, 11, 5556–5564. [Google Scholar] [CrossRef]
- Samadi, M.; Sarikhani, N.; Zirak, M.; Zhang, H.; Zhang, H.L.; Moshfegh, A.Z. Group 6 transition metal dichalcogenide nanomaterials: Synthesis, applications and future perspectives. Nanoscale Horiz. 2018, 3, 90–204. [Google Scholar] [CrossRef]
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-layer MoS2 transistors. Nat. Nanotechnol. 2011, 6, 147–150. [Google Scholar] [CrossRef]
- Splendiani, A.; Sun, L.; Zhang, Y.; Li, T.; Kim, J.; Chim, C.Y.; Galli, G.; Wang, F. Emerging photoluminescence in monolayer MoS2. Nano Lett. 2010, 10, 1271–1275. [Google Scholar] [CrossRef]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically thin MoS2: A new direct-gap semiconductor. Phys. Rev. Lett. 2010, 105, 136805. [Google Scholar] [CrossRef] [Green Version]
- Yan, C.; Gong, C.; Wangyang, P.; Chu, J.; Hu, K.; Li, C.; Wang, X.; Du, X.; Zhai, T.; Li, Y.; et al. 2D group IVB transition metal dichalcogenides. Adv. Funct. Mater. 2018, 28, 1803305. [Google Scholar] [CrossRef]
- Abdulsalam, M.; Joubert, D.P. Optical spectrum and excitons in bulk and monolayer MX2 (M = Zr, Hf; X = S, Se). Phys. Status Solidi B 2016, 253, 705–711. [Google Scholar] [CrossRef]
- Jiang, H. Structural and electronic properties of ZrX2 and HfX2 (X = S and Se) from first principles calculations. J. Chem. Phys. 2011, 134, 204705. [Google Scholar] [CrossRef] [PubMed]
- Greenway, D.L.; Nitsche, R. Preparation and optical properties of group IV-VI2 chalcogenides having the CdI2 structure. J. Phys. Chem. Solids 1965, 26, 1445–1458. [Google Scholar] [CrossRef]
- Roubi, L.; Garlone, G. Resonance Raman spectrum of HfS2 and ZrS2. Phys. Rev. B 1988, 37, 6808–6812. [Google Scholar] [CrossRef] [PubMed]
- Terashima, K.; Imai, I. Indirect absorption edge of ZrS2 and HfS2. Solid State Commun. 1987, 63, 315–318. [Google Scholar] [CrossRef]
- Gaiser, C.; Zandt, T.; Krapf, A.; Serverin, R.; Janowitz, C.; Manzke, R. Band-gap engineering with HfSxSe2−x. Phys. Rev. B 2004, 69, 075205. [Google Scholar] [CrossRef]
- Lee, P.A.; Said, G.; Davis, R.; Lim, T.H. On the optical properties of some layer compound. J. Phys. Chem. Solids 1969, 30, 2719–2729. [Google Scholar] [CrossRef]
- Moustafa, M.; Zandt, T.; Janowitz, C.; Manzke, R. Growth and band gap determination of the ZrSxSe2−x single crystal series. Phys. Rev. B 2009, 80, 035206. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, X.; Wang, Z. Recent advances in properties, synthesis and applications of two-dimensional HfS2. J. Nanosci. Nanotechnol. 2018, 18, 7319–7334. [Google Scholar] [CrossRef]
- Kaur, H.; Yadav, S.; Srivastava, A.K.; Singh, N.; Rath, S.; Schneider, J.J.; Sinha, O.P.; Srivastava, R. High-yield synthesis and liquid-exfoliation of two-dimensional belt-like hafnium disulphide. Nano Res. 2018, 11, 343–353. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Meng, J.; Zhang, X.; Guo, G.; Yin, Z.; Liu, H.; Cheng, L.; Gao, M.; You, J.; Wang, R. Selective direct growth of atomic layered HfS2 on hexagonal boron nitride for high performance photodetectors. Chem. Mater. 2018, 30, 3819–3826. [Google Scholar] [CrossRef]
- Li, N.; Wang, Y.; Sun, H.; Hu, J.; Zheng, M.; Ye, S.; Wang, Q.; Li, Y.; He, D.; Wang, J.; et al. Resistive switching behaviors and mechanisms of HfS2 film memory devices studied by experiments and density functional theory calculations. Appl. Phys. Lett. 2020, 116, 063503. [Google Scholar] [CrossRef]
- Yin, J.; Zhu, F.; Lai, J.; Chen, H.; Zhang, M.; Zhang, J.; Wang, J.; He, T.; Zhang, B.; Yuan, J.; et al. Hafnium sulfide nanosheets for ultrafast photonic device. Adv. Opt. Mater. 2018, 7, 1801303. [Google Scholar] [CrossRef]
- Hoat, D.M.; Ponce-Pérez, R.; Vu, T.V.; Rivas-Silva, J.F.; Cocoletzi, G.H. Theoretical analysis of the HfS2 monolayer electronic structure and optical properties under vertical strain effects. Optik 2021, 225, 165718. [Google Scholar] [CrossRef]
- Fu, L.; Wang, F.; Wu, B.; Wu, N.; Huang, W.; Wang, H.; Jin, C.; Zhuang, L.; He, J.; Fu, L.; et al. Van der Waals epitaxial growth of atomic layered HfS2 crystals for ultrasensitive near-infrared phototransistors. Adv. Mater. 2017, 29, 1700439. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Huang, Y.; Chen, B.; Xia, Y.; Lei, W.; Wang, Z.; Wang, Q.; Wang, F.; Yin, L.; He, J. Toward high-performance top-gate ultrathin HfS2 field-effect transistors by interface engineering. Small 2016, 12, 3106–3111. [Google Scholar] [CrossRef]
- Xu, K.; Wang, Z.; Wang, F.; Huang, Y.; Wang, F.; Yin, L.; Jiang, C.; He, J. Ultrasensitive phototransistors based on few-layered HfS2. Adv. Mater. 2015, 27, 7881–7887. [Google Scholar] [CrossRef]
- Kanazawa, T.; Amemiya, T.; Ishikawa, A.; Upadhyaya, V.; Tsuruta, K.; Tanaka, T.; Miyamoto, Y. Few-layer HfS2 transistors. Sci. Rep. 2016, 6, 22277. [Google Scholar] [CrossRef] [PubMed]
- Mattinen, M.; Popov, G.; Vehkamäki, M.; King, P.J.; Mizohata, K.; Jalkanen, P.; Räisänen, J.; Leskelä, M.; Ritala, M. Atomic layer deposition of emerging 2D semiconductors, HfS2 and ZrS2, for optoelectronics. Chem. Mater. 2019, 31, 5713–5724. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Huang, Z.; Zhang, W.; Li, Y. Two-dimensional semiconductors with possible high room temperature mobility. Nano Res. 2014, 7, 1731–1737. [Google Scholar] [CrossRef] [Green Version]
- Suh, J.; Park, T.E.; Lin, D.Y.; Fu, D.; Park, J.; Jung, H.J.; Chen, Y.; Ko, C.; Jang, C.; Sun, Y.; et al. Doping against the native propensity of MoS2: Degenerate hole doping by cation substitution. Nano Lett. 2014, 14, 6976–6982. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Feng, S.; Wang, J.; Azcatl, A.; Lu, N.; Addou, R.; Wang, N.; Zhou, C.; Lerach, J.O.; Bojan, V.; et al. Manganese doping of monolayer MoS2: The substrate is critical. Nano Lett. 2015, 15, 6586–6591. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, Z.; Lupini, A.; Shi, G.; Lin, J.; Najmaei, S.; Lin, Z.; Elias, A.L.; Berkdemir, A.; You, G.; et al. Band gap engineering and layer-by-layer mapping of selenium-doped molybdenum disulfide. Nano Lett. 2014, 14, 442–449. [Google Scholar] [CrossRef]
- Mouri, S.; Miyauchi, Y.; Matsuda, K. Tunable photoluminescence of monolayer MoS2 via chemical doping. Nano Lett. 2013, 13, 5944–5948. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Xing, T.; Zhong, M.; Huang, L.; Lei, N.; Zhang, J.; Li, J.; Wei, Z. A two-dimensional Fe-doped SnS2 magnetic semiconductor. Nat. Commun. 2017, 8, 1958. [Google Scholar] [CrossRef]
- Zhao, X.; Xia, C.; Wang, T.; Dai, X.; Yang, L. Characteristics of n- and p-type dopants in 1T-HfS2 monolayer. J. Alloy. Compd. 2016, 689, 302–306. [Google Scholar] [CrossRef]
- Lu, H.; Guo, Y.; Robertson, J. Band edge states, intrinsic defects, and dopants in monolayer HfS2 and SnS2. Appl. Phys. Lett. 2018, 112, 062105. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.; Ahuja, R. Enhanced optoelectronic and thermoelectric properties by intrinsic structural defects in monolayer HfS2. ACS Appl. Energy Mater. 2019, 2, 6891–6903. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, T.; Wang, G.; Dai, X.; Xia, C.; Yang, L. Electronic and magnetic properties of 1T-HfS2 by doping transition-metal atoms. Appl. Surf. Sci. 2016, 383, 151–158. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, X.; Wu, N.; Xin, Q.; Liu, X.; Wang, T.; Wei, S. Ferromagnetic properties of Mn-doped HfS2 monolayer under strain. Solid State Commun. 2017, 268, 15–19. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, X.; Wang, T.; Wei, S. Effect of structural defects on electronic and magnetic properties in pristine and Cr-doped HfS2 monolayer. J. Alloy. Compd. 2018, 731, 303–309. [Google Scholar] [CrossRef]
- Ali, A.; Zhang, J.M.; Muhammad, I.; Wei, X.M.; Ahmad, I.; Rehman, M.U. Changing the electronic and magnetic properties of monolayer HfS2 by doping and vacancy defects: Insight from first-principles calculations. Phys. Status Solidi B 2020, 257, 1900768. [Google Scholar] [CrossRef]
- Obodo, K.O.; Gebreyesus, G.; Ouma, C.N.M.; Obodo, J.T.; Ezeonu, S.O.; Rai, D.P.; Bouhafs, B. Controlling the electronic and optical properties of HfS2 monolayers via lanthanide substitutional doping: A DFT+U study. RSC Adv. 2020, 10, 15670. [Google Scholar] [CrossRef] [Green Version]
- Lucovsky, G.; White, R.M.; Benda, J.A.; Revelli, J.F. Infrared-reflectance spectra of layered group-IV and group-VI transition-metal dichalcogenides. Phys. Rev. B 1973, 7, 3859–3870. [Google Scholar] [CrossRef]
- Traving, M.; Seydel, T.; Kipp, L.; Skibowski, M.; Starrost, F.; Krasovskii, E.E.; Perlov, A.; Schattke, W. Combined photoemission and inverse photoemission study of HfS2. Phys. Rev. B 2001, 63, 035107. [Google Scholar] [CrossRef] [Green Version]
- Neal, S.N.; Li, S.; Birol, T.; Musfeldt, J.L. Chemical bonding and Born charge in 1T-HfS2. NPJ 2D Mater. Appl. 2021, 5, 45. [Google Scholar] [CrossRef]
- Iwasaki, T.; Kuroda, N.; Nishina. Y. Anisotropy of lattice dynamical properties in ZrS2 and HfS2. J. Phys. Soc. Jpn. 1982, 51, 2233–2240. [Google Scholar] [CrossRef]
- Uchida, S.; Tanaka, S. Optical phonon modes and localized effective charges of transition-metal dichalcogenides. J. Phys. Soc. Jpn. 1978, 45, 153–161. [Google Scholar] [CrossRef]
- Chen, J. Phonons in bulk and monolayer HfS2 and possibility of phonon-mediated superconductivity: A first-principles study. Solid State Commun. 2016, 237–238, 14–18. [Google Scholar] [CrossRef]
- Cingolani, A.; Lugara, M.; Scamarcio, G.; Levy, F. The Raman scattering in hafnium disulfide. Solid State Comm. 1987, 62, 121–123. [Google Scholar] [CrossRef]
- Cingolani, A.; Lugara, M.; Levy, F. Resonance Raman scattering in HfSe, and HfS. Phys. Scripta 1988, 37, 389–391. [Google Scholar] [CrossRef]
- Zheng, B.; Chen, Y.; Wang, Z.; Qi, F.; Huang, Z.; Hao, X.; Li, P.; Zhang, W.; Li, Y. Vertically oriented few-layered HfS2 nanosheets: Growth mechanism and optical properties. 2D Mater. 2016, 3, 035024. [Google Scholar] [CrossRef]
- Ibáñez, J.; Woźniak, T.; Dybala, F.; Oliva, R.; Hernández, S.; Kudrawiec, R. High-pressure Raman scattering in bulk HfS2: Comparison of density functional theory methods in layered MS2 compounds (M = Hf, Mo) under compression. Sci. Rep. 2018, 8, 12757. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.F.; Jian, W.B.; Wang, C.P.; Suen, Y.W.; Wu, Z.Y.; Chen, F.R.; Kai, J.J.; Lin, J.J. Contact to ZnO and intrinsic resistances of individual ZnO nanowires with a circular cross section. Appl. Phys. Lett. 2007, 90, 223117. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.T.; Young, S.L.; Kung, C.Y.; Chen, H.Z.; Kao, M.C.; Chang, M.C.; Ou, C.R. Variable-range hopping and thermal activation conduction of Y-doped ZnO nanocrystalline films. IEEE Trans. Nanotechnol. 2014, 13, 425–430. [Google Scholar] [CrossRef] [Green Version]
- Pleshchev, V.G.; Selezneva, N.V. Electrical and magnetic properties of hafnium disulfide intercalated with iron atoms. Phys. Solid State 2018, 60, 250–255. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).