Cavitated Charcoal—An Innovative Method for Affecting the Biochemical Properties of Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Cavitation of Charcoal
2.2. Chemical Composition of Charcoal after Cavitation
2.3. Scanning Electron Microscopy, X-ray Diffractometry and Infrared Spectroscopy of Dry Residue Charcoal after Cavitation
2.4. Growth Experiment
2.5. Biochemical Properties of Soil
2.6. Chemical Properties of Soil
2.7. Statistical Analysis
3. Results
3.1. Properties of Cavitated Charcoal and Soils
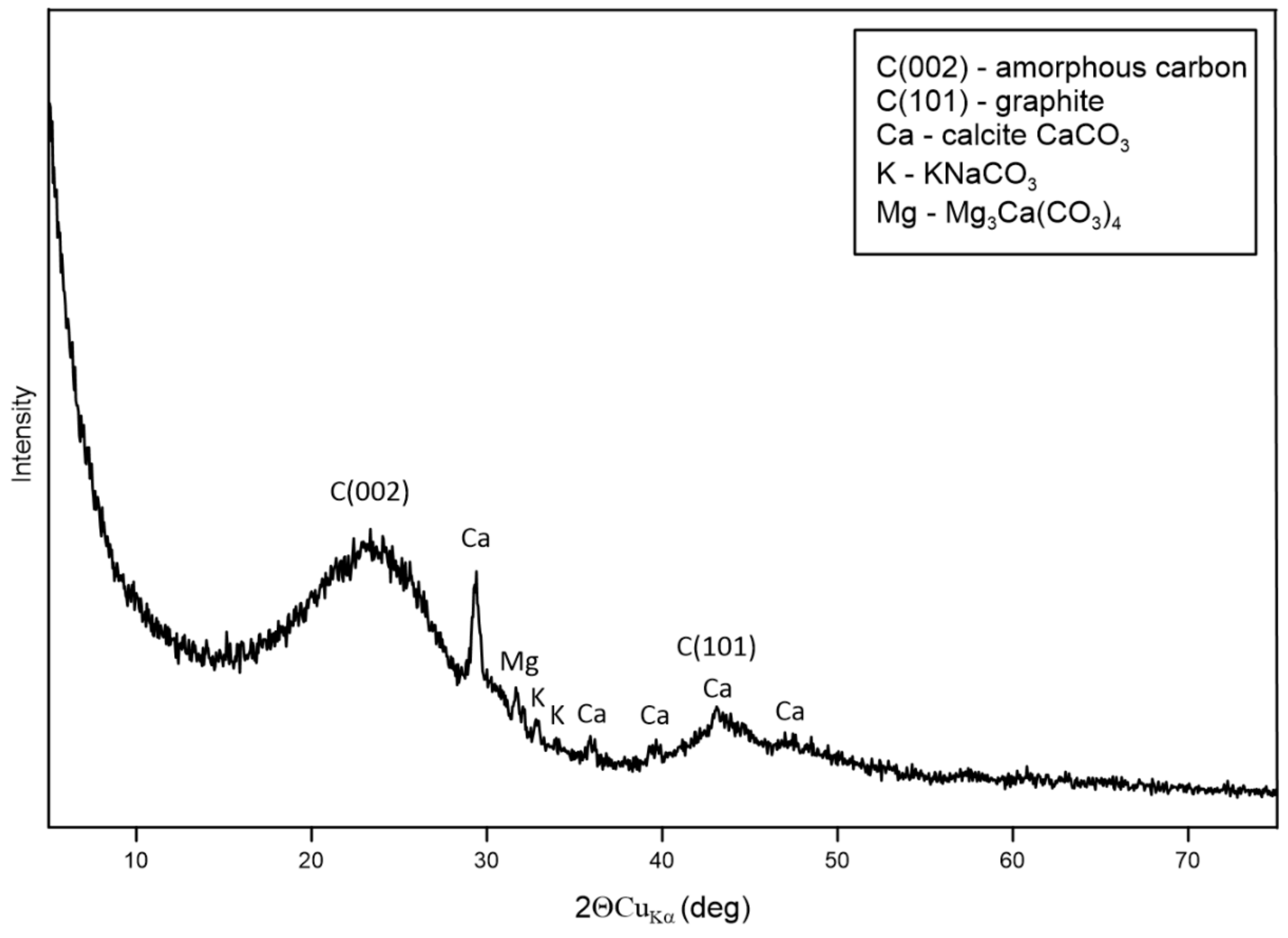
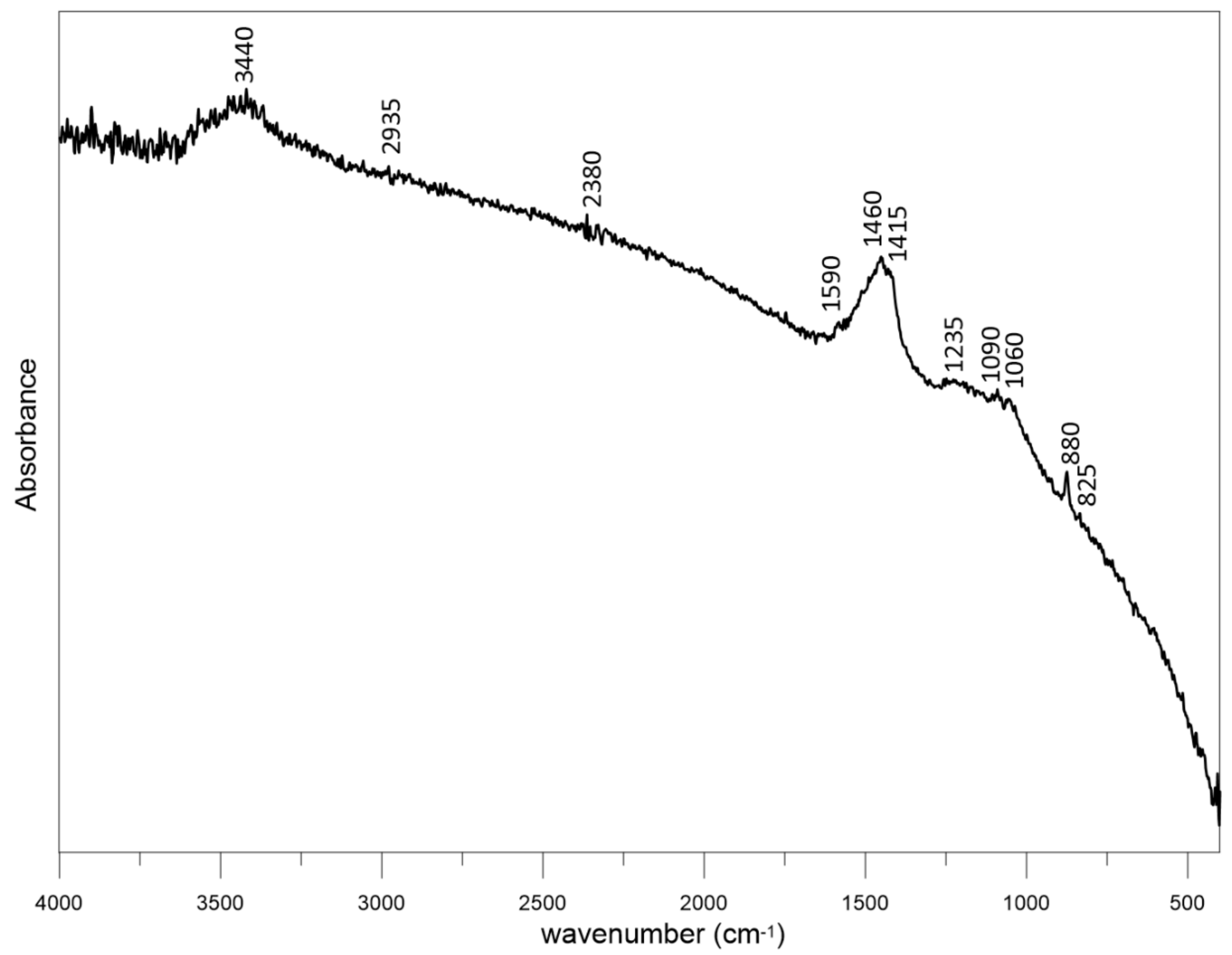
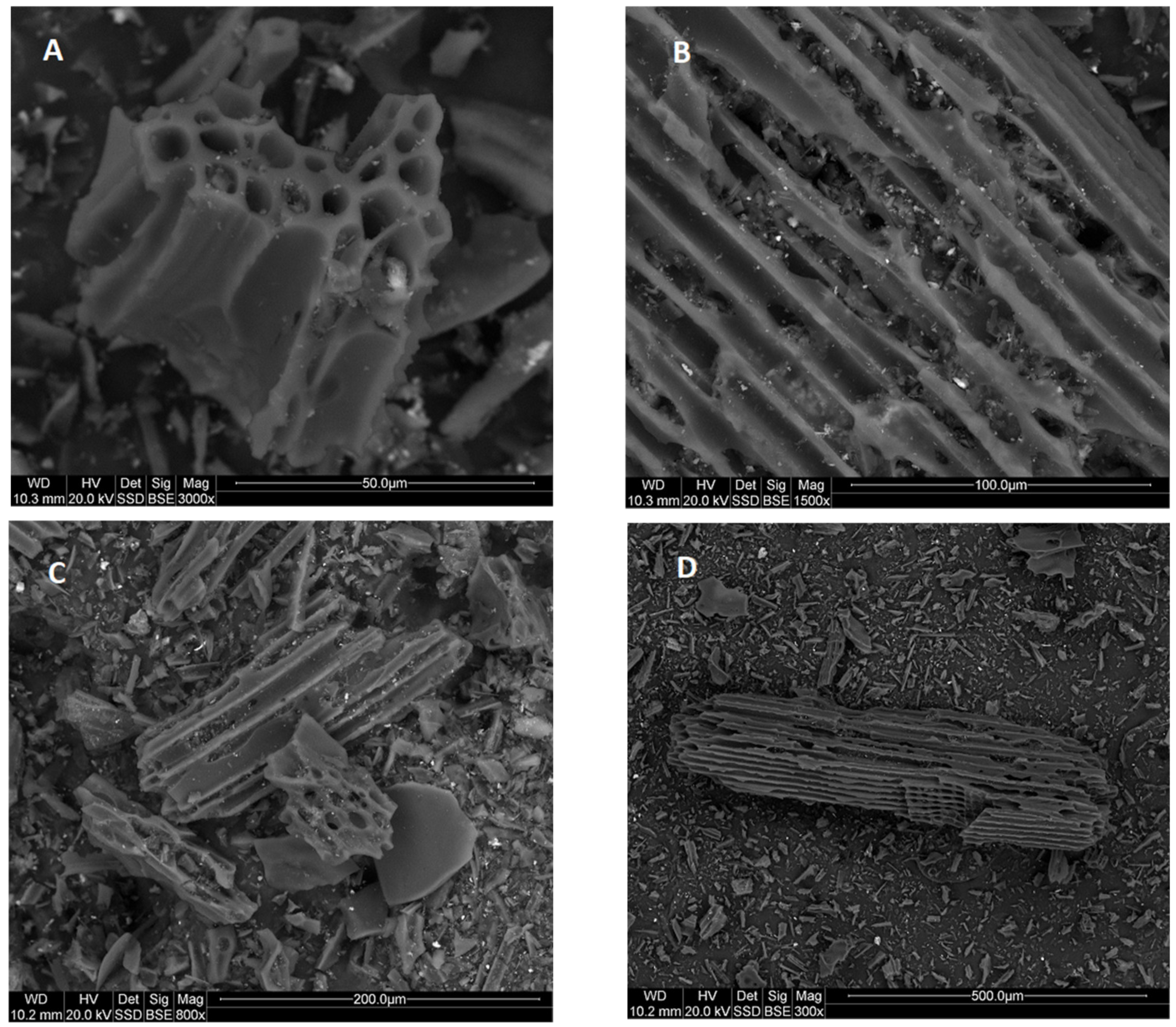
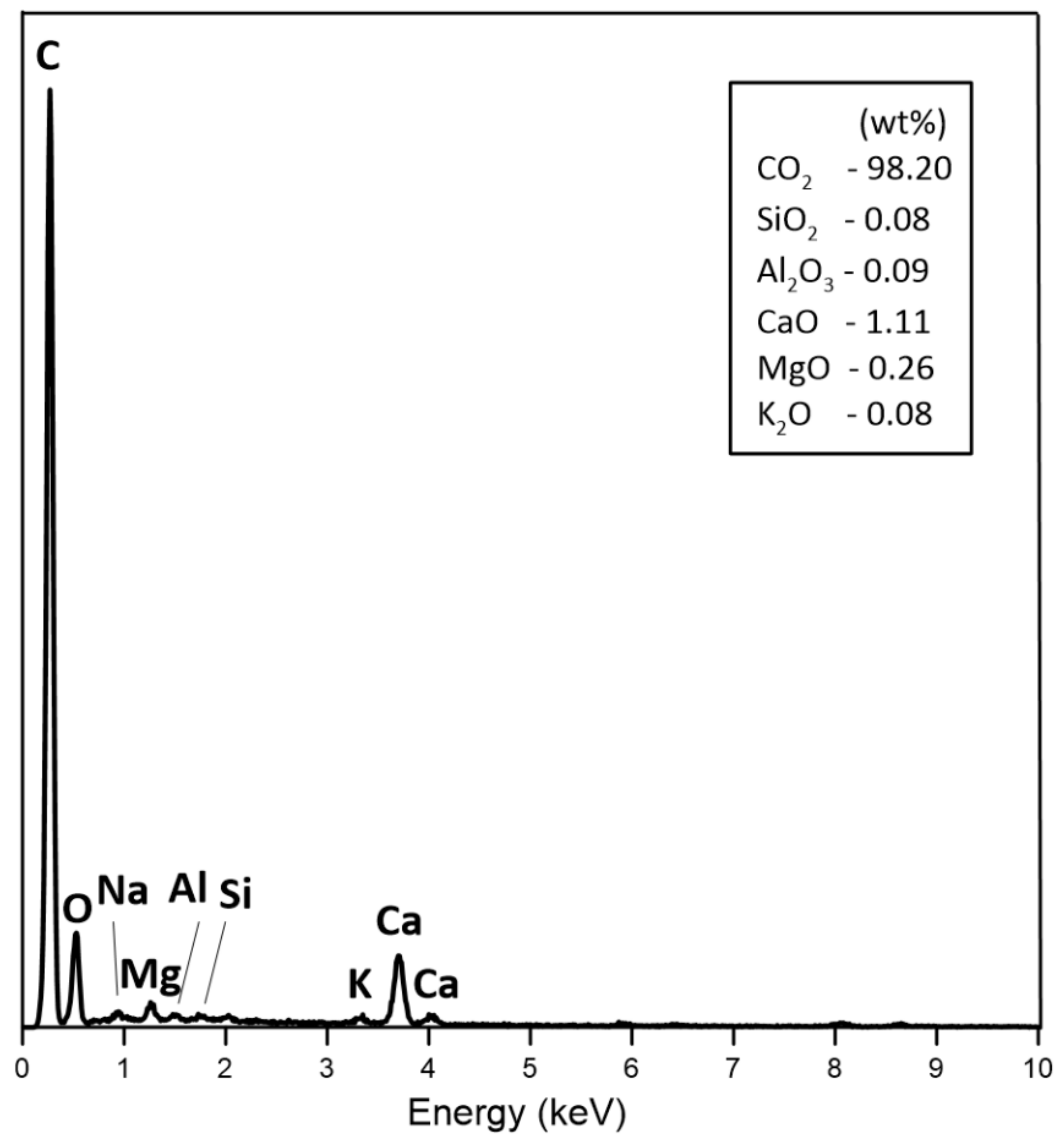
3.2. Physical Analysis of Dry Residue CHAR-C
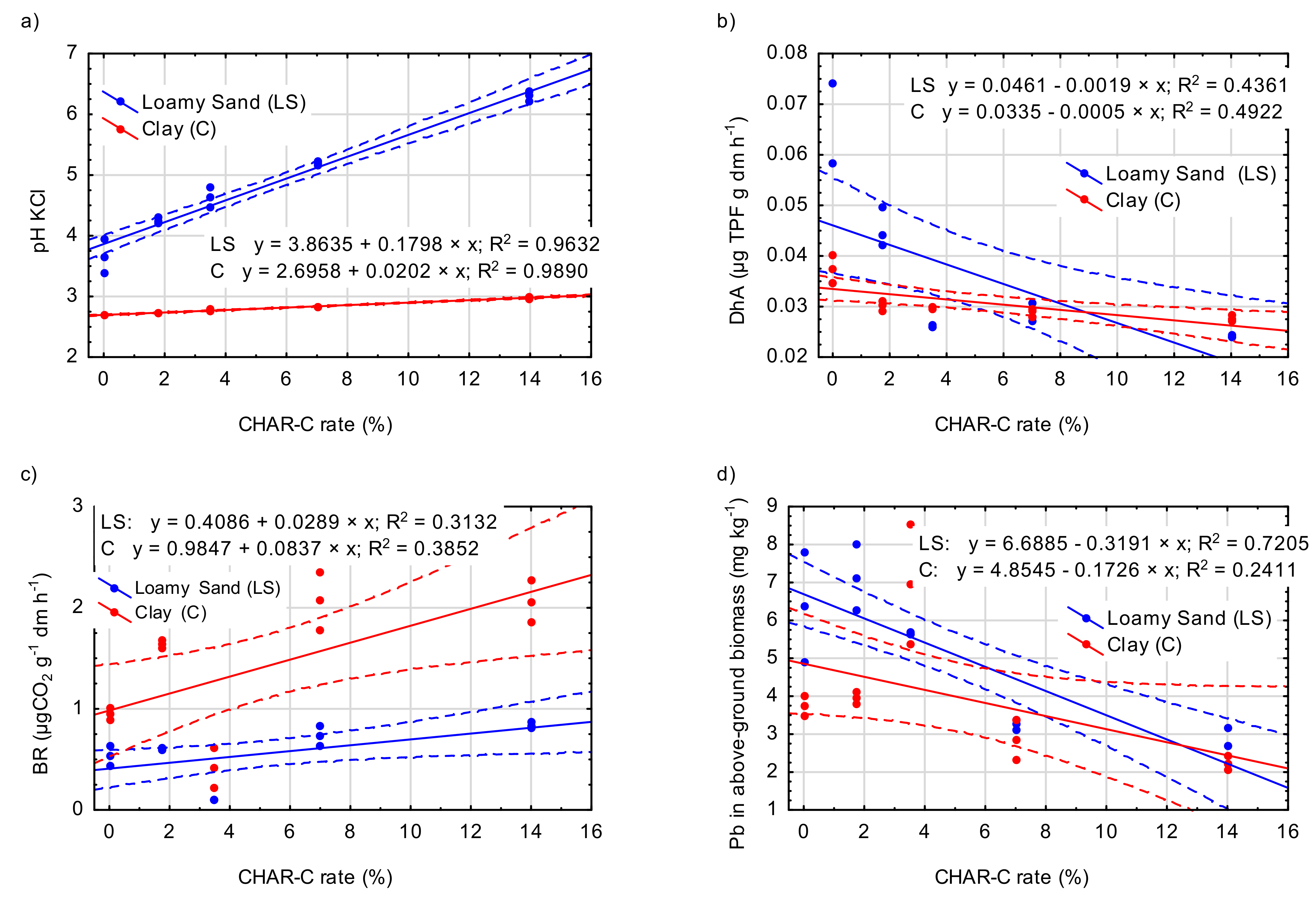
3.3. Effect of the Addition of Cavitated Charcoal on Selected Chemical Properties of Soils
3.4. Effect of the Addition of Cavitated Charcoal on the Content of Selected Heavy Metals in Soils
3.5. Effect of the Addition of Cavitated Charcoal on the Value of Selected Biochemical Parameters of Soils
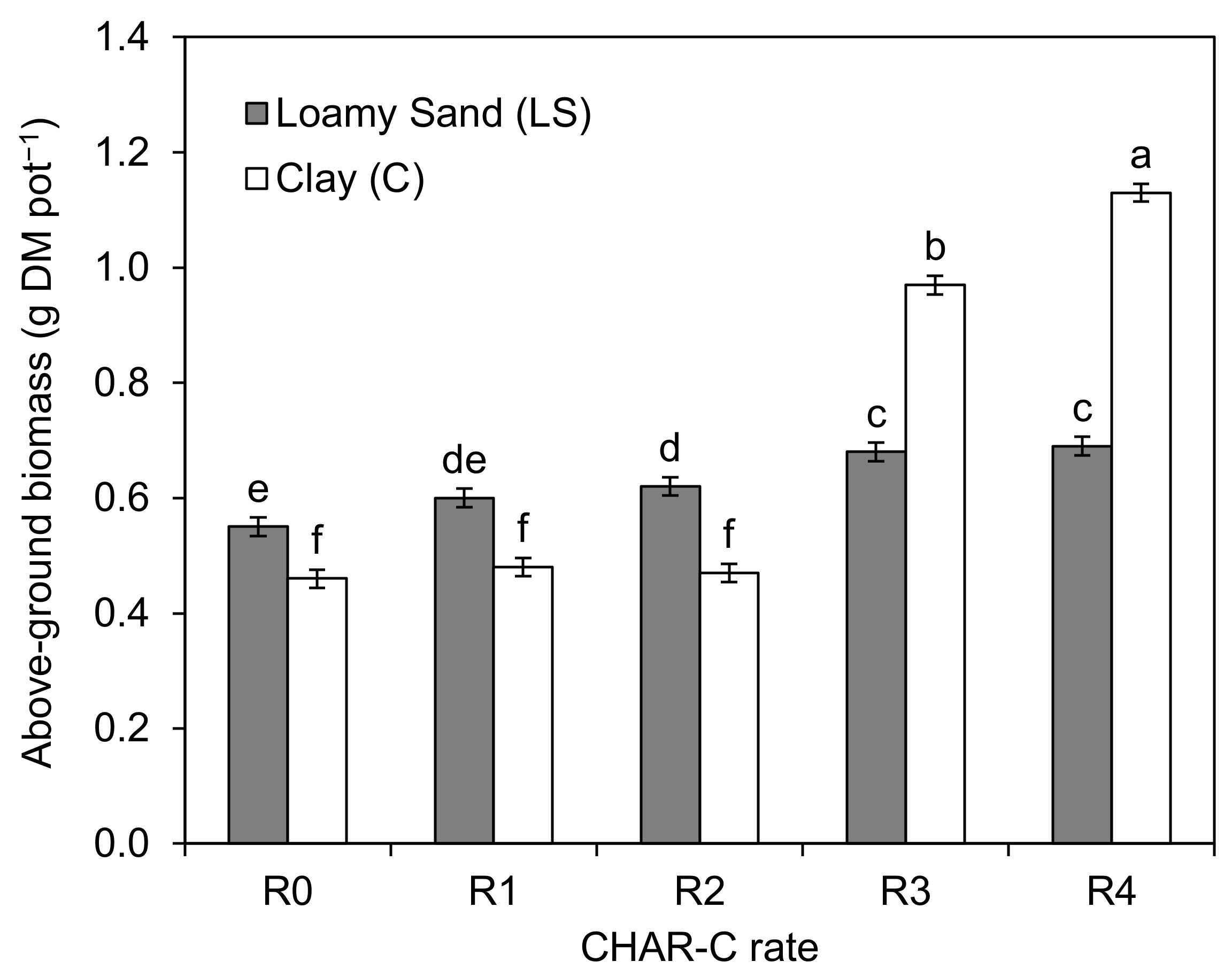
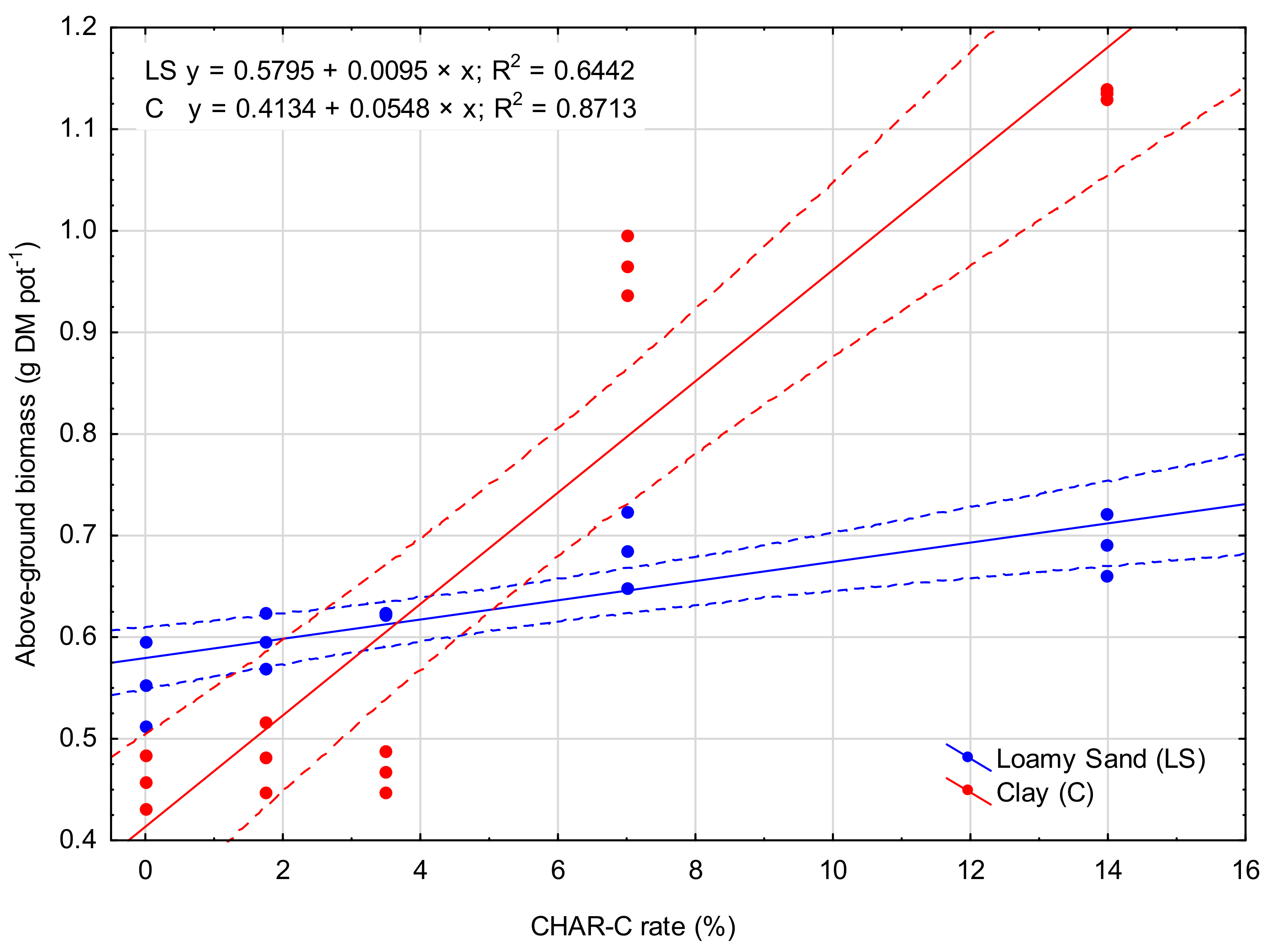
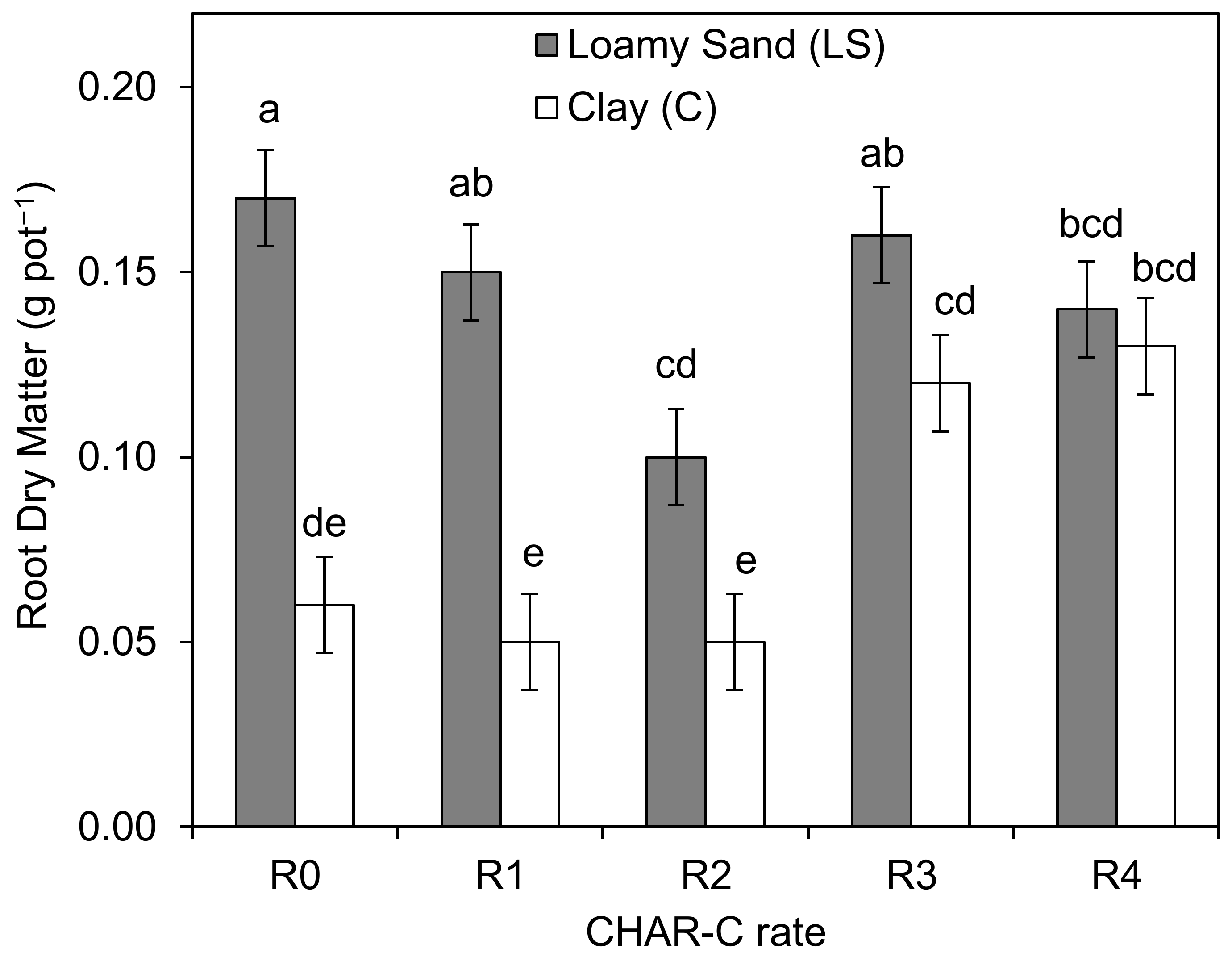
3.6. Effect of the Addition of Cavitated Charcoal on the Amount and Content of Selected Heavy metals in S. saccharatum (L.) Biomass
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Glaser, B. Prehistorically modified soils of central Amazonia: A model for sustainable agriculture in the twenty-first century. Philos. Trans. R. Soc. B Biol. Sci. 2006, 362, 187–196. [Google Scholar] [CrossRef]
- Deenik, J.L.; McClellan, T.; Uehara, G.; Antal, M.J.; Campbell, S. Charcoal Volatile Matter Content Influences Plant Growth and Soil Nitrogen Transformations. Soil Sci. Soc. Am. J. 2010, 74, 1259–1270. [Google Scholar] [CrossRef]
- Oliveira, F.R.; Patel, A.K.; Jaisi, D.P.; Adhikari, S.; Lu, H.; Khanal, S.K. Environmental application of biochar: Current status and perspectives. Bioresour. Technol. 2017, 246, 110–122. [Google Scholar] [CrossRef]
- Li, B.; Ding, S.; Fan, H.; Ren, Y. Experimental Investigation into the Effect of Pyrolysis on Chemical Forms of Heavy Metals in Sewage Sludge Biochar (SSB), with Brief Ecological Risk Assessment. Materials 2021, 14, 447. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Lehmann, J.; Thies, J.E.; Burton, S.D. Stability of black carbon in soils across a climatic gradient. J. Geophys. Res. Space Phys. 2008, 113. [Google Scholar] [CrossRef]
- Tan, Z.; Lin, C.S.; Ji, X.; Rainey, T.J. Returning biochar to fields: A review. Appl. Soil Ecol. 2017, 116, 1–11. [Google Scholar] [CrossRef]
- Gluba, Ł.; Rafalska-Przysucha, A.; Szewczak, K.; Łukowski, M.; Szlązak, R.; Vitková, J.; Kobyłecki, R.; Bis, Z.; Wichliński, M.; Zarzycki, R.; et al. Effect of Fine Size-Fractionated Sunflower Husk Biochar on Water Retention Properties of Arable Sandy Soil. Materials 2021, 14, 1335. [Google Scholar] [CrossRef] [PubMed]
- Kerré, B.; Willaert, B.; Cornelis, Y.; Smolders, E. Long-term presence of charcoal increases maize yield in Belgium due to increased soil water availability. Eur. J. Agron. 2017, 91, 10–15. [Google Scholar] [CrossRef]
- Bell, M.; Worrall, F. Charcoal addition to soils in NE England: A carbon sink with environmental co-benefits? Sci. Total Environ. 2011, 409, 1704–1714. [Google Scholar] [CrossRef]
- Cui, H.; Fan, Y.; Fang, G.; Zhang, H.; Su, B.; Zhou, J. Leachability, availability and bioaccessibility of Cu and Cd in a contaminated soil treated with apatite, lime and charcoal: A five-year field experiment. Ecotoxicol. Environ. Saf. 2016, 134, 148–155. [Google Scholar] [CrossRef]
- Abiven, S.; Andreoli, R. Charcoal does not change the decomposition rate of mixed litters in a mineral cambisol: A controlled conditions study. Biol. Fertil. Soils 2011, 47, 111–114. [Google Scholar] [CrossRef][Green Version]
- Fatima, S.; Riaz, M.; Al-Wabel, M.I.; Arif, S.M.; Yasmin, T.; Hussain, Q.; Roohi, M.; Fahad, S.; Ali, K.; Muhammad, A. Higher biochar rate strongly reduced decomposition of soil organic matter to enhance C and N sequestration in nutrient-poor alkaline calcareous soil. J. Soils Sediments 2021, 21, 148–162. [Google Scholar] [CrossRef]
- Pluchon, N.; Gundale, M.J.; Nilsson, M.; Kardol, P.; Wardle, D.A. Stimulation of boreal tree seedling growth by wood-derived charcoal: Effects of charcoal properties, seedling species and soil fertility. Funct. Ecol. 2014, 28, 766–775. [Google Scholar] [CrossRef]
- Dieguez-Alonso, A.; Funke, A.; Anca-Couce, A.; Rombolà, A.G.; Ojeda, G.; Bachmann, J.; Behrendt, F. Towards Biochar and Hydrochar Engineering—Influence of Process Conditions on Surface Physical and Chemical Properties, Thermal Stability, Nutrient Availability, Toxicity and Wettability. Energies 2018, 11, 496. [Google Scholar] [CrossRef]
- O’Connor, D.; Peng, T.; Zhang, J.; Tsang, D.C.; Alessi, D.S.; Shen, Z.; Bolan, N.S.; Hou, D. Biochar application for the remediation of heavy metal polluted land: A review of in situ field trials. Sci. Total Environ. 2018, 619–620, 815–826. [Google Scholar] [CrossRef]
- Wystalska, K.; Kwarciak-Kozłowska, A. The Effect of Biodegradable Waste Pyrolysis Temperatures on Selected Biochar Properties. Materials 2021, 14, 1644. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.M.; Alvim-Ferraz, M.C.; Almeida, M.F.; Rivera-Utrilla, J.; Sánchez-Polo, M. Waste materials for activated carbon preparation and its use in aqueous-phase treatment: A review. J. Environ. Manag. 2007, 85, 833–846. [Google Scholar] [CrossRef]
- Anemana, T.; Óvári, M.; Varga, M.; Mihály, J.; Uzinger, N.; Rékási, M.; Yao, J.; Tatár, E.; Streli, C.; Záray, G.; et al. Granular activated charcoal from peanut (Arachis hypogea) shell as a new candidate for stabilization of arsenic in soil. Microchem. J. 2019, 149, 104030. [Google Scholar] [CrossRef]
- Kloss, S.; Zehetner, F.; Wimmer, B.; Buecker, J.; Rempt, F.; Soja, G. Effects on soil fertility and crop growth under greenhouse conditions. J. Plant Nutr. Soil Sci. 2014, 177, 3–15. [Google Scholar] [CrossRef]
- Steiner, C.; Teixeira, W.G.; Lehmann, J.; Nehls, T.; de Macêdo, J.L.V.; Blum, W.E.H.; Zech, W. Long term effects of manure, charcoal and mineral fertilization on crop production and fertility on a highly weathered Central Amazonian upland soil. Plant Soil 2007, 291, 275–290. [Google Scholar] [CrossRef]
- Jassal, R.S.; Johanson, M.S.; Molodovskaya, M.; Black, A.; Jollymore, A.; Sveinson, K. Nitrogen enrichment potential of biochar in relation to pyrolysis temperature and feedstock quality. J. Environ. Manag. 2015, 152, 140–144. [Google Scholar] [CrossRef]
- Gondek, K.; Mierzwa-Hersztek, M.; Kopeć, M.; Mróz, T. The Influence of Biochar Enriched with Magnesium and Sulfur on the Amount of Perennial Ryegrass Biomass and Selected Chemical Properties and Biological of Sandy Soil. Commun. Soil Sci. Plant Anal. 2018, 49, 1257–1265. [Google Scholar] [CrossRef]
- Świechowski, K.; Stępień, P.; Syguła, E.; Koziel, J.; Białowiec, A. Lab-Scale Study of Temperature and Duration Effects on Carbonized Solid Fuels Properties Produced from Municipal Solid Waste Components. Materials 2021, 14, 1191. [Google Scholar] [CrossRef]
- Montanarella, L.; Lugato, E. The Application of Biochar in the EU: Challenges and Opportunities. Agronomy 2013, 3, 462–473. [Google Scholar] [CrossRef]
- Lenik, K.; Ozonek, J. The evaluation of the cavitation number in hydrodynamic cavitation including the influence of the orifice plate geometry. Automat. Robot. Measur. 2013, 6, 60–63. [Google Scholar]
- Nakashima, K.; Ebi, Y.; Shibasaki-Kitakawa, N.; Soyama, H.; Yonemoto, T. Hydrodynamic Cavitation Reactor for Efficient Pretreatment of Lignocellulosic Biomass. Ind. Eng. Chem. Res. 2016, 55, 1866–1871. [Google Scholar] [CrossRef]
- Lewandowski, G.; Milchert, E. Modern installation of dry distillation of wood. Chemist 2011, 65, 1301–1306. [Google Scholar]
- Oleszczuk, N.; Castro, J.; DaSilva, M.; Korn, M.; Welz, B.; Vale, M. Method development for the determination of manganese, cobalt and copper in green coffee comparing direct solid sampling electrothermal atomic absorption spectrometry and inductively coupled plasma optical emission spectrometry. Talanta 2007, 73, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Regulation of the Minister of the Environment of 1 September 2016 on the method of conducting the assessment of soil surface pollution. J. Laws 2016, 1395, 86.
- Thalmann, A. Methods of dehydrogenase activity determination with triphenyltetrazoliumchlorid (TTC). Landwirtsch. Forsch. 1968, 21, 249–258. (In German) [Google Scholar]
- Zantua, M.I.; Bremner, J.M. Comparison of methods of assaying urease activity in soils. Soil Biol. Biochem. 1975, 7, 291–295. [Google Scholar] [CrossRef]
- ISO 16072:2002. Soil Quality—Laboratory Methods for Determination of Microbial Soil Respiration; International Organization for Standardization: Geneva, Switzerland, 2002. [Google Scholar]
- ISO PN-EN ISO 14240-1:2011. Soil Quality—Determination of Soil Microbial Biomass—Part 1: Substrate-Induced Respiration Method (ISO 14240-1:1997); International Organization for Standardization: Geneva, Switzerland, 2011. [Google Scholar]
- ISO 17155:2012. Soil Quality—Determination of Abundance and Activity of Soil Microflora Using Respiration Curves; International Organization for Standardization: Geneva, Switzerland, 2012. [Google Scholar]
- Operating Instructions Vario MAX Cube; Elementar Analysensysteme GmbH: Langenselbold, Germany, 2013; p. 407.
- Houba, V.J.G.; Temminghoff, E.J.M.; Gaikhorst, G.A.; Van Vark, W. Soil analysis procedures using 0.01 M calcium chloride as extraction reagent. Commun. Soil Sci. Plant Anal. 2000, 31, 1299–1396. [Google Scholar] [CrossRef]
- Gondek, K.; Mierzwa-Hersztek, M.; Baran, A.; Szostek, M.; Pieniążek, R.; Pieniążek, M.; Stanek-Tarkowska, J.; Noga, T. The effect of low-temperature conversion of plant materials on chemical composition and ecotoxicity of biochars. Waste Biom. Valor. 2017, 8, 599–609. [Google Scholar] [CrossRef]
- Mehrasbi, M.R.; Farahmandkia, Z.; Taghibeigloo, B.; Taromi, A. Adsorption of Lead and Cadmium from Aqueous Solution by Using Almond Shells. Water Air Soil Pollut. 2009, 199, 343–351. [Google Scholar] [CrossRef]
- Rajak, V.K.; Sunil Kumar, N.V.; Thombre, A.M. Synthesis of activated charcoal from saw-dust and characterization for adsorptive separation of oil from oil-in-water emulsion. Chem. Eng. Commun. 2018, 205, 897–913. [Google Scholar] [CrossRef]
- Shang, H.; Lu, Y.; Zhao, F.; Chao, C.; Zhang, B.; Zhang, H. Preparing high surface area porous carbon from biomass by carbonization in a molten salt medium. RSC Adv. 2015, 5, 75728–75734. [Google Scholar] [CrossRef]
- Hardy, B.; Cornelis, J.T.; Houben, D.; Lambert, R.; Dufey, J.E. The effect of pre-industrial charcoal kilns on chemical properties of forest soil of Wallonia, Belgium. Eur. J. Soil Sci. 2016, 67, 206–216. [Google Scholar] [CrossRef]
- Mastrolonardo, G.; Calderaro, C.; Cocozza, C.; Hardy, B.; Dufey, J.; Cornelis, J.-T. Long-Term Effect of Charcoal Accumulation in Hearth Soils on Tree Growth and Nutrient Cycling. Front. Environ. Sci. 2019, 7, 51. [Google Scholar] [CrossRef]
- Hardy, B.; Dufey, J.E. The resistance of centennial soil charcoal to the “Walkley-Black” oxidation. Geoderma 2017, 303, 37–43. [Google Scholar] [CrossRef]
- Kerré, B.; Bravo, C.T.; Leifeld, J.; Cornelissen, G.; Smolders, E. Historical soil amendment with charcoal increases sequestration of non-charcoal carbon: A comparison among methods of black carbon quantification. Eur. J. Soil Sci. 2016, 67, 324–331. [Google Scholar] [CrossRef]
- Yang, S.; Chen, X.; Jiang, Z.; Ding, J.; Sun, X.; Xu, J. Effects of Biochar Application on Soil Organic Carbon Composition and Enzyme Activity in Paddy Soil under Water-Saving Irrigation. Int. J. Environ. Res. Public Health 2020, 17, 333. [Google Scholar] [CrossRef]
- Ma, L.; Lv, N.; Ye, J.; Ru, S.-B.; Li, G.-F.; Hou, Z.-A. Effects of biochar on organic carbon content and fractions of gray desert soil. Chin. J. Eco Agric. 2012, 20, 976–981. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, G.; Lü, Y.; Shao, H. Effects of biochar amendment on soil physical and chemical properties: Current status and knowledge gaps. Adv. Earth Sci. 2014, 29, 68–79. [Google Scholar]
- Gong, H.; Li, Y.; Li, S. Effects of the interaction between biochar and nutrients on soil organic carbon sequestration in soda saline-alkali grassland: A review. Glob. Ecol. Conserv. 2021, 26, e01449. [Google Scholar] [CrossRef]
- Steinbeiss, S.; Gleixner, G.; Antonietti, M. Effect of biochar amendment on soil carbon balance and soil microbial activity. Soil Biol. Biochem. 2009, 41, 1301–1310. [Google Scholar] [CrossRef]
- Hirsch, F.; Raab, T.; Ouimet, W.; Dethier, D.; Schneider, A.; Raab, A. Soils on Historic Charcoal Hearths: Terminology and Chemical Properties. Soil Sci. Soc. Am. J. 2017, 81, 1427–1435. [Google Scholar] [CrossRef]
- Liao, J.; Liu, X.; Hu, A.; Song, H.; Chen, X.; Zhang, Z. Effects of biochar-based controlled release nitrogen fertilizer on nitrogen-use efficiency of oilseed rape (Brassica napus L.). Sci. Rep. 2020, 10, 11063. [Google Scholar] [CrossRef] [PubMed]
- Kalu, S.; Oyekoya, G.N.; Ambus, P.; Tammeorg, P.; Simojoki, A.; Pihlatie, M.; Karhu, K. Effects of two wood-based biochars on the fate of added fertilizer nitrogen—A 15N tracing study. Biol. Fertil. Soils 2021, 57, 457–470. [Google Scholar] [CrossRef]
- Miura, A.; Shiratani, E. Practical use of charcoal as a remedioation material for cadmium-polluted soil. Environ. Ecol. Res. 2018, 6, 130–136. [Google Scholar] [CrossRef][Green Version]
- Mierzwa-Hersztek, M.; Gondek, K.; Klimkowicz-Pawlas, A.; Chmiel, M.; Dziedzic, K.; Taras, H. Assessment of soil quality after biochar application based on enzymatic activity and microbial composition. Int. Agrophys. 2019, 33, 331–336. [Google Scholar] [CrossRef]
- Gondek, K.; Mierzwa-Hersztek, M. Effect of low-temperature biochar derived from pig manure and poultry litter on mobile and organic matter-bound forms of Cu, Cd, Pb and Zn in sandy soil. Soil Use Manag. 2016, 32, 357–367. [Google Scholar] [CrossRef]
- Han, G.; Zhou, G.; Xu, Z.; Yang, Y.; Liu, J.; Shi, K. Soil temperature and biotic factors drive the seasonal variation of soil respiration in a maize (Zea mays L.) agricultural ecosystem. Plant Soil 2007, 291, 15–26. [Google Scholar] [CrossRef]
- Mierzwa-Hersztek, M.; Klimkowicz-Pawlas, A.; Gondek, K. Influence of poultry litter and poultry litter biochar on soil microbial respiration and nitrifying bacteria activity. Waste Biomass Valoriz. 2018, 9, 379–389. [Google Scholar] [CrossRef]
- Eisentraeger, A.; Maxam, G.; Rila, J.-P.; Dott, W. A Stepwise Procedure for Assessment of the Microbial Respiratory Activity of Soil Samples Contaminated with Organic Compounds. Ecotoxicol. Environ. Saf. 2000, 47, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Asirifi, I.; Werner, S.; Heinze, S.; Saba, C.; Lawson, I.; Marschner, B. Short-Term Effect of Biochar on Microbial Biomass, Respiration and Enzymatic Activities in Wastewater Irrigated Soils in Urban Agroecosystems of the West African Savannah. Agronomy 2021, 11, 271. [Google Scholar] [CrossRef]
- Beheshti, M.; Etesami, H.; Alikhani, H.A. Effect of different biochars amendment on soil biological indicators in a calcareous soil. Environ. Sci. Pollut. Res. 2018, 25, 14752–14761. [Google Scholar] [CrossRef]
- Tian, J.; Wang, J.; Dippold, M.; Gao, Y.; Blagodatskaya, E.; Kuzyakov, Y. Biochar affects soil organic matter cycling and microbial functions but does not alter microbial community structure in a paddy soil. Sci. Total Environ. 2016, 556, 89–99. [Google Scholar] [CrossRef]
- Vithanage, M.; Bandara, T.; Al-Wabel, M.I.; Abduljabbar, A.; Usman, A.R.A.; Ahmad, M.; Ok, Y.S. Soil Enzyme Activities in Waste Biochar Amended Multi-Metal Contaminated Soil; Effect of Different Pyrolysis Temperatures and Application Rates. Commun. Soil Sci. Plant Anal. 2018, 49, 635–643. [Google Scholar] [CrossRef]
- Lammirato, C.; Miltner, A.; Kaestner, M. Effects of wood char and activated carbon on the hydrolysis of cellobiose by β-glucosidase from Aspergillus niger. Soil Biol. Biochem. 2011, 43, 1936–1942. [Google Scholar] [CrossRef]
- Ameloot, N.; De Neve, S.; Jegajeevagan, K.; Yildiz, G.; Buchan, D.; Funkuin, Y.N.; Prins, W.; Bouckaert, L.; Sleutel, S. Short-term CO2 and N2O emissions and microbial properties of biochar amended sandy loam soils. Soil Biol. Biochem. 2013, 57, 401–410. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Gasco, G.; Gutierrez, B.A.R.; Méndez, A. Soil biochemical activities and the geometric mean of enzyme activities after application of sewage sludge and sewage sludge biochar to soil. Biol. Fertil. Soils 2012, 48, 511–517. [Google Scholar] [CrossRef]
- Futa, B.; Oleszczuk, P.; Andruszczak, S.; Kwiecińska-Poppe, E.; Kraska, P. Effect of Natural Aging of Biochar on Soil Enzymatic Activity and Physicochemical Properties in Long-Term Field Experiment. Agronomy 2020, 10, 449. [Google Scholar] [CrossRef]
- Mierzwa-Herstek, M.; Gondek, K.; Bajda, T.; Kopeć, M. Use of biochar and a zeolite as adsorbents of mineral pollutions. Chem. Ind. 2019, 98, 1969–1972. [Google Scholar]
- Li, Q.; Li, H.; Fu, Q.; Li, T.; Liu, D.; Hou, R.; Cui, S.; Ji, Y.; Cai, Y. Effects of different biochar application methods on soybean growth indicator variability in a seasonally frozen soil area. Catena 2020, 185, 104307. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Solomon, D.; Kinyangi, J.; Grossman, J.; O’Neill, B.; Skjemstad, J.O.; Thies, J.; Luizão, F.J.; Petersen, J.; et al. Black Carbon Increases Cation Exchange Capacity in Soils. Soil Sci. Soc. Am. J. 2006, 70, 1719–1730. [Google Scholar] [CrossRef]
- Laird, D.; Fleming, P.; Wang, B.; Horton, R.; Karlen, D. Biochar impact on nutrient leaching from a Midwestern agricultural soil. Geoderma 2010, 158, 436–442. [Google Scholar] [CrossRef]
- Xu, P.; Sun, C.X.; Ye, X.Z.; Xiao, W.D.; Zhang, Q.; Wang, Q. The effect of biochar and crop straws on heavy metal bioavailability and plant accumulation in a Cd and Pb polluted soil. Ecotoxicol. Environ. Saf. 2016, 132, 94–100. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Zhang, M.; Inyang, M.; Zimmerman, A.R. Effect of biochar amendment on sorption and leaching of nitrate, ammonium, and phosphate in a sandy soil. Chemosphere 2012, 89, 1467–1471. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Y.; Song, L.; Song, X.; Hänninen, H.; Wu, J. Biochar enhances nut quality of Torreya grandis and soil fertility under simulated nitrogen deposition. For. Ecol. Manag. 2017, 391, 321–329. [Google Scholar] [CrossRef]
- Morales, M.M.; Comerford, N.; Guerrini, I.A.; Falcão, N.P.S.; Reeves, J.B. Sorption and desorption of phosphate on biochar and biochar-soil mixtures. Soil Use Manag. 2013, 29, 306–314. [Google Scholar] [CrossRef]
| Parameter | Unit | Value |
|---|---|---|
| pH H2O | – | 8.02 |
| Electrical conductivity (EC) | mS cm−1 | 4.11 |
| Dry matter | g dm−3 | 84.6 |
| Organic matter | g dm−3 | 77.4 |
| N–NH4 | mg dm−3 | 56.0 |
| N–NO3 | mg dm−3 | 5.00 |
| P | mg dm−3 | 247 |
| K | mg dm−3 | 1214 |
| Ca | mg dm−3 | 2343 |
| Mg | mg dm−3 | 551 |
| Na | mg dm−3 | 21.0 |
| Cu | mg dm−3 | 3.52 |
| Zn | mg dm−3 | 27.5 |
| Cd | mg dm−3 | 0.302 |
| Pb | mg dm−3 | 0.22 |
| Parameter | Unit | Loamy Sand (LS) | Clay (C) |
|---|---|---|---|
| pH H2O | – | 5.90 ± 0.03 | 5.08 ± 0.04 |
| pH KCl | – | 4.55 ± 0.02 | 3.55 ± 0.06 |
| Electrical conductivity (EC) | µS cm−1 | 24 ± 0 | 34 ± 3 |
| Hydrolytic acidity (Hh) | mmol(+) kg−1 | 26.80 ± 3.44 | 130.95 ± 2.01 |
| Sum of alkaline cation (S) | mmol(+) kg−1 | 51.38 ± 7.66 | 120.68 ± 18.38 |
| Total Carbon | g kg−1 | 4.30 ± 0.87 | 26.56 ± 0.59 |
| Total Nitrogen | g kg−1 | 0.38 ± 0.07 | 2.75 ± 0.03 |
| Total Sulphur | g kg−1 | 2.32 ± 0.69 | 0.15 ± 0.01 |
| Total Magnesium | g kg−1 | 0.28 ± 0.01 | 2.81 ± 0.02 |
| Total Potassium | g kg−1 | 0.31 ± 0.03 | 3.34 ± 0.02 |
| Total Phosphorus | g kg−1 | 0.16 ± 0.00 | 0.68 ± 0.00 |
| Total Copper | mg kg−1 | 1.99 ± 0.16 | 9.67 ± 0.45 |
| Total Cadmium | mg kg−1 | 0.45 ± 0.17 | 0.68 ± 0.04 |
| Total Lead | mg kg−1 | 22.6 ± 0.91 | 33.0 ± 0.39 |
| Total Zinc | mg kg−1 | 25.3 ± 0.5 | 117.4 ± 0.4 |
| Sand | g kg−1 | 830 | 170 |
| Silt | g kg−1 | 90 | 310 |
| Clay | g kg−1 | 80 | 520 |
| Treatments | Soil Types | Charcoal Rate (% v/w) |
|---|---|---|
| LS-0 | Loamy sand | 0 |
| LS-1 | Loamy sand | 1.76 |
| LS-2 | Loamy sand | 3.50 |
| LS-3 | Loamy sand | 7.00 |
| LS-4 | Loamy sand | 14.00 |
| C-0 | Clay | 0 |
| C-1 | Clay | 1.76 |
| C-2 | Clay | 3.50 |
| C-3 | Clay | 7.00 |
| C-4 | Clay | 14.00 |
| Treatment | pH H2O | pH KCl | EC uS cm−1 | CTot g kg−1 | NTot g kg−1 | |
|---|---|---|---|---|---|---|
| Loamy Sand (LS) | LS-0 | 5.53 ± 0.03 e | 3.66 ± 0.02 d | 25 ± 1 b | 4.1 ± 0.4 c | 0.39 ± 0.03 a |
| LS-1 | 5.73 ± 0.03 d | 4.26 ± 0.03 d | 16 ± 0 b | 5.8 ± 0.5 b,c | 0.41 ± 0.05 a | |
| LS-2 | 6.10 ± 0.04 c | 4.63 ± 0.03 c | 24 ± 2 b | 6.2 ± 0.8 b | 0.37 ± 0.04 a | |
| LS-3 | 6.38 ± 0.06 b | 5.20 ± 0.05 b | 35 ± 4 a,b | 6.6 ± 0.6 b | 0.35 ± 0.04 a | |
| LS-4 | 7.34 ± 0.05 a | 6.30 ± 0.06 a | 59 ± 3 a | 12.2 ± 0.7 a | 0.39 ± 0.06 a | |
| Clay (C) | C-0 | 3.60 ± 0.02 c | 2.69 ± 0.03 c | 148 ± 6 c | 24.4 ± 0.9 b | 2.74 ± 0.03 a |
| C-1 | 3.55 ± 0.02 c | 2.74 ± 0.03 c | 109 ± 5 d | 22.4 ± 0.9 b | 2.54 ± 0.06 b,c | |
| C-2 | 3.55 ± 0.02 c | 2.78 ± 0.02 c | 148 ± 7 c | 22.6 ± 0.8 b | 2.47 ± 0.04 c | |
| C-3 | 4.06 ± 0.03 b | 2.84 ± 0.03 a,b | 267 ± 12 b | 27.7 ± 0.7 a | 2.59 ± 0.07 b | |
| C-4 | 4.41 ± 0.03 a | 2.98 ± 0.03 a | 310 ± 9 a | 29.0 ± 0.5 a | 2.48 ± 0.06 c | |
| Treatment | Total Content | Extracted with 0.01 mol dm−3 CaCl2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CdTot | CuTot | PbTot | ZnTot | Cd | Cu | Pb | Zn | ||
| Loamy Sand (LS) | LS-0 | 0.25 ± 0.05 a,b | 2.20 ± 0.23 a | 23.1 ± 0.84 a | 24.7 ± 0.4 a | 0.05 ± 0.00 a | nd | 0.05 ± 0.00 a,b | 0.98 ± 0.04 a |
| LS-1 | 0.23 ± 0.05 c | 2.50 ± 0.15 a | 22.9 ± 0.93 a,b | 27.4 ± 0.5 a | 0.04 ± 0.00 a | nd | 0.04 ± 0.00 a,b | 0.80 ± 0.06 b | |
| LS-2 | 0.28 ± 0.04 a | 2.44 ± 0.13 a | 23.2 ± 0.75 a | 26.6 ± 0.4 a | 0.03 ± 0.00 b | nd | 0.06 ± 0.00 a | 0.55 ± 0.07 b | |
| LS-3 | 0.24 ± 0.05 a,b | 2.45 ± 0.26 a | 21.7 ± 0.87 b | 26.7 ± 0.4 a | 0.03 ± 0.00 b | nd | 0.04 ± 0.00 a,b | 0.34 ± 0.04 c | |
| LS-4 | 0.25 ± 0.07 a,b | 2.65 ± 0.18 a | 22.9b ± 085 b | 27.2 ± 0.6 a | 0.01 ± 0.00 c | nd | 0.03 ± 0.00 b | 0.21 ± 0.05 c | |
| Clay (C) | C-0 | 0.75 ± 0.09 a | 10.2 ± 0.36 a | 31.8 ± 0.41 a | 109 ± 0.7 b | 0.32 ± 0.02 a | 0.06 ± 0.00a | 0.10 ± 0.01 a | 5.64 ± 0.03 a |
| C-1 | 0.66 ± 0.08 b | 9.67 ± 0.46 a | 31.6 ± 0.38 a | 109 ± 0.6 b | 0.30 ± 0.03 b | 0.01 ± 0.00a | 0.10 ± 0.01 a | 5.29 ± 0.04 a | |
| C-2 | 0.66 ± 0.09 b | 10.1 ± 0.54 a | 30.9 ± 0.32 a | 109 ± 0.7 b | 0.30 ± 0.002 b | nd | 0.08 ± 0.02 a,b | 5.16 ± 0.04 a | |
| C-3 | 0.79 ± 0.14 a | 10.3 ± 0.49 a | 30.7 ± 0.37 a | 112 ± 0.4 a | 0.27 ± 0.002 c | nd | 0.06 ± 0.01 b | 4.67 ± 0.05 b | |
| C-4 | 0.68 ± 0.10 b | 10.0 ± 0.39 a | 30.5 ± 0.36 a | 111 ± 0.5 a,b | 0.23 ± 0.01 d | nd | 0.07 ± 0.01 b | 3.46 ± 0.06 c | |
| Treatment | BR (µgCO2 g−1 dm h−1) | SIR (µgCO2 g−1 dm h−1) | QR Ratio | DhA (µg TPF g dm h−1) | Ure (mg NH4 kg−1 h−1) | |
|---|---|---|---|---|---|---|
| Loamy Sand (LS) | LS-0 | 0.53 ± 0.06 c | 4.7 ± 0.6 c | 0.11 ± 0.01 c | 0.056 ± 0.009 a | 0.8 ± 0.0 c |
| LS-1 | 0.60 ± 0.09 b | 4.5 ± 0.6 c | 0.14 ± 0.02 a | 0.045 ± 0.010 a,b | 7.7 ± 0.2 a | |
| LS-2 | 0.61 ± 0.06 b | 5.3 ± 0.8 b | 0.11 ± 0.01 c | 0.026 ± 0.008 c | 6.5 ± 0.6 b | |
| LS-3 | 0.73 ± 0.07 a,b | 6.4 ± 0.7 a,b | 0.11 ± 0.01 c | 0.028 ± 0.009 c | 0.8 ± 0.1 c | |
| LS-4 | 0.84 ± 0.08 a | 6.6 ± 0.9 a | 0.13 ± 0.03 b | 0.024 ± 0.06 c | 0.8 ± 0.1 c | |
| Clay (C) | C-0 | 0.94 ± 0.11 c | 8.3 ± 0.6 c | 0.11 ± 0.03 d | 0.037 ± 0.004 a | 3.7 ± 0.6 d |
| C-1 | 1.64 ± 0.15 b | 7.8 ± 0.7 c | 0.22 ± 0.02 a | 0.030 ± 0.008 b | 12.9 ± 0.4 a | |
| C-2 | 1.41 ± 0.13 b | 10.6 ± 0.8 b | 0.13 ± 0.04 c | 0.030 ± 0.005 b | 11.9 ± 0.7 a | |
| C-3 | 2.07 ± 0.14 a | 13.3 ± 0.8 a | 0.16 ± 0.03 b | 0.029 ± 0.006 c | 8.8 ± 0.6 c | |
| C-4 | 2.06 ± 0.10 a | 14.8 ± 0.9 a | 0.14 ± 0.03 b | 0.028 ± 0.005 c | 10.1 ± 0.5 b |
| Treatment | Above-Ground Biomass | Roots | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cd | Pb | Zn | Cu | Cd | Cu | Zn | Pb | ||
| Loamy Sand (LS) | LS-0 | 1.92 ± 0.32 d | 6.35 ± 0.21 c,d | 120 ± 0.9 a,b | 14.0 ± 0.6 a,b,c | 10.9 ± 0.5 b,c,d | 31.1 ± 0.8 c,d | 606 ± 32 b,c | 29.5 ± 0.7 a |
| LS-1 | 2.66 ± 0.42 d | 7.13 ± 0.32 d | 153 ± 1.3 b | 12.1 ± 0.9 a,b | 7.7 ± 0.5 b,c,d | 18.8 ± c1.2 d | 531 ± 21 c | 16.8 ± 0.6 a,b | |
| LS-2 | 1.97 ± 0.43 d | 5.66 ± 0.45 c | 149 ± 1.6 a,b | 12.4 ± 0.4 a,b | 11.7 ± 0.8 b,c,d | 14.2 ± 0.7 c,d | 679 ± 26 b,c | 19.9 ± 0.5 a,b,c | |
| LS-3 | 1.13 ± 0.39 e | 3.25 ± 0.26 a,b | 112 ± 0.8 a,b | 11.9 ± 0.8 a,b | 6.7 ± 0.04 c,d | 13.0 ± 0.5 d | 200 ± 28 d | 20.6 ± 0.6 a,b | |
| LS-4 | 0.68 ± 0.29 e | 2.62 ± 0.39 a,b | 83 ± 1.3 a | 11.0 ± 0.7 a | 4.6 ± 0.7 d | 20.1 ± 0.4 c,d | 125 ± 16 d | 17.0 ± 0.8 b,c | |
| Clay (C) | C-0 | 10.4 ± 0.84 c | 3.75 ± 0.34 b | 360 ± 1.6 d | 14.8 ± 0.8 a,b,c | 9.8 ± 0.6 b,c,d | 35.3 ± 0.9 b,c,d | 91 ± 12 b,c | 19.7 ± 0.9 a,b,c |
| C-1 | 9.7 ± 0.95 c | 3.96 ± 0.42 b | 260 ± 2.1 c | 16.6 ± 0.7 a,b,c | 14.7 ± 0.4 b,c | 65.5 ± 0.8 a | 1060 ± 38 a | 24.9 ± 0.5 a,b | |
| C-2 | 9.8 ± 0,75 c | 3.95 ± 0.048 b | 232 ± 1.5 c,d | 21.7 ± 0.6 d | 11.7 ± 0.7 a,b,c,d | 38.6 ± 0.9 b,c | 760 ± 25 b,c | 21.2 ± 0.6 a,b | |
| C-3 | 11.4 ± 0.68 b | 2.83 ± 0.27 a,b | 285 ± 2.3 c | 18.7 ± 0.8 c,d | 6.7 ± 0.5 c,d | 32.4 ± 0.9 c,d | 807 ± 29 b | 9.4 ± 0.8 c | |
| C-4 | 14.6 ± 0.91 a | 2.24 ± 0.36 a | 300 ± 2.9 c,d | 16.2 ± 0.8 b,c | 36.7 ± 0.6 a | 56.7 ± 0.7 a,b | 699 ± 26 b,c | 18.0 ± 0.09 b,c | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gondek, K.; Mierzwa-Hersztek, M.; Grzymała, W.; Głąb, T.; Bajda, T. Cavitated Charcoal—An Innovative Method for Affecting the Biochemical Properties of Soil. Materials 2021, 14, 2466. https://doi.org/10.3390/ma14092466
Gondek K, Mierzwa-Hersztek M, Grzymała W, Głąb T, Bajda T. Cavitated Charcoal—An Innovative Method for Affecting the Biochemical Properties of Soil. Materials. 2021; 14(9):2466. https://doi.org/10.3390/ma14092466
Chicago/Turabian StyleGondek, Krzysztof, Monika Mierzwa-Hersztek, Wojciech Grzymała, Tomasz Głąb, and Tomasz Bajda. 2021. "Cavitated Charcoal—An Innovative Method for Affecting the Biochemical Properties of Soil" Materials 14, no. 9: 2466. https://doi.org/10.3390/ma14092466
APA StyleGondek, K., Mierzwa-Hersztek, M., Grzymała, W., Głąb, T., & Bajda, T. (2021). Cavitated Charcoal—An Innovative Method for Affecting the Biochemical Properties of Soil. Materials, 14(9), 2466. https://doi.org/10.3390/ma14092466









