On Relationships between Gas-Phase Chemistry and Reactive Ion Etching Kinetics for Silicon-Based Thin Films (SiC, SiO2 and SixNy) in Multi-Component Fluorocarbon Gas Mixtures
Abstract
1. Introduction
- The main contribution to the chemical etching pathway for all above-mentioned materials under typical RIE conditions (p < 50 mTorr, ion bombardment energy ~200–400 eV) belongs to F atoms. The role of CFx (x = 1–3) radicals as etchant species is almost negligible; it works only through the ion-induced defluorination of deposited fluorocarbon polymer film [9,16].
- The steady-state thickness of polymer film, , under the same processing conditions normally decreases in the sequence of Si–Si3N4–SiO2 [15,16,17], as follows from an opposite order of corresponding etching rates [16,17]. The lowest value is due to the etching of polymer by oxygen atoms on the film/SiO2 interface [9,17,18,19,20], while the condition > is provided by the higher sticking probability of polymerizing radicals to the Si surface [16]. However, this rule may not work for all gas systems and processing conditions. As an example, Ref. [16] reports on the highest etching rate for Si3N4 in CHF3 plasma at ion energies above 150 eV.
- Both etching and polymerization kinetics may be effectively adjusted by mixing fluorocarbon gas with Ar and/or O2 [9,17,20,21,22,23]. Corresponding mechanisms work through changes in (a) the formation/decay balance for F atoms and polymerizing radicals in a gas phase; and (b) the physical and chemical decomposition rates of the fluorocarbon polymer film.
- The chemical interaction of F atoms with Si has no threshold energy and occurs spontaneously with the formation of highly volatile SiF4 at typical process temperatures [12,13,14]. That is why the “pure” Si + F reaction kinetics exhibits weak sensitivity to ion bombardment with energies below ~100 eV [11,12] (in fact, to the ion-stimulated desorption of reaction products) as well as being characterized by the exponent-like dependence of its etching rate on surface temperature [9]. At the same time, the RIE kinetics of silicon in high-polymerizing plasmas exhibits sufficient sensitivity to both ion flux and energy, in addition to the F atom density. The reasons are (a) the contribution of the sputter etching pathway; and (b) the change in that influences the Si + F reaction probability through the access of F atoms to the etched surface [15,16,24].
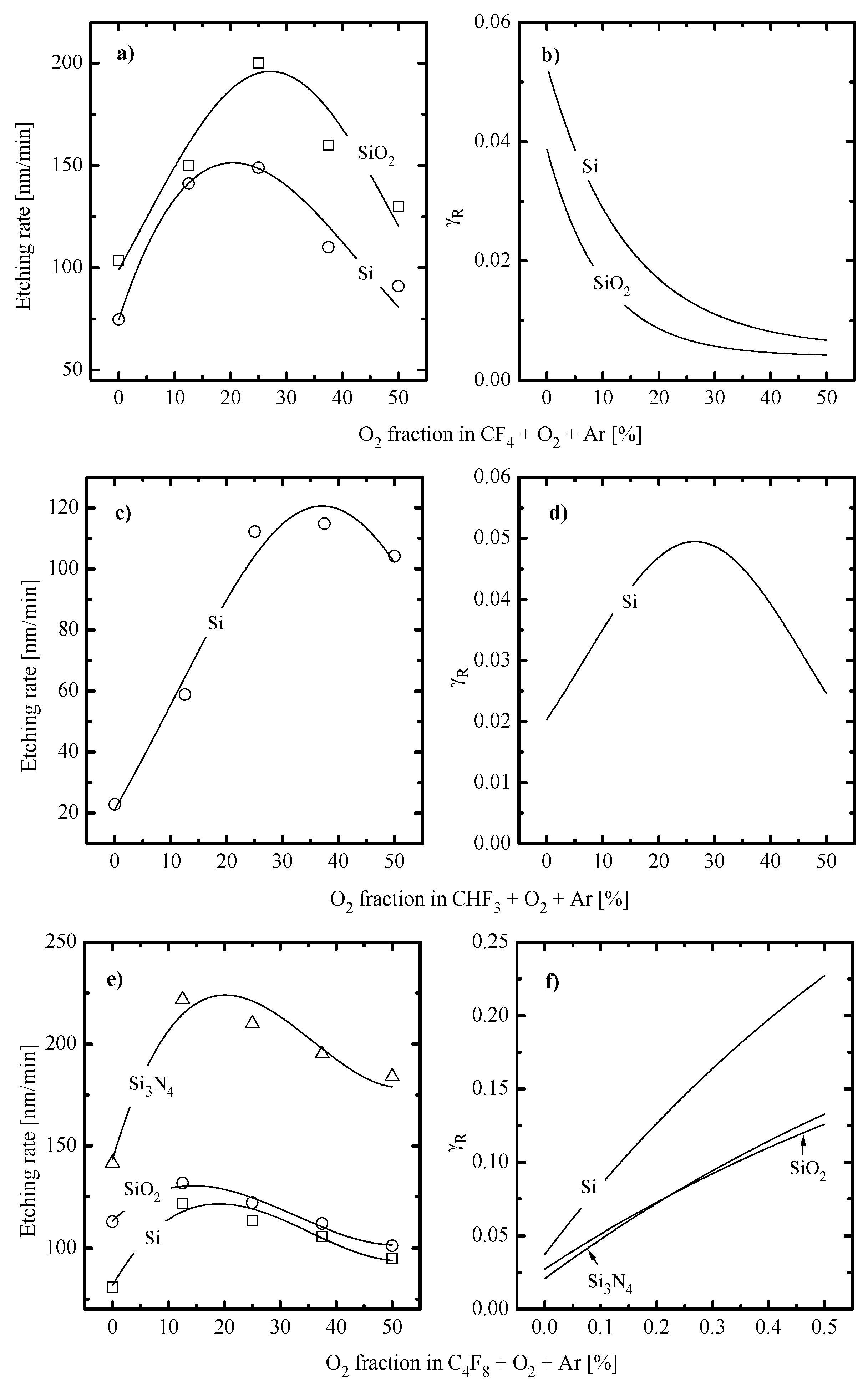
- The chemical interaction of F atoms with SiC also has a threshold-less nature, since the strength of the Si–C bond of ~447 kJ/mol is lower compared with both Si–F (~552 kJ/mol [25]) and C–F (~514 kJ/mol [25]). Depending on the combination of processing conditions (type of fluorocarbon gas, additive components and ion bombardment energy), the SiC etching kinetics may correspond to either the neutral-flux-limited or the ion-flux-limited etching regime. Evidence of the first one is illustrated by the non-monotonic SiC etching rate vs. O2 fraction in the CF4 + O2 plasma [26,27] that corresponds to the change in F atom density [28,29,30]. Accordingly, the possibility of the ion-driven etching regime is indicated by the decreasing SiC etching rate vs. gas pressure [31]. Similarly to other materials, the SiC etching process in high-polymerizing fluorocarbons is affected by the deposition of polymer film [27,31].
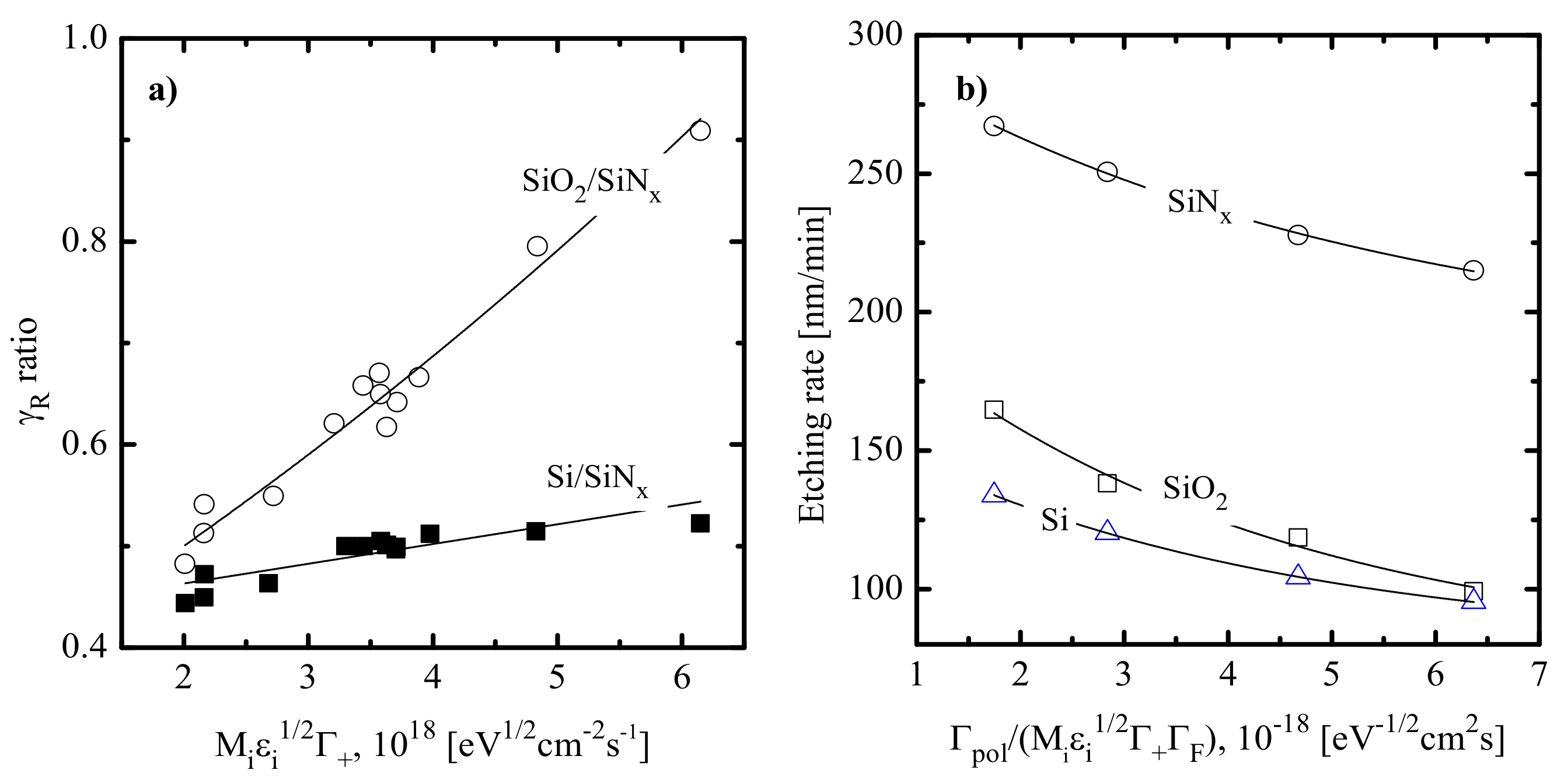
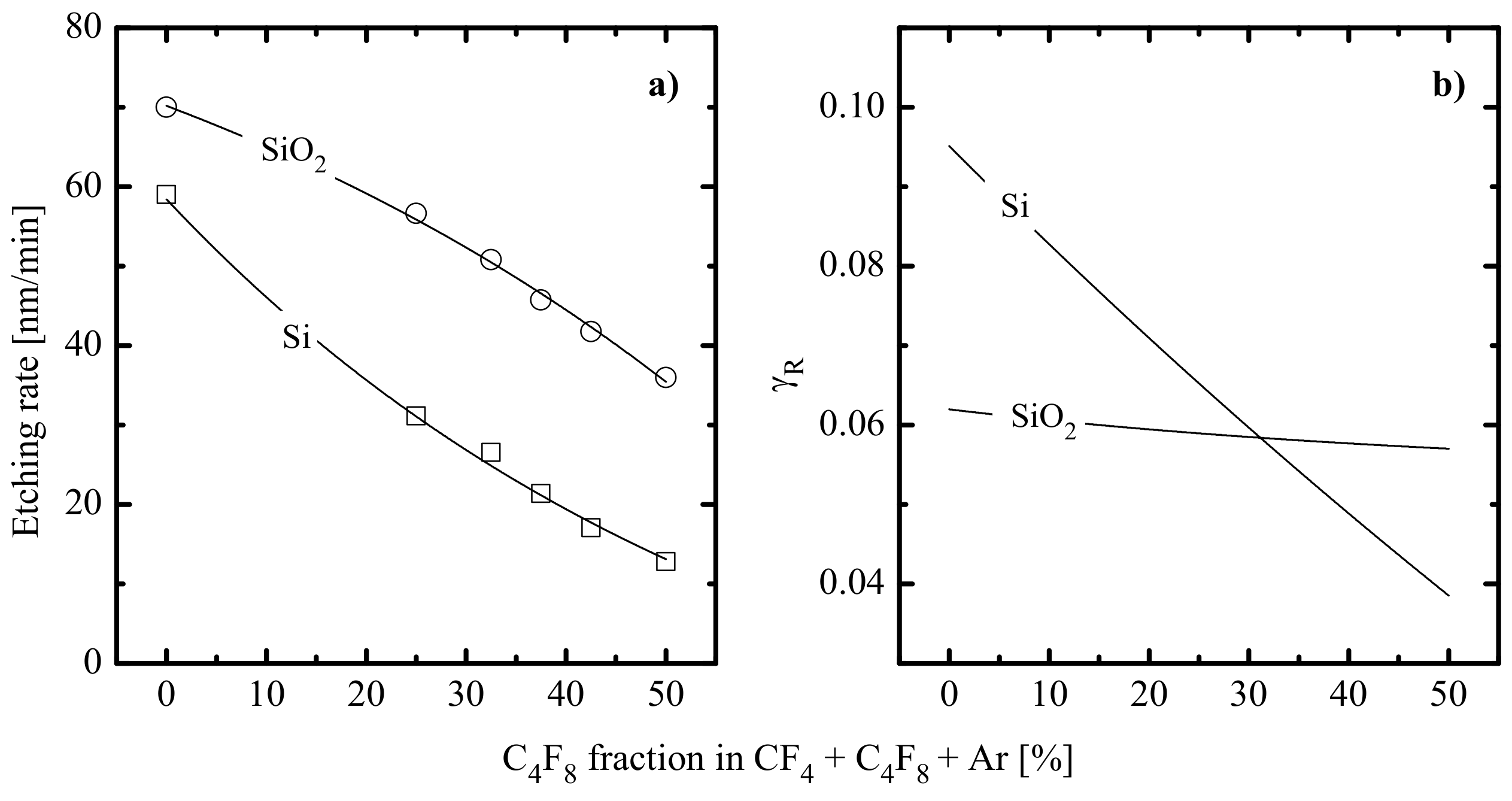
- The chemical interaction of F atoms with Si3N4 can also follow a spontaneous mechanism (as the strength of the Si–N bond of ~470 kJ/mol [25] is smaller compared with that for Si–F) but shows much higher sensitivity to the ion bombardment intensity compared with Si [17]. This is due to the ion-induced destruction of Si–N bonds that creates energetically favorable adsorption sites for F atoms. Under typical RIE conditions, the Si3N4 etching process exhibits the features of a neutral-flux-limited regime controlled by F atom flux. The last conclusion follows from (a) an increase in Si3N4 etching with increasing gas pressure and input power [17,32] and (b) the non-monotonic (with a maximum at 30–40% O2) Si3N4 etching rate in the CF4 + O2 plasma [18,33,34] that corresponds to the similar non-monotonic behavior of F atom density [28,29,30].
- The chemical interaction of F atoms with SiO2 cannot occur spontaneously because of the sufficient energy threshold (as the Si-F bond energy is lower than the Si–O one, ~799 kJ/mol [25]). That is why the dry etching of SiO2 requires mandatory ion bombardment (to produce adsorption sites for F atoms through Si–O bonds breaking as well as to desorb low volatile non-saturated SiFx compounds [9]) and is controlled by ion flux at ion energies below ~200 eV. At the same time, higher ion energies are generally enough to provide domination of the chemical etching pathway controlled by F atom flux [10,14]. The last feature is confirmed by the non-monotonic (with a maximum at 30–40% O2) SiO2 etching rate in the CF4 + O2 plasma [21,23], similar to those obtained for Si [9,12] and Si3N4 [18,33,34].
- To understand how the chemical nature of the fluorocarbon component and corresponding value influences electron- and ion-related plasma parameters (electron temperature, plasma density, ion flux and energy) that determine both electron impact kinetics and ion–surface interaction efficiency;
- To figure out differences in steady-state densities of F atoms and polymerizing radicals under the same processing conditions in light of their formation/decay kinetics as well as to evaluate the ability of additive gases (rather, the impact of their mixing ratios) to adjust gas-phase compositions in corresponding gas systems;
- To compare RIE performances for both non-oxygenated and oxygenated gas systems with respect to various silicon-based materials in terms of etching rates and etching selectivity as well as to suggest corresponding etching mechanisms and limiting stages through correlations between fluxes of active species and obtained etching kinetics.
2. Materials and Methods
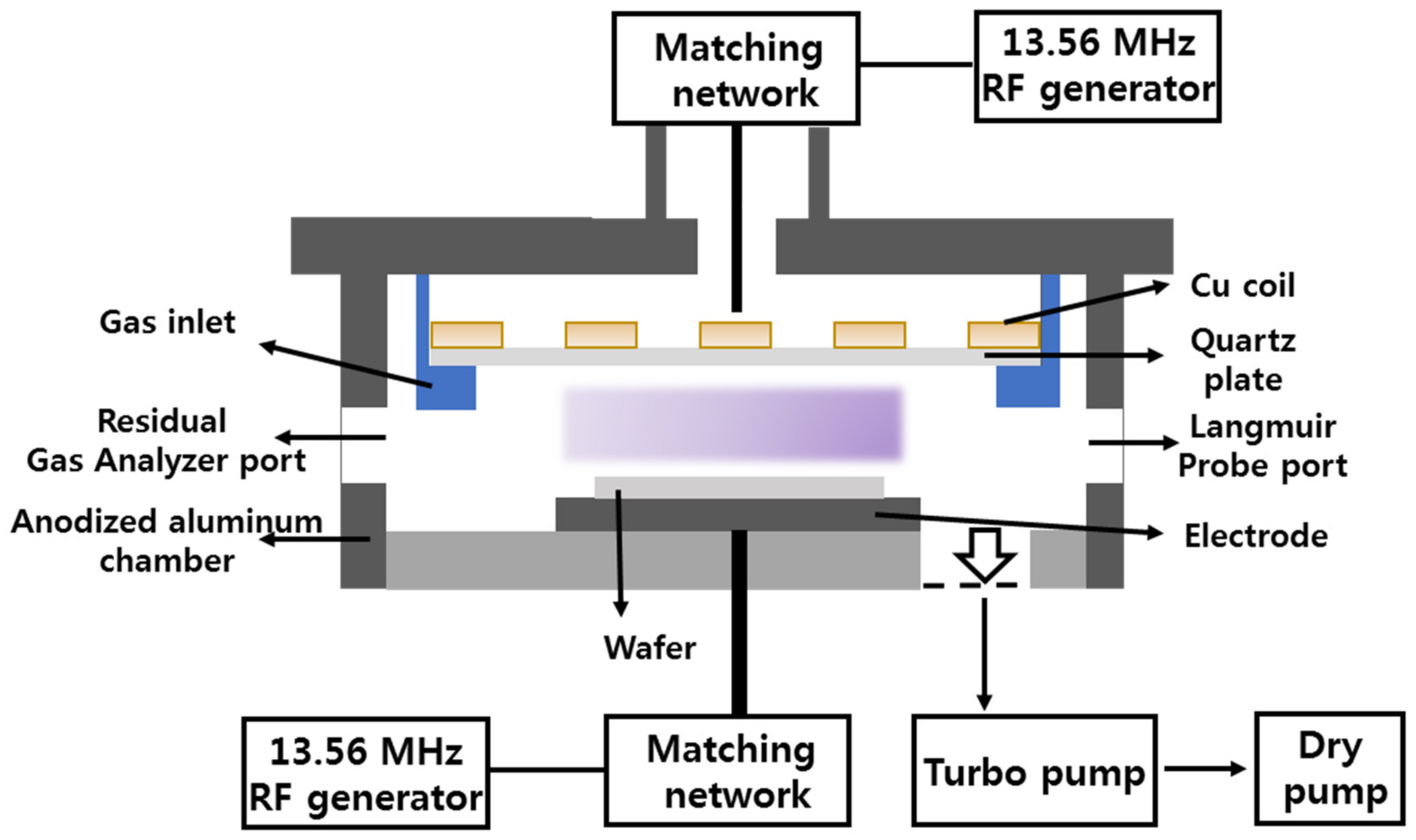
2.1. Experimental Setup and Procedures
2.2. Approaches for Analysis of Gas-Phase Chemistry
- The electron energy distribution function (EEDF) has a nearly Maxwellian shape. The applicability of Maxwellian EEDFs to describe electron-impact kinetics in low-pressure CF4–, CHF3– and C4F8– based plasmas has been demonstrated in several works [28,55,56,57,58]. This allows one to obtain electron-impact rate coefficients as [55,56,58] based on measured values.
- The dependence of gas temperature, , on gas mixing ratios at = const. may be neglected. The indirect proof for this suggestion is the nearly constant temperature of the external chamber wall obtained for different gas mixing ratios at fixed plasma on time. Since experimental data on were not available in this study, we took 600–700 K as the typical value for close processing conditions, reactor type and geometry [47,48,49,50,51,52,53]. This allowed us to operate with constant rate coefficients for gas-phase atom molecular reactions, which were taken from the NIST(National Institute of Standards and Technology) Chemical Kinetics Database [59].
- The heterogeneous loss of atoms and radicals is described by first-order recombination kinetics with [36,37,38], where is the diffusion length [9] and is the thermal velocity for a particle with a mass of . Recombination probabilities were taken from Refs. [55,56,58,60] where these were obtained experimentally or adjusted by a plasma modeling procedure. We also suggested that all recombination probabilities are independent of gas mixing ratios because of the stable chamber wall conditions. The reasons are (a) no re-deposition of reaction products due to the low sample size; and (b) the nearly constant wall temperature. The indirect proof for the last suggestion is the nearly constant external wall temperature, as was mentioned above.
- The electronegativity of low-pressure CF4–, CHF3– and C4F8– based plasmas, even with addition of O2, is low enough to assume [28,55,56,57,58], where is the electron density and is the total density of positive ions. The latter was obtained from measured without accounting for the influence of negative ions on the ion Bohm velocity:
2.3. Approaches for Analysis of Etching Kinetics
- Under reactive ion etching conditions (i.e., when the ion bombardment energy exceeds the sputtering threshold for the target surface), the measured etching rate, , may be represented in the form of two summands, [9,61]. These represent physical (sputtering by ion bombardment) and chemical (ion-assisted chemical reaction) etching pathways, respectively.
- The ion-assisted chemical reaction has the rate of [37,38], where is the effective reaction probability which accounts for the net effect from all heterogeneous stages, and is the fluorine atom flux. In general, the effective reaction probability [38,39] depends on surface temperature (through the sticking coefficient ) as well as on any factor influencing the fraction of free adsorption sites for F atoms . In polymerizing plasmas, decreases with increasing thickness of the fluorocarbon polymer film when the latter comes to be enough to provide << 1, where is the flux of F atoms on the polymer film/etched surface interface [39].
- The physical sputtering (as well as any other ion-related etching effect with a purely physical nature: breaking of chemical bonds between surface atoms, ion-stimulated desorption of reaction products) has the rate of [9,63], where ~ [35,36,37] is the sputtering yield, is the effective ion molar mass, is the ion bombardment energy, is the floating potential and is the flux of positive ions. Accordingly, the relative change in the corresponding process rate may be traced by the parameter [37,38]. Obviously, in the case of const., one can simply use .
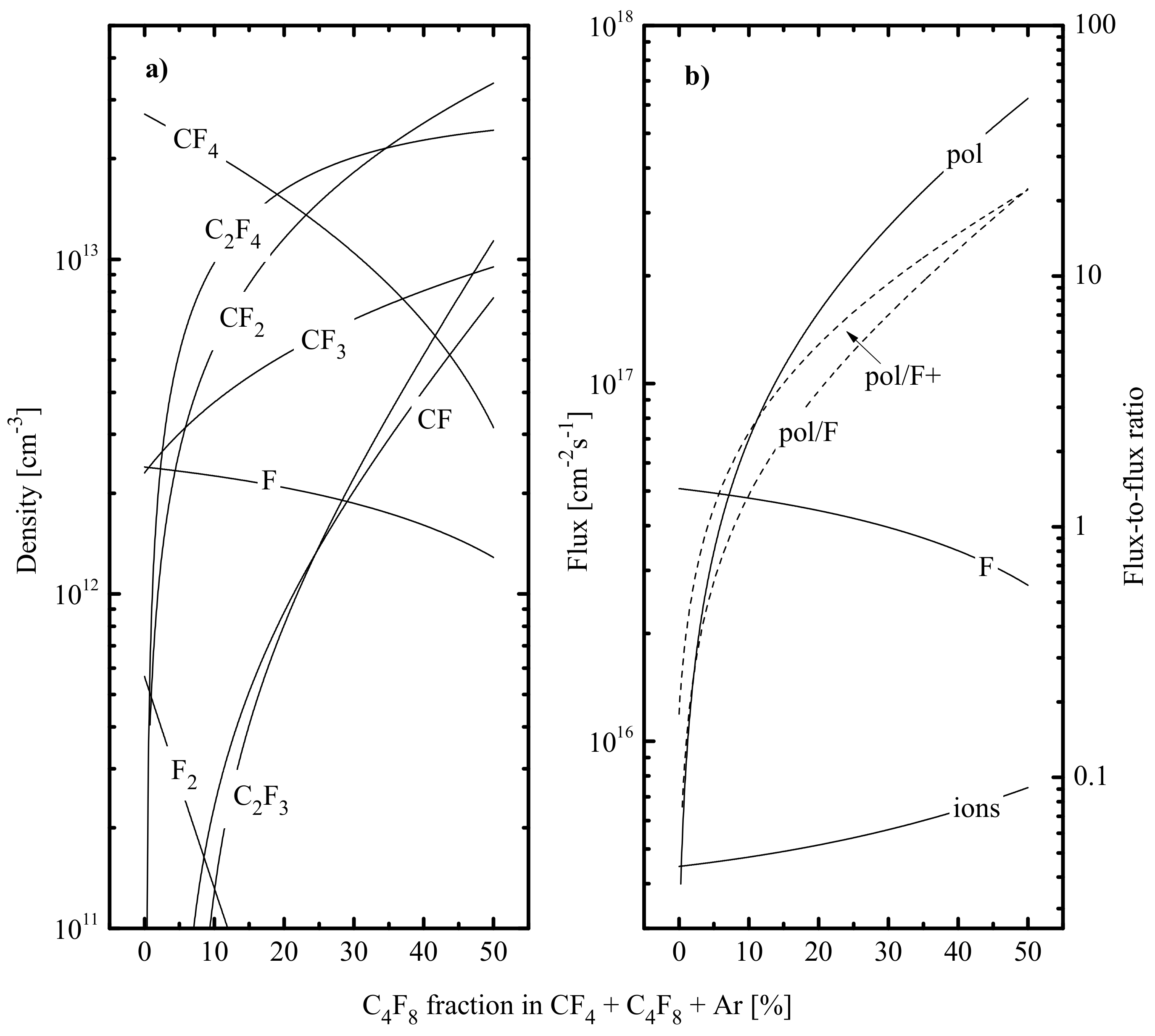
- The growth of the fluorocarbon polymer film is provided by the CFx (x = 1, 2) radicals, and the polymerization ability increases in fluorine-poor plasmas [16,24]. Accordingly, the polymer deposition rate may be traced by the ratio [35,36,37], where is the total flux of polymerizing radicals ( in CF4- and C4F8- based plasmas while in CHF3- based plasmas). Accordingly, parameters and characterize the change in the polymer film thickness due to physical (destruction by ion bombardment) and chemical (etching by O atoms) destruction pathways, respectively [35,36,40,48].
3. Results
3.1. Non-Oxygenated Gas Systems
- -
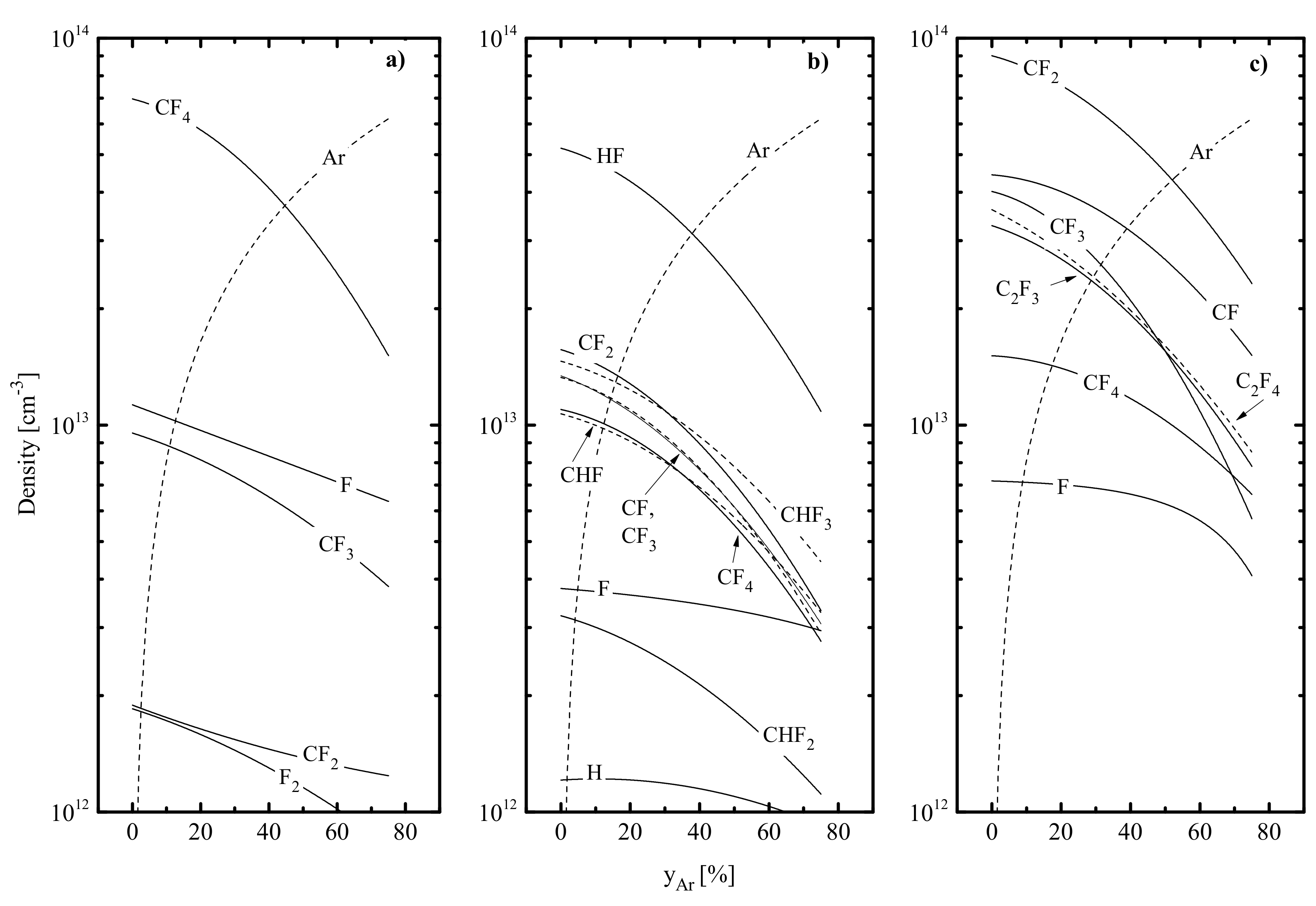
- The electron temperature increases in the sequence of CF4–C4F8–CHF3, while the shapes of curves in corresponding gas mixtures are somewhat different (Table 1). The maximum value of in the CHF3 plasma results from low electron energy losses for the ionization of the dominant neutral component HF (Hydrogen Fluoride) compared with that for CF4 molecules in the CF4 plasma and CF2 radicals in the C4F8 plasma (Figure 2). Such a conclusion directly follows from the comparison of rate coefficients () and threshold energies () for R1: HF + e → HF+ + 2e ( = 16.1 eV, = 4.6 × 10−10 cm3/s at = 3 eV); R2: CF4 + e → CF3+ + F + 2e ( = 15.9 eV, = 1.6 × 10−9 cm3/s at = 3 eV) and R3: CF2 + e → CF2+ + 2e ( = 10.0 eV, = 2.1 × 10−9 cm3/s at = 3 eV). The condition (CF4) < (C4F8) is connected with higher electron energy losses for vibrational and electronic excitations for CF4 compared with CF2. An increase in in the CF4 + Ar gas mixture causes a nearly proportional decrease in electron energy losses for vibrational and electronic excitations of CFx species as well as leads to the opposite change in energy losses for ionization due to R4: Ar + e → Ar+ + 2e ( = 15.6 eV, = 3.0 × 10−9 cm3/s at = 5 eV). Accordingly, the change in CF4/Ar mixing ratio at = const. results in ≈ const. At the same time, an increase in in CHF3 + Ar and C4F8 + Ar plasmas is characterized by much a stronger increase in electron energy losses for ionization (due to << and ) that produces a decrease in (Table 1).
- -
- The plasma density increases in the sequence of C4F8–CF4–CHF3 (Table 1) due to the same order of total ionization frequencies, as follows from < < . Similar changes in both and vs. Ar fraction in the feed gas in all three gas systems result from an increase in total ionization frequencies toward Ar-rich plasmas (2.7 × 104–6.5 × 104 s−1 for CF4 + Ar; 1.2 × 105–1.7 × 105 s−1 for CHF3 + Ar and 9.5 × 104–2.1 × 105 s−1 for C4F8 + Ar at 0–75% Ar). Mechanisms providing such a phenomenon are the condition > , and as well as the contribution of R5: Arm + e → Ar+ + 2e, where Arm = Ar (3P0,1,2) are the metastable Ar atoms. Accordingly, it is analogous for ion current densities (0.95–1.63 mA/cm2 for CF4 + Ar; 1.55–2.20 mA/cm2 for CHF3 + Ar and 1.05–2.36 mA/cm2 for C4F8 + Ar at 0–75% Ar) that determine ion fluxes to the etched surface . The local disturbance of this rule for CF4 + Ar and C4F8 + Ar plasmas at < 30% ( > in spite of < ) is connected with differences in masses and transport coefficients for dominant positive ions.
- -
- The negative dc bias at = const. exhibits a growth in the sequence of CHF3–CF4–C4F8 as well as decreases with increasing Ar fraction in a feed gas (Table 1). This reasonably causes the same order and behaviors of ion bombardment energies (285–211 eV for CF4 + Ar, 261–208 eV for CHF3 + Ar and 307–221 eV for C4F8 + Ar at 0–75% Ar). Quite close values of in all three gas systems allow one to conclude that corresponding differences in the efficiency of ion bombardment are mostly related to those in ion fluxes. From Figure 3, it can be understood that highest and lowest values for the parameter under the condition of < 60% are in CHF3 + Ar and CF4 + Ar plasmas, respectively. At the same time, an increase in Ar fraction above 60% changes the situation due to the rapid increase in in the C4F8 + Ar plasma.
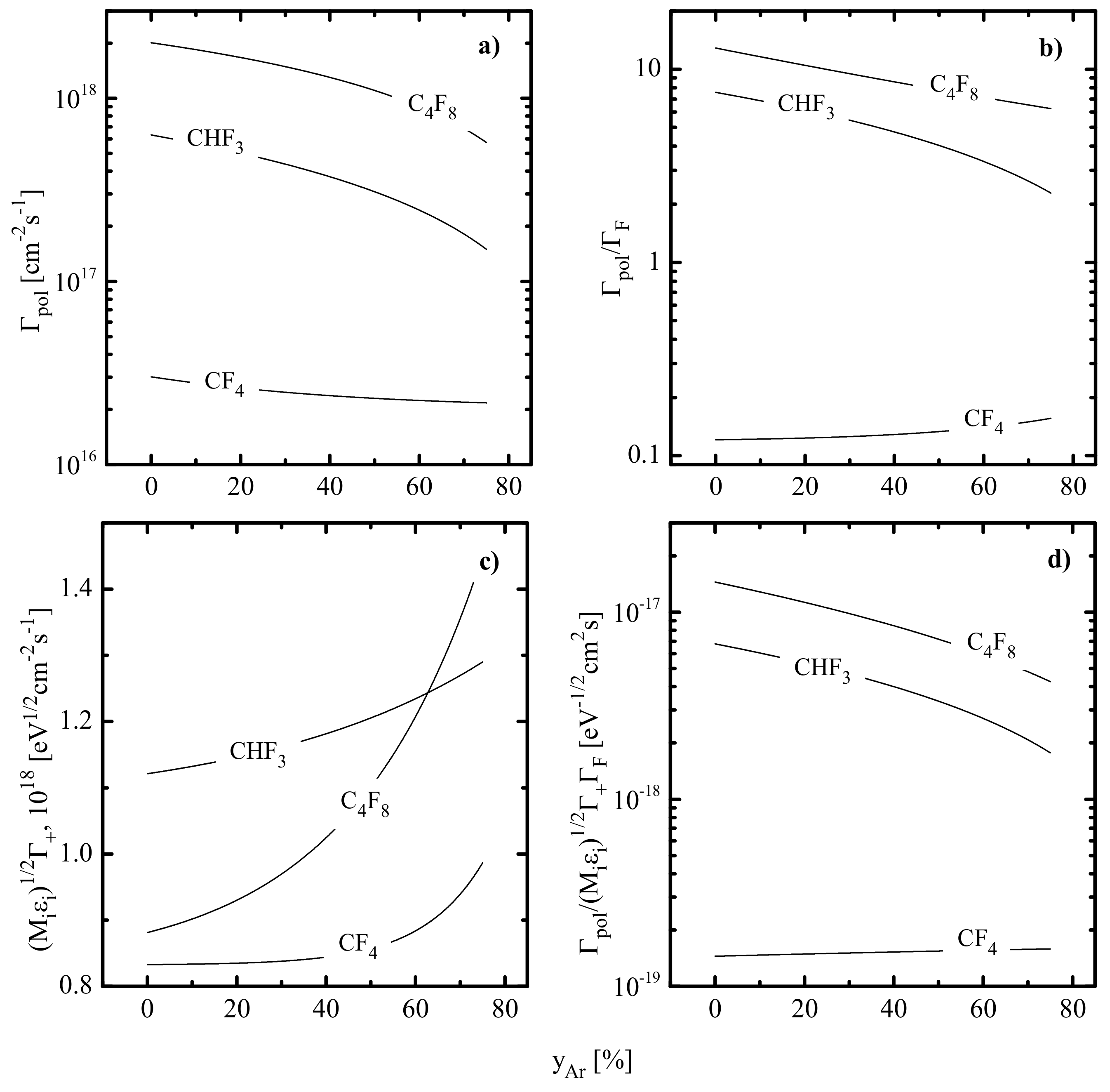
- The total density of polymerizing radicals ( for CF4 + Ar and C4F8 + Ar plasmas while for the CHF3 + Ar plasma) increases in the sequence of CF4–CHF3–C4F8, and the gap between first and last gas systems reaches two orders of magnitude. Such a phenomenon is because the C4F8+Ar plasma provides the maximum production rate of CF2 radicals by R16, R18 and R20. An increase in causes a nearly proportional decrease in in CHF3 + Ar (by ~4.2 times at 0–75% Ar) and C4F8 + Ar (by ~3.8 times at 0–75% Ar) plasmas but produces a much slower effect in the case of the CF4 + Ar gas system (by ~1.4 times at 0–75% Ar). The reason is the increasing dissociation frequencies for multi-atomic fluorocarbon species (16.2–34.4 s−1 for CF4 in R6 and R7, 28.8–51.0 s−1 for CF3 in R6 and 40.2–74.1 s−1 for CF in R6 at 0–75% Ar) due to an increase in both electron temperature and electron density. Accordingly, similar behaviors were also obtained for the total flux of polymerizing radicals to the etched surface (Figure 3a).
- The density of fluorine atoms decreases in the sequence of CF4–C4F8–CHF3, and the gap between first and last gas systems reaches around three times. The lowest value in the CHF3 plasma results from the (a) low F atom formation rate because < and (b) high F decay rate in R11 that exceeds the total effect from R8 and R9. The much smaller difference in between CF4 + Ar and C4F8 + Ar plasmas (that is in good agreement with Ref. [68]) is only because the last system provides slightly higher F atom decay frequencies due to the contribution of R20. It can be understood, also, that as the Ar fraction in a feed increases, the F atoms’ density in all three gas systems decreases slower compared with . In the CF4 + Ar plasma ( = 1.1 × 1013 cm−3–6.4 × 1012 cm−3, or by ~1.8 times at 0–75% Ar), the reason for this phenomenon is the same behavior of the F atom formation rate in R6 and R7 due to increasing process frequencies, as was mentioned above. Oppositely, CHF3 + Ar and C4F8 + Ar plasmas exhibit nearly constant frequencies for R6, R7 and R14 (due to opposite changes in electron temperature and electron density) and, thus, a nearly proportional decrease in F atom formation rate with increasing Ar fraction in the feed gas. As such, the slow change in in these gas systems (3.8 × 1012 cm−3–3.0 × 1012 cm−3, or by ~1.3 times in CHF3 + Ar and 7.1 × 1012 cm−3–4.1 × 1012 cm−3, or by ~1.7 times in C4F8 + Ar at 0–75% Ar) is not connected with electron impact kinetics but results from rapidly decreasing F atom decay frequencies in gas-phase reactions R11 and R20. Similar tendencies were also obtained for the flux of fluorine atoms to the etched surface .
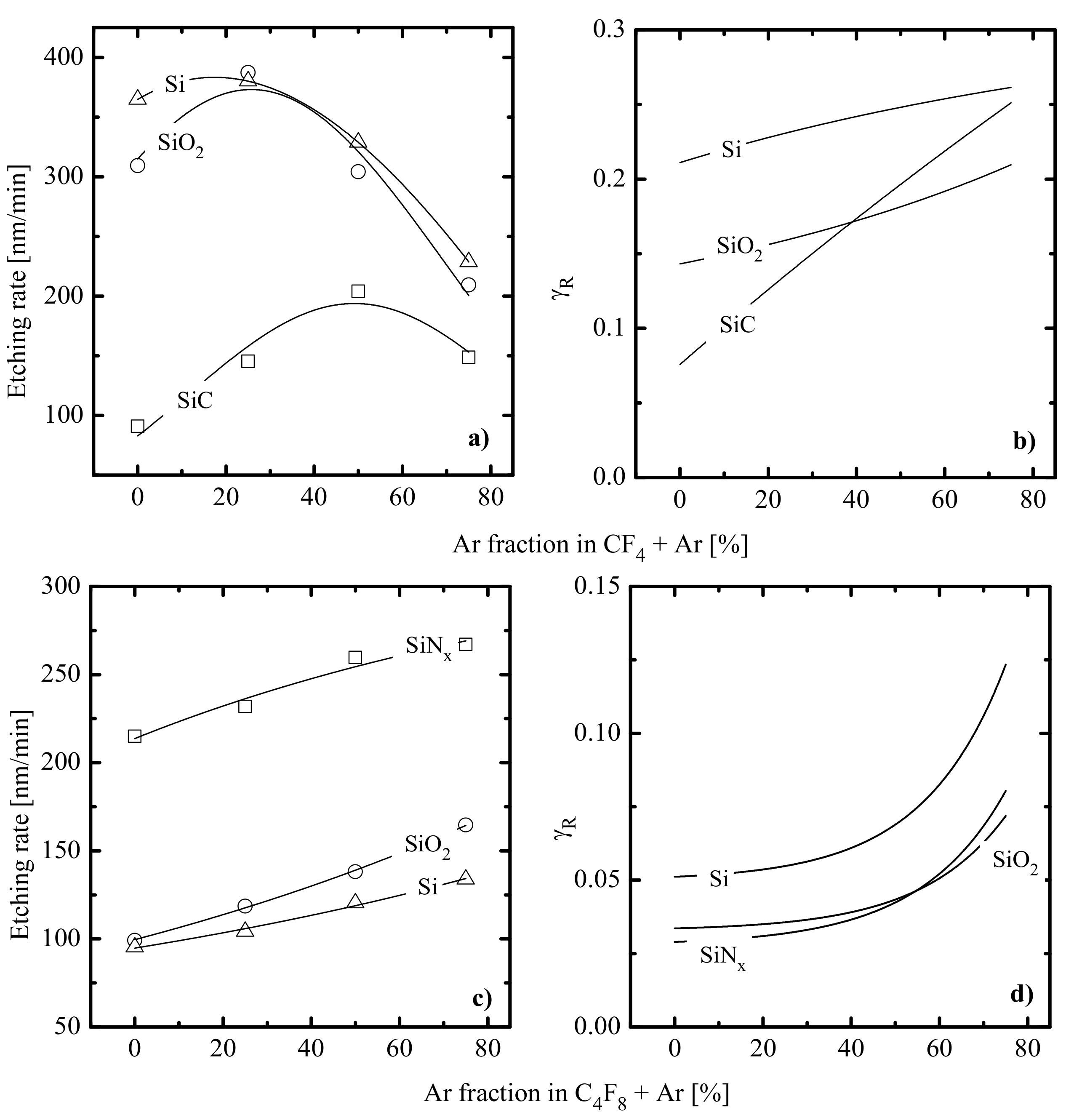
- Experiments with pure Ar plasma indicated that etching rates for all four materials (in fact, rates of physical sputtering, ) are quite close and do not exceed 20 nm/min. The correction of corresponding values according to the change of in fluorocarbon-containing plasmas (see Figure 3c) confirmed that the range of 0–75% Ar is characterized by << and . As such, the dominant etching pathway in all cases is the ion-assisted chemical reaction.
- Quite similar curves within the given gas system may be caused by the fact that corresponding etching processes are driven by identical active species and have the same limiting stage. A similar conclusion was made in the experimental study of Standaert et al. [16] for Si, SiO2 and Si3N4 in both CHF3 and C4F8 plasmas. At the same time, the evident dissimilarity in etching rate behaviors in Figure 4a,c points to different etching mechanisms in CF4- and C4F8-based plasmas. This may be, for example, the ion-assisted chemical reaction under the condition of thin or even non-continuous polymer film (in the case of low-polymerizing CF4 + Ar plasma) or the etching regime controlled by the polymer thickness through the transport of etchant species to the film/etched surface interface (in the case of high-polymerizing C4F8 + Ar plasma).
3.2. Oxygenated Gas Systems
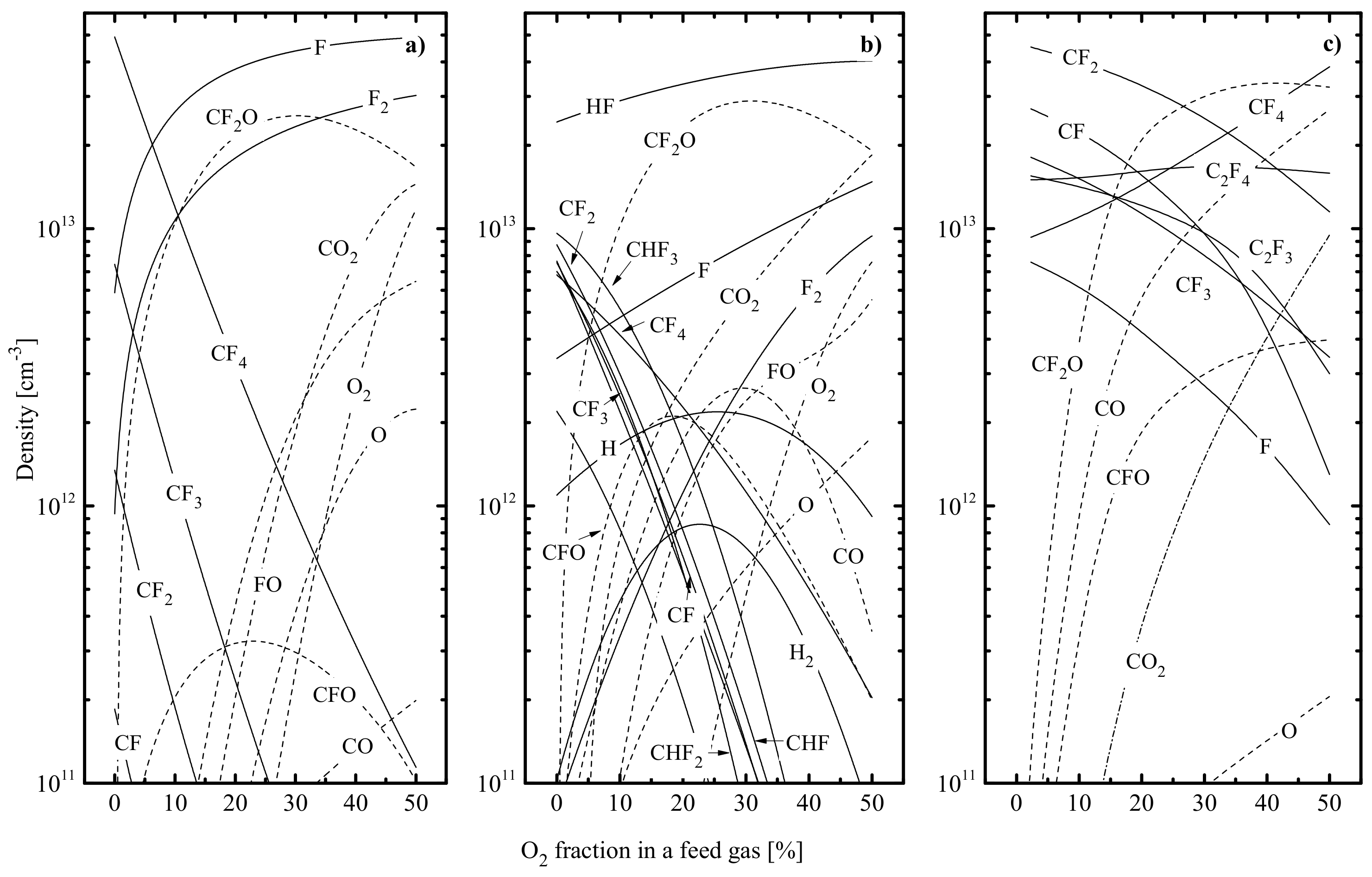
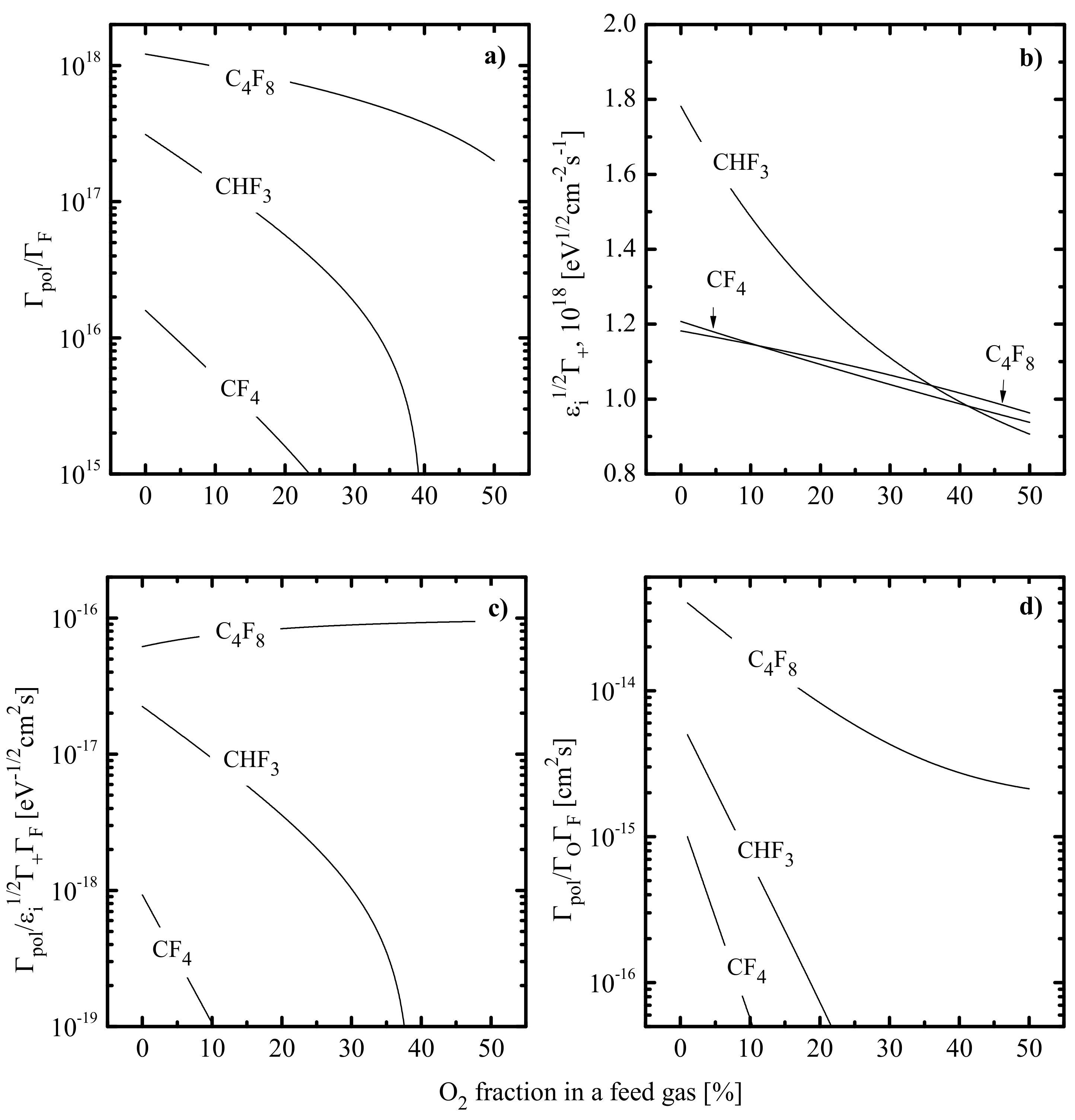
- A decrease in toward O2-rich plasmas results from an increase in electron energy losses in low-threshold inelastic processes, such as vibrational and electronic excitations of both O2 itself and molecular products of plasma chemical reactions—CO, CO2, FO and CFxO (Figure 8). In particular, the lowest excitation potential for Ar is ~11.6 eV, while O2 is characterized by the almost continuous energy loss spectrum from ~0.2 eV. Such a situation is due to the vibration excitation R23: O2 (V = 0) + e → O2 (V > 0) + e ( = 0.16 eV) as well as by the formation of metastable states in R24: O2 + e → O2 (a1∆) + e ( = 0.98 eV) and R25: O2 + e → O2(b1∑) + e ( = 1.64 eV).
- A decrease in plasma density toward higher values is provided by a combination of two evident mechanisms. First, the decreasing lowers the overall ionization efficiency through the change in ionization rate coefficients, , for all neutral gas-phase components. The high sensitivity of , to is because typical threshold energies (≈ 12–15 eV [9]) exceed the mean electron energy (3/2. Secondly, an increase in introduces many electronegative species in the form of O- and F-containing reaction products. This probably accelerates losses of both electrons and positive ions in the dissociative attachment and ion–ion recombination processes. Ion current densities and ion fluxes in all three gas systems also exhibit decreasing tendencies, according to the behavior of .
- An increase in negative dc bias is mainly due to the opposite change in ion flux. The reason is that the lower ion flux leads to weaker compensation for the excess negative charge provided by bias power source at = const. The corresponding increase in ion bombardment energy does not overlap with the change in , so the parameter characterizing the intensity of ion bombardment at ≈ const. also shows a decrease toward O2-rich plasmas (Figure 9).
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baliga, B.J. Trends in power semiconductor devices. IEEE Trans. Electron Devices 1996, 43, 1717–1731. [Google Scholar] [CrossRef]
- Chelnokov, V. SiC bipolar devices. Mater. Sci. Eng. B 1992, 11, 103–111. [Google Scholar] [CrossRef]
- Chow, T.; Ghezzo, M. SiC power devices. MRS Online Proc. Libr. 1996, 423, 9–21. [Google Scholar] [CrossRef]
- Sze, S.M. VLSI Technology; McGraw-Hill: New York, NY, USA, 1988. [Google Scholar]
- Chu, T. Dielectric materials in semiconductor devices. J. Vac. Sci. Technol. 1969, 6, 25–33. [Google Scholar] [CrossRef]
- Niklasson, G.; Eriksson, T.; Brantervik, K. Dielectric properties of silicon oxynitride films. Appl. Phys. Lett. 1989, 54, 965–967. [Google Scholar] [CrossRef]
- Wörhoff, K.; Hilderink, L.; Driessen, A.; Lambeck, P. Silicon oxynitride: A versatile material for integrated optics applications. J. Electrochem. Soc. 2002, 149, F85. [Google Scholar] [CrossRef]
- Alayo, M.; Criado, D.; Gonçalves, L.; Pereyra, I. Deposition and characterization of silicon oxynitride for integrated optical applications. J. Non Cryst. Solids 2004, 338, 76–80. [Google Scholar] [CrossRef]
- Lieberman, M.A.; Lichtenberg, A.J. Principles of Plasma Discharges and Materials Processing; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Coburn, J.W. Plasma Etching and Reactive Ion Etching; American Vacuum Society: New York, NY, USA; American Institute of Physics Inc.: College Park, MD, USA, 1982. [Google Scholar]
- Sugano, T.; Kim, H.-G. Applications of Plasma Processes to VLSI Technology; Wiley-Interscience: Hoboken, NJ, USA, 1985. [Google Scholar]
- Reece Roth, J. Industrial Plasma Engineering; IOP: Bristol, UK, 1995. [Google Scholar]
- Wolf, S. Silicon Processing for the VLSI Era; LATTICE: Portland, OR, USA, 1995; pp. 559–581. [Google Scholar]
- Van Roosmalen, A.J.; Baggerman, J.; Brader, S. Dry Etching for VLSI; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Schaepkens, M.; Standaert, T.; Rueger, N.; Sebel, P.; Oehrlein, G.; Cook, J. Study of the SiO2-to-Si3N4 Etch Selectivity Mechanism in Inductively Coupled Fluorocarbon Plasmas and a Comparison with the SiO2-to-Si. J. Vac. Sci. Technol. A 1999, 17, 26. [Google Scholar] [CrossRef]
- Standaert, T.; Hedlund, C.; Joseph, E.; Oehrlein, G.; Dalton, T. Role of fluorocarbon film formation in the etching of silicon, silicon dioxide, silicon nitride, and amorphous hydrogenated silicon carbide. J. Vac. Sci. Technol. A 2004, 22, 53–60. [Google Scholar] [CrossRef]
- Lee, H.K.; Chung, K.S.; Yu, J.S. Selective etching of thick Si3N4, SiO2 and Si by using CF4/O2 and C2F6 gases with or without O2 or Ar addition. J. Korean Phys. Soc. 2009, 54, 1816–1823. [Google Scholar] [CrossRef]
- Kastenmeier, B.; Matsuo, P.; Oehrlein, G. Highly selective etching of silicon nitride over silicon and silicon dioxide. J. Vac. Sci. Technol. A 1999, 17, 3179–3184. [Google Scholar] [CrossRef]
- Lele, C.; Liang, Z.; Linda, X.; Dongxia, L.; Hui, C.; Tod, P. Role of CF2 in the etching of SiO2, Si3N4 and Si in fluorocarbon plasma. J. Semicond. 2009, 30, 033005. [Google Scholar] [CrossRef]
- Matsui, M.; Tatsumi, T.; Sekine, M. Relationship of etch reaction and reactive species flux in C4F8/Ar/O2 plasma for SiO2 selective etching over Si and Si3N4. J. Vac. Sci. Technol. A 2001, 19, 2089–2096. [Google Scholar] [CrossRef]
- Li, X.; Ling, L.; Hua, X.; Fukasawa, M.; Oehrlein, G.S.; Barela, M.; Anderson, H.M. Effects of Ar and O 2 additives on SiO2 etching in C4F8-based plasmas. J. Vac. Sci. Technol. A 2003, 21, 284–293. [Google Scholar] [CrossRef]
- Li, X.; Ling, L.; Hua, X.; Oehrlein, G.S.; Wang, Y.; Anderson, H. Characteristics of C4F8 plasmas with Ar, Ne, and He additives for SiO2 etching in an inductively coupled plasma (ICP) reactor. J. Vac. Sci. Technol. A 2003, 21, 1955–1963. [Google Scholar] [CrossRef]
- Sankaran, A.; Kushner, M.J. Etching of porous and solid SiO2 in Ar∕c-C4F8, O2∕c-C4F8 and Ar∕O2∕c-C4F8 plasmas. J. Appl. Phys. 2005, 97, 023307. [Google Scholar] [CrossRef]
- Stoffels, W.; Stoffels, E.; Tachibana, K. Polymerization of fluorocarbons in reactive ion etching plasmas. J. Vac. Sci. Technol. A 1998, 16, 87–95. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2004; Volume 85. [Google Scholar]
- Padiyath, R.; Wright, R.L.; Chaudhry, M.; Babu, S. Reactive ion etching of monocrystalline, polycrystalline, and amorphous silicon carbide in CF4/O2 mixtures. Appl. Phys. Lett. 1991, 58, 1053–1055. [Google Scholar] [CrossRef]
- Cao, L.; Li, B.; Zhao, J.H. Etching of SiC using inductively coupled plasma. J. Electrochem. Soc. 1998, 145, 3609. [Google Scholar] [CrossRef]
- Kimura, T.; Noto, M. Experimental study and global model of inductively coupled CF4∕O2 discharges. J. Appl. Phys. 2006, 100, 063303. [Google Scholar] [CrossRef]
- Venkatesan, S.P.; Trachtenberg, I.; Edgar, T.F. Modeling of silicon etching in CF4/O2 and CF4/H2 plasmas. J. Electrochem. Soc. 1990, 137, 2280. [Google Scholar] [CrossRef]
- Schoenborn, P.; Patrick, R.; Baltes, H.P. Numerical simulation of a CF4/O2 plasma and correlation with spectroscopic and etch rate data. J. Electrochem. Soc. 1989, 136, 199. [Google Scholar] [CrossRef]
- Hong, J.; Shul, R.; Zhang, L.; Lester, L.; Cho, H.; Hahn, Y.; Hays, D.; Jung, K.; Pearton, S.; Zetterling, C.-M.; et al. Plasma chemistries for high density plasma etching of SiC. J. Electron. Mater. 1999, 28, 196–201. [Google Scholar] [CrossRef]
- Ayari-Kanoun, A.; Jaouad, A.; Souifi, A.; Drouin, D.; Beauvais, J. Silicon nitride nanotemplate fabrication using inductively coupled plasma etching process. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. Mater. Process. Measur. Phenom. 2011, 29, 051802. [Google Scholar] [CrossRef]
- Chen, L.; Xu, L.; Li, D.; Lin, B. Mechanism of selective Si3N4 etching over SiO2 in hydrogen-containing fluorocarbon plasma. Microelectron. Eng. 2009, 86, 2354–2357. [Google Scholar] [CrossRef]
- Kastenmeier, B.; Matsuo, P.; Beulens, J.; Oehrlein, G. Chemical dry etching of silicon nitride and silicon dioxide using CF4/O2/N2 gas mixtures. J. Vac. Sci. Technol. A 1996, 14, 2802–2813. [Google Scholar] [CrossRef]
- Efremov, A.; Lee, J.; Kim, J. On the control of plasma parameters and active species kinetics in CF4+ O2+Ar gas mixture by CF4/O2 and O2/Ar mixing ratios. Plasma Chem. Plasma Process. 2017, 37, 1445–1462. [Google Scholar] [CrossRef]
- Lee, B.J.; Efremov, A.; Nam, Y.; Kwon, K.-H. Plasma Parameters and Silicon Etching Kinetics in C4F8+ O2+Ar Gas Mixture: Effect of Component Mixing Ratios. Plasma Chem. Plasma Process. 2020, 40, 1365–1380. [Google Scholar] [CrossRef]
- Lee, J.; Efremov, A.; Yeom, G.Y.; Lim, N.; Kwon, K.-H. Application of Si and SiO2 etching mechanisms in CF4/C4F8/Ar inductively coupled plasmas for nanoscale patterns. J. Nanosci. Nanotechnol. 2015, 15, 8340–8347. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, J.; Efremov, A.; Kwon, K.-H. On the relationships between plasma chemistry, etching kinetics and etching residues in CF4+ C4F8+Ar and CF4+CH2F2+Ar plasmas with various CF4/C4F8 and CF4/CH2F2 mixing ratios. Vacuum 2018, 148, 214–223. [Google Scholar] [CrossRef]
- Lim, N.; Efremov, A.; Kwon, K.-H. Gas-phase chemistry and etching mechanism of SiNx thin films in C4F8+ Ar inductively coupled plasma. Thin Solid Films 2019, 685, 97–107. [Google Scholar] [CrossRef]
- Son, J.; Efremov, A.; Chun, I.; Yeom, G.Y.; Kwon, K.-H. On the LPCVD-formed SiO2 etching mechanism in CF4/Ar/O2 inductively coupled plasmas: Effects of gas mixing ratios and gas pressure. Plasma Chem. Plasma Process. 2014, 34, 239–257. [Google Scholar] [CrossRef]
- Lee, B.J.; Efremov, A.; Lee, J.; Kwon, K.-H. Etching Kinetics and Mechanisms of SiC Thin Films in F-, Cl-and Br-Based Plasma Chemistries. Plasma Chem. Plasma Process. 2019, 39, 325–338. [Google Scholar] [CrossRef]
- Efremov, A.; Murin, D.; Betelin, V.; Kwon, K.-H. Special Aspects of the Kinetics of Reactive Ion Etching of SiO2 in Fluorine-, Chlorine-, and Bromine-Containing Plasma. Rus. Microelectron. 2020, 49, 94–102. [Google Scholar] [CrossRef]
- Efremov, A.; Murin, D.; Kwon, K.-H. On the effect of the ratio of concentrations of fluorocarbon components in a CF4+ C4F8+ Ar mixture on the parameters of plasma and SiO2/Si etching selectivity. Rus. Microelectron. 2018, 47, 239–246. [Google Scholar] [CrossRef]
- Shun’ko, E.V. Langmuir Probe in Theory and Practice; Universal-Publishers: Irvine, CA, USA, 2009. [Google Scholar]
- Johnson, E.; Malter, L. A floating double probe method for measurements in gas discharges. Phys. Rev. 1950, 80, 58. [Google Scholar] [CrossRef]
- Seo, J.K.; Ko, K.-h.; Choi, W.S.; Park, M.; Lee, J.H.; Yi, J.-S. The effect of deposition RF power on the SiC passivation layer synthesized by an RF magnetron sputtering method. J. Cryst. Growth 2011, 326, 183–185. [Google Scholar]
- Efremov, A.; Lee, J.; Kwon, K.-H. A comparative study of CF4, Cl2 and HBr+Ar inductively coupled plasmas for dry etching applications. Thin Solid Films 2017, 629, 39–48. [Google Scholar] [CrossRef]
- Chun, I.; Efremov, A.; Yeom, G.Y.; Kwon, K.-H. A comparative study of CF4/O2/Ar and C4F8/O2/Ar plasmas for dry etching applications. Thin Solid Films 2015, 579, 136–143. [Google Scholar] [CrossRef]
- Efremov, A.; Kim, D.-P.; Kim, C.-I. Effect of gas mixing ratio on gas-phase composition and etch rate in an inductively coupled CF4/Ar plasma. Vacuum 2004, 75, 133–142. [Google Scholar] [CrossRef]
- Lim, N.; Efremov, A.; Yeom, G.Y.; Kwon, K.-H. On the etching characteristics and mechanisms of HfO2 thin films in CF4/O2/Ar and CHF3/O2/Ar plasma for nano-devices. J. Nanosci. Nanotechnol. 2014, 14, 9670–9679. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Efremov, A.; Kwon, K.-H.; Morgunov, A.; Shabadarova, D. Comparative study of CF4-and CHF3-based plasmas for dry etching applications. In Proceedings of the SPIE 20224, International Conference on Micro- and Nano-Electronics 2016, Zvenigorod, Russia, 2–7 October; 2016. 102241W. [Google Scholar]
- Efremov, A.; Murin, D.; Kwon, K.-H. Plasma Parameters and Kinetics of Active Particles in the Mixture CHF3+ O2+ Ar. Rus. Microelectron. 2020, 49, 233–243. [Google Scholar] [CrossRef]
- Efremov, A.; Murin, D.; Kwon, K.-H. Parameters of plasma and kinetics of active particles in CF4 (CHF3)+ Ar mixtures of a variable initial composition. Rus. Microelectron. 2018, 47, 371–380. [Google Scholar] [CrossRef]
- Efremov, A.; Murin, D.; Kwon, K.-H. Concerning the Effect of Type of Fluorocarbon Gas on the Output Characteristics of the Reactive-Ion Etching Process. Rus. Microelectron. 2020, 49, 157–165. [Google Scholar] [CrossRef]
- Kimura, T.; Ohe, K. Probe measurements and global model of inductively coupled Ar/CF4 discharges. Plasma Sources Sci. Technol. 1999, 8, 553. [Google Scholar] [CrossRef]
- Ho, P.; Johannes, J.E.; Buss, R.J.; Meeks, E. Modeling the plasma chemistry of C2F6 and CHF3 etching of silicon dioxide, with comparisons to etch rate and diagnostic data. J. Vac. Sci. Technol. A 2001, 19, 2344–2367. [Google Scholar] [CrossRef]
- Rauf, S.; Ventzek, P.L. Model for an inductively coupled Ar/c-C4F8 plasma discharge. J. Vac. Sci. Technol. A 2002, 20, 14–23. [Google Scholar] [CrossRef]
- Kokkoris, G.; Goodyear, A.; Cooke, M.; Gogolides, E. A global model for C4F8 plasmas coupling gas phase and wall surface reaction kinetics. J. Phys. D Appl. Phys. 2008, 41, 195211. [Google Scholar] [CrossRef]
- NIST Chemical Kinetics Database. 2019. Available online: https://kinetics.nist.gov/kinetics/index.jsp (accessed on 15 January 2021).
- Vasenkov, A.V.; Li, X.; Oehrlein, G.S.; Kushner, M.J. Properties of c-C4F8 inductively coupled plasmas. II. Plasma chemistry and reaction mechanism for modeling of Ar/c-C4F8/O2 discharges. J. Vac. Sci. Technol. A 2004, 22, 511–530. [Google Scholar] [CrossRef]
- Winters, H.F.; Coburn, J.; Chuang, T. Surface processes in plasma-assisted etching environments. J. Vac. Sci. Technol. B Microelectron. Process. Phenom. 1983, 1, 469–480. [Google Scholar] [CrossRef]
- Gray, D.C.; Tepermeister, I.; Sawin, H.H. Phenomenological modeling of ion-enhanced surface kinetics in fluorine-based plasma etching. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 1993, 11, 1243–1257. [Google Scholar] [CrossRef]
- Chapman, B.N. Glow Discharge Processes: Sputtering and Plasma Etching; Wiley: Hoboken, NJ, USA, 1980. [Google Scholar]
- Rakhimova, T.V.; Braginsky, O.V.; Klopovskiy, K.S.; Kovalev, A.S.; Lopaev, D.V.; Proshina, O.V.; Rakhimov, A.T.; Shamiryan, D.; Vasilieva, A.N.; Voloshin, D.G. Experimental and Theoretical Studies of Radical Production in RF CCP Discharge at 81-MHz Frequency in Ar/CF4 and Ar/CHF3 Mixtures. IEEE Trans. Plasma Sci. 2009, 37, 1683–1696. [Google Scholar] [CrossRef]
- Proshina, O.; Rakhimova, T.; Zotovich, A.; Lopaev, D.; Zyryanov, S.; Rakhimov, A. Multifold study of volume plasma chemistry in Ar/CF4 and Ar/CHF3 CCP discharges. Plasma Sources Sci. Technol. 2017, 26, 075005. [Google Scholar] [CrossRef]
- Kimura, T.; Ohe, K. Model and probe measurements of inductively coupled CF4 discharges. J. Appl. Phys. 2002, 92, 1780–1787. [Google Scholar] [CrossRef]
- Takahashi, K.; Hori, M.; Goto, T. Characteristics of fluorocarbon radicals and CHF3 molecule in CHF3 electron cyclotron resonance downstream plasma. Jpn. J. Appl. Phys. 1994, 33, 4745. [Google Scholar] [CrossRef]
- Sasaki, K.; Kawai, Y.; Kadota, K. Determination of fluorine atom density in reactive plasmas by vacuum ultraviolet absorption spectroscopy at 95.85 nm. Rev. Sci. Instrum. 1999, 70, 76–81. [Google Scholar] [CrossRef]
- Lee, C.; Graves, D.; Lieberman, M.A. Role of etch products in polysilicon etching in a high-density chlorine discharge. Plasma Chem. Plasma Process. 1996, 16, 99–120. [Google Scholar] [CrossRef]
- Efremov, A.M.; Kim, D.-P.; Kim, C.-I. Simple model for ion-assisted etching using Cl2-Ar inductively coupled plasma: Effect of gas mixing ratio. IEEE Trans. Plasma Sci. 2004, 32, 1344–1351. [Google Scholar] [CrossRef]
- Biyikli, N.; Haider, A.; Deminskyi, P.; Yilmaz, M. Self-aligned nanoscale processing solutions via selective atomic layer deposition of oxide, nitride, and metallic films. In Low-Dimensional Materials and Devices 2017; International Society for Optics and Photonics: Bellingham, WA, USA, 2017; p. 103490M. [Google Scholar]
- Cunge, G.; Kogelschatz, M.; Joubert, O.; Sadeghi, N. Plasma–wall interactions during silicon etching processes in high-density HBr/Cl2/O2 plasmas. Plasma Sources Sci. Technol. 2005, 14, S42. [Google Scholar] [CrossRef]
- Tinck, S.; Boullart, W.; Bogaerts, A. Modeling Cl2/O2/Ar inductively coupled plasmas used for silicon etching: Effects of SiO2 chamber wall coating. Plasma Sources Sci. Technol. 2011, 20, 045012. [Google Scholar] [CrossRef]
- Lee, B.J.; Efremov, A.; Kim, J.; Kim, C.; Kwon, K.-H. Peculiarities of Si and SiO2 Etching Kinetics in HBr+Cl2+O2 Inductively Coupled Plasma. Plasma Chem. Plasma Process. 2019, 39, 339–358. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Efremov, A.; Kim, C.; Lee, H.W.; Kwon, K.-H. Etching mechanisms and surface conditions for SiOxNy thin films in CF4+CHF3+O2 inductively coupled plasma. Plasma Chem. Plasma Process. 2019, 39, 1127–1144. [Google Scholar] [CrossRef]
- Nam, Y.; Efremov, A.; Lee, B.J.; Kwon, K.-H. Plasma Parameters and Etching Characteristics of SiOxNy Films in CF4+O2+ X (X= C4F8 or CF2Br2) Gas Mixtures. Materials 2020, 13, 5476. [Google Scholar] [CrossRef] [PubMed]
| CF4 + Ar | CHF3 + Ar | C4F8 + Ar | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Te (eV) | n+ (cm−3) | −Udc (V) | Te (eV) | n+ (cm−3) | −Udc (V) | Te (eV) | n+ (cm−3) | −Udc (V) | |
| 0 | 3.6 | 4.4 × 1010 | 262 | 5.2 | 5.1 × 1010 | 230 | 4.7 | 3.9 × 1010 | 278 |
| 25 | 3.6 | 4.5 × 1010 | 249 | 4.9 | 5.7 × 1010 | 205 | 4.5 | 4.4 × 1010 | 249 |
| 75 | 3.8 | 5.8 × 1010 | 188 | 4.7 | 6.9 × 1010 | 180 | 3.8 | 8.3 × 1010 | 198 |
(%) | Te (eV) | J+ (mA/cm2) | n+ ≈ ne (cm−3) | −Udc (V) | (eV) | |
|---|---|---|---|---|---|---|
| 0 | 3.5 | 0.73 | 2.9 × 1010 | 121 | 141 | 5.4 × 1016 |
| 25 | 3.9 | 0.83 | 3.3 × 1010 | 148 | 169 | 6.8 × 1016 |
| 50 | 4.3 | 1.21 | 4.0 × 1010 | 165 | 190 | 1.0 × 1017 |
| CF4 + O2 + Ar | CHF3 + O2 + Ar | C4F8 + O2 + Ar | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Te (eV) | n+ (cm−3) | −Udc (V) | Te (eV) | n+ (cm−3) | −Udc (V) | Te (eV) | n+ (cm−3) | −Udc (V) | |
| 0 | 3.6 | 4.9 × 1010 | 215 | 4.8 | 6.2 × 1010 | 190 | 4.8 | 4.4 × 1010 | 212 |
| 25 | 3.5 | 3.9 × 1010 | 239 | 3.9 | 3.9 × 1010 | 234 | 4.0 | 4.0 × 1010 | 238 |
| 50 | 3.4 | 3.2 × 1010 | 250 | 3.0 | 3.0 × 1010 | 254 | 3.1 | 3.7 × 1010 | 269 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efremov, A.; Lee, B.J.; Kwon, K.-H. On Relationships between Gas-Phase Chemistry and Reactive Ion Etching Kinetics for Silicon-Based Thin Films (SiC, SiO2 and SixNy) in Multi-Component Fluorocarbon Gas Mixtures. Materials 2021, 14, 1432. https://doi.org/10.3390/ma14061432
Efremov A, Lee BJ, Kwon K-H. On Relationships between Gas-Phase Chemistry and Reactive Ion Etching Kinetics for Silicon-Based Thin Films (SiC, SiO2 and SixNy) in Multi-Component Fluorocarbon Gas Mixtures. Materials. 2021; 14(6):1432. https://doi.org/10.3390/ma14061432
Chicago/Turabian StyleEfremov, Alexander, Byung Jun Lee, and Kwang-Ho Kwon. 2021. "On Relationships between Gas-Phase Chemistry and Reactive Ion Etching Kinetics for Silicon-Based Thin Films (SiC, SiO2 and SixNy) in Multi-Component Fluorocarbon Gas Mixtures" Materials 14, no. 6: 1432. https://doi.org/10.3390/ma14061432
APA StyleEfremov, A., Lee, B. J., & Kwon, K.-H. (2021). On Relationships between Gas-Phase Chemistry and Reactive Ion Etching Kinetics for Silicon-Based Thin Films (SiC, SiO2 and SixNy) in Multi-Component Fluorocarbon Gas Mixtures. Materials, 14(6), 1432. https://doi.org/10.3390/ma14061432






