Dry Etching Performance and Gas-Phase Parameters of C6F12O + Ar Plasma in Comparison with CF4 + Ar
Abstract
1. Introduction
- (1)
- The dominant role in the chemical etching pathway for Si and SiO2 under typical RIE conditions ( < 50 mTorr, ion bombardment energy ~200–400 eV) belongs to F atoms [5]. Fluorocarbon gases with < 3 (where and are coefficients in the CxHyFz formula) exhibit high polymerization ability that results in the deposition of fluorocarbon polymer film on the treated surface. This lowers absolute etching rates but results in the highly-anisotropic etching of silicon (due to the passivation of side walls by the fluorocarbon polymer layer) as well as in advanced SiO2/Si etching selectivity (due to different thicknesses of polymer films on oxygen-free and oxygen-containing surfaces) [8,9,11].
- (2)
- Both etching and polymerization kinetics may be effectively adjusted by mixing of fluorocarbon gas with Ar and/or O2 [10,13,14,15,16]. Corresponding mechanisms do work through changes in both gas-phase chemistry (formation/decay balance for F atoms and polymerizing radicals) [5,17] and heterogeneous processes kinetics (physical and chemical decomposition of the fluorocarbon polymer film) [14,15,16,17].
- (3)
- The chemical interaction of F atoms with Si and SiO2 exhibits different mechanisms and thus, may be controlled by different limiting stages. In the case of Si, spontaneous chemical reaction mostly produces the high volatile SiF4 [4,5]. That is why the Si + F reaction rate in non- or low-polymerizing plasmas is characterized by low sensitivity to the intensity of ion bombardment as well as exhibits the nearly exponential dependence on surface temperature [2,3]. Oppositely, the SiO2 + F reaction has the sufficient threshold energy (as the Si-O bond of ~799 kJ/mol is stronger than the Si-F of ~552 kJ/mol [18]) and occurs only as the ion-assisted process. The role of ion bombardment includes the production of adsorption sites for F atoms and the sputtering of low volatile non-saturated SiFx compounds [5,7]. At the same time, ion energies above ~200 eV are generally enough to provide the reaction-rate-limited etching regime controlled by the F atom flux [7,19].
2. Materials and Methods
2.1. Experimental Setup and Procedures
2.2. Approaches for the Analysis of Etching Kinetics
- (1)
- Under typical reactive-ion etching conditions ( < 50 mTorr and > 200 V that provide an excess of ion bombardment energy over sputtering thresholds [3,4,5,6] for target materials), the experimentally obtained etching rate is the superposition of two parallel etching pathways, such as physical sputtering and ion-assisted chemical reactions. Accordingly, one can simply suggest [5,36,37].
- (2)
- The rate of physical sputtering, , may be found as [5,36], where ~ [30,32,33,34] is the sputtering yield, is the ion bombardment energy, is the floating potential, and is the flux of positive ions. As such, the relative change in with variations of processing conditions may be traced by the parameter characterizing the ion momentum flux [32,33,34].
- (3)
- The rate of ion-assisted chemical reaction, , is represented by the multiplication of [17,30,34], where [17] is the effective reaction probability, is the sticking probability for etchant species on the free adsorption site, and is the fraction of adsorption sites occupied by reaction products, and is the thermal flux of F atoms with the gas-phase density of . In general case, the situation → 0 corresponds to the reaction-rate-limited process regime where is only the exponential function of surface temperature. Oppositely, the condition → 1 points out on the ion-flux-limited process regime. Here, even if the nearly constant surface temperature provides ≈ const, the trend of is controlled by the change in through the fraction of free adsorption sites for F atoms . In polymerizing plasmas, may also be sensitive to fluorocarbon polymer thickness if the latter provides << 1, where is the flux of F atoms on the polymer film/etched surface interface [5,9,13].
3. Results and Discussion
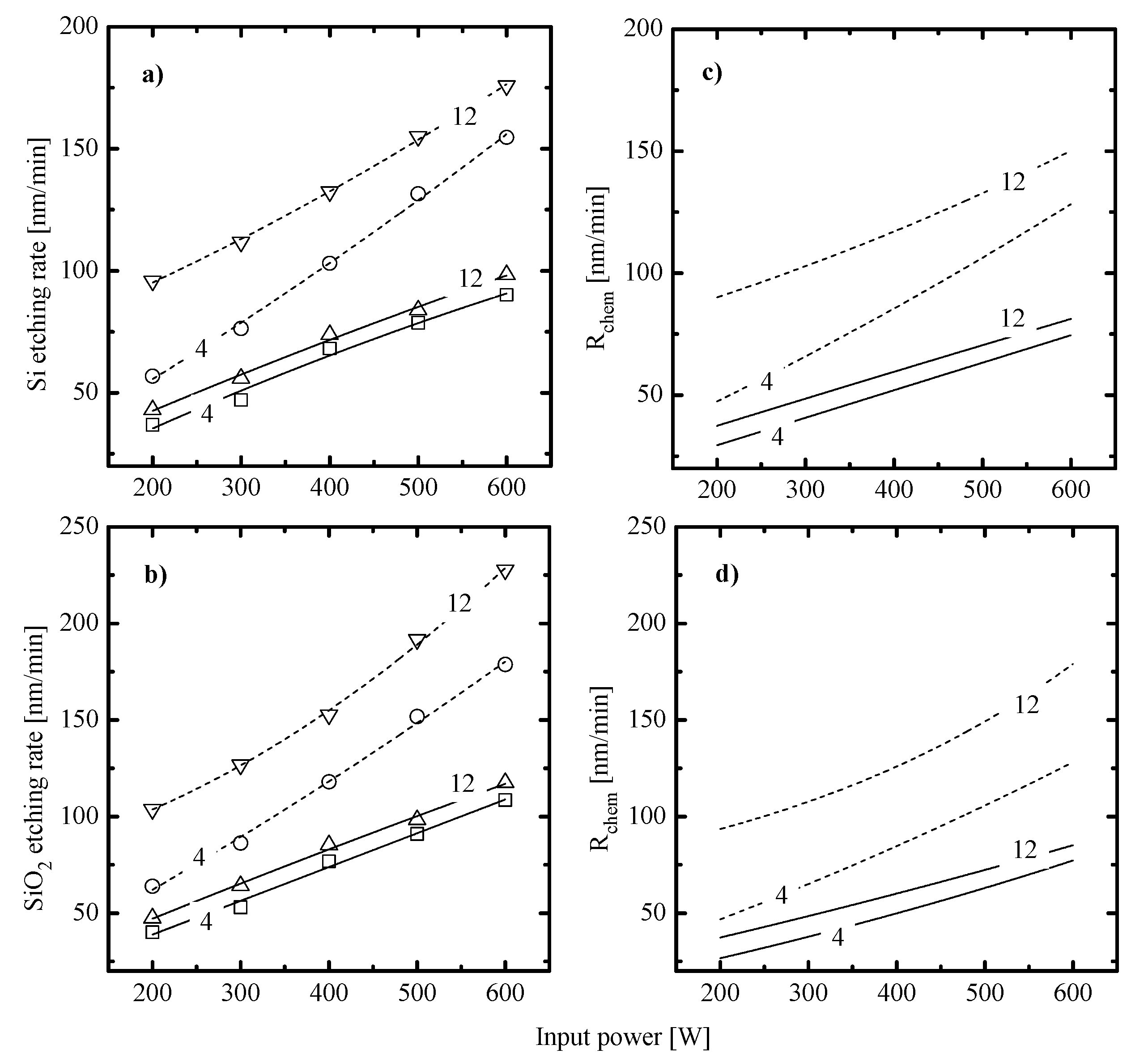
- (1)
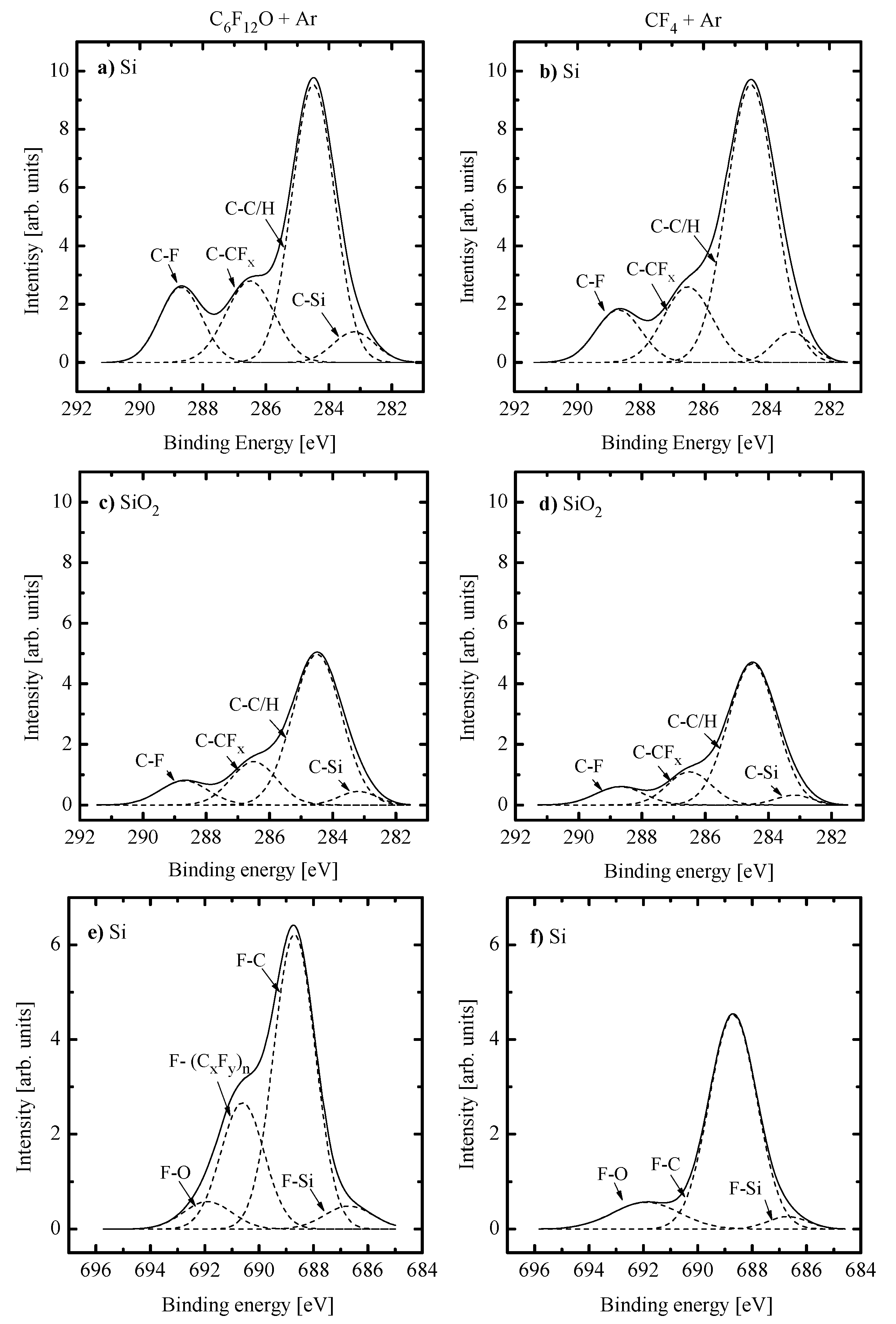
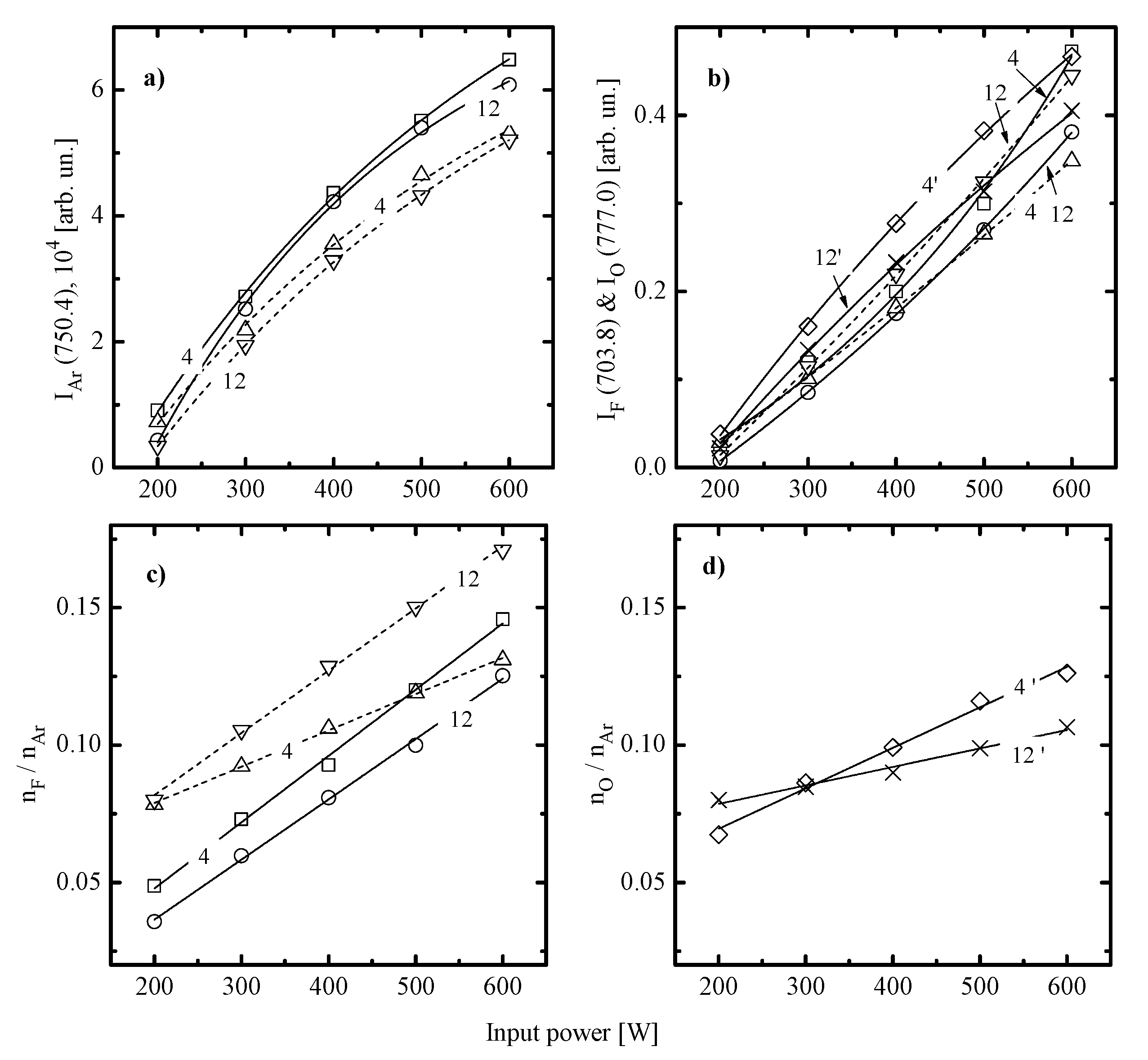
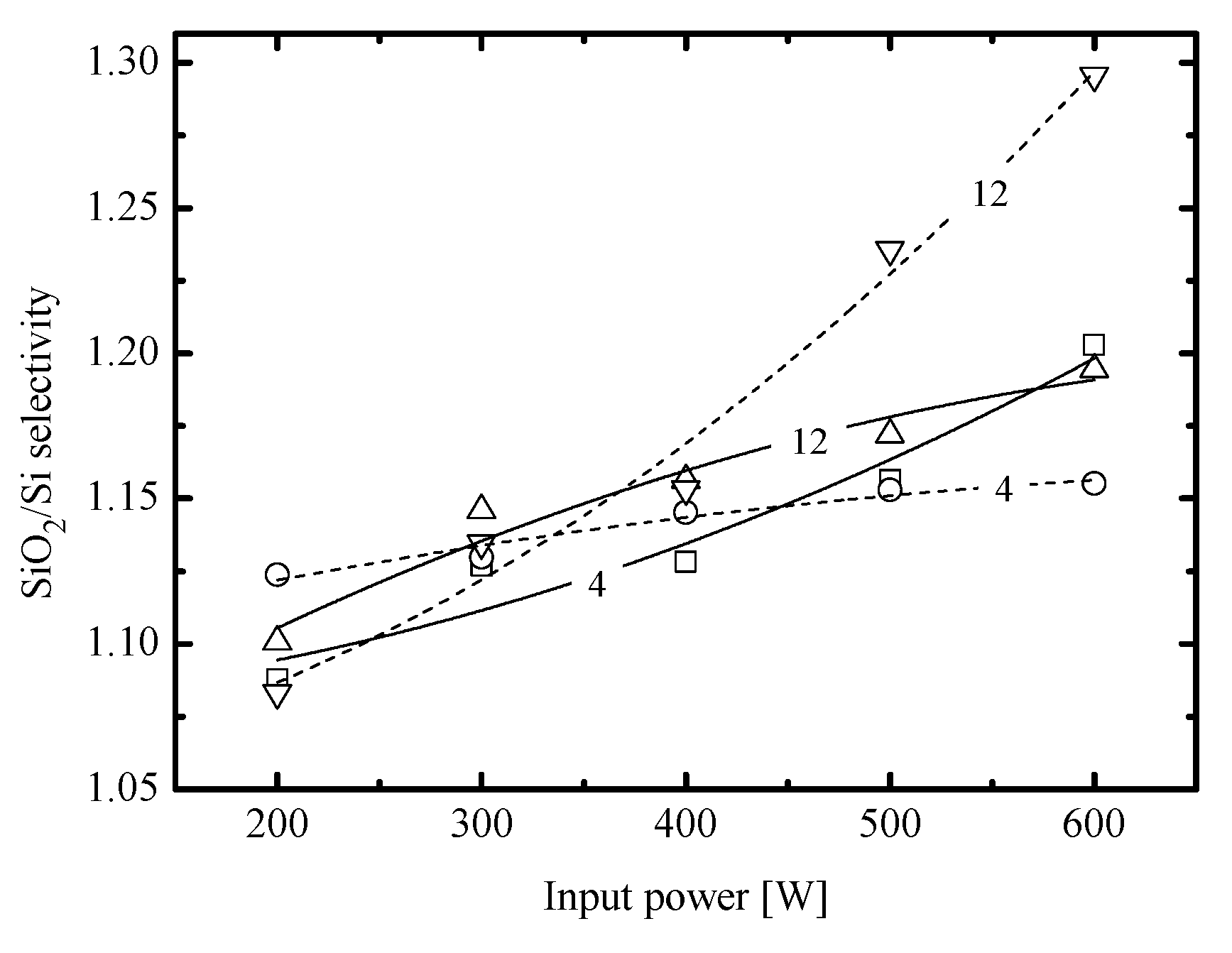
- Similar changes of etching rate for each material in C6F12O- and CF4-based plasmas vs. input power and gas pressure may be attributed to similar etching regimes. In general, this may be either the polymer-thickness-controlled etching process (through the transport of etchant species to the film/etched surface interface) or the chemical reaction itself under the condition of thin or even non-continuous polymer film. In our case, the second variant looks more favorable because of the low polymerizing ability of CF4 plasma [3,4,5,7,8,17] as well as the similar feature of the C6F12O + Ar gas system. As follows from Figure 3a–d, the latter exhibits the only small increase in the amount of carbon-containing compounds on both Si and SiO2 surfaces. In addition, one can see that both gas systems provide the lower amount of fluorocarbon polymer on SiO2 compared with that on Si. This well-known effect is due to the etching of polymer by O atoms on the film/SiO2 interface [5,6,7,8].
- (2)
- Similar changes of Si and SiO2 etching rates in each gas system indicate that corresponding chemical etching pathways are driven by identical active species and have one and the same limiting stage. In particular, an increase in both Si and SiO2 etching rates vs. gas pressure surely points out on the absence of ions-driven limiting stages in corresponding heterogeneous reaction schemes. In fact, this means that the SiO2 + F reaction kinetics is not limited by the ion-assisted destruction of oxide bonds SiOx(s.) → Si(s.) + xO to produce adsorption sites for F atoms. Probably, such situation is due to the quite high ion bombardment energy used in this study. As such, all experimental curves in Figure 2a,b reflect changes in the Si(s.) + xF → SiFx reaction kinetics while systematically lower etching rates in C6F12O + Ar plasma may preliminary be attributed to corresponding differences in gas-phase densities of F atoms.
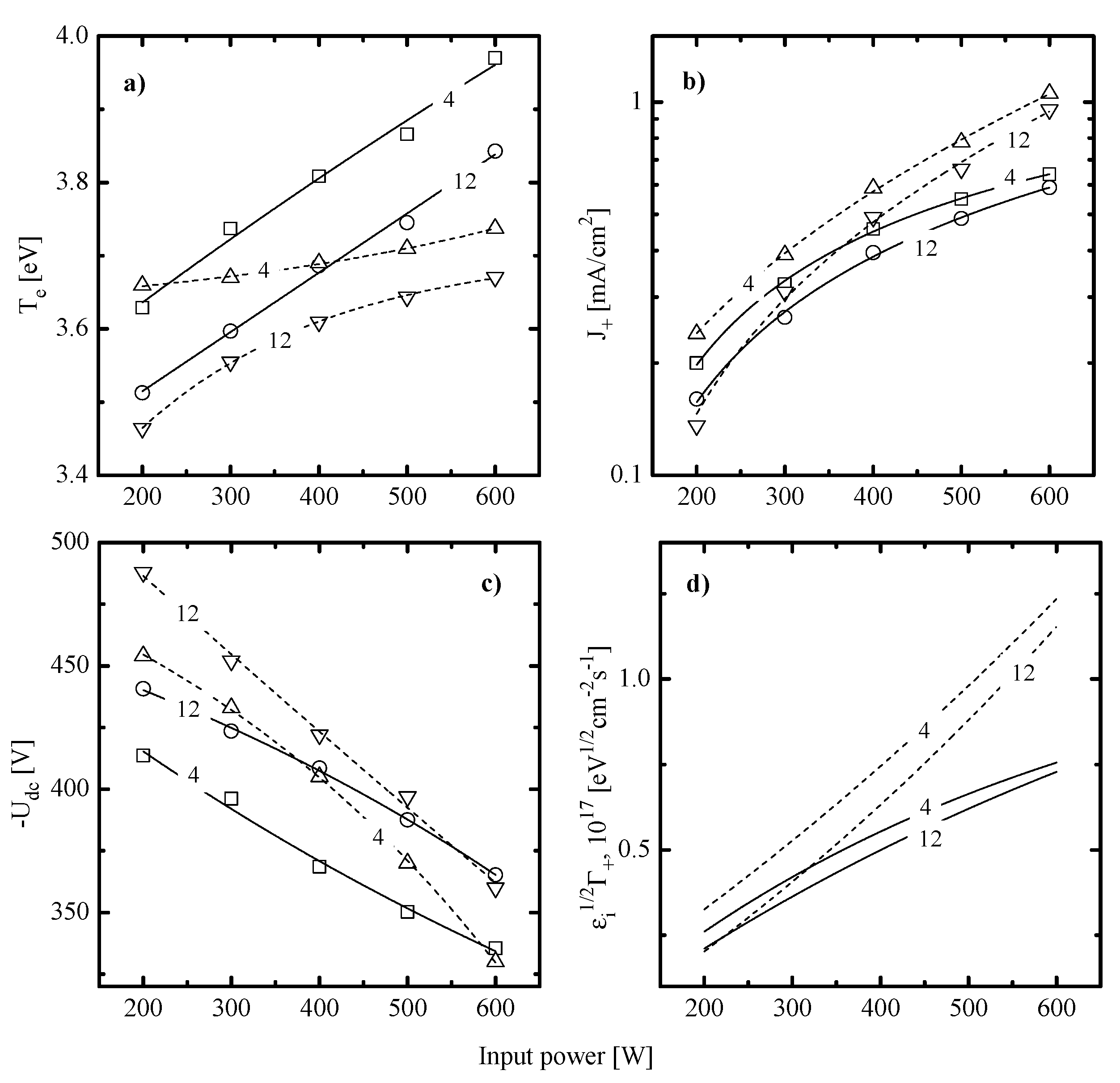
- The electron temperature (Figure 4a) increases toward higher input powers at = const and decreases toward higher pressures at = const. The first phenomenon is probably due to a decrease in electron energy losses for vibrational and electronic excitations of dominant neutral particles. The evident reason is an increase in densities of less saturated radicals and atomic species due to the acceleration of electron-impact dissociation for multi-atomic components. The faster growth of in the C6F12O + Ar plasma as well as higher electron temperatures at > 400 W may result from the multichannel fragmentation mechanism with including CxFy + O/O(1D) → Cx-nFy-mO + CnFm and CFx + O/O(1D) → CFx-1O + F reactions [32,33]. As such, one can easily imagine the situation when the given gas system provides higher densities of less saturated species compared with CF4 + Ar under identical processing conditions. The decreasing tendency for is surely connected with an increase in the overall electron energy loss due to increasing electron-neutral collision frequency [5].
- The ion current density (Figure 4b) in both gas systems mainly reflects the change of and thus, depends on the positive ion formation/decay balance. Particularly, an increase in at = const surely results in increasing total ionization rates and thus, causes the same response from the side of . It should be noted that a bit lower values in the C6F12O + Ar plasma at > 400 W are in formal agreement with above suggestion on higher densities of less saturated species. At least, one can simply assume that the smaller particle is featured by the lower ionization rate coefficients because of the lower process cross-section and/or higher ionization threshold . An increase in gas pressure at = const results in decreasing ionization rate coefficients (because of the same change in and mean electron energy that provides decreasing fraction of electrons with > ) as well as accelerates the loss of positive ions in bulk plasma (because of increasing plasma electronegativity and negative ion density). As such, the decreasing tendency for is probably due to corresponding changes in both and ion Bohm velocity.
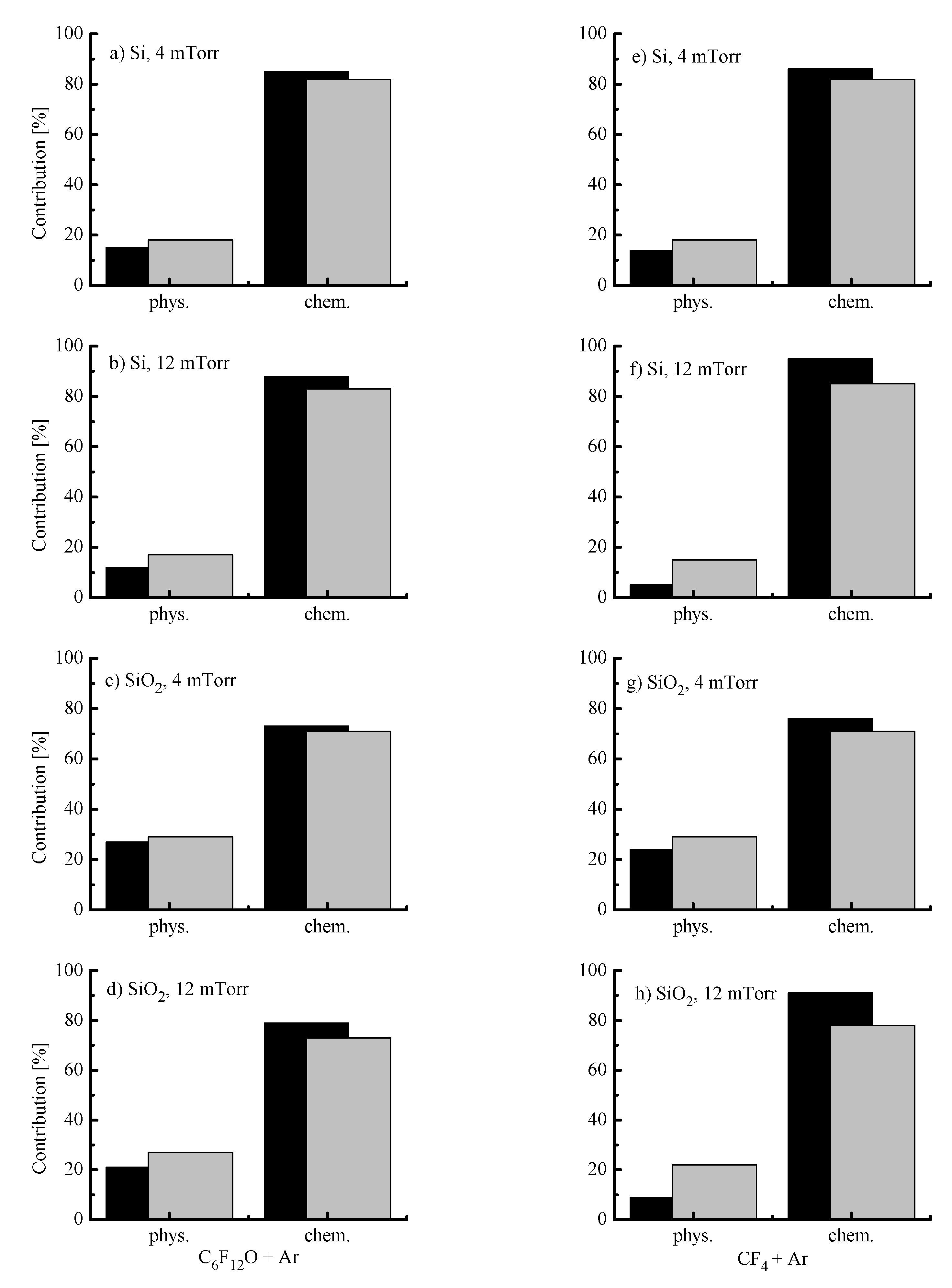
- The negative dc bias at constant bias power (Figure 4c) always shows opposite trends compared with . The reason is that the positive ion flux partly compensates for the negative charge produced by bias source. In both gas systems, the growth of ion flux vs. input power overlaps the weaker decrease in and provides the intensification of the physical etching pathway. The last conclusion directly follows from the change in values shown in Figure 4d. Accordingly, the combination of decreasing ion flux and the nearly constant with increasing gas pressure lowers the ion bombardment intensity and thus, suppresses the physical etching pathway. Similar effects have been reported for various gas systems [2,5,6].
- (1)
- In both gas systems, the behavior of qualitatively correlates with the corresponding trend of F atom density while lower Si and SiO2 etching rates in the C6F12O + Ar plasma generally correspond to lower values. This directly points to a similar limiting stage in a form of Si(s.) + xF → SiFx reaction as well as probably means the non-principal influence of fluorocarbon polymer film on the etching kinetics.
- (2)
- In both gas systems, the change in appears to be quantitatively different than that for F atom density. In particular, the CF4 + Ar plasma at the low-pressure represents the special case when for both Si and SiO2 increases faster compared with F atom density. Such situation corresponds to an increase in effective reaction probability that correlates with the behavior of . This allows one to suggest that, under the given set of processing conditions, an increase in ion momentum flux accelerates chemical reaction. At the same time, the high-pressure CF4 + Ar plasma, as well as the C6F12O + Ar plasma at any pressure within 4–12 mTorr, demonstrate the slower increase in compared with F atom density. Accordingly, one can speak about decreasing effective reaction probability that contradicts with changes in ion momentum fluxes. In order to explain this phenomenon, one can simply suggest an increase in the amount of deposited polymer (which can be a true if the flux of polymerizing radicals growths faster compared with ) or heterogeneous reactions with a participation of oxygen atoms. The last mechanism may suppress in C6F12O + Ar plasma through the formation of oxide bonds and blocking of adsorption sites for F atoms.
- (3)
- The situation ≠ const obtained for all processing gases and conditions at constant surface temperature generally means the sensitivity of to gas-phase plasma parameters, such as particle and/or energy fluxes. Here, though the main trend for is determined by the F atom flux, the change in affects the slope and/or the shape of corresponding curve and thus, provides the ability for additional process control. As such, the understanding of factors influencing is the key point for understanding the etching mechanism itself.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sze, S.M. VLSI Technology; McGraw-Hill: New York, NY, USA, 1988. [Google Scholar]
- Sugano, T. Applications of Plasma Processes to VLSI Technology; John Wiley & Sons Inc.: Hoboken, NJ, USA, 1990. [Google Scholar]
- Rooth, J.R. Industrial Plasma Engineering; IOP Publishing Ltd.: Philadelphia, PA, USA, 1995. [Google Scholar]
- Wolf, S.; Tauber, R.N. Silicon Processing for the VLSI Era; Lattice Press: New York, NY, USA, 2000; Volume 1. [Google Scholar]
- Lieberman, M.A.; Lichtenberg, A.J. Principles of Plasma Discharges and Materials Processing; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Coburn, J.W. Plasma Etching and Reactive Ion Etching; AVS: New York, NY, USA, 1982. [Google Scholar]
- Stoffels, W.W.; Stoffels, E.; Tachibana, K. Polymerization of fluorocarbons in reactive ion etching plasmas. J. Vac. Sci. Technol. A 1998, 16, 87–95. [Google Scholar] [CrossRef]
- Schaepkens, M.M.; Standaert, T.E.F.M.; Rueger, N.R.; Sebel, P.P.; Oehrlein, G.G.; Cook, J.M. Study of the SiO2-to-Si3N4 etch selectivity mechanism in inductively coupled fluorocarbon plasmas and a comparison with the SiO2-to-Si mechanism. J. Vac. Sci. Technol. A 1999, 17, 26–37. [Google Scholar] [CrossRef]
- Standaert, T.E.F.M.; Hedlund, C.; Joseph, E.A.; Oehrlein, G.S.; Dalton, T.J. Role of fluorocarbon film formation in the etching of silicon, silicon dioxide, silicon nitride, and amorphous hydrogenated silicon carbide. J. Vac. Sci. Technol. A 2004, 22, 53. [Google Scholar] [CrossRef]
- Lee, H.K.; Chung, K.S.; Yu, J.S. Selective Etching of Thick Si3N4, SiO2 and Si by Using CF4/O2 and C2F6 Gases with or without O2 or Ar Addition. J. Korean Phys. Soc. 2009, 54, 1816–1823. [Google Scholar] [CrossRef]
- Kastenmeier, B.E.E.; Matsuo, P.J.; Oehrlein, G.S. Highly selective etching of silicon nitride over silicon and silicon dioxide. J. Vac. Sci. Technol. A 1999, 17, 3179–3184. [Google Scholar] [CrossRef]
- Lele, C.; Liang, Z.; Linda, X.; Dongxia, L.; Hui, C.; Tod, P. Role of CF2 in the etching of SiO2, Si3N4 and Si in fluorocarbon plasma. J. Semicond. 2009, 30, 033005. [Google Scholar] [CrossRef]
- Matsui, M.; Tatsumi, T.; Sekine, M. Relationship of etch reaction and reactive species flux in C4F8/Ar/O2 plasma for SiO2 selective etching over Si and Si3N4. J. Vac. Sci. Technol. 2001, A19, 2089–2096. [Google Scholar] [CrossRef]
- Li, X.; Ling, L.; Hua, X.; Fukasawa, M.; Oehrlein, G.S.; Barela, M.; Anderson, H.M. Effects of Ar and O2 additives on SiO2 etching in C4F8-based plasmas. J. Vac. Sci. Technol. 2003, A21, 284–293. [Google Scholar] [CrossRef]
- Li, X.; Hua, X.; Ling, L.; Oehrlein, G.S.; Wang, Y.; Anderson, H.M. Characteristics of C4F8 plasmas with Ar, Ne, and He additives for SiO2 etching in an inductively coupled plasma (ICP) reactor. J. Vac. Sci. Technol. 2003, A21, 1955–1963. [Google Scholar] [CrossRef]
- Sankaran, A.; Kushner, M.J. Etching of porous and solid SiO2 in Ar/c-C4F8, O2/c-C4F8 and Ar/O2/c-C4F8 plasmas. J. Appl. Phys. 2005, 97, 023307. [Google Scholar] [CrossRef]
- Lee, J.; Efremov, A.; Yeom, G.Y.; Lim, N.; Kwon, K.H. Application of Si and SiO2 Etching Mechanisms in CF4/C4F8/Ar Inductively Coupled Plasmas for Nanoscale Patterns. J. Nanosci. Nanotechnol. 2015, 15, 8340–8347. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lide, D.R. Handbook of Chemistry and Physics; CRC Press: New York, NY, USA, 1998. [Google Scholar]
- Van Roosmalen, A.J.; Baggerman, J.A.G.; Brader, S.J.H. Dry Etching for VLSI; Springer: Berlin/Heidelberg, Germany, 1991. [Google Scholar]
- Tran-Quinn, T.; Lakritz, M. Unsaturated Fluorocarbons in the Etching Process, Environmental Benefit, Technical Hurdles. In Proceedings of the 2008 IEEE/SEMI Advanced Semiconductor Manufacturing Conference, Cambridge, MA, USA, 5–7 May 2008; pp. 37–42. [Google Scholar] [CrossRef]
- Muhle, J.; Ganesan, A.L.; Miller, B.R.; Salameh, P.K.; Harth, C.M.; Greally, B.R.; Rigby, M.; Porter, L.W.; Steele, L.P.; Trudinger, C.M.; et al. Perfluorocarbons in the global atmosphere: Tetrafluoromethane, hexafluoroethane, and octafluoro-propane. Atmos. Chem. Phys. 2010, 10, 5145–5164. [Google Scholar] [CrossRef]
- Kiehlbauch, M.W.; Graves, D.B. Temperature resolved modeling of plasma abatement of perfluorinated compounds. J. Appl. Phys. 2001, 89, 2047–2057. [Google Scholar] [CrossRef]
- Bolaji, B.; Huan, Z. Ozone depletion and global warming: Case for the use of natural refrigerant—A review. Renew. Sustain. Energy Rev. 2013, 18, 49–54. [Google Scholar] [CrossRef]
- Krishnan, N.; Smati, R.; Raoux, S.; Dornfeld, D. Alternatives to reduce perfluorinated compound (PFC) emissions from semi-conductor dielectric etch processes: Meeting environmental commitments while minimizing costs. In Proceedings of the International Symposium on Electronics and the Environment (IEEE), Boston, MA, USA, 19–22 May 2003. [Google Scholar] [CrossRef]
- Mocella, M.T. PFC Emission Control Options for Plasma Processing Tools: A Current Assessment; Cambridge University Press: Cambridge, UK, 1996; Volume 447, pp. 29–34. [Google Scholar]
- Tian, S.; Zhang, X.; Wang, Y.; Rao, X.; Ye, F.; Li, Y.; Xiao, S. Partial discharge characteristics of C6F12O/CO2 mixed gas at power frequency AC voltage. AIP Adv. 2019, 9, 095057. [Google Scholar] [CrossRef]
- Lee, J.; Nam, Y.; Lee, J.; Lee, H.W.; Kwon, K.-H. Etching characteristics of thin SiON films using a liquefied perfluorocarbon precursor of C6F12O with a low global warming potential. Plasma Sci. Technol. 2020, 22, 105505. [Google Scholar] [CrossRef]
- Veselov, D.S.; Bakun, A.D.; Voronov, Y.A. Reactive ion etching of silicon using low-power plasma etcher. J. Phys. Conf. Ser. 2016, 748, 012017. [Google Scholar] [CrossRef]
- Ashraf, M.; Sundararajan, S.V.; Grenc, G. Low-power, low-pressure reactive-ion etching process for silicon etching with ver-tical and smooth walls for mechanobiology application. J. Micro Nanolith. MEMS MOEMS 2017, 16, 034501. [Google Scholar] [CrossRef]
- Lee, J.; Kwon, K.H.; Efremov, A. On the Relationships Between Plasma Chemistry, Etching Kinetics and Etching Residues in CF4 + C4F8 + Ar and CF4 + CH2F2 + Ar Plasmas with Various CF4/C4F8 and CF4/CH2F2 Mixing Ratios. Vacuum 2018, 148, 214–223. [Google Scholar] [CrossRef]
- Shun’ko, E.V. Langmuir Probe in Theory and Practice; Universal Publishers: Boca Raton, FL, USA, 2008. [Google Scholar]
- Efremov, A.; Lee, J.; Kim, J. On the Control of Plasma Parameters and Active Species Kinetics in CF4+ O2+ Ar Gas Mixture by CF4/O2 and O2/Ar Mixing Ratios. Plasma Chem. Plasma Process. 2017, 37, 1445–1462. [Google Scholar] [CrossRef]
- Chun, I.; Efremov, A.; Yeom, G.Y.; Kwon, K.H. A comparative study of CF4/O2/Ar and C4F8/O2/Ar plasmas for dry etching applications. Thin Solid Films 2015, 579, 136–143. [Google Scholar] [CrossRef]
- Lim, N.; Efremov, A.; Kwon, K.H. Gas-phase chemistry and etching mechanism of SiNx thin films in C4F8 + Ar inductively coupled plasma. Thin Solid Films 2019, 685, 97–107. [Google Scholar] [CrossRef]
- Lopaev, D.V.; Volynets, A.V.; Zyryanov, S.M.; Zotovich, A.I.; Rakhimov, A.T. Actinometry of O, N and F atoms. J. Phys. D Appl. Phys. 2017, 50, 075202. [Google Scholar] [CrossRef]
- Winters, H.F. Surface processes in plasma-assisted etching environments. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 1983, 1, 469. [Google Scholar] [CrossRef]
- Gray, D.C.; Tepermeister, I.; Sawin, H.H. Phenomenological modeling of ion-enhanced surface kinetics in fluorine-based plasma etching. J. Vac. Sci. Technol. 1993, B11, 1243–1257. [Google Scholar] [CrossRef]
- Zalm, P.C. Energy dependence of the sputtering yield of silicon bombarded with neon, argon, krypton, and xenon ions. J. Appl. Phys. 1983, 54, 2660. [Google Scholar] [CrossRef]
- Seah, M.P.; Nunney, T.S. Sputtering yields of compounds using argon ions. J. Phys. D Appl. Phys. 2010, 43, 253001. [Google Scholar] [CrossRef]
- Kimura, T.; Ohe, K. Model and probe measurements of inductively coupled CF4 discharges. J. Appl. Phys. 2002, 92, 1780–1787. [Google Scholar] [CrossRef]
- Sasaki, K.; Kawai, Y.; Kadota, K. Determination of fluorine atom density in reactive plasmas by vacuum ultraviolet absorption spectroscopy at 95.85 nm. Rev. Sci. Instrum. 1999, 70, 76–81. [Google Scholar] [CrossRef]
- NIST X-Ray Photoelectron Spectroscopy Database; Version 4.1; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2012.
- Cunge, G.; Kogelschatz, M.; Joubert, O.; Sadeghi, N. Plasma–wall interactions during silicon etching processes in high-density HBr/Cl2/O2 plasmas. Plasma Sources Sci. Technol. 2005, 14, S42–S52. [Google Scholar] [CrossRef]
- Tinck, S.; Boullart, W.; Bogaerts, A. Modeling Cl2/O2/Ar inductively coupled plasmas used for silicon etching: Effects of SiO2 chamber wall coating. Plasma Sources Sci. Technol. 2011, 11, 045012. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, N.; Choi, Y.S.; Efremov, A.; Kwon, K.-H. Dry Etching Performance and Gas-Phase Parameters of C6F12O + Ar Plasma in Comparison with CF4 + Ar. Materials 2021, 14, 1595. https://doi.org/10.3390/ma14071595
Lim N, Choi YS, Efremov A, Kwon K-H. Dry Etching Performance and Gas-Phase Parameters of C6F12O + Ar Plasma in Comparison with CF4 + Ar. Materials. 2021; 14(7):1595. https://doi.org/10.3390/ma14071595
Chicago/Turabian StyleLim, Nomin, Yeon Sik Choi, Alexander Efremov, and Kwang-Ho Kwon. 2021. "Dry Etching Performance and Gas-Phase Parameters of C6F12O + Ar Plasma in Comparison with CF4 + Ar" Materials 14, no. 7: 1595. https://doi.org/10.3390/ma14071595
APA StyleLim, N., Choi, Y. S., Efremov, A., & Kwon, K.-H. (2021). Dry Etching Performance and Gas-Phase Parameters of C6F12O + Ar Plasma in Comparison with CF4 + Ar. Materials, 14(7), 1595. https://doi.org/10.3390/ma14071595






