Flake (NH4)6Mo7O24/Polydopamine as a High Performance Anode for Lithium Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Synthesis
2.2. Material Characterizations
2.3. Electrochemical Measurements
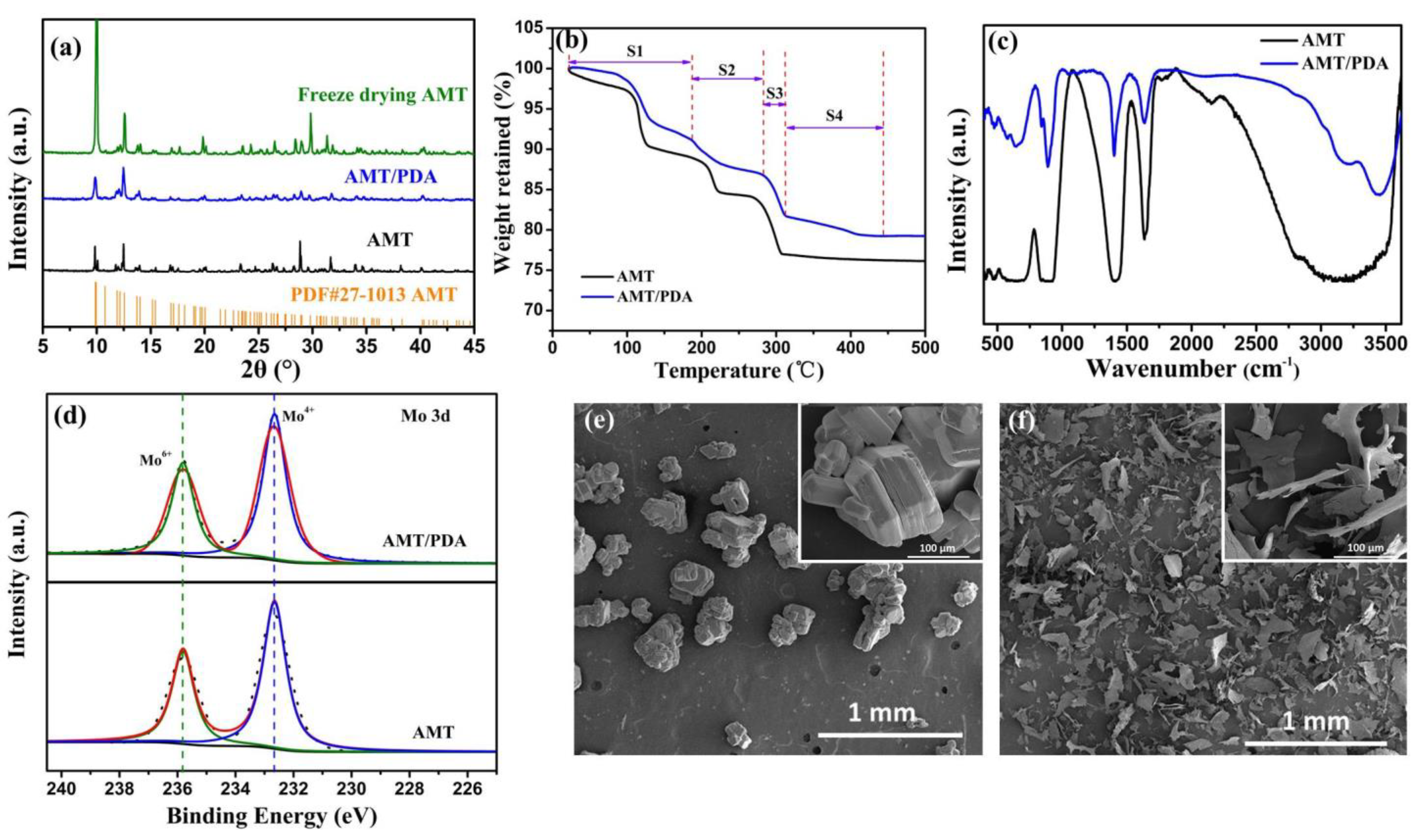
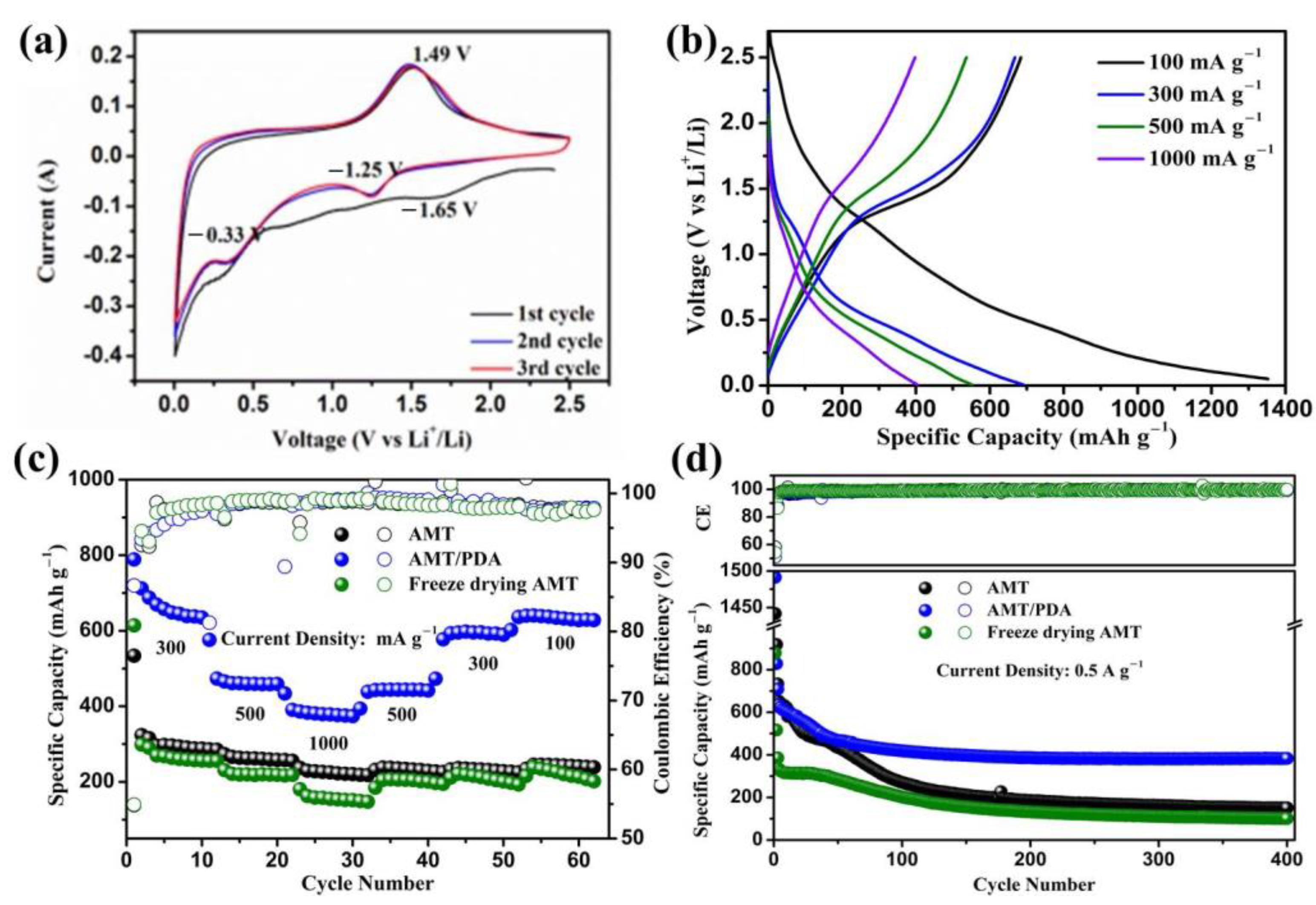
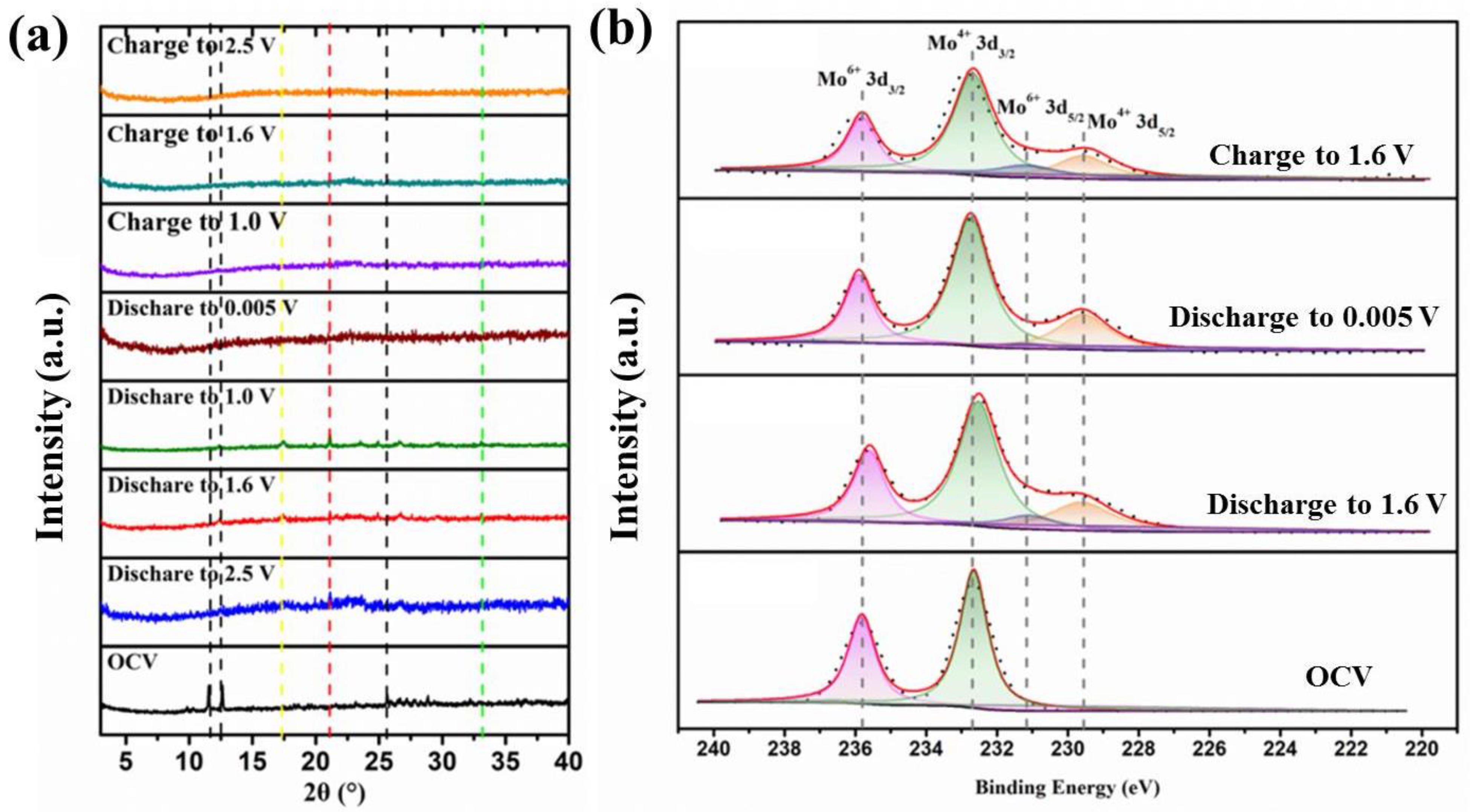
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, D.; Li, H.; Li, R.; Hu, Y.; Hu, X. In situ Growth of Copper Rhodizonate Complexes on Reduced Graphene Oxide for High-Performance Organic Lithium-Ion Batteries. Chem. Commun. 2018, 54, 11415–11418. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Cao, D.; She, Y.; Wang, H.; Shi, J.-W.; Leung, M.K.H.; Niu, C. Atomic Layer Deposition of TiO2 Shells on MoO3 Nanobelts Allowing Enhanced Lithium Storage Performance. Chem. Commun. 2018, 54, 7782–7785. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and Challenges Facing Rechargeable Lithium Batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Li, N.; Xie, Y.; Peng, S.; Xiong, X.; Han, K. Ultra-Lightweight Ti3C2Tx MXene Modified Separator for Li–S Batteries: Thickness Regulation Enabled Polysulfide Inhibition and Lithium Ion Transportation. J. Energy Chem. 2020, 42, 116–125. [Google Scholar] [CrossRef]
- Luo, L.; Wu, J.; Xu, J.; Dravid, V.P. Atomic Resolution Study of Reversible Conversion Reaction in Metal Oxide Electrodes for Lithium-Ion Battery. ACS Nano 2014, 8, 11560–11566. [Google Scholar] [CrossRef] [PubMed]
- Chandrasiri, K.W.D.K.; Nguyen, C.C.; Zhang, Y.; Parimalam, B.S.; Lucht, B.L. Systematic Investigation of Alkali Metal Ions as Additives for Graphite Anode in Propylene Carbonate Based Electrolytes. Electrochim. Acta 2017, 250, 285–291. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Zhang, Y.; Liu, H.; Meng, X.; Yang, J.; Geng, D.; Wang, D.; Li, R.; Sun, X. High Concentration Nitrogen Doped Carbon Nanotube Anodes with Superior Li+ Storage Performance for Lithium Reachargeable Battery Application. J. Power Sources 2012, 197, 238–245. [Google Scholar] [CrossRef]
- Han, T.-H.; Lee, Y.; Choi, M.-R.; Woo, S.-H.; Bae, S.-H.; Hong, B.H.; Ahn, J.-H.; Lee, T.-W. Extremely Efficient Flexible Organic Light-Emitting Diodes with Modified Graphene Anode. Nat. Photonics 2012, 6, 105–110. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, Z.X.; Zheng, M.B.; Wang, T.H.; Yang, J.Z.; Yuan, F.S.; Lu, X.Y.; Liu, L.; Sun, D.P. Amorphous Fe2O3 Nanoshells Coated on Carbonized Bacterial Cellulose Nanofibers as a Flexible Anode for High-Performance Lithium Ion Batteries. J. Power Sources 2016, 307, 649–656. [Google Scholar] [CrossRef]
- Li, L.; Zhou, G.M.; Shan, X.Y.; Pei, S.F.; Li, F.; Cheng, H.M. Co3O4 Mesoporous Nanostructures@graphene membrane as an Integrated Anode for Long-Life Lithium-Ion Batteries. J. Power Sources 2014, 255, 52–58. [Google Scholar] [CrossRef]
- Jiang, H.; Zhou, X.; Liu, G.; Zhou, Y.; Ye, H.; Liu, Y.; Han, K. Free-Standing Si/Graphene Paper Using Si Nanoparticles Synthesized by Acid-Etching Al-Si Alloy Powder for High-Stability Li-Ion Battery Anodes. Electrochim. Acta 2016, 188, 777–784. [Google Scholar] [CrossRef]
- Lee, Y.; Lim, H.; Kim, S.-O.; Kim, H.-S.; Kim, K.J.; Lee, K.-Y.; Choi, W. Thermal Stability of Sn Anode Material with Non-Aqueous Electrotyles in Sodium-Ion Batteries. J. Mater. Chem. A 2018, 6, 20383–20392. [Google Scholar] [CrossRef]
- Ressler, T.; Wienold, J.; Jentoft, R.E.; Neisius, T. Bulk Structural Investigation of the Reduction of MoO3 with Propene and the Oxidation of MoO2 with Oxygen. J. Catal. 2002, 210, 67–83. [Google Scholar] [CrossRef]
- Wang, H.; Skeldon, P.; Thompson, G.E.; Wood, G.C. Synthesis and Characterization of Molybdenum Disulphide Formed from Ammonium Tetrathiomolybdate. J. Mater. Sci. 1997, 32, 497–502. [Google Scholar] [CrossRef]
- Luo, W.; Hu, X.; Sun, Y.; Huang, Y. Electrospinning of Carbon-Coated MoO2 Nanofibers with Enhanced Lithium-Storage Properties. Phys. Chem. Chem. Phys. 2011, 13, 16735–16740. [Google Scholar] [CrossRef]
- Chang, K.; Chen, W.X. L-Cysteine-Assisted Synthesis of Layered MoS2/Graphene Composites with Excellent Electrochemical Performances for Lithium Ion Batteries. ACS Nano 2011, 5, 4720–4728. [Google Scholar] [CrossRef]
- Das, B.; Reddy, M.V.; Krishnamoorthi, C.; Tripathy, S.; Mahendiran, R.; Rao, G.V.S.; Chowdari, B.V.R. Carbothermal Synthesis, Spectral and Magnetic Characterization and Li-Cyclability of the Mo-Cluster Compounds, LiYMo3O8 and Mn2Mo3O8. Electrochim. Acta 2009, 54, 3360–3373. [Google Scholar] [CrossRef]
- Mai, L.Q.; Yang, F.; Zhao, Y.-L.; Xu, X.; Xu, L.; Luo, Y.-Z. Hierarchical MnMoO4/CoMoO4 Heterostructured Nanowires with Enhanced Supercapacitor Performance. Nat. Commun. 2011, 2, 381. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, X.; Luo, W.; Shu, J.; Huang, Y. Self-Assembly of Hybrid Fe2Mo3O8–Reduced Graphene Oxide Nanosheets with Enhanced Lithium Storage Properties. J. Mater. Chem. A 2013, 1, 4468–4474. [Google Scholar] [CrossRef]
- Guo, L.; Liu, Q.; Li, G.; Shi, J.; Liu, J.; Wang, T.; Jiang, G. A Mussel-Inspired Polydopamine Coating as a Versatile Platform for the in situ Synthesis of Graphene-Based Nanocomposites. Nanoscale 2012, 4, 5864–5867. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Guo, H.; Yang, Y.; Wang, Z.; Li, X.; Zhou, Y. N-Doped Carbon Layer Derived from Polydopamine to Improve the Electrochemical Performance of Spray-Dried Si/Graphite Composite Anode Material for Lithium Ion Batteries. J. Alloy. Compd. 2016, 689, 130–137. [Google Scholar] [CrossRef]
- Sun, T.; Li, Z.-J.; Wang, H.-G.; Bao, D.; Meng, F.-I.; Zhang, X.-B. A Biodegradable Polydopamine-Derived Electrode Material for High-Capacity and Long-Life Lithium-Ion and Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2016, 55, 10662–10666. [Google Scholar] [CrossRef]
- Dong, X.; Ding, B.; Guo, H.; Dou, H.; Zhang, X. Superlithiated Polydopamine Derivative for High-Capacity and High-Rate Anode for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 38101–38108. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Gu, C.; Zheng, W.; Dai, F.; Wang, X.; Zheng, Z. Facile Synthesis of novel Size-Controlled Antibacterial Hybrid Spheres Using Silver Nanoparticles Loaded with Poly-Dopamine Spheres. RSC Adv. 2015, 5, 13470–13477. [Google Scholar] [CrossRef]
- Bie, Y.; Yang, J.; Liu, X.; Wang, J.; Nuli, Y.; Lu, W. Polydopamine Wrapping Silicon Cross-linked with Polyacrylic Acid as High-Performance Anode for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 2899–2904. [Google Scholar] [CrossRef]
- Hao, J.; Zhang, J.; Xia, G.; Liu, Y.; Zheng, Y.; Zhang, W.; Tang, Y.; Pang, W.K.; Guo, Z. Heterostructure Manipulation via in Situ Localized Phase Transformation for High-Rate and Highly Durable Lithium Ion Storage. ACS Nano 2018, 12, 10430–10438. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, D.O.; Watson, G.W.; Payne, D.J.; Athinsjon, G.R.; Edgell, R.G.; Law, D.S.L. Theoretical and Experimental Study of the Electronic Structures of MoO3 and MoO2. J. Phys. Chem. C 2010, 114, 4636–4645. [Google Scholar] [CrossRef]
- Xiu, L.; Wang, Z.; Yu, M.; Wu, X.; Qiu, J. Aggregation-Resistant 3D MXene-Based Architecture as Efficient Bifunctional Electrocatalyst for Overall Water Splitting. ACS Nano 2018, 12, 8017–8028. [Google Scholar] [CrossRef] [PubMed]
- Sacci, R.L.; Dudney, N.J.; More, K.L.; Parent, L.R.; Arslan, I.; Brwoning, N.D.; Unocic, R.R. Direct Visualization of Initial SEI Morphology and Growth Kinetics During Lithium Deposition by in situ Electrochemical Transmission Electron Microscopy. Chem. Commun. 2014, 50, 2104–2107. [Google Scholar] [CrossRef]
- Ju, Z.; Zhang, E.; Zhao, Y.; Xing, Z.; Zhuang, Q.; Qiang, Y.; Qian, Y. One-Pot Hydrothermal Synthesis of FeMoO4 Nanocubes as an Anode Material for Lithium-Ion Batteries with Excellent Electrochemical Performance. Small 2015, 11, 4753–4761. [Google Scholar] [CrossRef]
- Jiang, J.; Yang, W.; Wang, H.; Zhao, Y.; Guo, J.; Zhao, J.; Beidaghi, M.; Gao, L. Electrochemical Performances of MoO2/C Nanocomposite for Sodium Ion Storage: An Insight into Rate Dependent Charge/Discharge Mechanism. Electrochim. Acta 2017, 240, 379–387. [Google Scholar] [CrossRef]
- Chen, M.; Cao, W.; Wang, L.; Ma, X.; Han, K. Chessboard-Like Silicon/Graphite Anodes with High Cycling Stability toward Practical Lithium-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 775–783. [Google Scholar] [CrossRef]
- Lu, J.; Chen, Z.; Pan, F.; Cui, Y.; Amine, K. High-Performance Anode Materials for Rechargeable Lithium-Ion Batteries. J. Electrochem. Energy Rev. 2018, 1, 35–53. [Google Scholar] [CrossRef]
- Zhang, H.; He, H.; Luan, J.; Huang, X.; Tang, Y.; Wang, H. Adjusting the Yolk–Shell Structure of Carbon Spheres to Boost The Capacitive K+ Storage Ability. J. Mater. Chem. A 2018, 6, 23318–23325. [Google Scholar] [CrossRef]
- Kim, H.-S.; Cook, J.B.; Lin, H.; Ko, J.S.; Tolbert, S.H.; Ozolins, V.; Dunn, B. Oxygen Vacancies Enhance Pseudocapacitive Charge Storage Properties of MoO3−x. Nat. Mater. 2017, 16, 454–460. [Google Scholar] [CrossRef]
- Cheng, X.; Li, Y.; Sang, L.; Ma, J.; Shi, H.; Liu, X.; Lu, J.; Zhang, Y. Boosting the Electrochemical Performance of MoO3 Anode for Long-Life Lithium Ion Batteries: Dominated by an Ultrathin TiO2 Passivation Layer. Electrochimica Acta 2018, 269, 241–249. [Google Scholar] [CrossRef]
- Wang, M.-S.; Wang, G.-L.; Wang, S.; Zhang, J.; Wang, J.; Zhong, W.; Tang, F.; Yang, Z.-L.; Zheng, J.; Li, X. In Situ Catalytic Growth 3D multi-Layers Graphene Sheets Coated Nano-Silicon Anode for High Performance Lithium-Ion Batteries. Chem. Eng. J. 2019, 356, 895–903. [Google Scholar] [CrossRef]
- Zhang, H.; Zong, P.; Chen, M.; Jin, H.; Bai, Y.; Li, S.; Ma, F.; Xu, H.; Lian, K. In Situ Synthesis of Multilayer Carbon Matrix Decorated with Copper Particles: Enhancing the Performance of Si as Anode for Li-Ion Batteries. ACS Nano 2019, 13, 3054–3062. [Google Scholar] [CrossRef]
- Chen, T.-R.; Sheng, T.; Wu, Z.-G.; Li, J.-T.; Wang, E.-H.; Wu, C.-J.; Li, H.-T.; Guo, X.-D.; Zhong, B.-H.; Huang, L.; et al. Cu2+ Dual-Doped Layer-Tunnel Hybrid Na0.6Mn1–xCuxO2 as a Cathode of Sodium-Ion Battery with Enhanced Structure Stability, Electrochemical Property, and Air Stability. ACS Appl. Mater. Interfaces 2018, 10, 10147–10156. [Google Scholar] [CrossRef]
- Fu, Y.; Wei, Q.; Zhang, G.; Wang, X.; Zhang, J.; Hu, Y.; Wang, D.; Zuin, L.; Zhou, T.; Wu, Y.; et al. High-Performance Reversible Aqueous Zn-Ion Battery Based on Porous MnOx Nanorods Coated by MOF-Derived N-Doped Carbon. Adv. Energy Mater. 2018, 8, 1801445. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Xiong, X.; Han, K. Flake (NH4)6Mo7O24/Polydopamine as a High Performance Anode for Lithium Ion Batteries. Materials 2021, 14, 1115. https://doi.org/10.3390/ma14051115
Xie Y, Xiong X, Han K. Flake (NH4)6Mo7O24/Polydopamine as a High Performance Anode for Lithium Ion Batteries. Materials. 2021; 14(5):1115. https://doi.org/10.3390/ma14051115
Chicago/Turabian StyleXie, Ying, Xiang Xiong, and Kai Han. 2021. "Flake (NH4)6Mo7O24/Polydopamine as a High Performance Anode for Lithium Ion Batteries" Materials 14, no. 5: 1115. https://doi.org/10.3390/ma14051115
APA StyleXie, Y., Xiong, X., & Han, K. (2021). Flake (NH4)6Mo7O24/Polydopamine as a High Performance Anode for Lithium Ion Batteries. Materials, 14(5), 1115. https://doi.org/10.3390/ma14051115






