Thermoelectric and Transport Properties of Permingeatite Cu3SbSe4 Prepared Using Mechanical Alloying and Hot Pressing
Abstract
1. Introduction
2. Experimental Procedure
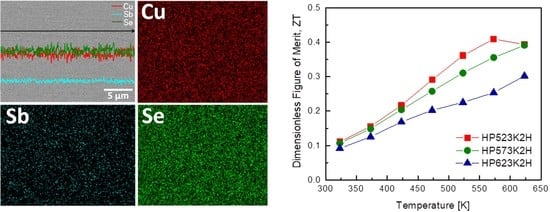
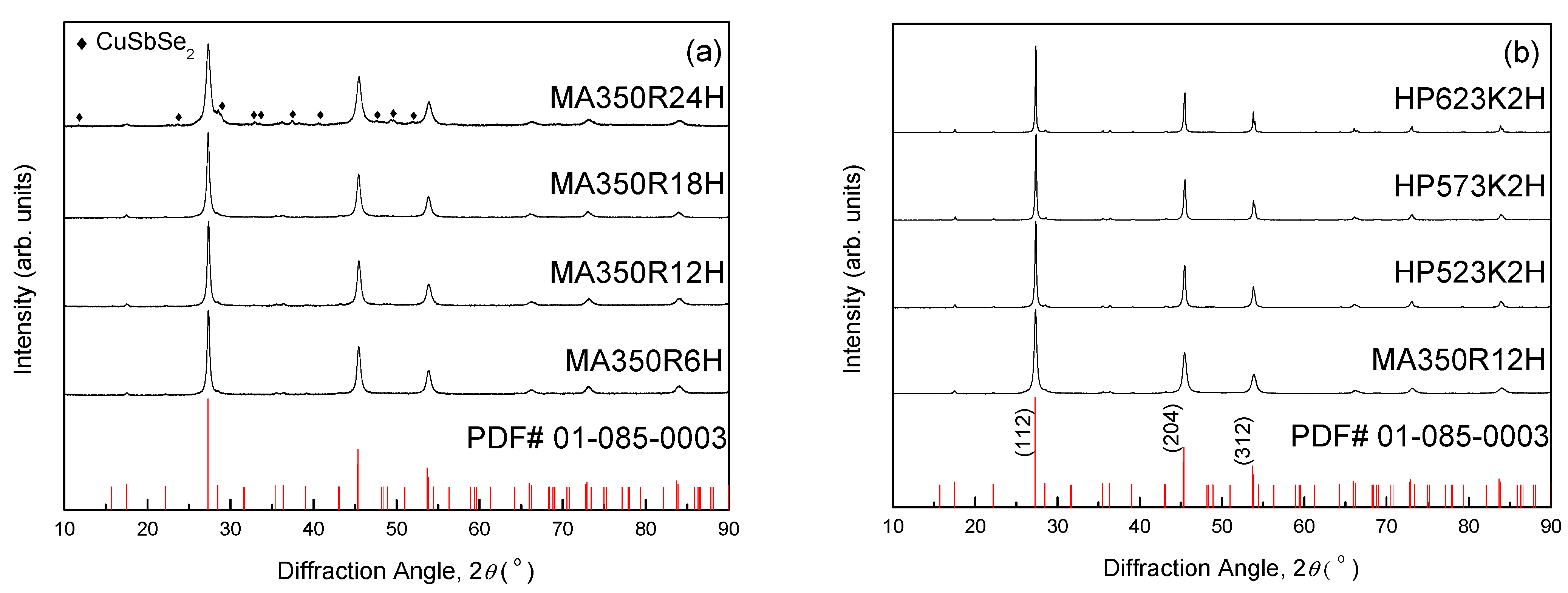
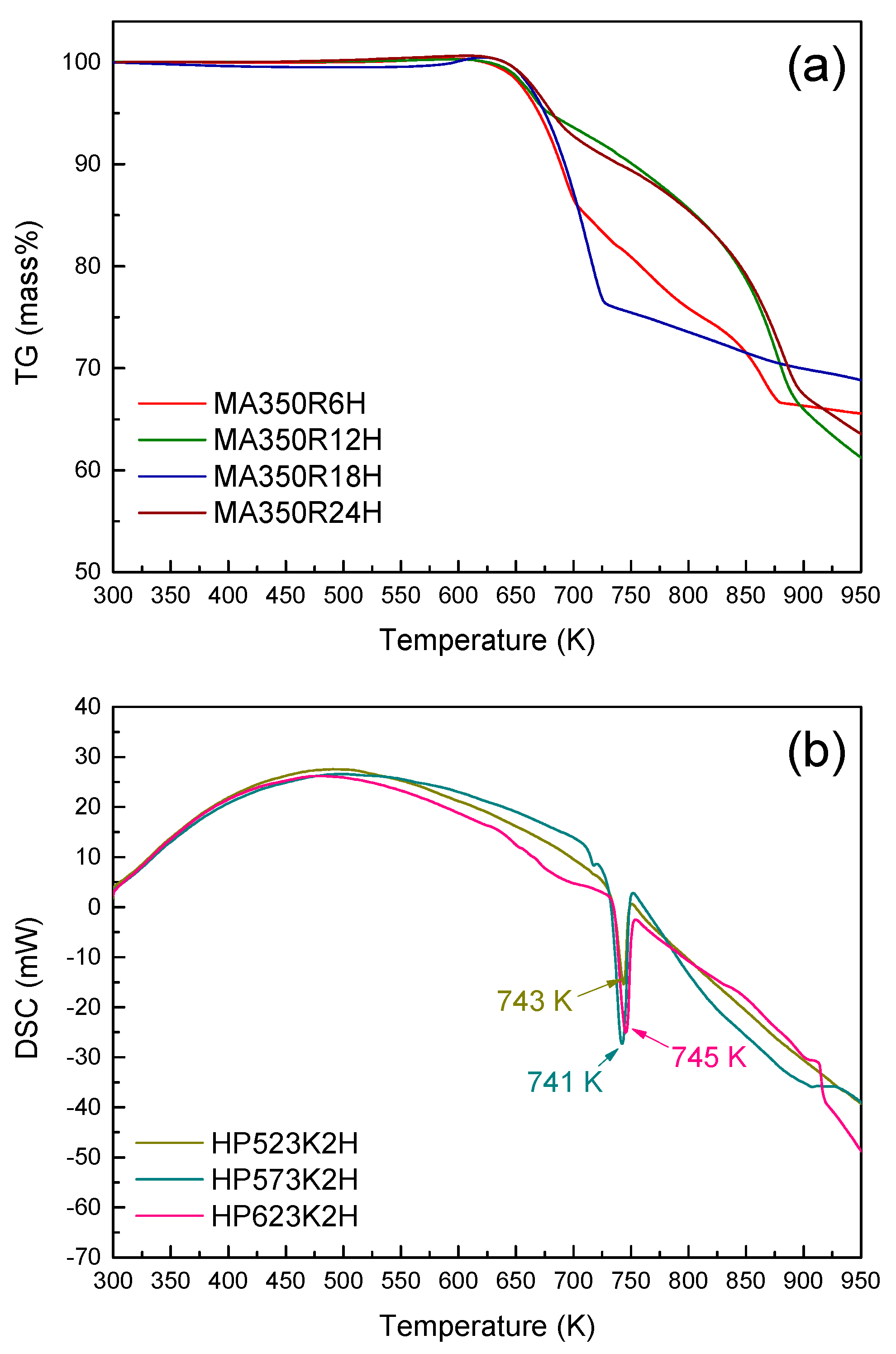
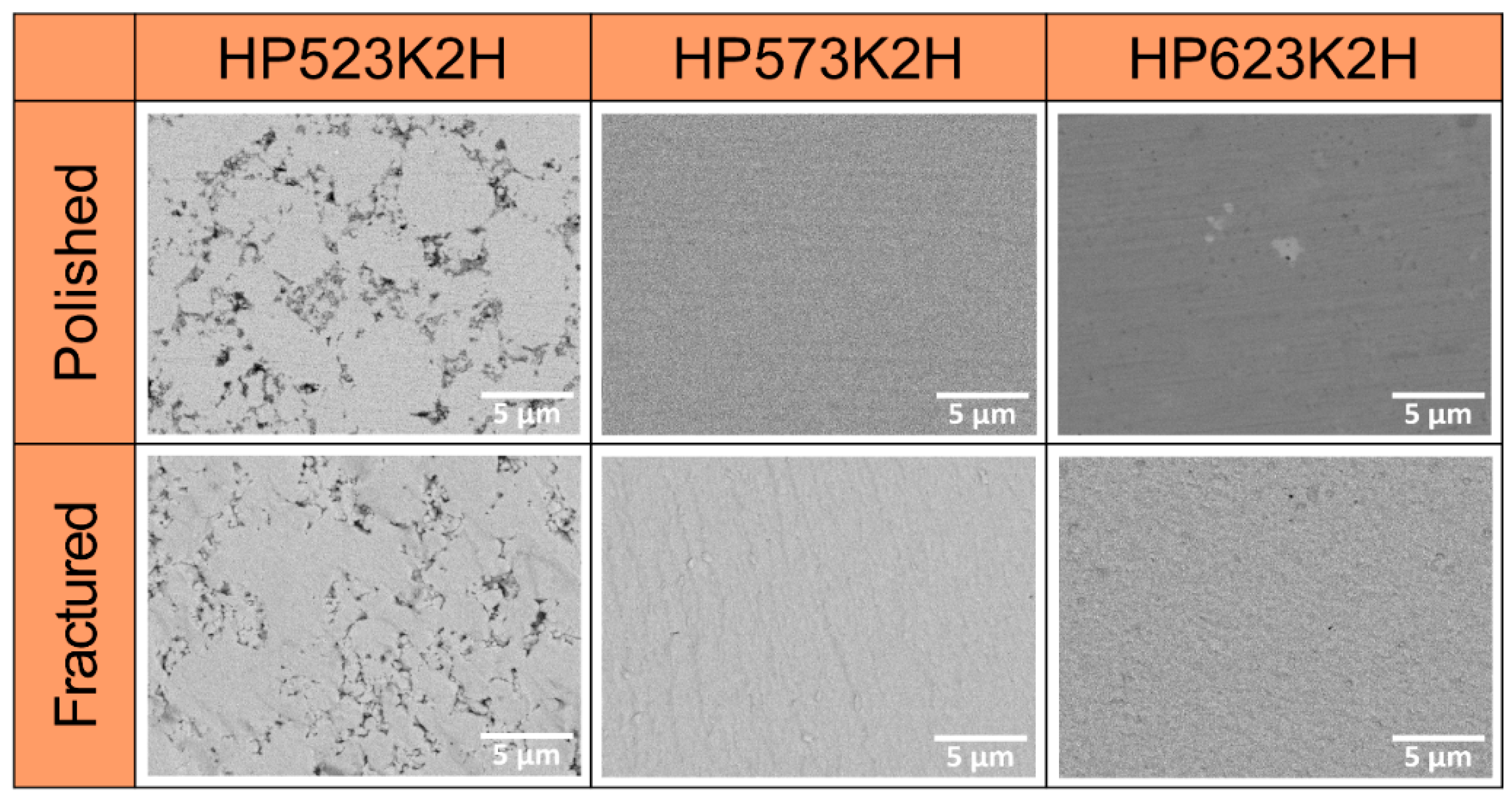
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kwak, S.G.; Lee, G.E.; Kim, I.H. Electronic transport and thermoelectric properties of Cu12-xZnxSb4S13 tetrahedrites prepared by mechanical alloying and hot pressing. Korean J. Met. Mater. 2019, 57, 328–333. [Google Scholar] [CrossRef]
- Cha, Y.E.; Kim, I.H. Synthesis and thermoelectric properties of partially double-filled skutterudites (La1−zYbz)0.8Fe4−xCoxSb12. Korean J. Met. Mater. 2019, 57, 801–807. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Z.G.; Dargusch, M.S.; Zou, J. High performance thermoelectric materials: Progress and their applications. Adv. Energy Mater. 2018, 8, 1701797. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, J.; Jiang, Q.; Zhou, Z.; Li, X.; Ren, Y.; Xin, J.; Basit, A.; He, X.; Chu, W.; et al. Simultaneous optimization of the overall thermoelectric properties of Cu3SbSe4 by band engineering and phonon blocking. J. Alloys Compd. 2017, 724, 597–602. [Google Scholar] [CrossRef]
- Yang, C.; Huang, F.; Wu, L.; Xu, K. New stannite-like p-type thermoelectric material Cu3SbSe4. J. Phys. D Appl. Phys. 2011, 44, 295404. [Google Scholar] [CrossRef]
- Wei, T.R.; Wang, H.; Gibbs, Z.M.; Wu, C.F.; Snyder, G.J.; Li, J.F. Thermoelectric properties of Sn-doped p-type Cu3SbSe4: A compound with large effective mass and small band gap. J. Mater. Chem. A 2014, 2, 13527–13533. [Google Scholar] [CrossRef]
- Zhao, D.; Wu, D.; Bo, L. Enhanced thermoelectric properties of Cu3SbSe4 compounds via gallium doping. Energies 2017, 10, 1524. [Google Scholar] [CrossRef]
- Li, X.Y.; Li, D.; Xin, H.X.; Zhang, J.; Song, C.J.; Qin, X.Y. Effects of bismuth doping on the thermoelectric properties of Cu3SbSe4 at moderate temperatures. J. Alloys Compd. 2013, 561, 105–108. [Google Scholar] [CrossRef]
- Kumar, A.; Dhama, P.; Saini, D.S.; Banerji, P. Effect of Zn substitution at a Cu site on the transport behavior and thermoelectric properties in Cu3SbSe4. RSC Adv. 2016, 6, 5528–5534. [Google Scholar] [CrossRef]
- Choi, J.S.; Oh, T.S.; Hyun, D.B. Sintering characteristics and thermoelectric properties of n-type PbTe fabricated by mechanical alloying and hot pressing. Korean J. Met. Mater. 1998, 36, 606–613. [Google Scholar]
- Yu, Y.Q.; Zhang, B.P.; Ge, Z.H.; Shang, P.P.; Chen, Y.X. Thermoelectric properties of Ag-doped bismuth sulfide polycrystals prepared by mechanical alloying and spark plasma sintering. Mater. Chem. Phys. 2011, 131, 216–222. [Google Scholar] [CrossRef]
- Wojciechowski, S. New trends in the development of mechanical engineering materials. J. Mater. Process. Technol. 2000, 106, 230–235. [Google Scholar] [CrossRef]
- Lee, G.E.; Pi, J.H.; Kim, I.H. Preparation and thermoelectric properties of famatinite Cu3SbS4. J. Electron. Mater. 2020, 49, 2781–2788. [Google Scholar] [CrossRef]
- Patil, U.; Hong, S.J.; Suryanarayana, C. An unusual phase transformation during mechanical alloying of an Fe-based bulk metallic glass composition. J. Alloys Compd. 2005, 389, 121–126. [Google Scholar] [CrossRef]
- Zhou, A.J.; Zhao, X.B.; Zhu, T.J.; Dasgupta, T.; Stiewe, C.; Hassdorf, R.; Mueller, E. Mechanochemical decomposition of higher manganese silicides in the ball milling process. Intermetallics 2010, 18, 2051–2056. [Google Scholar] [CrossRef]
- Shin, D.K.; Jang, K.W.; Ur, S.C.; Kim, I.H. Thermoelectric properties of higher manganese silicides prepared by mechanical alloying and hot pressing. J. Electron. Mater. 2013, 42, 1756–1761. [Google Scholar] [CrossRef]
- Chen, X.; Shi, L.; Zhou, J.; Goodenough, J.B. Effects of ball milling on microstructures and thermoelectric properties of higher manganese silicides. J. Alloys Compd. 2015, 641, 30–36. [Google Scholar] [CrossRef]
- Scott, W.; Kench, J.R. Phase diagram and properties of Cu3SbSe4 and other A3IBVC4VI compounds. Mater. Res. Bull. 1973, 8, 1257–1268. [Google Scholar] [CrossRef]
- Wei, T.R.; Li, F.; Li, J.F. Enhanced thermoelectric performance of nonstoichiometric compounds Cu3-xSbSe4 by Cu deficiencies. J. Electron. Mater. 2014, 43, 2229–2238. [Google Scholar] [CrossRef]
- Skoug, E.J.; Cain, J.D.; Majsztrik, P.; Kirkham, M.; Lara-Curzio, E.; Morelli, D.T. Doping effects on the thermoelectric properties of Cu3SbSe4. Sci. Adv. Mater. 2011, 3, 602–606. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Y.; Li, S.; Wan, D.; Huang, F. CuSbSe2-assisted sintering of CuInSe2 at low temperature. J. Mater. Sci. 2012, 47, 7085–7089. [Google Scholar] [CrossRef]
- Li, D.; Li, R.; Qin, X.Y.; Song, C.J.; Xin, H.X.; Wang, L.; Zhang, J.; Guo, G.L.; Zou, T.H.; Liu, Y.F.; et al. Co-precipitation synthesis of nanostructured Cu3SbSe4 and its Sn-doped sample with high thermoelectric performance. Dalt. Trans. 2014, 43, 1888–1896. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qin, X.; Yang, X.; Liu, Y.; Zhang, J.; Dou, Y.; Song, C.; Xin, H. Enhanced thermoelectric performance through carrier scattering at heterojunction potentials in BiSbTe based composites with Cu3SbSe4 nanoinclusions. J. Mater. Chem. C 2015, 3, 7045–7052. [Google Scholar] [CrossRef]
- Li, Y.; Qin, X.; Li, D.; Li, X.; Liu, Y.; Zhang, J.; Song, C.; Xin, H. Transport properties and enhanced thermoelectric performance of aluminum doped Cu3SbSe4. RSC Adv. 2015, 5, 31399–31403. [Google Scholar] [CrossRef]
- Do, D.T.; Mahanti, S.D. Theoretical study of defects Cu3SbSe4: Search for optimum dopants for enhancing thermoelectric properties. J. Alloys Compd. 2015, 625, 346–354. [Google Scholar] [CrossRef]
- Xie, D.; Zhang, B.; Zhang, A.; Chen, Y.; Yan, Y.; Yang, H.; Wang, G.; Wang, G.; Han, X.; Han, G.; et al. High thermoelectric performance of Cu3SbSe4 nanocrystals with Cu2-xSe in situ inclusions synthesized by a microwave-assisted solvothermal method. Nanoscale 2018, 10, 14546–14553. [Google Scholar] [CrossRef] [PubMed]
- Berger, L.I. Semiconductor Materials; CRC Press: Boca Raton, FL, USA, 1996; p. 267. [Google Scholar]
- Zhou, T.; Wang, L.; Zheng, S.; Hong, M.; Fang, T.; Bai, P.P.; Chang, S.; Cui, W.; Shi, X.; Zhao, H.; et al. Self-assembled 3D flower-like hierarchical Ti-doped Cu3SbSe4 microspheres with ultralow thermal conductivity and high zT. Nano Energy 2018, 49, 221–229. [Google Scholar] [CrossRef]
- Kim, H.S.; Gibbs, Z.M.; Tang, Y.; Wang, H.; Snyder, G.J. Characterization of Lorenz number with Seebeck coefficient measurement. APL Mater. 2015, 3, 041506. [Google Scholar] [CrossRef]
- Kumar, A.; Dhama, P.; Banerji, P. Effect of Cu deficiency on the transport behavior and thermoelectric properties in Cu3SbSe4. AIP Conf. Proc. 2017, 1832, 110050. [Google Scholar]
- Wu, Y.; Qiao, X.; Fan, X.; Zhang, X.; Cui, S.; Wan, J. Facile synthesis of monodisperse Cu3SbSe4 nanoparticles and thermoelectric performance of Cu3SbSe4 nanoparticle based materials. J. Nano. Res. 2015, 17, 1–7. [Google Scholar] [CrossRef]
- Tyagi, K.; Gahtori, B.; Bathula, S.; Toutam, V.; Sharma, S.; Singh, N.K.; Dhar, A. Thermoelectric and mechanical properties of spark plasma sintered Cu3SbSe3 and Cu3SbSe4: Promising thermoelectric materials. Appl. Phys. Lett. 2014, 105, 261902. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, J.; Jiang, Q.; Fu, L.; Xiao, Y.; Luo, Y.; Zhou, Z. Improvement of thermoelectric properties of Cu3SbSe4 compound by In doping. Mater. Des. 2016, 98, 150–154. [Google Scholar] [CrossRef]
- Bo, L.; Wang, W.; Wang, Y.; Wang, L.; Zuo, M.; Zhao, D. Effects of Co doping on the thermoelectric properties of Cu3SbSe4 at moderate temperature. Mater. Sci. For. 2020, 993, 899–905. [Google Scholar]

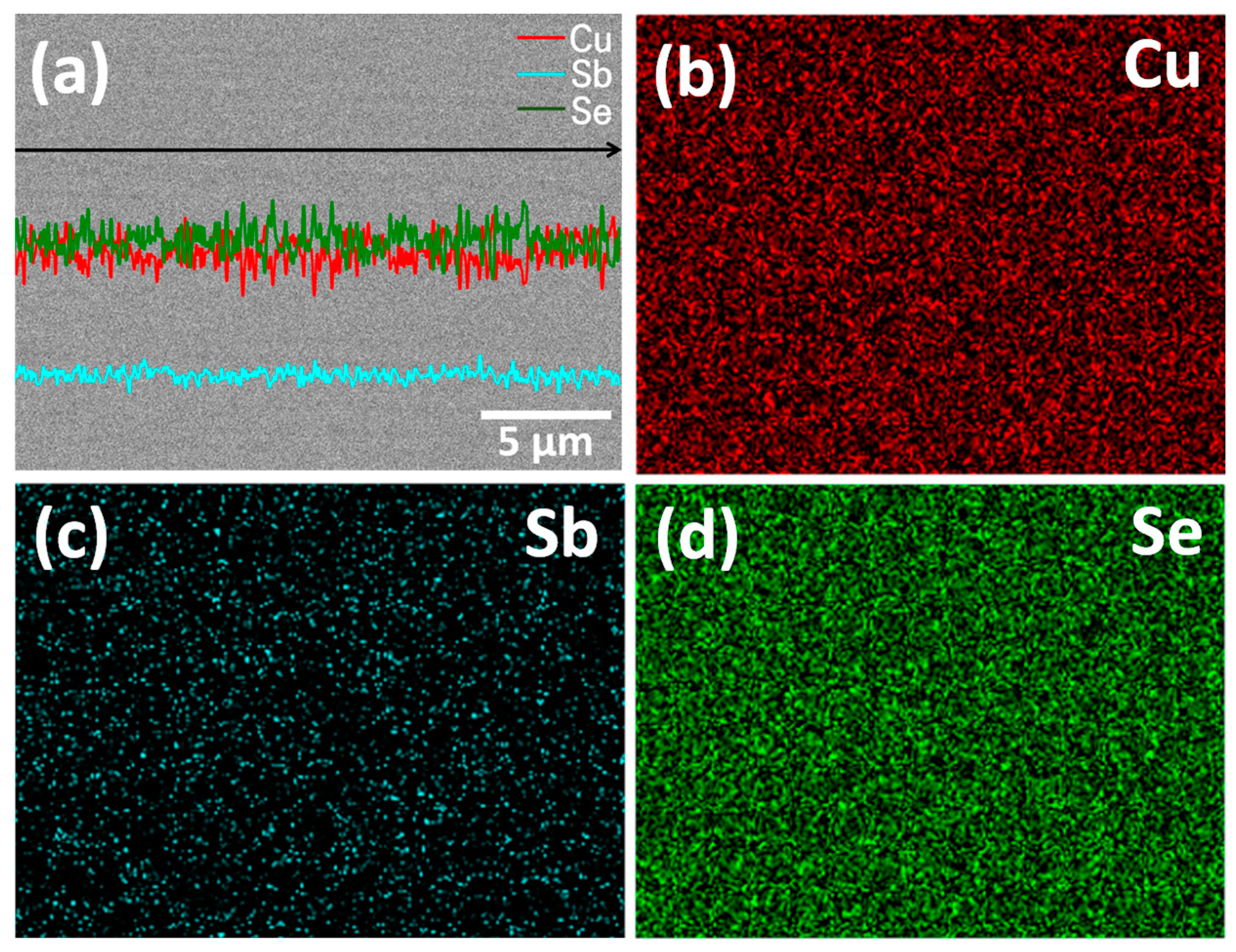
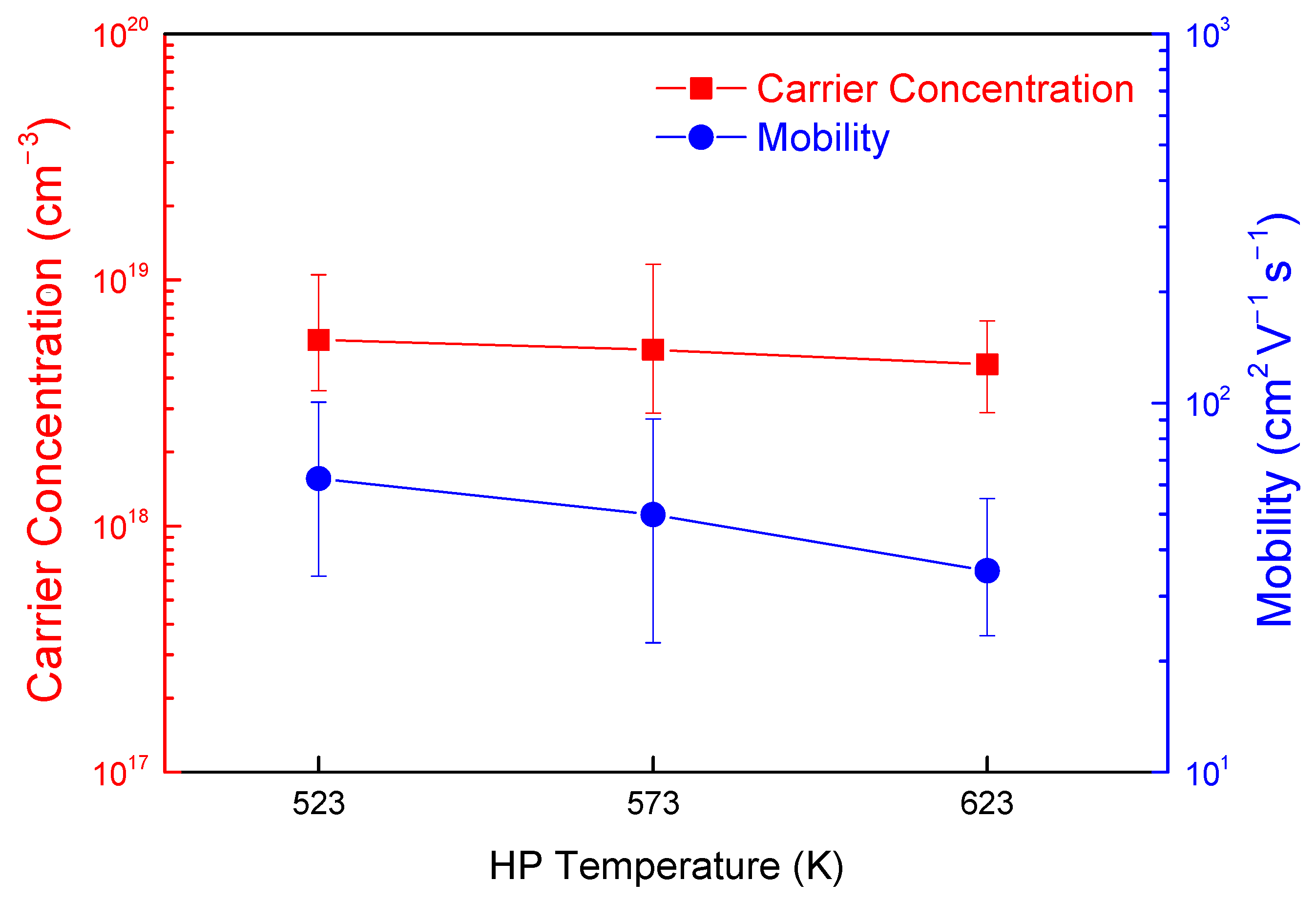

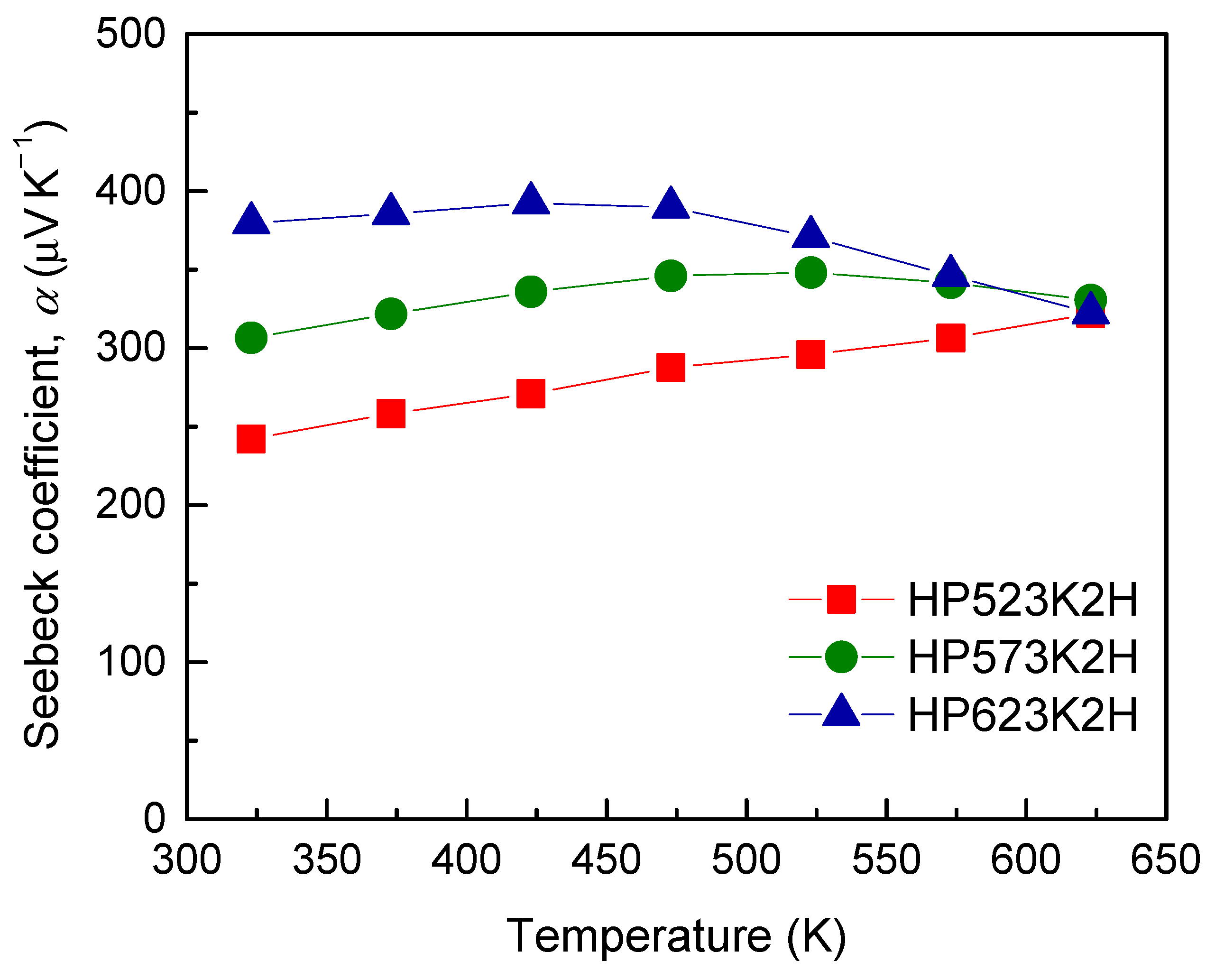
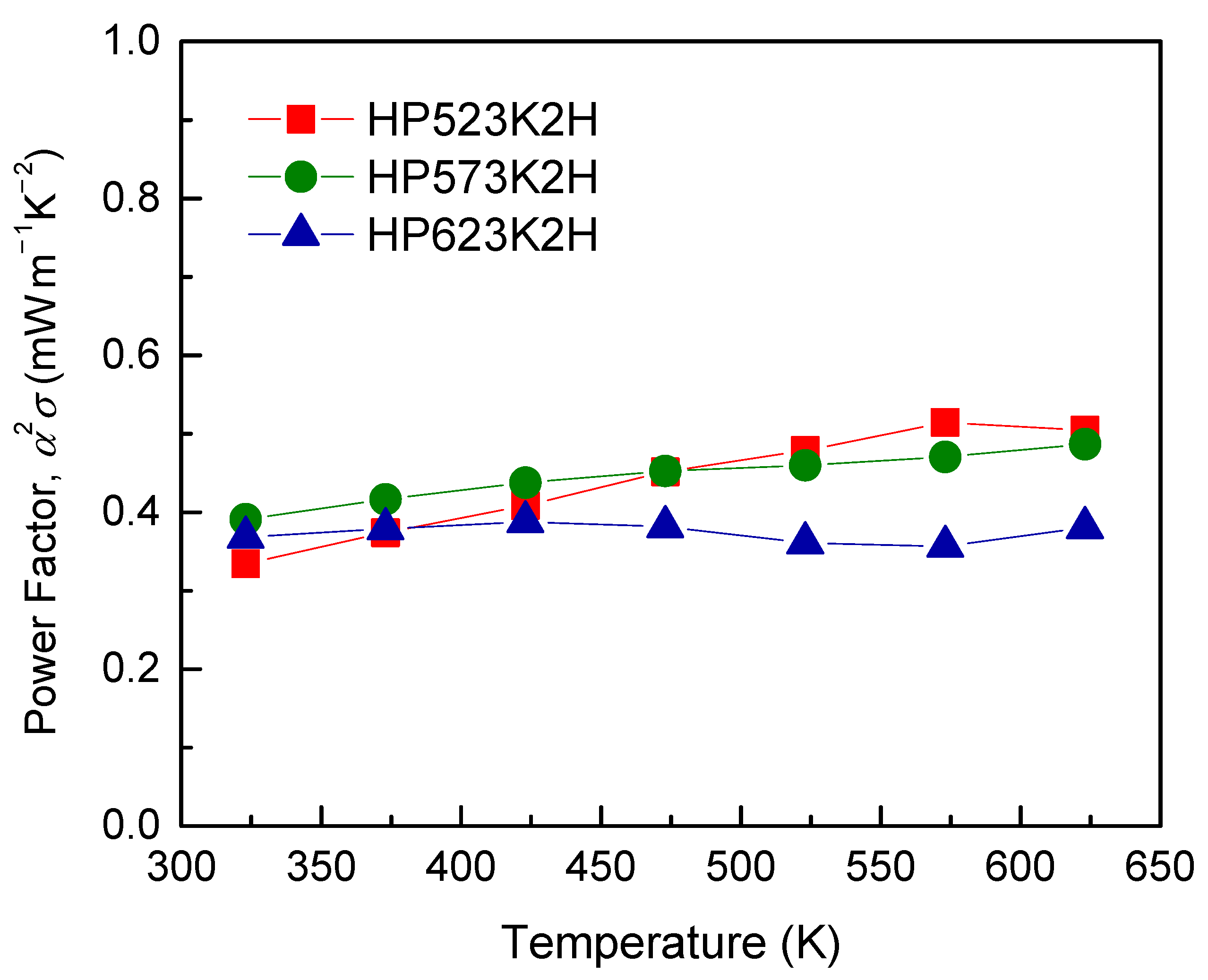
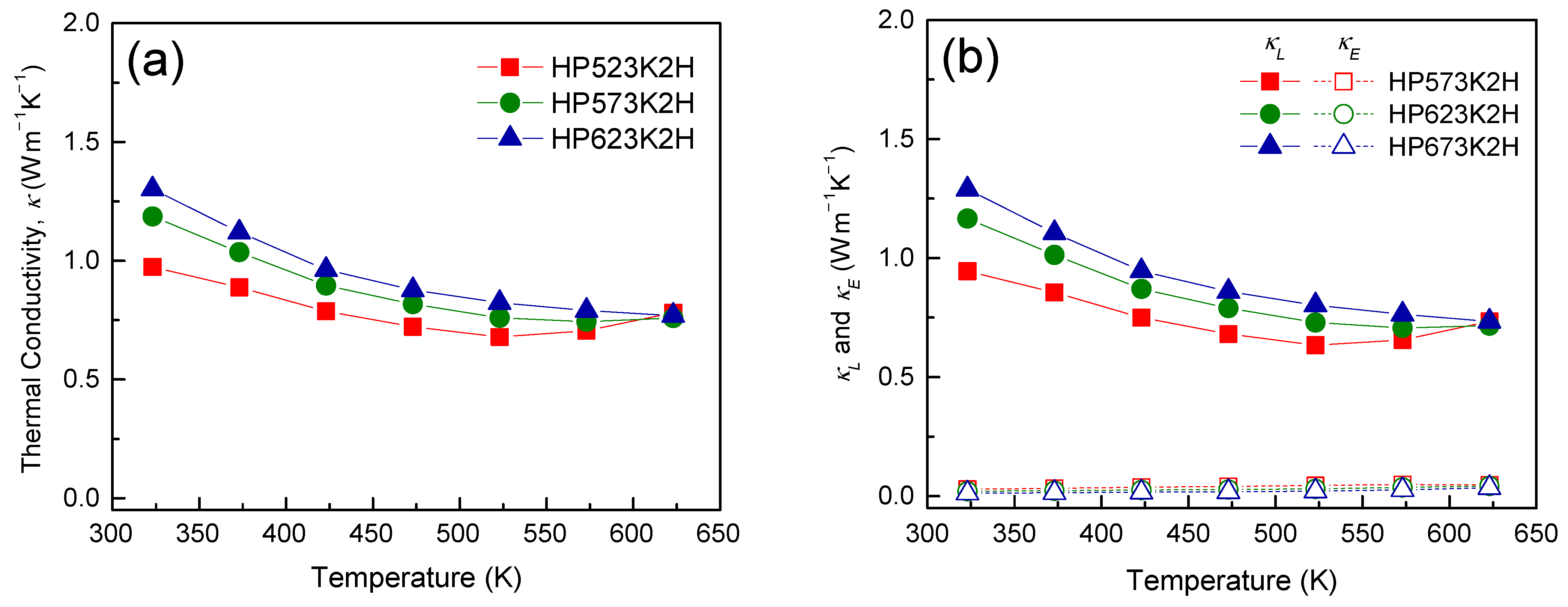
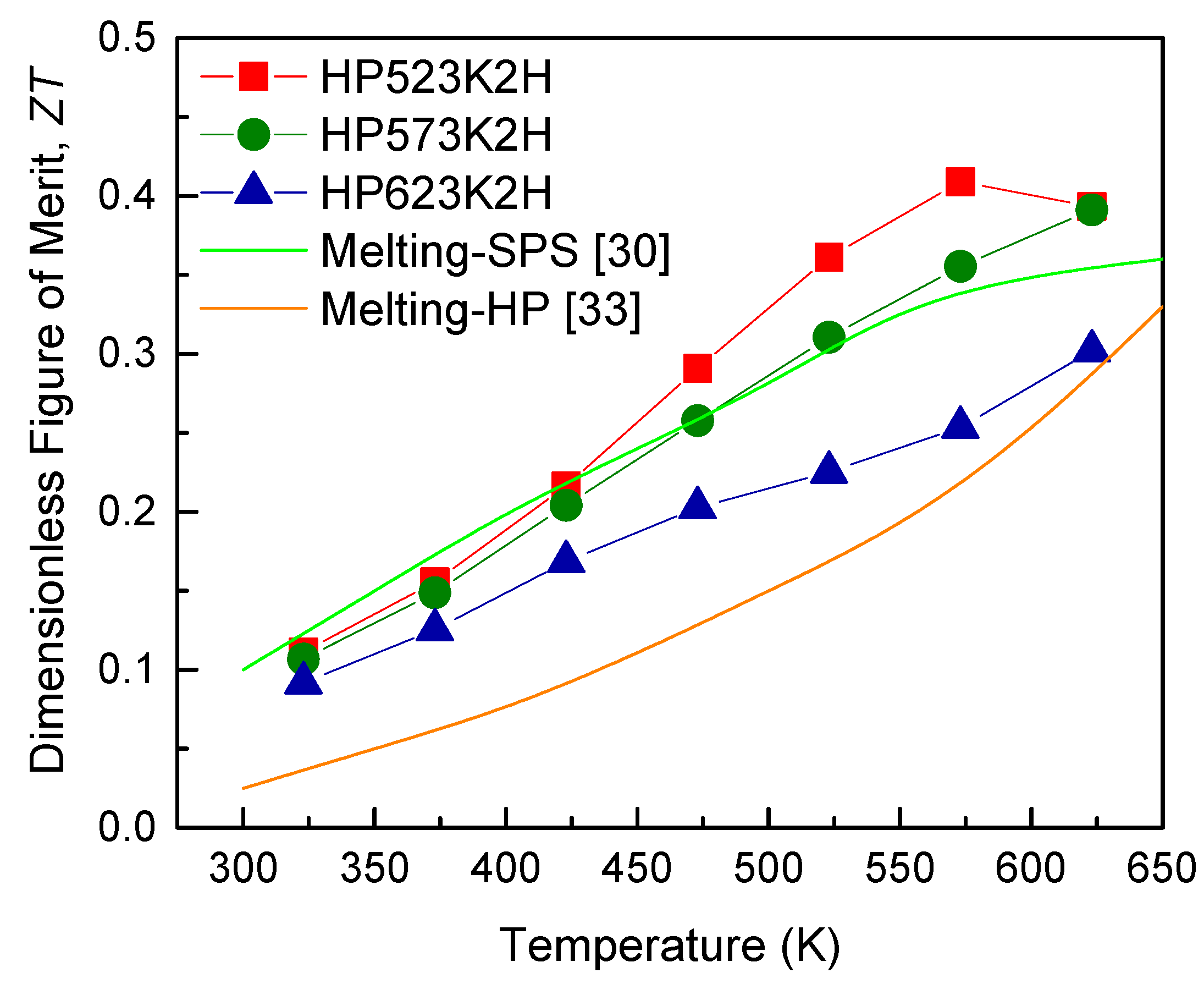
| Specimen | Composition | Relative Density (%) | Lattice Constant (nm) | FWHM(112) (°) | Lorenz Number (10−8 V2 K−2) | ||
|---|---|---|---|---|---|---|---|
| Nominal | Actual | a | c | ||||
| MA350R12H HP523K2H HP573K2H HP623K2H | Cu3SbSe4 | Cu3.31Sb0.97Se3.72 Cu3.27Sb0.93Se3.80 Cu3.14Sb0.97Se3.89 Cu3.29Sb0.93Se3.78 | - 89.9 97.8 97.7 | 0.5651 0.5646 0.5649 0.5650 | 1.1252 1.1243 1.1243 1.1243 | 0.355 0.234 0.203 0.171 | - 1.62 1.57 1.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, G.-E.; Kim, I.-H. Thermoelectric and Transport Properties of Permingeatite Cu3SbSe4 Prepared Using Mechanical Alloying and Hot Pressing. Materials 2021, 14, 1116. https://doi.org/10.3390/ma14051116
Lee G-E, Kim I-H. Thermoelectric and Transport Properties of Permingeatite Cu3SbSe4 Prepared Using Mechanical Alloying and Hot Pressing. Materials. 2021; 14(5):1116. https://doi.org/10.3390/ma14051116
Chicago/Turabian StyleLee, Go-Eun, and Il-Ho Kim. 2021. "Thermoelectric and Transport Properties of Permingeatite Cu3SbSe4 Prepared Using Mechanical Alloying and Hot Pressing" Materials 14, no. 5: 1116. https://doi.org/10.3390/ma14051116
APA StyleLee, G.-E., & Kim, I.-H. (2021). Thermoelectric and Transport Properties of Permingeatite Cu3SbSe4 Prepared Using Mechanical Alloying and Hot Pressing. Materials, 14(5), 1116. https://doi.org/10.3390/ma14051116