Gelatin reduced Graphene Oxide Nanosheets as Kartogenin Nanocarrier Induces Rat ADSCs Chondrogenic Differentiation Combining with Autophagy Modification
Abstract
1. Introduction
2. Materials and Methods
2.1. Rat ADSCs Isolation and Culture
2.2. The Effects of KGN on Rat ADSCs
2.3. Synthesis of rGO@Ge and Evaluation on Its Nanocarrier Function
2.4. The Biocompatibility and Entry Pathways of rGO@Ge in ADSCs
2.5. ADSCs Chondrogenesis Induced by KGN-Loaded rGO@Ge
2.6. Analysis on the Intracellular ROS and Autophagy Changes in ADSCs Treated with Different K/Gs Media
2.7. Immunofluorescence (IF), Alcian Blue (Ab) and Toluidine Blue (Tb) Stainings
2.8. Real-Time Quantitative PCR Analysis
2.9. Western Blotting Analysis
2.10. Statistical Analysis
3. Results
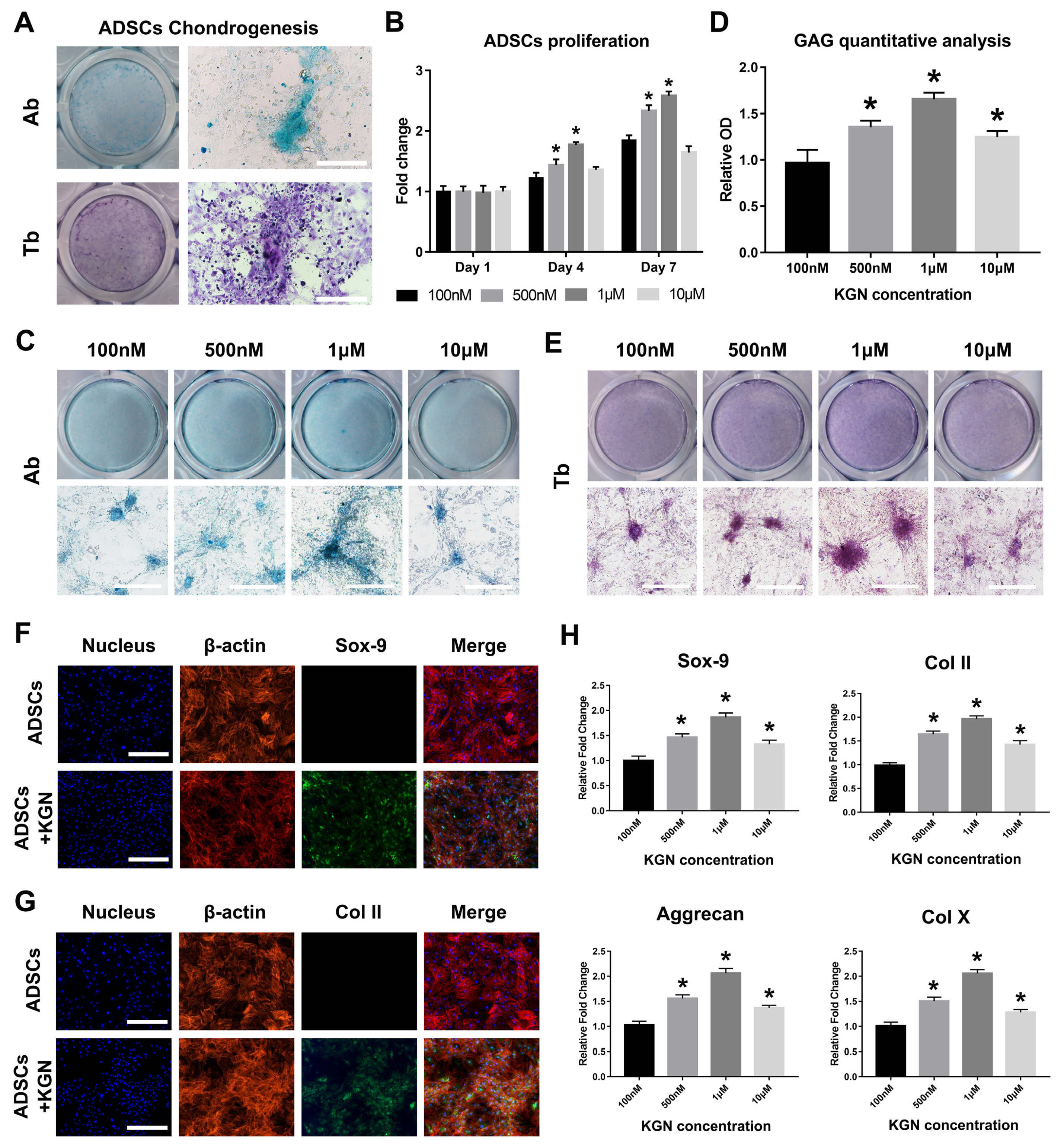
3.1. Promotion of ADSCs Chondrogenesis by KGN
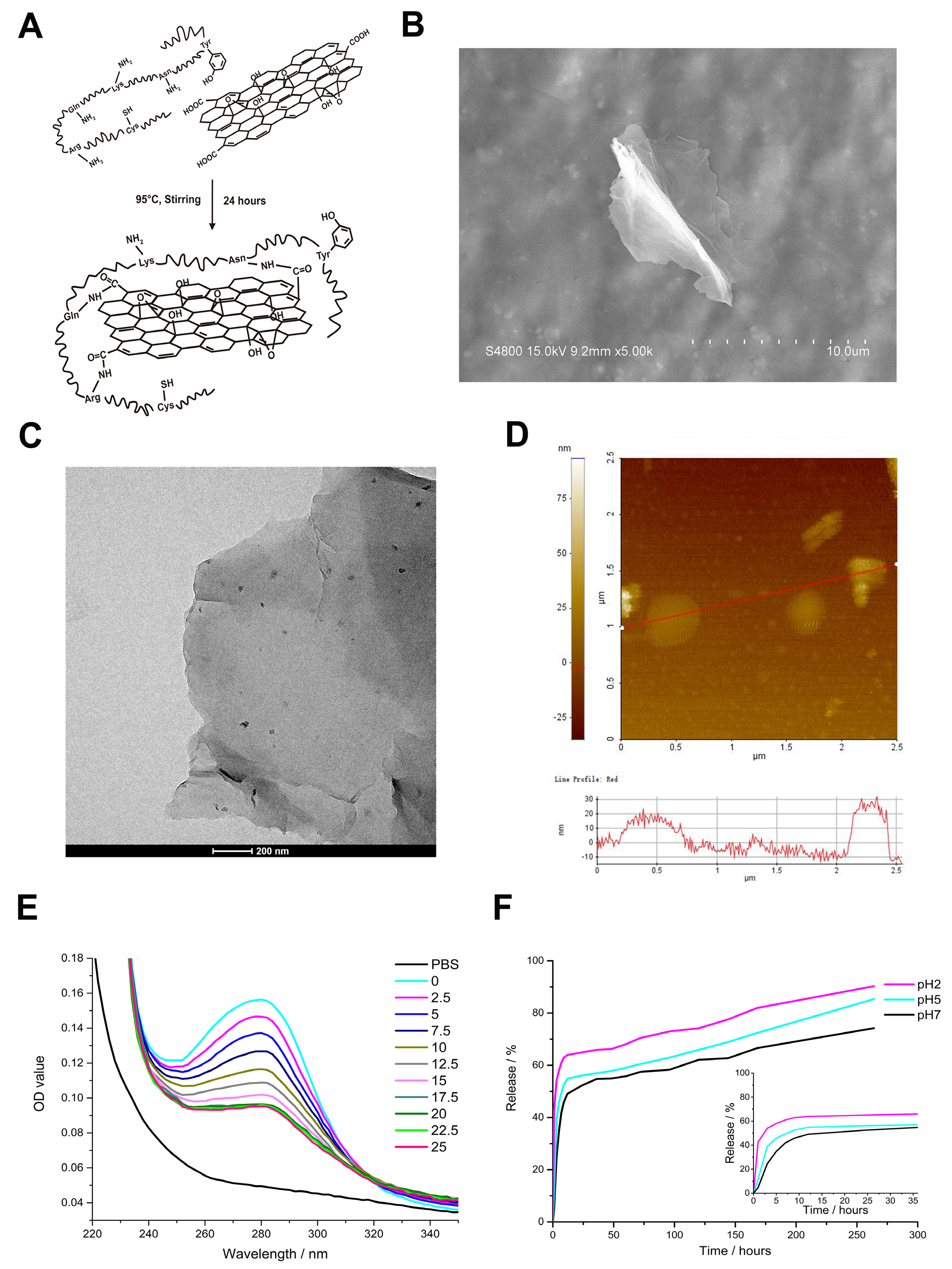
3.2. Nanocarrier Function and Biocompatibility of rGO@Ge
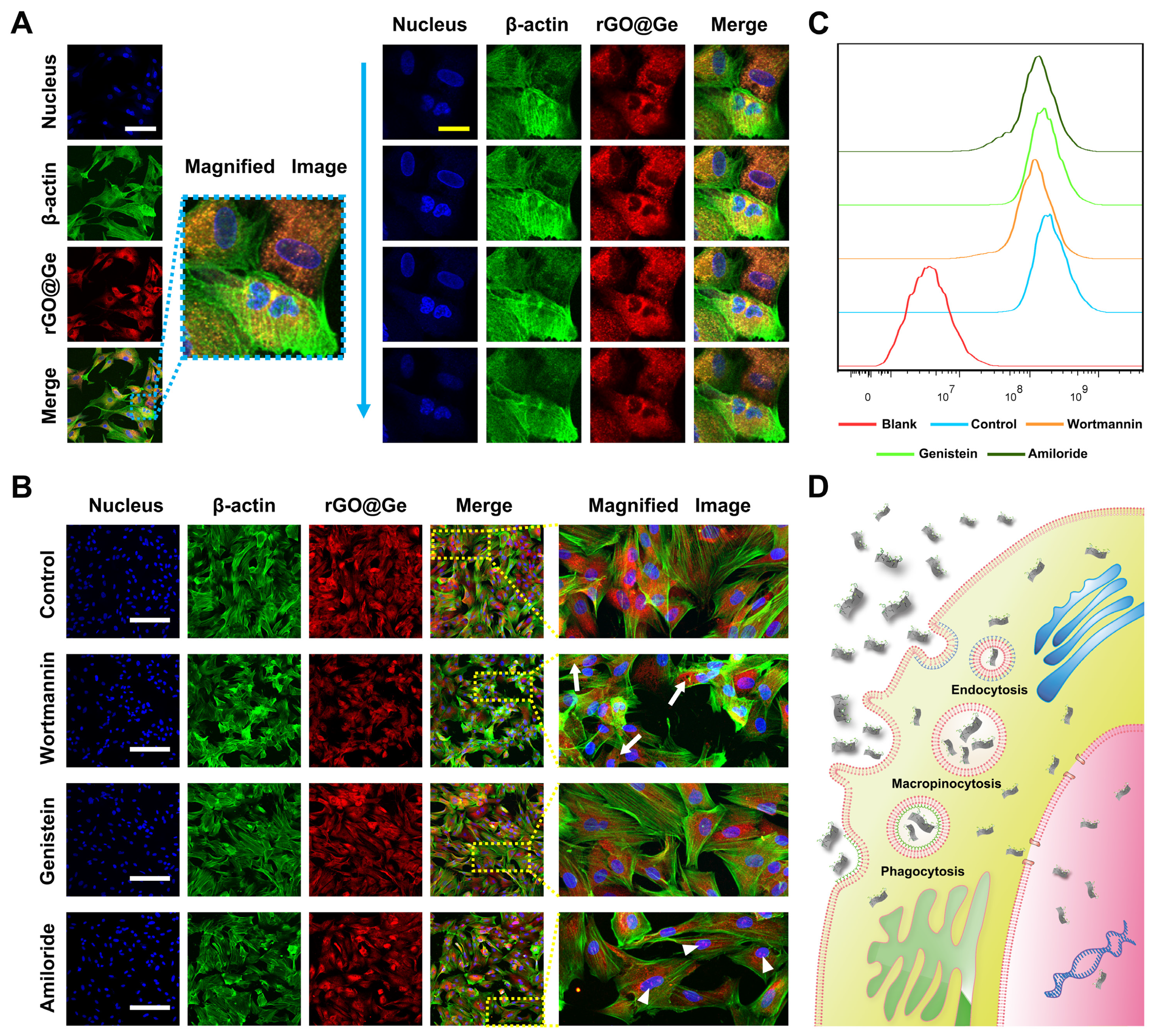
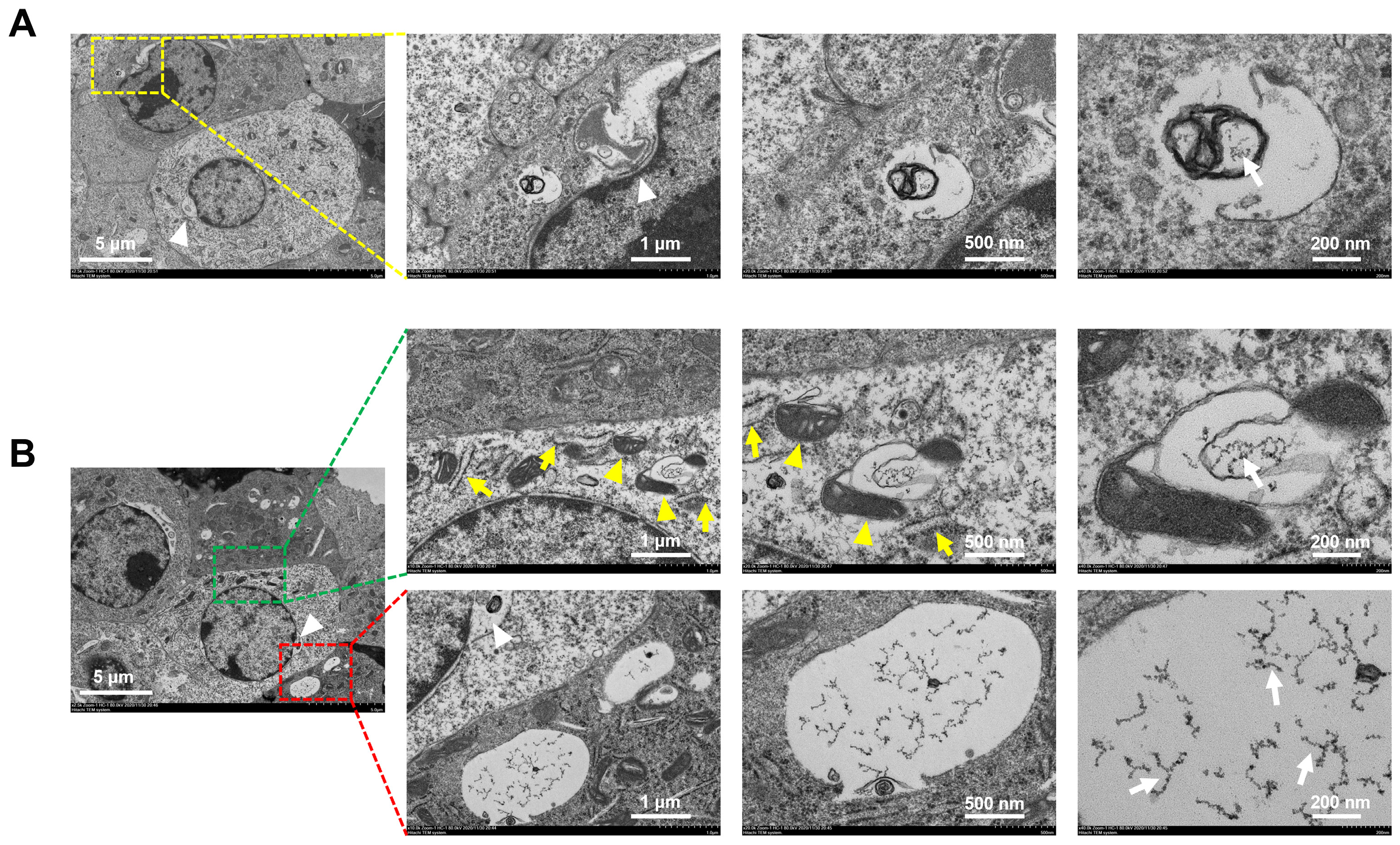
3.3. The Entry Pathway and Degradation of rGO@Ge in ADSCs
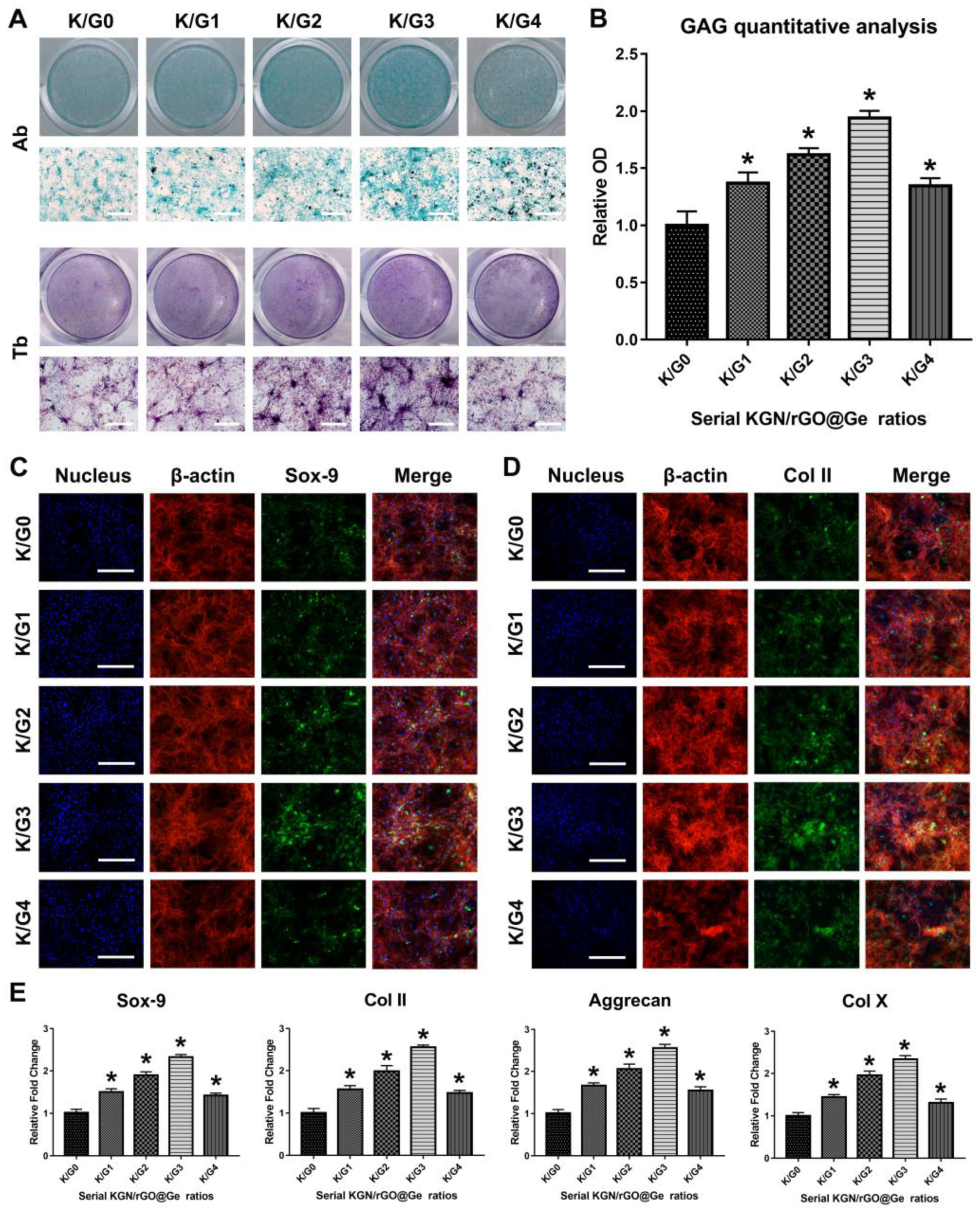
3.4. Promotion of ADSCs Chondrogenesis by KGN-Loaded rGO@Ge
3.5. The Effects of K/Gs on Intracellular ROS and Autophagy of ADSCs Correlated to Chondrogenesis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abramoff, B.; Caldera, F.E. Osteoarthritis: Pathology, Diagnosis, and Treatment Options. Med. Clin. N. Am. 2020, 104, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Redondo, M.L.; Beer, A.J.; Yanke, A.B. Cartilage Restoration: Microfracture and Osteochondral Autograft Transplantation. J. Knee Surg. 2018, 31, 231–238. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, J.; Han, J.; Zhang, W.; Ma, J. Mesenchymal stem cell related therapies for cartilage lesions and osteoarthritis. Am. J. Transl. Res. 2019, 11, 6275–6289. [Google Scholar] [PubMed]
- Finnson, K.W.; Chi, Y.; Bou-Gharios, G.; Leask, A.; Philip, A. TGF-b signaling in cartilage homeostasis and osteoarthritis. Front. Biosci. 2012, 4, 251–268. [Google Scholar] [CrossRef]
- Johnson, K.; Zhu, S.; Tremblay, M.S.; Payette, J.N.; Wang, J.; Bouchez, L.C.; Meeusen, S.; Althage, A.; Cho, C.Y.; Wu, X.; et al. A stem cell-based approach to cartilage repair. Science 2012, 336, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Zhang, X.; Gao, M.; Luo, K.; Fu, W.; Yin, M.; Wang, W.; Zhu, Z.; Zheng, J.; He, X. Kartogenin preconditioning commits mesenchymal stem cells to a precartilaginous stage with enhanced chondrogenic potential by modulating JNK and β-catenin-related pathways. FASEB J. 2019, 33, 5641–5653. [Google Scholar] [CrossRef]
- Burke, J.; Hunter, M.; Kolhe, R.; Isales, C.; Hamrick, M.; Fulzele, S. Therapeutic potential of mesenchymal stem cell based therapy for osteoarthritis. Clin. Transl. Med. 2016, 5, 27. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Graphene Nanomaterials: Synthesis, Biocompatibility, and Cytotoxicity. Int. J. Mol. Sci. 2018, 19, 3564. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Liu, H.; Fan, Y. Graphene-Based Materials in Regenerative Medicine. Adv. Healthc. Mater. 2015, 4, 1451–1468. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Lozano, N.; Wychowaniec, J.K.; Hodgkinson, T.; Richardson, S.M.; Kostarelos, K.; Hoyland, J.A. Graphene oxide: A growth factor delivery carrier to enhance chondrogenic differentiation of human mesenchymal stem cells in 3D hydrogels. Acta Biomater. 2019, 96, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Lin, H.; Sun, A.X.; Song, S.; Wang, B.; Yang, Y.; Dai, J.; Tuan, R.S. Acceleration of chondrogenic differentiation of human mesenchymal stem cells by sustained growth factor release in 3D graphene oxide incorporated hydrogels. Acta Biomater. 2020, 105, 44–55. [Google Scholar] [CrossRef]
- Compton, O.C.; Nguyen, S.T. Graphene oxide, highly reduced graphene oxide, and graphene: Versatile building blocks for carbon-based materials. Small 2010, 6, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Lee, T.; El-Said, W.A.; Choi, J.W. Graphene-Based Materials for Stem Cell Applications. Materials 2015, 8, 8674–8690. [Google Scholar] [CrossRef] [PubMed]
- Halim, A.; Luo, Q.; Ju, Y.; Song, G. A Mini Review Focused on the Recent Applications of Graphene Oxide in Stem Cell Growth and Differentiation. Nanomaterials 2018, 8, 736. [Google Scholar] [CrossRef]
- Yoon, H.H.; Bhang, S.H.; Kim, T.; Yu, T.; Hyeon, T.; Kim, B.-S. Dual Roles of Graphene Oxide in Chondrogenic Differentiation of Adult Stem Cells: Cell-Adhesion Substrate and Growth Factor-Delivery Carrier. Adv. Funct. Mater. 2014, 24, 6455–6464. [Google Scholar] [CrossRef]
- Yin, R.; Shen, P.; Lu, Z. A green approach for the reduction of graphene oxide by the ultraviolet/sulfite process. J. Colloid Interface Sci. 2019, 550, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Anagnostou, K.; Stylianakis, M.M.; Atsalakis, G.; Kosmidis, D.M.; Skouras, A.; Stavrou, I.J.; Petridis, K.; Kymakis, E. An extensive case study on the dispersion parameters of HI-assisted reduced graphene oxide and its graphene oxide precursor. J. Colloid Interface Sci. 2020, 580, 332–344. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Kim, H.D.; Han, S.S. Biocompatibility and hemocompatibility of hydrothermally derived reduced graphene oxide using soluble starch as a reducing agent. Colloids Surf. B Biointerfaces 2020, 185, 110579. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Piner, R.D.; Chen, X.; Wu, N.; Nguyen, S.B.T.; Ruoff, R.S. Stable aqueous dispersions of graphitic nanoplatelets via the reduction of exfoliated graphite oxide in the presence of poly(sodium 4-styrenesulfonate). J. Mater. Chem 2006, 16, 155. [Google Scholar] [CrossRef]
- Selvakumar, D.; Sivaram, H.; Alsalme, A.; Alghamdi, A.; Jayavel, R. Facile synthesize of free standing highly conducting flexible reduced graphene oxide paper. J. Mater. Sci. Mater. Electron. 2016, 27, 6232–6241. [Google Scholar] [CrossRef]
- Zhang, J.J.; Gu, M.M.; Zheng, T.T.; Zhu, J.J. Synthesis of Gelatin-Stabilized Gold Nanoparticles and Assembly of Carboxylic Single-Walled Carbon Nanotubes/Au Composites for Cytosensing and Drug Uptake. Anal. Chem. 2009, 81, 6641–6648. [Google Scholar] [CrossRef] [PubMed]
- Jiao, D.; Cao, L.; Liu, Y.; Wu, J.; Zheng, A.; Jiang, X. Synergistic Osteogenesis of Biocompatible Reduced Graphene Oxide with Methyl Vanillate in BMSCs. ACS Biomater. Sci. Eng. 2019, 5, 1920–1936. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Y.; Pang, W.P.; Hwang, S.M.; Tuan, H.Y.; Hu, Y.C. A graphene-based platform for induced pluripotent stem cells culture and differentiation. Biomaterials 2012, 33, 418–427. [Google Scholar] [CrossRef]
- Lotz, M.K.; Caramés, B. Autophagy and cartilage homeostasis mechanisms in joint health, aging and OA. Nat. Rev. Rheumatol. 2011, 7, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, Q.; Zhang, T.; Wang, X.; Cheng, K.; Gao, M.; Xia, P.; Li, X. Low-intensity pulsed ultrasound promotes chondrogenesis of mesenchymal stem cells via regulation of autophagy. Stem Cell Res. Ther. 2019, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Linares, J.; Matesanz, M.C.; Vila, M.; Feito, M.J.; Gonçalves, G.; Valletregí, M.; Marques, P.A.; Portolés, M.T. Endocytic mechanisms of graphene oxide nanosheets in osteoblasts, hepatocytes and macrophages. ACS Appl. Mater. Interfaces 2014, 6, 13697. [Google Scholar] [CrossRef]
- Zhao, Y.; Teng, B.; Sun, X.; Dong, Y.; Wang, S.; Hu, Y.; Wang, Z.; Ma, X.; Yang, Q. Synergistic Effects of Kartogenin and Transforming Growth Factor-β3 on Chondrogenesis of Human Umbilical Cord Mesenchymal Stem Cells In Vitro. Orthop. Surg. 2020. [Google Scholar] [CrossRef]
- Fan, W.; Yuan, L.; Li, J.; Wang, Z.; Chen, J.; Guo, C.; Mo, X.; Yan, Z. Injectable double-crosslinked hydrogels with kartogenin-conjugated polyurethane nano-particles and transforming growth factor β3 for in-situ cartilage regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110705. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, G.; Yan, J.; Chen, X.; He, F.; Zhu, C.; Zhang, J.; Lin, J.; Pan, G.; Yu, J.; et al. Upregulation of SIRT1 by Kartogenin Enhances Antioxidant Functions and Promotes Osteogenesis in Human Mesenchymal Stem Cells. Oxidative Med. Cell. Longev. 2018, 2018, 1368142. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, H.B.; Kim, Y.; Jeong, J.R.; Lee, M.H.; Kang, K. Neurite Guidance on Laser-Scribed Reduced Graphene Oxide. Nano Lett. 2018, 18, 7421–7427. [Google Scholar] [CrossRef] [PubMed]
- Norahan, M.H.; Pourmokhtari, M.; Saeb, M.R.; Bakhshi, B.; Soufi Zomorrod, M.; Baheiraei, N. Electroactive cardiac patch containing reduced graphene oxide with potential antibacterial properties. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109921. [Google Scholar] [CrossRef]
- Norahan, M.H.; Amroon, M.; Ghahremanzadeh, R.; Rabiee, N.; Baheiraei, N. Reduced graphene oxide: Osteogenic potential for bone tissue engineering. IET Nanobiotechnol. 2019, 13, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Montenegro, M.F.; Ntola, C.N.M.; Vranic, S.; Kostarelos, K.; Vogt, C.; Toprak, M.S.; Duan, T.; Leifer, K.; Bräutigam, L.; et al. Nitric oxide-dependent biodegradation of graphene oxide reduces inflammation in the gastrointestinal tract. Nanoscale 2020, 12, 16730–16737. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Sriram, P.; Barui, A.K.; Nethi, S.K.; Veeriah, V.; Chatterjee, S.; Suresh, K.I.; Patra, C.R. Graphene Oxides Show Angiogenic Properties. Adv. Healthc. Mater. 2015, 4, 1722–1732. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Z.; Zheng, Y.; Geng, Y.; Han, C.; Shi, Y.; Sun, H.; Zhang, C.; Chen, Y.; Zhang, L.; et al. Glycyrrhetinic Acid Functionalized Graphene Oxide for Mitochondria Targeting and Cancer Treatment In Vivo. Small 2018, 14. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, S.; Strojny, B.; Sawosz, E.; Wierzbicki, M.; Grodzik, M.; Kutwin, M.; Daniluk, K.; Chwalibog, A. Degradation of Mitochondria and Oxidative Stress as the Main Mechanism of Toxicity of Pristine Graphene on U87 Glioblastoma Cells and Tumors and HS-5 Cells. Int. J. Mol. Sci. 2019, 20, 650. [Google Scholar] [CrossRef] [PubMed]
- Pelin, M.; Fusco, L.; Martín, C.; Sosa, S.; Frontiñán-Rubio, J.; González-Domínguez, J.M.; Durán-Prado, M.; Vázquez, E.; Prato, M.; Tubaro, A. Graphene and graphene oxide induce ROS production in human HaCaT skin keratinocytes: The role of xanthine oxidase and NADH dehydrogenase. Nanoscale 2018, 10, 11820–11830. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.Y.; Laurent, S.; Chen, W.; Akhavan, O.; Imani, M.; Ashkarran, A.A.; Mahmoudi, M. Graphene: Promises, facts, opportunities, and challenges in nanomedicine. Chem. Rev. 2013, 113, 3407–3424. [Google Scholar] [CrossRef]
- Caramés, B.; Taniguchi, N.; Otsuki, S.; Blanco, F.J.; Lotz, M. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 2010, 62, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Luan, Z.; Zhang, X.; Zou, K.; Ma, S.; Yang, Z.; Feng, W.; He, M.; Jiang, L.; Li, J.; et al. Chondro-protective effects of polydatin in osteoarthritis through its effect on restoring dysregulated autophagy via modulating MAPK, and PI3K/Akt signaling pathways. Sci. Rep. 2019, 9, 13906. [Google Scholar] [CrossRef]
- Shan, M.; Qin, J.; Jin, F.; Han, X.; Guan, H.; Li, X.; Zhang, J.; Zhang, H.; Wang, Y. Autophagy suppresses isoprenaline-induced M2 macrophage polarization via the ROS/ERK and mTOR signaling pathway. Free Radic. Biol. Med. 2017, 110, 432–443. [Google Scholar] [CrossRef] [PubMed]



| Gene Name | Forward and Reverse Sequence |
|---|---|
| Sox-9-F | 5′-TCAACGGCTCCAGCAAGAACAAG-3′ |
| Sox-9-R | 5′-CTCCGCCTCCTCCACGAAGG-3′ |
| Col II-F | 5′-ACGCTCAAGTCGCTGAACAACC-3′ |
| Col II-R | 5′-ATCCAGTAGTCTCCGCTCTTCCAC-3′ |
| Aggrecan-F | 5′-CTGATCCACTGTCCAAGCACCATG-3′ |
| Aggrecan-R | 5′-ATCCACGCCAGGCTCCACTC-3′ |
| Col X-F | 5′-TGATCCTGGAGTGGGAGGAG-3′ |
| Col X-R | 5′-GGGATACCTGGTGGTCCAAT-3′ |
| β-actin-F | 5′-GTAAAGACCTCTATGCCAACA-3′ |
| β-actin-R | 5′-GGACTCATCGTACTCCTGCT-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, D.; Wang, J.; Yu, W.; Zhang, N.; Zhang, K.; Bai, Y. Gelatin reduced Graphene Oxide Nanosheets as Kartogenin Nanocarrier Induces Rat ADSCs Chondrogenic Differentiation Combining with Autophagy Modification. Materials 2021, 14, 1053. https://doi.org/10.3390/ma14051053
Jiao D, Wang J, Yu W, Zhang N, Zhang K, Bai Y. Gelatin reduced Graphene Oxide Nanosheets as Kartogenin Nanocarrier Induces Rat ADSCs Chondrogenic Differentiation Combining with Autophagy Modification. Materials. 2021; 14(5):1053. https://doi.org/10.3390/ma14051053
Chicago/Turabian StyleJiao, Delong, Jing Wang, Wenting Yu, Ning Zhang, Ke Zhang, and Yuxing Bai. 2021. "Gelatin reduced Graphene Oxide Nanosheets as Kartogenin Nanocarrier Induces Rat ADSCs Chondrogenic Differentiation Combining with Autophagy Modification" Materials 14, no. 5: 1053. https://doi.org/10.3390/ma14051053
APA StyleJiao, D., Wang, J., Yu, W., Zhang, N., Zhang, K., & Bai, Y. (2021). Gelatin reduced Graphene Oxide Nanosheets as Kartogenin Nanocarrier Induces Rat ADSCs Chondrogenic Differentiation Combining with Autophagy Modification. Materials, 14(5), 1053. https://doi.org/10.3390/ma14051053





