Solutions of Critical Raw Materials Issues Regarding Iron-Based Alloys
Abstract
1. Introduction
- -
- The substitution and minimizing of the use of CRMs and chromium, which could be also considered strategic, in iron-based alloys (steel and cast iron);
- -
- The use of iron-based materials to substitute other CRM-containing materials.
2. Substitution and Saving of Chromium in Stainless Steel
2.1. Stainless Steel under “Dry Corrosion” Conditions
2.2. Stainless Steel under “Wet Corrosion” Conditions
2.3. Cr as CRM in Stainless Steel—Summary
3. Substitution of Critical Raw Materials in Tool Steel
3.1. Rational Approach to the Substitution of CRMs in Tool Steel
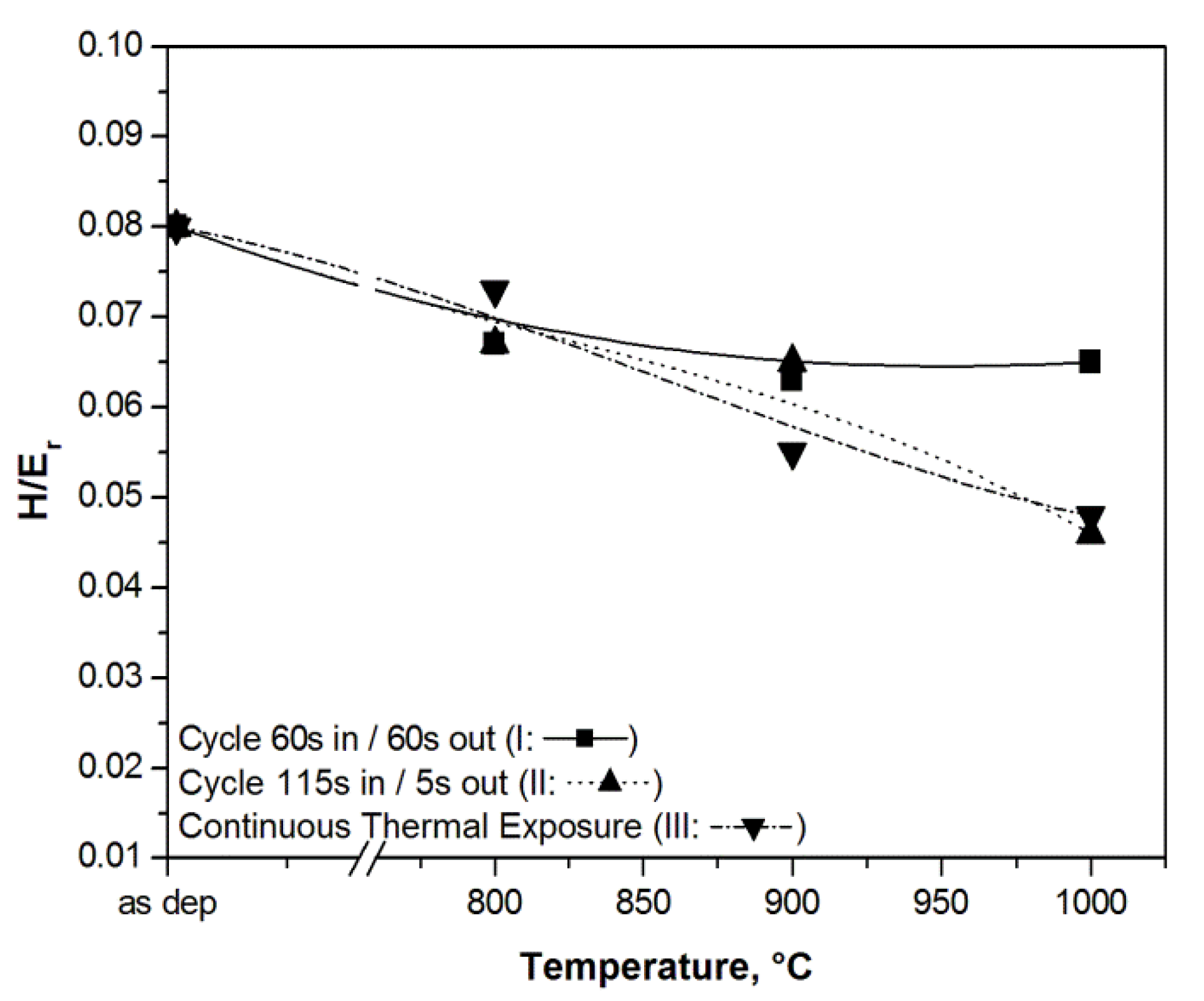
3.2. Hard Coatings of Tools as a Means of Saving CRMs
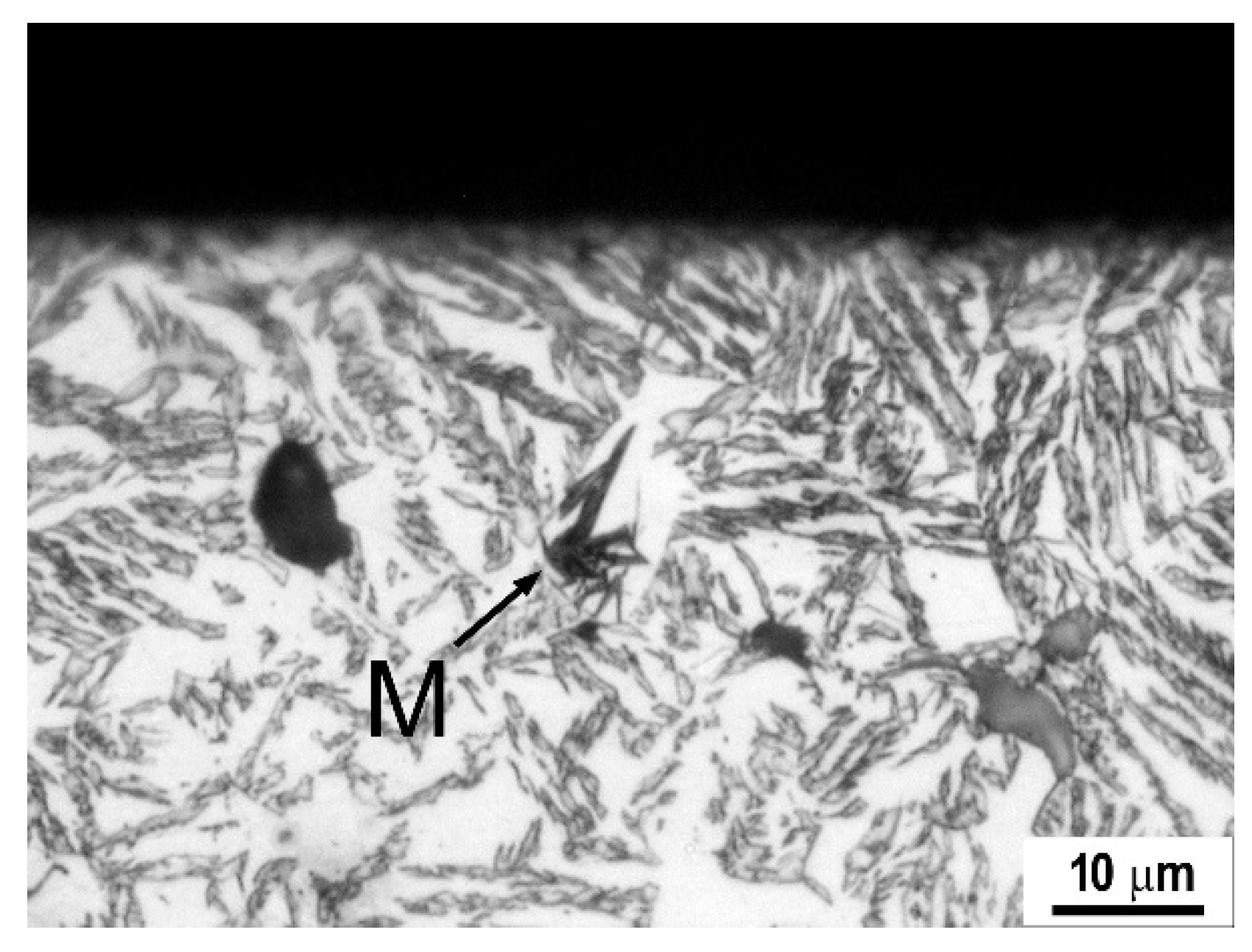
4. Advanced Cast Irons—Austempered Ductile Iron
4.1. Austempered Ductile Irons
4.2. Wear Properties of the ADI
4.3. Cavitation Properties of the ADI
4.4. Ballistic Properties of the ADI
4.5. Summary of the ADI Properties
5. Iron-Based Materials as Solutions for CRM Substitution in Other Tool Materials
- -
- Substitution of tungsten carbide;
- -
- Substitution of cobalt binder;
- -
- Design of a whole-new concept of CRM-free material.
- -
- Tools for extracting large rock blocks in quarries;
- -
- Tools for cutting rock blocks;
- -
- Tools for cutting and processing cladding and tiles;
- -
- Stone grinding and polishing tools.
6. Iron-Based High-Temperature Alloys as CRM Substitutes
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Commission. A New Industrial Strategy for Europe; COM (2020) 102 final; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- European Commission. Tackling the Challenges in Commodity Markets and On Raw Materials; COM (2011) 25 final; European Commission: Brussels, Belgium, 2011. [Google Scholar]
- European Commission. Policy and Strategy for Raw Materials; European Commission: Brussels, Belgium, 2008; Available online: https://ec.europa.eu/growth/sectors/raw-materials/policy-strategy_en (accessed on 1 January 2021).
- European Commission and Directorate_General_Joint_Research_Centre. Methodology for Establishing the EU List of Critical Raw Materials. Guidelines; European Commission: Brussels, Belgium, 2017. [Google Scholar]
- Study on the EU’s list of Critical Raw Materials—Final Report; European Union: Luxembourg, 2020; Available online: https://ec.europa.eu/docsroom/documents/42883/attachments/1/translations/en/renditions/native (accessed on 28 December 2020). [CrossRef]
- Pippel, E.; Woltersdorf, J.; Pöckl, G.; Lichtenegger, G. Microstructure and Nanochemistry of Carbide Precipitates in High-Speed Steel S 6-5-2-5. Mater. Charact. 1999, 43, 41–55. [Google Scholar] [CrossRef]
- Dobrzański, L.A.; Kasprzak, W. The influence of 5% cobalt addition on structure and working properties of the 9-2-2-5, 11-2-2-5 and 11-0-2-5 high-speed steels. J. Mater. Process. Technol. 2001, 109, 52–64. [Google Scholar] [CrossRef]
- Study on the EU’s list of Critical Raw Materials Non-Critical Raw Materials Factsheets; European Union: Luxembourg, 2020; Available online: https://ec.europa.eu/docsroom/documents/42883/attachments/3/translations/en/renditions/native (accessed on 28 December 2020). [CrossRef]
- Working Group on Defining Critical Raw Materials for EU. Report on Critical Raw Materials for EU. 2014. Available online: http://mima.geus.dk/report-on-critical-raw-materials_en.pdf (accessed on 28 December 2020).
- Study on the Review of the List of Critical Raw Materials—Criticality Assessments. 2017. Available online: https://op.europa.eu/en/publication-detail/-/publication/08fdab5f-9766-11e7-b92d-01aa75ed71a1 (accessed on 28 December 2020). [CrossRef]
- Study on the Review of the List of Critical Raw Materials—Non-Critical Raw Materials Factsheets; European Union: Luxembourg, 2017; Available online: https://op.europa.eu/en/publication-detail/-/publication/6f1e28a7-98fb-11e7-b92d-01aa75ed71a1/language-en (accessed on 28 December 2020). [CrossRef]
- Grilli, M.L.; Bellezze, T.; Gamsjäger, E.; Rinaldi, A.; Novak, P.; Balos, S.; Piticescu, R.R.; Ruello, M.L. Solutions for Critical Raw Materials under Extreme Conditions: A Review. Metals 2017, 10, 285. [Google Scholar] [CrossRef]
- Han, J.; Li, Y.; Jiang, Z.; Yang, Y.; Wang, X.; Wang, L.; Li, K. Summary of the Function of Sn in Iron and Steel. Adv. Mat. Res. 2013, 773, 406–411. [Google Scholar] [CrossRef]
- Davis, J.R. Stainless Steel—ASM Specialty Handbook; ASM International: Materials Park, OH, USA, 1994. [Google Scholar]
- Di Caprio, G. Gli Acciai Inossidabili, 4th ed.; Hoepli: Milan, Italy, 2003. [Google Scholar]
- Van Rooyen, G.T. The Potential of Chromium as an Alloying Element. In Proceedings of the 1st International Chromium Steel and Alloys Congress, Cape Town, South Africa, 8–11 March 1992; Volume 2, pp. 43–47. [Google Scholar]
- Metals Handobook, Corrosion, 9th ed.; ASM International: Metals Park, OH, USA, 1987; Volume 13.
- Cunat, P.J. Alloying Elements in Stainless Steel and Other Chromium-Containing Alloys; Euro Inox: Paris, France, 2004; Available online: https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.323.12&rep=rep1&type=pdf (accessed on 28 December 2020).
- Floreen, S. An Examination of Chromium Substitution in Stainless Steels. Metall. Trans. A 1982, 13A, 2003–2013. [Google Scholar] [CrossRef]
- Bittence, J.C. Can There Be “Stainless” Without Chromium? Mater. Eng. 1989, 89, 28–32. [Google Scholar]
- Substitution Alternatives for Strategic Materials. In Strategic Materials: Technologies to Reduce US Import Vulnerability; OTA-ITE-248; US Congress, Office of Technology Assessment: Washington, DC, USA, 1985; Chapter 7; pp. 263–328.
- Glenn, M.L.; Larson, D.E. Reduced-Chromium Stainless Steel Substitutes Containing Silicon and Aluminum; Report of Investigation 8918; United States Department of the Interior, Bureau of Mines: Albany, OR, USA, 1984. [Google Scholar]
- Bullard, S.J.; Larson, D.E.; Dunning, J.S. Oxidation and Corrosion Resistance of Two Fe-8Cr-16Ni-Si-Cu Alloys. Corrosion 1992, 48, 891–897. [Google Scholar] [CrossRef]
- Dunning, J.S.; Alman, D.E.; Rawers, J.C. Influence of Silicon and Aluminum Additions on the Oxidation Resistance of a Lean-Chromium Stainless Steel. Oxid. Met. 2002, 57, 409–425. [Google Scholar] [CrossRef]
- Engkvist, J.; Bexell, U.; Grehk, M.; Olsson, M. High temperature oxidation of FeCrAl-alloys-Influence of Al-concentration on oxide layer characteristics. Mater. Corros. 2009, 60, 876–881. [Google Scholar] [CrossRef]
- Wolff, I.M.; Iorio, L.E.; Rumpf, T.; Scheers, P.V.T.; Potgieter, J.H. Oxidation and corrosion behaviour of Fe-Cr and Fe-Cr-Al alloys with minor alloying additions. Mater. Sci. Eng. A 1998, 241, 264–276. [Google Scholar] [CrossRef]
- Jönsson, B.; Lu, Q.; Chandrasekaran, D.; Berglund, R.; Rave, F. Oxidation and Creep Limited Lifetime of Kanthal APMT®, a Dispersion Strengthened FeCrAlMo Alloy Designed for Strength and Oxidation Resistance at High Temperatures. Oxid. Met. 2013, 79, 29–39. [Google Scholar] [CrossRef]
- Pothen, F.; Goeschl, T.; Löschel, A.; Jaha, V. Strategic Trade Policy and Critical Raw Materials in Stainless Steel Production; Project Report; Zentrum für Europäische Wirtschaftsforschung: Mannheim, Germany, 2013. [Google Scholar]
- Cavallini, M.; Felli, F.; Fratesi, R.; Veniali, F. High temperature air oxidation behaviour of “poor man” high manganese-aluminum steels. Mater. Corros. 1982, 33, 386–390. [Google Scholar] [CrossRef]
- Casteletti, L.C.; Neto, A.L.; Totten, G.E.; Heck, S.C.; Fernandes, F.A.P. Use of Fe-31Mn-7.5Al-1.3Si-0.9C Alloy for Fabrication of Resistive Elements. J. ASTM Int. 2010, 7, 1–4. [Google Scholar]
- Bellezze, T.; Giuliani, G.; Roventi, G.; Fratesi, R.; Andreatta, F.; Fedrizzi, L. Corrosion behaviour of austenitic and duplex stainless steels in an industrial strongly acidic solution. Mater. Corros. 2016, 67, 831–838. [Google Scholar] [CrossRef]
- Chen, W.Y.C.; Stephens, J.R. Anodic Polarization Behaviour of Austenitic Stainless Steel Alloys with Lower Chromium Content. Corrosion 1979, 35, 443–451. [Google Scholar] [CrossRef]
- Glenn, M.L.; Bullard, S.J.; Larson, D.E.; Rhoads, S.C. Partial replacements of chromium in stainless steel. J. Mater. Energy Syst. 1985, 7, 75–81. [Google Scholar] [CrossRef]
- Hio, K.; Yamada, T.; Tsuchida, Y.; Nakajima, K.; Hosoi, Y. Effect of Chromium Content on Anodic Polarization Characteristics of Fe-Cr-Al and Fe-Cr-Si Alloys. Corrosion 2002, 58, 124–131. [Google Scholar] [CrossRef]
- Bellezze, T.; Giuliani, G.; Roventi, G. Study of stainless steels corrosion in a strong acid mixture. Part 1: Cyclic potentiodynamic polarization curves examined by means of an analytical method. Corros. Sci. 2018, 130, 113–125. [Google Scholar] [CrossRef]
- Sheirer, L.L.; Jarman, R.A.; Burnstein, G.T. (Eds.) Stainless Steels. In Corrosion—Metal/Environment Reactions, 3rd ed.; Butterworth-Heinemann Ltd.: Oxford, UK, 1994; Volume 1, pp. 47–70. [Google Scholar]
- Davis, J.R.; Davis and Associates (Eds.) Atmospheric and Aqueous Corrosion. In ASM Speciality Handbook—Stainless Steels; ASTM International: Novelty, OH, USA, 1994; p. 133. [Google Scholar]
- Abdul-Azim, A.A.; Rahem Ghanem, W.A.E.; Abou-Shahba, R.M. Corrosion behaviour of low-Cr high·Al stainless steels in 65% boiling HNO3. Steel Res. 1994, 65, 350–353. [Google Scholar] [CrossRef]
- Reformatskaya, I.I.; Rodionova, I.G.; Podobaev, A.N.; Ashcheulova, I.I.; Trofimova, E.V. Silicon as an Alloying Element in Ferrite Stainless Steels Containing 8–13% Cr. Prot. Met. 2006, 42, 549–554. [Google Scholar] [CrossRef]
- Hodgkiess, T.; Chia, P.S. Assessment of lower-alloy stainless steels for use in desalination plant. Desalination 1991, 84, 267–278. [Google Scholar] [CrossRef]
- Basile, F.; Lorthioir, G. Quantitative analysis, by cathodic reduction, of passive layers on Fe-17Cr alloy and its application to substituted alloys. Brit. Corros. J. 1993, 28, 31–36. [Google Scholar] [CrossRef]
- Wan, J.; Ran, Q.; Li, J.; Xu, Y.; Xiao, X.; Yu, H.; Jiang, L. A new resource-saving, low chromium and low nickel duplex stainless steel 15Cr-xAl-2Ni-yMn. Mater. Des. 2014, 53, 43–50. [Google Scholar] [CrossRef]
- Cavallini, M.; Felli, F.; Fratesi, R.; Veniali, F. Aqueous solution corrosion behaviour of “poor man” high manganese-aluminum steels. Mater. Corros. 1982, 33, 281–284. [Google Scholar] [CrossRef]
- Abuzriba, M.B.; Musa, S.M. Substitution for chromium and nickel in Austenitic stainless steels. In Springer Proceedings in Physics, Proceedings of the 2nd International Multidisciplinary Microscopy and Microanalysis Congress Oludeniz, Turkey, 16–19 October 2014; Springer: Cham, Switzerland, 2015; Volume 164, pp. 205–214. [Google Scholar] [CrossRef]
- Moon, J.; Ha, H.-Y.; Kim, K.-W.; Park, S.-J.; Lee, T.-H.; Kim, S.-D.; Jang, J.H.; Jo, H.-H.; Hong, H.-U.; Lee, B.H.; et al. A new class of lightweight, stainless steels with ultra-high strength and large ductility. Sci. Rep. 2020, 10, 12140. [Google Scholar] [CrossRef] [PubMed]
- Tandon, V.; Patil, A.P.; Rathod, R.C. Enhanced corrosion resistance of Cr-Mn ASS by low temperature salt bath nitriding technique for the replacement of convectional Cr-Ni ASS. Anti-Corros. Methods Mater. 2019, 66, 439–445. [Google Scholar] [CrossRef]
- Li, C.; Bell, T. Corrosion properties of plasma nitrided AISI 410 martensitic stainless steel in 3.5% NaCl and 1% HCl aqueous solutions. Corros. Sci. 2006, 48, 2036–2049. [Google Scholar] [CrossRef]
- Sakasegawa, H.; Tanigawa, H.; Ando, M. Corrosion-resistant coating technique for oxide-dispersion-strengthened ferritic/martensitic steel. J. Nucl. Sci. Technol. 2014, 51, 737–743. [Google Scholar] [CrossRef]
- Bobzin, K.; Zhao, L.; Öte, M.; Königstein, T. Development of a FeCrMnBC-based economical wear and corrosion resistant coating. Surf. Coat. Technol. 2019, 362, 12–20. [Google Scholar] [CrossRef]
- Kotrba, A.; Quan, T.; Wei, W.; Detweiler, Z.; Keifer, D.; Bullard, D. Spatially Optimized Diffusion Alloys: A Novel Multi-Layered Steel Material for Exhaust Applications. SAE Int. 2020, 2, 2135–2141. [Google Scholar] [CrossRef]
- Bellezze, T.; Roventi, G.; Quaranta, A.; Fratesi, R. Improvement of pitting corrosion resistance of AISI 444 stainless steel to make it a possible substitute for AISI 304L and 316L in hot natural waters. Mater. Corros. 2008, 59, 727–731. [Google Scholar] [CrossRef]
- Parsons, S.; Poyntz-Wright, O.; Kent, A.; McManus, M.C. Green chemistry for stainless steel corrosion resistance: Life cycle assessment of citric acid versus nitric acid passivation. Mater. Today Sustain. 2019, 3–4, 100005. [Google Scholar] [CrossRef]
- Balzar, D.; Ledbetter, H. Accurate Modeling of Size and Strain Broadening in the Rietveld Refinement: The “Double-Voigt” Approach, Advances in X-Ray Analysis 38; Plenum Press: New York, NY, USA, 1995; pp. 397–404. [Google Scholar]
- Wiessner, M.; Gamsjäger, E.; Van Der Zwaag, S.; Angerer, P. Effect of reverted austenite on tensile and impact strength in a martensitic stainless steel ? An in-situ X-ray diffraction study. Mater. Sci. Eng. A 2017, 682, 117–125. [Google Scholar] [CrossRef]
- Wießner, M.; Leisch, M.; Emminger, H.; Kulmburg, A. Phase transformation study of a high speed steel powder by high temperature X-ray diffraction. Mater. Charact. 2008, 59, 937–943. [Google Scholar]
- Novák, P.; Michalcová, A.; Marek, I.; Mudrová, M.; Saksl, K.; Bednarčík, J.; Zikmund, P.; Vojtěch, D. On the formation of intermetallics in Fe Al system an in situ XRD study. Intermetallics 2013, 32, 127–136. [Google Scholar]
- Wiessner, M.; Angerer, P.; Van der Zwaag, S.; Gamsjäger, E. Transient Phase Fraction and Dislocation Density Estimation from In-Situ X-Ray Diffraction Data with a Low Signal-to-Noise Ratio Using a Bayesian Approach to the Rietveld Analysis. Mater. Charact. 2021, 172, s.110860. [Google Scholar] [CrossRef]
- Karagöz, S.; Fischmeister, H.F. Cutting Performance and Microstructure of High Speed Steels: Contributions of Matrix Strengthening and Undissolved Carbides. Met. Mater. Trans. A 1998, 29, 205–216. [Google Scholar] [CrossRef]
- Li, K.; Yu, B.; Misra, R.D.K.; Han, G.; Liu, S.; Shang, C.J. Strengthening of cobalt-free 19Ni3Mo1.5Ti maraging steel through high-density and low lattice misfit nanoscale precipitates. Mater. Sci. Eng. A 2018, 715, 174–185. [Google Scholar] [CrossRef]
- Fathy, A.; Mattar, T.; EI-Faramawy, H.; Bleck, W. Mechanical properties of new low-nickel cobalt-free maraging steels. Steel Res. 2002, 73, 549–556. [Google Scholar] [CrossRef]
- Cheng, L.; Böttger, A.; De Keijser, T.H.; Mittemeijer, E.J. Lattice parameters of iron-carbon and iron-nitrogen martensites and austenites. Scr. Metall. Mater. 1990, 24, 509–514. [Google Scholar] [CrossRef]
- Krisement, O. Kalorimetrische Untersuchungen zur Kinetik des Martensitanlassens. Archiv für Eisenhüttenwesen 1957, 27, 731–742. [Google Scholar] [CrossRef]
- Dobrzanski, L.A.; Zarychta, A.; Ligarski, M. High-Speed Steels with Addition of Niobium or Titanium. J. Mater. Process. Technol. 1997, 63, 531. [Google Scholar] [CrossRef]
- Mirzaee, M.; Momeni, A.; Keshmiri, H.; Razavinejad, R. Effect of Titanium and Niobium on Modifying the Microstructure of Cast K100 Tool Steel. Met. Mater. Trans. B 2014, 45, 2304–2314. [Google Scholar] [CrossRef]
- Pavlickova, M.; Vojtech, D.; Stolar, P.; Jurci, P. Properties of rapidly solidified niobium-alloyed tool steel. Kovove Materialy 2002, 40, 171–183. [Google Scholar]
- Pavlíčková, M.; Vojtěch, D.; Novák, P.; Gemperlová, J.; Gemperle, A.; Zárubová, N.; Jurči, P.; Lejček, P. Influence of Thermal Treatment on Microstructure and Hardness of Niobium Alloyed PM/Tool Steel. Instrum. Sci. Technol. 2004, 32, 207–219. [Google Scholar] [CrossRef]
- Novák, P.; Vojtěch, D.; Šerák, J.; Knotek, V.; Bartová, B. Duplex surface treatment of the Nb-alloyed PM tool steel. Surf. Coatings Technol. 2006, 201, 3342–3349. [Google Scholar] [CrossRef]
- Shim, K.H.; Lee, S.K.; Kang, B.S.; Hwang, S.M. Investigation of blanking of thin sheet metal using ductile fracture criterion and its experimental verification. J. Mater. Process. Technol. 2004, 155–156, 1935–1942. [Google Scholar] [CrossRef]
- Monteil, G.; Greban, F.; Roizard, X. In situ punch wear measurement in a blanking tool by means of thin layer activation. Wear 2008, 265, 626–633. [Google Scholar] [CrossRef]
- Mayrhofer, P.H.; Mitterer, C.; Hultman, L.; Clemens, H. Microstructural design of hard coatings. Prog. Mater. Sci. 2006, 51, 1032–1114. [Google Scholar] [CrossRef]
- Hovsepian, P.E.; Lewis, D.B.; Münz, W.-D. Recent progress in large scale manufacturing of multilayer/superlattice hard coatings. Surf. Coat. Technol. 2000, 133–134, 166–175. [Google Scholar] [CrossRef]
- Tkadletz, M.; Schalk, N.; Daniel, R.; Keckes, J.; Czettl, C.; Mitterer, C. Advanced characterization methods for wear resistant hard coatings: A review on recent progress. Surf. Coat. Technol. 2016, 285, 31–46. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Kim, S.K.; Lee, D.B. High-temperature oxidation of nano-multilayered TiAlCrSiN thin films in air. Surf. Coatings Technol. 2009, 204, 697–704. [Google Scholar] [CrossRef]
- Kalss, W.; Reiter, A.; Derflinger, V.; Gey, C.; Endrino, J.L. Modern coatings in high performance cutting applications. Int. J. Refract. Met. Hard Mater. 2006, 24, 399–404. [Google Scholar] [CrossRef]
- Endrino, J.L.; Derflinger, V. The influence of alloying elements on the phase stability and mechanical properties of AlCrN coatings. Surf. Coatings Technol. 2005, 200, 2114–2122. [Google Scholar] [CrossRef]
- Kim, D.G.; Seong, T.Y.; Baik, Y.J. Effects of annealing on the microstructures and mechanical properties of TiN/AlN nano-multilayer films prepared by ion-beam assisted deposition. Surf. Coat. Technol. 2002, 153, 79–83. [Google Scholar] [CrossRef]
- Shinn, M.; Hultman, L.; Barnett, S.A. Growth, structure and microhardness of epitaxial TiN/ NbN superlattices. J. Mater. Res. 1992, 7, 901–911. [Google Scholar] [CrossRef]
- Ali, F.; Park, B.S.; Kwak, J.S. Effect of number of bi-layers on properties of TiN/TiAlN multilayer coatings. J. Ceram. Process Res. 2013, 14, 476–479. [Google Scholar]
- Yang, Q.; He, C.; Zhao, L.R.; Immarigeon, J.P. Preferred orientation and hardness enhancement of TiN/CrN superlattice coatings deposited by reactive magnetron sputtering. Scr. Mater. 2002, 46, 293–297. [Google Scholar] [CrossRef]
- Lin, J.; Moore, J.J.; Mishra, B.; Pinkas, M.; Zhang, X.; Sproul, W.D. CrN/AlN superlattice coatings synthesized by pulsed closed field unbalanced magnetron sputtering with different CrN layer thicknesses. Thin Solid Films 2009, 517, 5798–5804. [Google Scholar] [CrossRef]
- Reiter, A.E.; Derflinger, T.V.H.; Hanselmann, B.; Bachmann, T.; Sartory, B. Investigation of the properties of Al1-xCrxN coatings prepared by cathodic arc evaporation. Surf. Coat. Technol. 2005, 200, 2114–2122. [Google Scholar] [CrossRef]
- Jakubéczyová, D.; Hvizdoš, P.; Selecká, M. Investigation of thin layers deposited by two PVD techniques on high speed steel produced by powder metallurgy. Appl. Surf. Sci. 2012, 258, 5105–5110. [Google Scholar] [CrossRef]
- Cabibbo, M.; Ricci, P.; Cecchini, R.; Rymuza, Z.; Sullivan, J.; Dub, S.; Cohen, S. An international round-robin calibration protocol for nanoindentation measurements. Micron 2012, 43, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Cabibbo, M.; Clemente, N.; El Mehtedi, M.; Hamouda, A.H.; Musharavati, F.; Santecchia, E.; Spigarelli, S. Constitutive analysis for the quantification of hardness decay in a superlattice CrN/NbN hard-coating. Surf. Coat. Technol. 2015, 275, 155–166. [Google Scholar] [CrossRef]
- Santecchia, E.; Hamouda, A.M.S.; Musharavati, F.; Zalnezhad, E.; Cabibbo, M.; Spigarelli, S. Wear resistance investigation of titanium nitride-based coatings. Ceram. Int. Part A 2015, 41, 10349–10379. [Google Scholar] [CrossRef]
- Fabrizi, A.; Cecchini, R.; Kiryukhantsev-Korneev, P.V.; Sheveyko, A.N.; Spigarelli, S.; Cabibbo, M. Comparative investigation of oxidation resistance and thermal stability of nanostructured Ti-B-N and Ti-Si-B-N coatings. Prot. Met. Phys. Chem. Surf. 2017, 53, 452–459. [Google Scholar] [CrossRef]
- Santecchia, E.; Cabibbo, M.; Hamouda, A.M.S.; Musharavati, F.; Popelka, A.; Spigarelli, S. Investigation of the Temperature-Related Wear Performance of Hard Nanostructured Coatings Deposited on a S600 High Speed Steel. Metals 2019, 9, 332. [Google Scholar] [CrossRef]
- Kawate, M.; Hashimoto, A.K.; Suzuki, T. Oxidation resistance of Cr1-xAlxN and Ti1-xAlxN films. Surf. Coat. Technol. 2003, 165, 163–167. [Google Scholar] [CrossRef]
- Choi, P.-P.; Povstugar, I.; Ahn, J.-P.; Kostka, A.; Raabe, D. Thermal stability of TiAlN/CrN multilayer coatings studied by atom probe tomography. Ultramicroscopy 2011, 111, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Barshilia, H.; Prakash, M.S.; Jain, A.; Rajam, K.S. Structure, hardness and thermal stability of TiAlN and nanolayered TiAlN/CrN multilayer films. Vacuum 2005, 77, 169–179. [Google Scholar] [CrossRef]
- Forsén, R.; Johansson, M.P.; Odén, M.; Ghafoor, N. Effects of Ti alloying of AlCrN coatings on thermal stability and oxidation resistance. Thin Solid Films 2013, 534, 394–402. [Google Scholar] [CrossRef]
- Beake, B.D.; Fox-Rabinovich, G.S. Progress in high temperature nanomechanical testing of coatings for optimising their performance in high speed machining. Surf. Coat. Technol. 2014, 255, 102–111. [Google Scholar] [CrossRef]
- 48th Census of World Casting Production. Modern. Cast. 2014, 104, 17–21.
- Sidjanin, L.; Smallman, E.R.; Young, J.M. Electron Microstructure and Mechanical Properties of Silicon and Aluminium Ductile Irons. Acta Met. Mater. 1994, 42, 3149–3156. [Google Scholar] [CrossRef]
- Sidjanin, L.; Rajnovic, D.; Eric, O.; Smallman, R.E. Austempering study of unalloyed and alloyed ductile irons. Mater. Sci. Technol. 2010, 26, 567–571. [Google Scholar] [CrossRef]
- Eric, O.; Sidjanin, L.; Rajnovic, D.; Balos, S. The Austempering Kinetics of Cu-Ni Alloyed Austempered Ductile Iron. Met. Mater. Int. 2014, 20, 1131–1138. [Google Scholar] [CrossRef]
- Rajnovic, D.; Eric, O.; Sidjanin, L. The standard processing window of alloyed ADI materials. Kovove Mater. 2012, 50, 199–208. [Google Scholar] [CrossRef][Green Version]
- Rajnovic, D.; Eric, O.; Sidjanin, L. Transition temperature and fracture mode of as-cast and austempered ductile iron. J. Microsc. 2008, 232, 605–610. [Google Scholar] [CrossRef]
- Martinez, R.A. Fracture surfaces and the associated failure mechanisms in ductile iron with different matrices and load bearing. Eng. Fract. Mech. 2010, 77, 2749–2762. [Google Scholar] [CrossRef]
- Eric, O.; Rajnović, D.; Zec, S.; Sidjanin, L.; Jovanović, T. Microstructure and fracture of alloyed austempered ductile iron. Mater. Charact. 2006, 57, 211–217. [Google Scholar] [CrossRef]
- Harding, R.A. The production, properties and automotive applications for austempered ductile iron. Kovove Mater. 2007, 45, 1–16. [Google Scholar]
- Goergen, F.; Mevissen, D.; Masaggia, S.; Veneri, E.; Brimmers, J.; Brecher, C. Contact Fatigue Strength of Austempered Ductile Iron (ADI) in Gear Applications. Metals 2020, 10, 1147. [Google Scholar] [CrossRef]
- Balos, S.; Rajnovic, D.; Dramicanin, M.; Labus, D.; Cekic, O.E.; Grbovic-Novakovic, J.; Sidjanin, L. Abrasive wear behaviour of ADI material with various retained austenite content. Int. J. Cast Metals Res. 2016, 29, 187–193. [Google Scholar] [CrossRef]
- Dojcinovica, M.; Cekic, O.E.; Rajnovic, D.; Sidjanin, L.; Balos, S. Effect of austempering temperature on cavitation behaviour of unalloyed ADI material. Mater. Charact. 2013, 82, 66–72. [Google Scholar] [CrossRef]
- Rajnovic, D.; Balos, S.; Sidjanin, L.; Cekic, O.E.; Grbovic Novakovic, J. Tensile properties of ADI material in water and gaseous environments. Mater. Charact. 2015, 101, 26–33. [Google Scholar] [CrossRef]
- Janjatovic, P.; Cekic, O.E.; Sidjanin, L.; Balos, S.; Dramicanin, M.; Grbovic Novakovic, J.; Rajnovic, D. The Effect of Water Concentration in Ethyl Alcohol on the Environmentally Assisted Embrittlement of Austempered Ductile Irons. Metals 2021, 11, 94. [Google Scholar] [CrossRef]
- Balos, S.; Radisavljevic, I.; Rajnovic, D.; Dramicanin, M.; Tabakovic, S.; Cekic, O.E.; Sidjanin, L. Geometry, mechanical and ballistic properties of ADI material perforated plates. Mater. Des. 2015, 83, 66–74. [Google Scholar] [CrossRef]
- Balos, S.; Radisavljevic, I.; Rajnovic, D.; Janjatovic, P.; Dramicanin, M.; Eric-Cekic, O.; Sidjanin, L. Ballistic Behaviour of Austempered Compacted Graphite Iron Perforated Plates. Def. Sci. J. 2019, 69, 571–576. [Google Scholar] [CrossRef]
- Novák, P.; Vanka, T.; Nová, K.; Stoulil, J.; Průša, F.; Kopeček, J.; Haušild, P.; Laufek, F. Structure and Properties of Fe–Al–Si Alloy Prepared by Mechanical Alloying. Materials 2019, 12, 2463. [Google Scholar] [CrossRef]
- Ringwood, A.E. Diamond Compacts and Process for Making Same. U.S. Patent 4948388, 14 August 1990. Available online: https://www.freepatentsonline.com/4948388.html (accessed on 15 January 2021).
- Jaworska, L. Diamond-Ceramic Bonding Phase Composites for Application in Cutting Tools. Ceram. Mater. 2011, 63, 131–137. [Google Scholar]
- Cygan, S.; Jaworska, L.; Putyra, P.; Ratuszek, W.; Cyboroń, J.; Klimczyk, P. Thermal Stability and Coefficient of Friction of the Diamond Composites with the Titanium Compound Bonding Phase. J. Mater. Eng. Perform. 2017, 26, 2593–2598. [Google Scholar] [CrossRef]
- Tönshoff, H.K.; HillmannApmann, H.; Asche, j. Diamond tools in stone and civil engineering industry: Cutting principles, wear and applications. Diam. Relat. Mater. 2002, 11, 736–741. [Google Scholar] [CrossRef]
- Dormishi, A.; Ataei, M.; Mikaeil, R.; Khalokakaei, R.; Haghshenas, S.S. Evaluation of gang saws’ performance in the carbonate rock cutting process using feasibility of intelligent approaches. Eng. Sci. Technol. Int. J. 2019, 22, 990–1000. [Google Scholar] [CrossRef]
- Ersoy, A.; Atici, U. Performance characteristics of circular diamond saws in cutting different types of rocks. Diam. Relat. Mater. 2004, 13, 22–37. [Google Scholar] [CrossRef]
- Konstanty, J. Production parameters and materials selection of powder metallurgy diamond tools. Powder Metall. 2006, 49, 299–306. [Google Scholar] [CrossRef]
- Büttner, A. Diamond tools and stone. Ind. Diam. Rev. 1974, 3, 89–93. [Google Scholar]
- Chalus, P.A.D. Metal powders for optimum grain retention. Ind. Diam. Rev. 1994, 54, 170–172. [Google Scholar]
- Bullen, G.J. Choosing the best grit for the job. Ind. Diam. Rev. 1982, 1, 7–12. [Google Scholar]
- Konstanty, J. The materials science of stone sawing. Ind. Diam. Rev. 1991, 1, 27–31. [Google Scholar]
- Wright, D.N.; Tagg, W.R.J. The development of a rock classification system for use with diamond tools. Ind. Diam. Rev. 1998, 4, 113–119. [Google Scholar]
- Molinari, A.; Marchetti, F.; Gialanella, S.; Scardi, P.; Tiziani, A. Study of the Diamond-Matrix Interface in Hot-pressed Cobalt-based Tools. Mater. Sci. Eng. A 1990, 130, 257–262. [Google Scholar] [CrossRef]
- Hsieh, Y.Z.; Lin, S.T. Diamond tool bits with iron alloys as the binding matrixes. Mater. Chem. Phys. 2001, 72, 121–125. [Google Scholar] [CrossRef]
- Spriano, S.; Chen, Q.; Settineri, L.; Bugliosi, S. Low content and free Cobalt matrixes for diamond tools. Wear 2005, 259, 1190–1196. [Google Scholar] [CrossRef]
- Del Villar, M.; Muro, P.; Sanchez, J.M.; Iturriza, I.; Castro, F. Consolidation of diamond tools using Cu-Co-Fe based alloys as metallic binders. Powder Metall. 2001, 44, 82–90. [Google Scholar] [CrossRef]
- Lison, D.; Buchet, J.P.; Swennen, B.; Molders, J.; Lauwerys, R. Biological monitoring of workers exposed to cobalt metal, salt, oxides, and hard metal dust. Occup. Environ. Med. 1994, 51, 447–450. [Google Scholar] [CrossRef]
- Goerting, K.; Brewin, P. European New Chemicals Policy Response of The Hard Materials Industry. In Proceedings of the European Conference on Hard Materials and Diamond Tooling—Euro PM 2002, EPMA, Lausanne, Switzerland, 7–9 October 2002; pp. 9–18. [Google Scholar]
- Weber, G.; Weiss, C. DIAMIX—A family of bonds based on DIABASE-V21. Ind. Diam. Rev. 2005, 65, 27–28. [Google Scholar]
- Bonneau, M. NEXT and NEXT Pre-mixed Powders. Diam. Appl. Technol. 1999, 18, 45–52. [Google Scholar]
- Clark, I.E. Cobalite HDR-a new prealloyed matrix powder for diamond construction tools. Ind. Diam. Rev. 2002, 3, 177–182. [Google Scholar]
- Eurotungstene. Keen®—A new concept in prealloyed powders. Ind. Diam. Rev. 2005, 3, 45–47. [Google Scholar]
- Kamphuis, B.; Serneels, A. Cobalt and nickel free bond powder for diamond tools: Cobalite® CNF. Ind. Diam. Rev. 2004, 1, 26–32. [Google Scholar]
- De Oliveira, H.C.P.; Cabral, S.C.; Guimaries, R.S.; Bobrovnitchii, G.S.; Filgueira, M. Processing and characterization of a cobalt based alloy for use in diamond cutting tools. Materialwissenschaft 2009, 40, 907–909. [Google Scholar] [CrossRef]
- Palumbo, M.; Curiotto, S.; Battezzati, L. Thermodynamic analysis of the stable and metastable Co-Cu and Co-Cu-Fe phase diagrams. Calphad 2006, 30, 171–178. [Google Scholar] [CrossRef]
- Huang, X.; Mashimo, T. Metastable BCC and FCC alloy bulk bodies in Fe-Cu system prepared by mechanical alloying and shock compression. J. Alloys Compd. 1999, 288, 299–305. [Google Scholar] [CrossRef]
- Gaffet, E.; Harmelin, M.; Faudot, F. Far-from-equilibrium phase transition induced by mechanical alloying in the Cu-Fe system. J. Alloys Compd. 1993, 194, 23–30. [Google Scholar] [CrossRef]
- Menapace, C.; Bocchi, E.; Costa, P.; Molinari, A. Microstructural and mechanical characterization of iron and copper based powders for diamond tools. In Proceedings of the 2004 Powder Metallurgy World Congress, European Powder Metallurgy Association, Vienna, Austria, 17–21 October 2004; Volume 3, p. 681. [Google Scholar]
- Menapace, C.; Costa, P.; Molinari, A. Wear and Cutting Properties of New Diamond Inserts Based on Iron and Copper Powders. In Proceedings of the European Powder Metallurgy Congress and Exhibition, Prague, Czech Republic, 2–5 October 2005; pp. 311–316. [Google Scholar]
- De Oliveira, L.J.; Bobrovnitchii, G.S.; Filgueira, M. Processing and characterization of impregnated diamond cutting tools using a ferrous metal matrix. Int. J. Refract. Hard Met. 2007, 25, 328–335. [Google Scholar] [CrossRef]
- Meszaros, M.; Vadasdi, K. Process and equipment for electrochemical etching of Diamond-containing Co-WC tools and recovery of diamond from used steel tools. Int. J. Refract. Metals Hard Mater. 1994, 14, 229–234. [Google Scholar] [CrossRef]
- Baroura, L.; Boukhobza, A.; Derardja, A.; Fedaoui, K. Study of Microstructure and Mechanical Properties of Sintered Fe-Cu Alloys. Int. J. Eng. Res. Afr. 2018, 34, 5–12. [Google Scholar]
- Sung, C.M.; Tai, M.F. Reactivities of transition metals with carbon: Implications to the mechanism of diamond synthesis under high pressure. Int. J. Refract. Hard Met. Hard Mater. 1997, 15, 237–256. [Google Scholar] [CrossRef]
- Tillmann, W.; Ferreira, M.; Steffen, A.; Rüster, K.; Möller, J.; Bieder, S.; Paulus, M.; Tolan, M. Carbon reactivity of binder metals in diamond-metal composites—Characterization by scanning electron microscopy and X-ray diffraction. Diam. Relat. Mater. 2013, 38, 118–123. [Google Scholar] [CrossRef]
- Jaworska, L.; Szutkowska, M.; Klimczyk, P.; Sitarz, M.; Bucko, M.; Rutkowski, P.; Figiel, P.; Lojewska, J. Oxidation, graphitization, and thermal resistance of PCD materials with the various bonding phases of up to 800 °C. Int. J. Refract. Met. Hard Mater. 2014, 45, 109–116. [Google Scholar] [CrossRef]
- De Oliveira, L.J.; Cabral, S.C.; Filgueira, M. Study of the TiC Coating on Powder Metallurgy Diamonds Tool’s Performance. Mater. Res. 2015, 18, 441–447. [Google Scholar] [CrossRef][Green Version]
- Borowiecka-Jamrozek, J.; Lachowski, J. Modelling of retention of a diamond particle in matrices based on Fe and Cu. In Proceedings of the XXI International Polish-Slovak Conference “Machine Modeling and Simulations”, Hucisko, Poland, 6–8 September 2016. [Google Scholar] [CrossRef]
- Borowiecka-Jamrozek, J.; Konstanty, J.; Lachowski, J. The application of a ball-milled Fe-Cu-Ni powder mixture to fabricate sintered diamond tools. Arch. Foundry Eng. 2018, 18, 5–8. [Google Scholar]
- Konstanty, J.; Romański, A.; Baczek, E.; Tyrala, D. New Wear Resistant Iron-Base Matrix Materials for The Fabrication of Sintered Diamond Tools. Arch. Met. Mater. 2015, 60, 633–637. [Google Scholar] [CrossRef]
- Konstanty, J.; Romanski, A. New nanocrystalline Matrix Materials for Sintered Diamond Tools. Mater. Sci. Appl. 2012, 3, 779–783. [Google Scholar] [CrossRef][Green Version]
- Borowiecka-Jamrozek, J. Sintered Fe-Cu-Re alloys produced from commercially available powders. Arch. Met. Mater. 2017, 62, 1713–1720. [Google Scholar] [CrossRef]
- Mechnik, V.A.; Bondarenko, N.A.; Kolodnitskyi, V.M.; Zakiev, V.I.; Zakiev, I.M.; Ignatovich, S.R.; Yutskevych, S.S. Mechanical and Tribological Properties of Fe-Cu-Ni-Sn Materials with Different Amounts of CrB2 Used as Matrices for Diamond-Containing Composites. J. Superhard Mater. 2020, 42, 251–263. [Google Scholar] [CrossRef]
- Tyrala, D.; Romanski, A.; Konstanty, J. The Effects of Powder Composition on Microstructure and Properties of Hot-Pressed Matrix Materials for Sintered Diamond Tools. J. Mater. Eng. Perform. 2020, 29, 1467–1472. [Google Scholar] [CrossRef]
- Loginov, P.A.; Sidorenko, D.A.; Bychkova, M.Y.; Zaitsev, A.A.; Levashov, E.A. Performance of diamond drill bits with hybrid nanoreinforced Fe-Ni-Mo binder. Int. J. Adv. Manuf. Technol. 2020, 102, 2041–2047. [Google Scholar] [CrossRef]
- Kratochvíl, P. The history of the search and use of heat resistant Pyroferal© alloys based on FeAl. Intermetallics 2008, 16, 587–591. [Google Scholar] [CrossRef]
- Vodičková, V.; Švec, M.; Hanus, P.; Novák, P.; Záděra, A.; Keller, V.; Prokopčáková, P.P. The Effect of Simultaneous Si and Ti/Mo Alloying on High-Temperature Strength of Fe3Al-Based Iron Aluminides. Molecules 2020, 25, 4268. [Google Scholar] [CrossRef]
- Novák, P.; Nová, K. Oxidation Behavior of Fe-Al, Fe-Si and Fe-Al-Si Intermetallics. Materials 2019, 12, 1748. [Google Scholar] [CrossRef] [PubMed]
- Novák, P.; Zelinková, M.; Šerák, J.; Michalcová, A.; Novák, M.; Vojtěch, D. Oxidation resistance of SHS Fe-Al-Si alloys at 800 °C in air. Intermetallics 2011, 19, 1306–1312. [Google Scholar] [CrossRef]
- Novák, P.; Jaworska, L.; Cabibbo, M. Intermetallics as innovative CRM-free materials. IOP Conference Series: Mater. Sci. Eng. 2018, 329, 012013. [Google Scholar] [CrossRef]
- Šerák, J.; Vojtěch, D.; Novák, P.; Šefl, V.; Janoušek, T. Možnosti snížení obsahu železa ve slitinách AlSiCuMgFe. Slévárenství 2008, 56, 343–345. [Google Scholar]
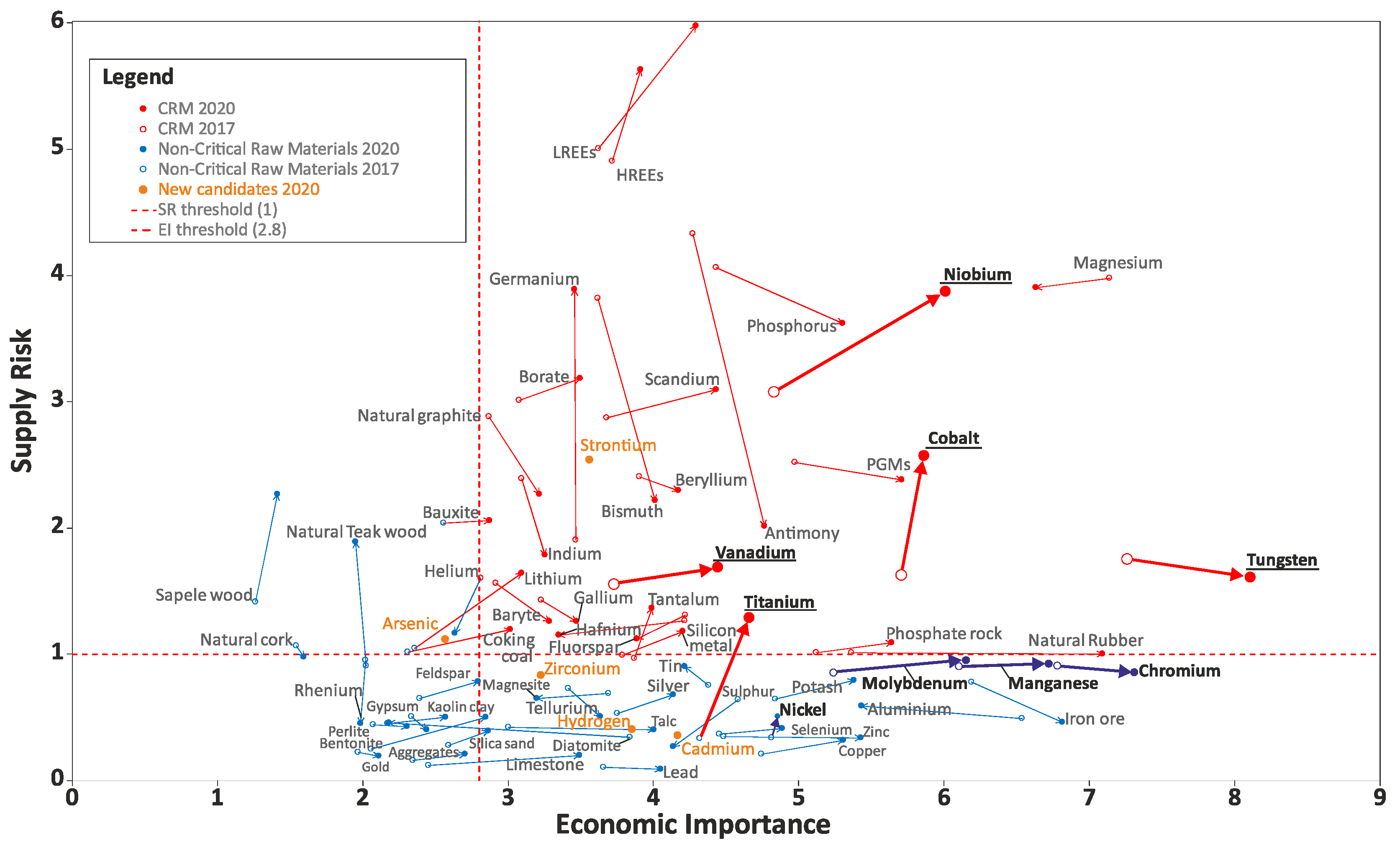
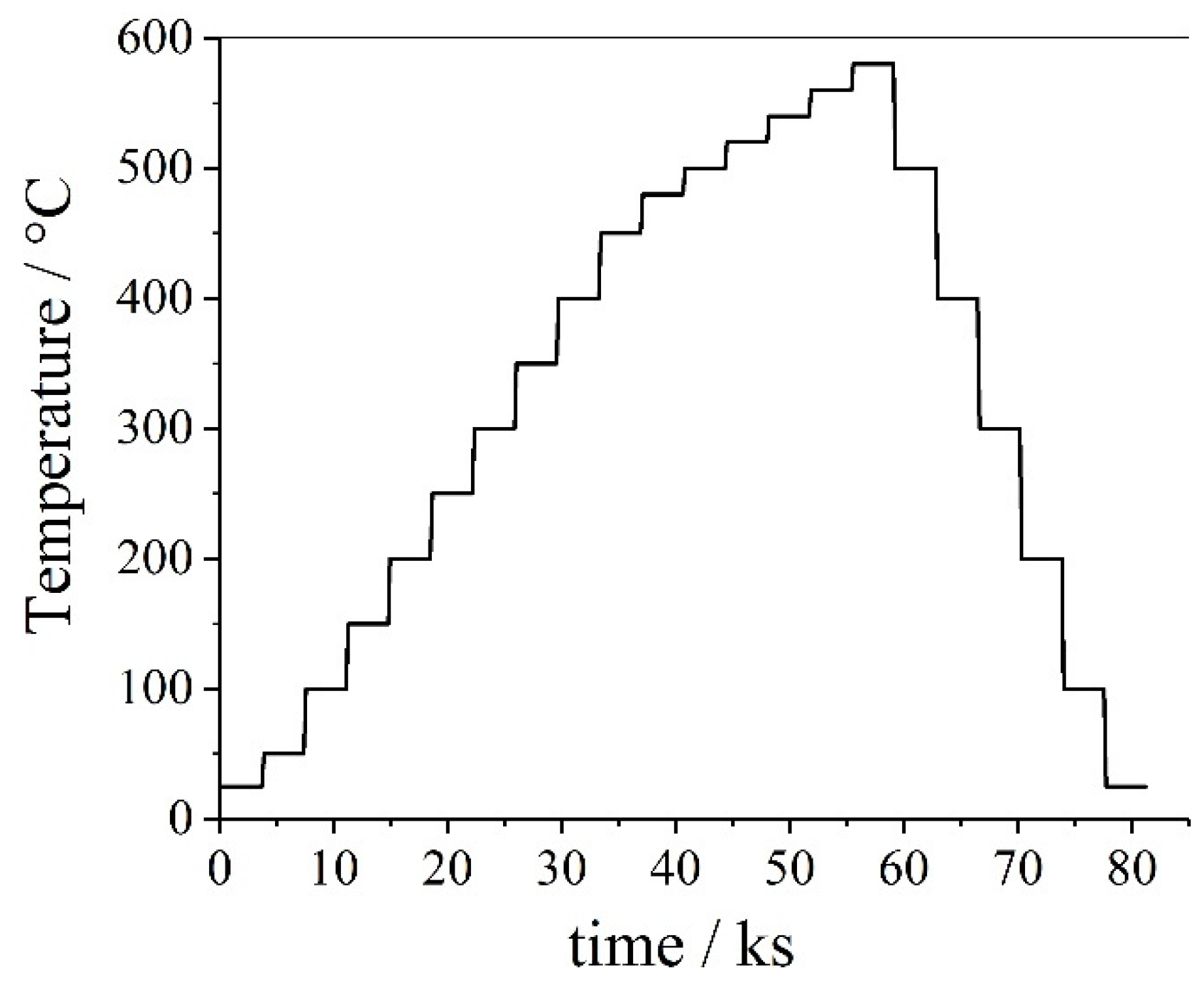
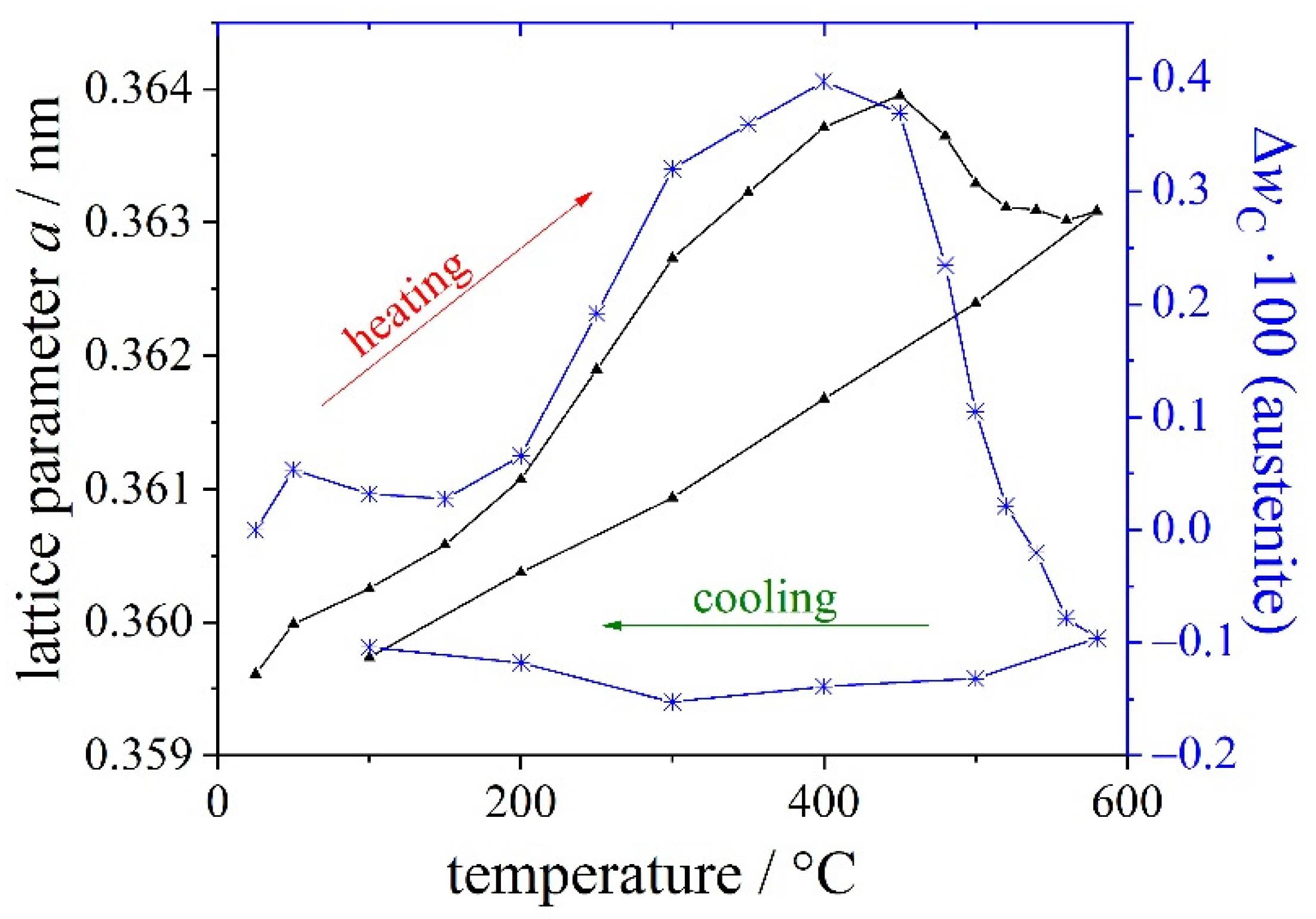
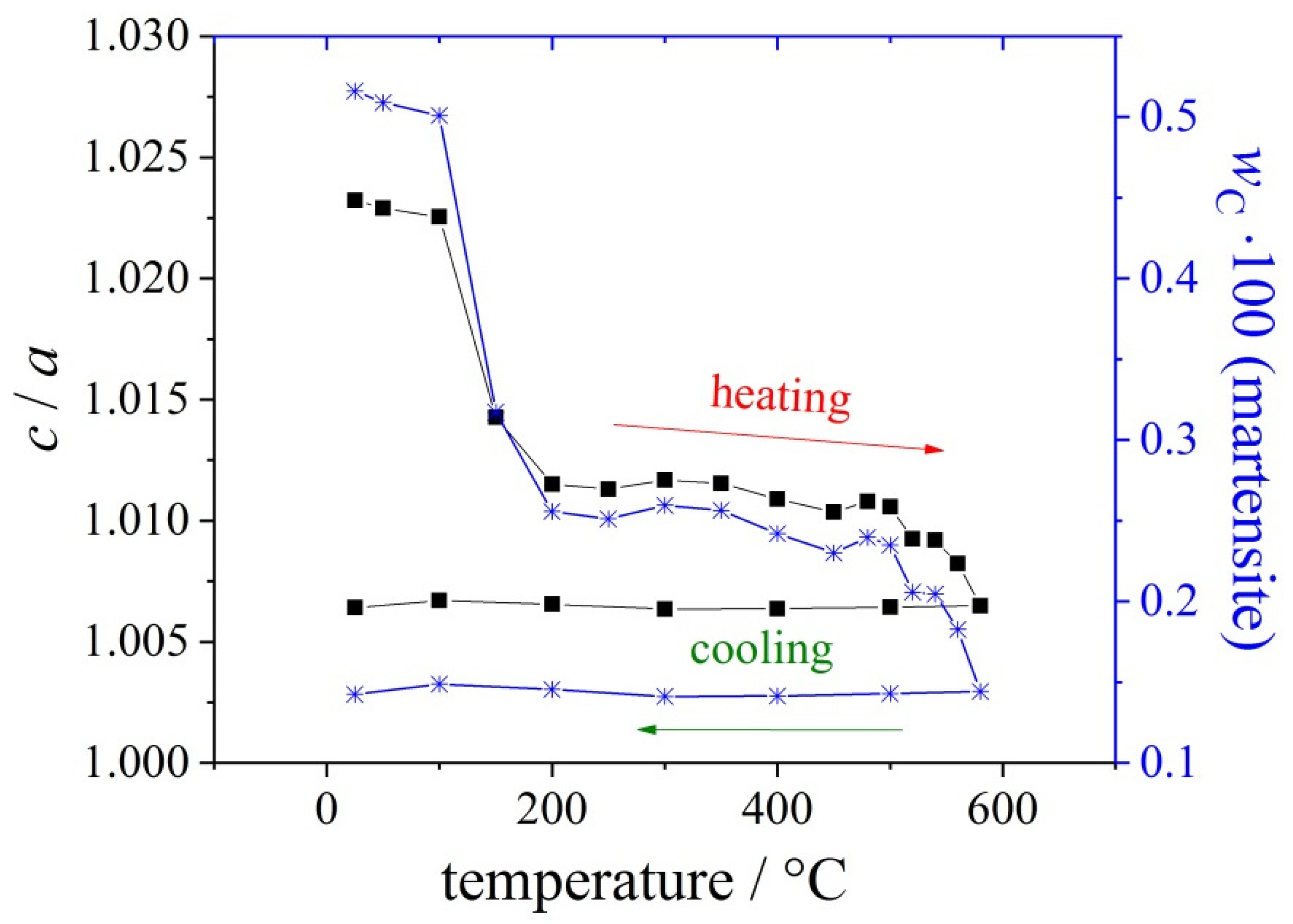

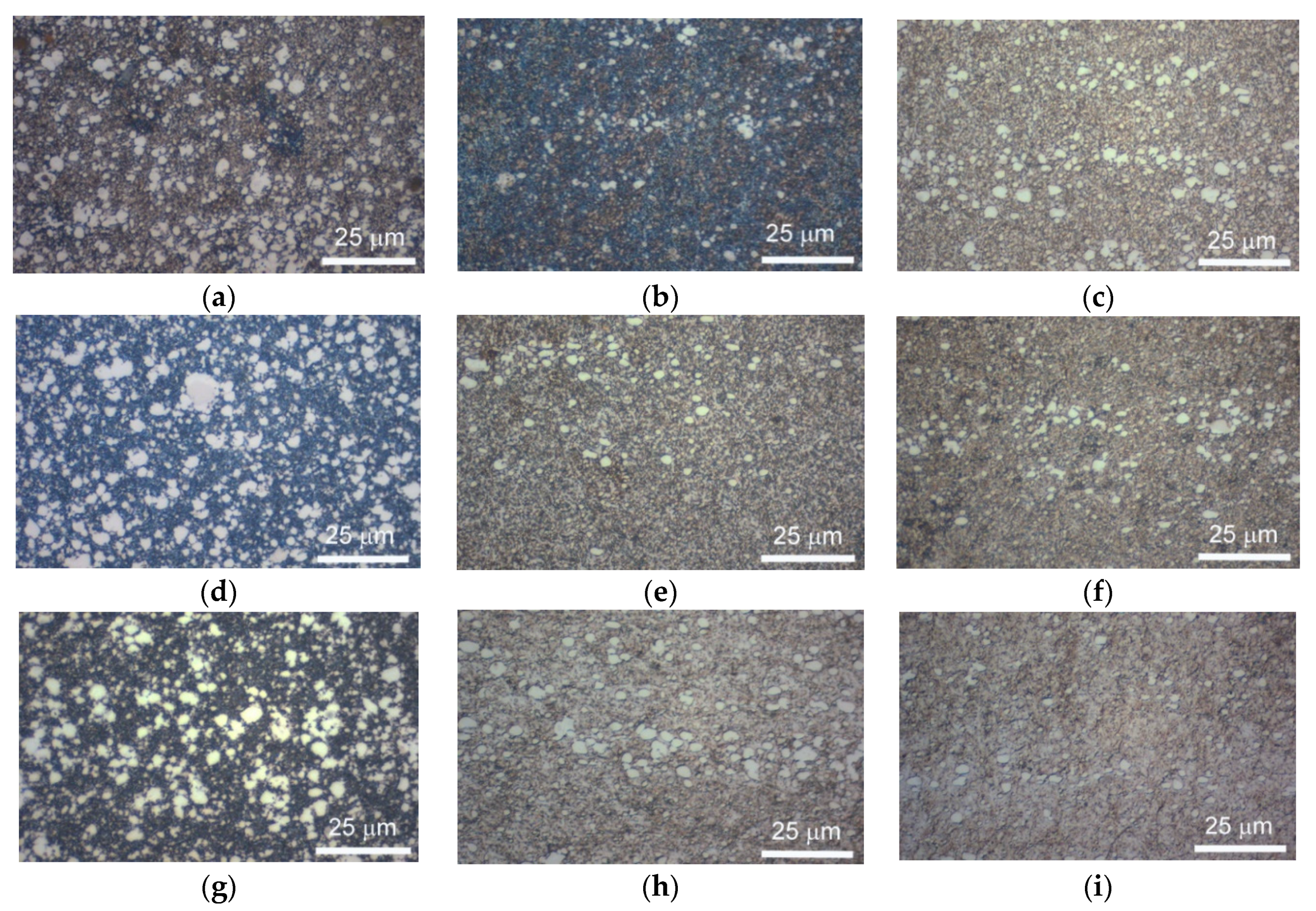

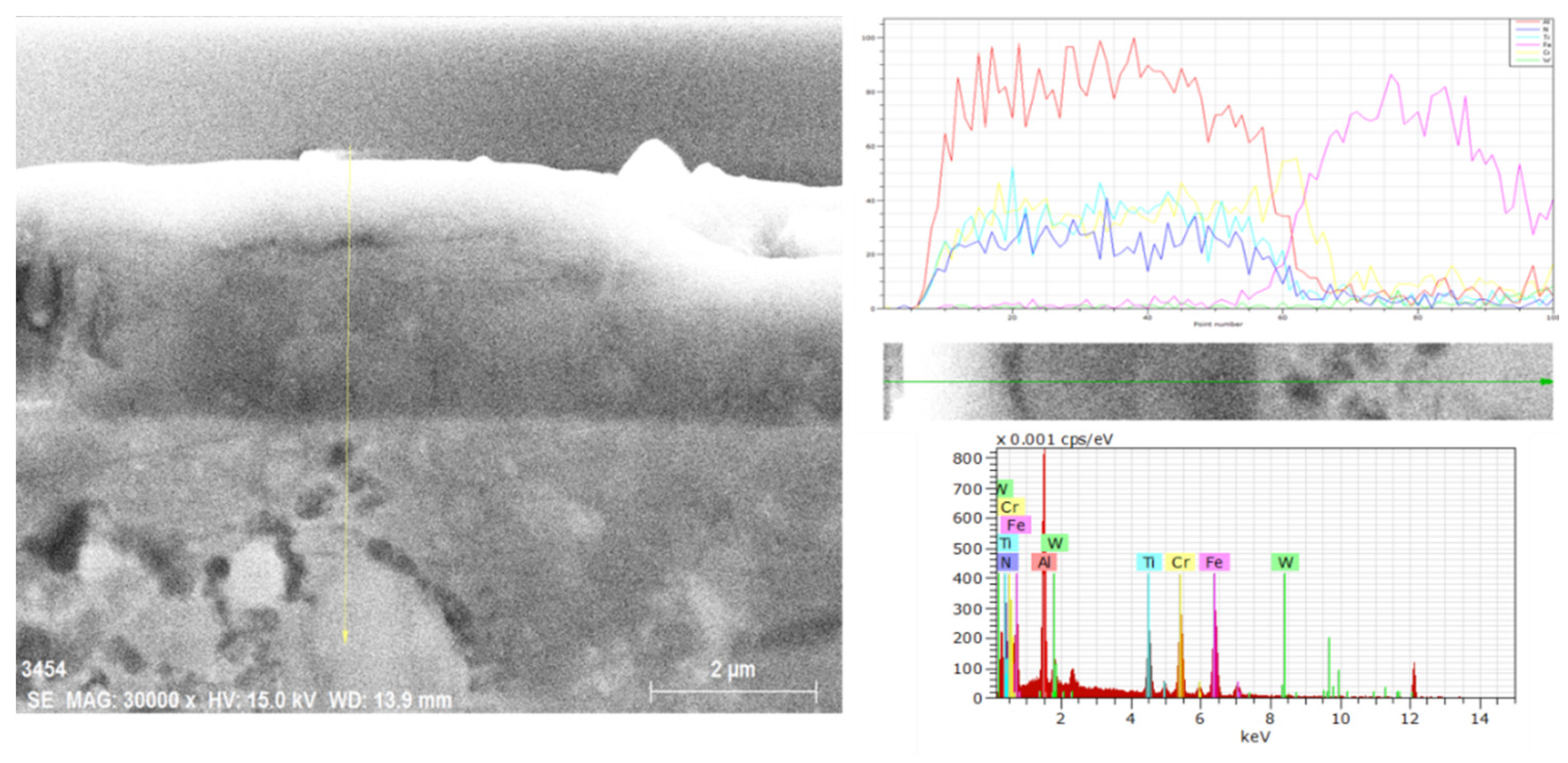
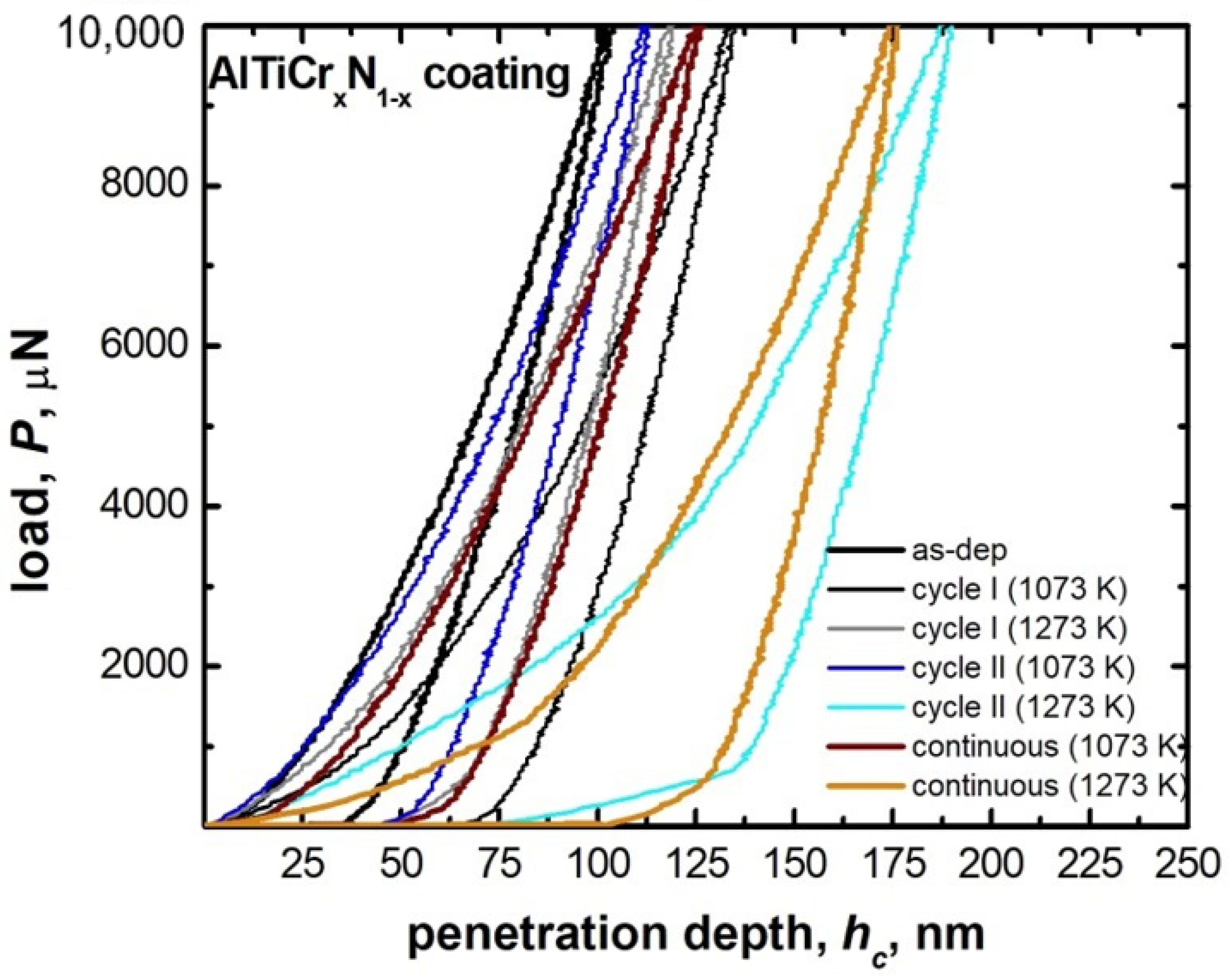
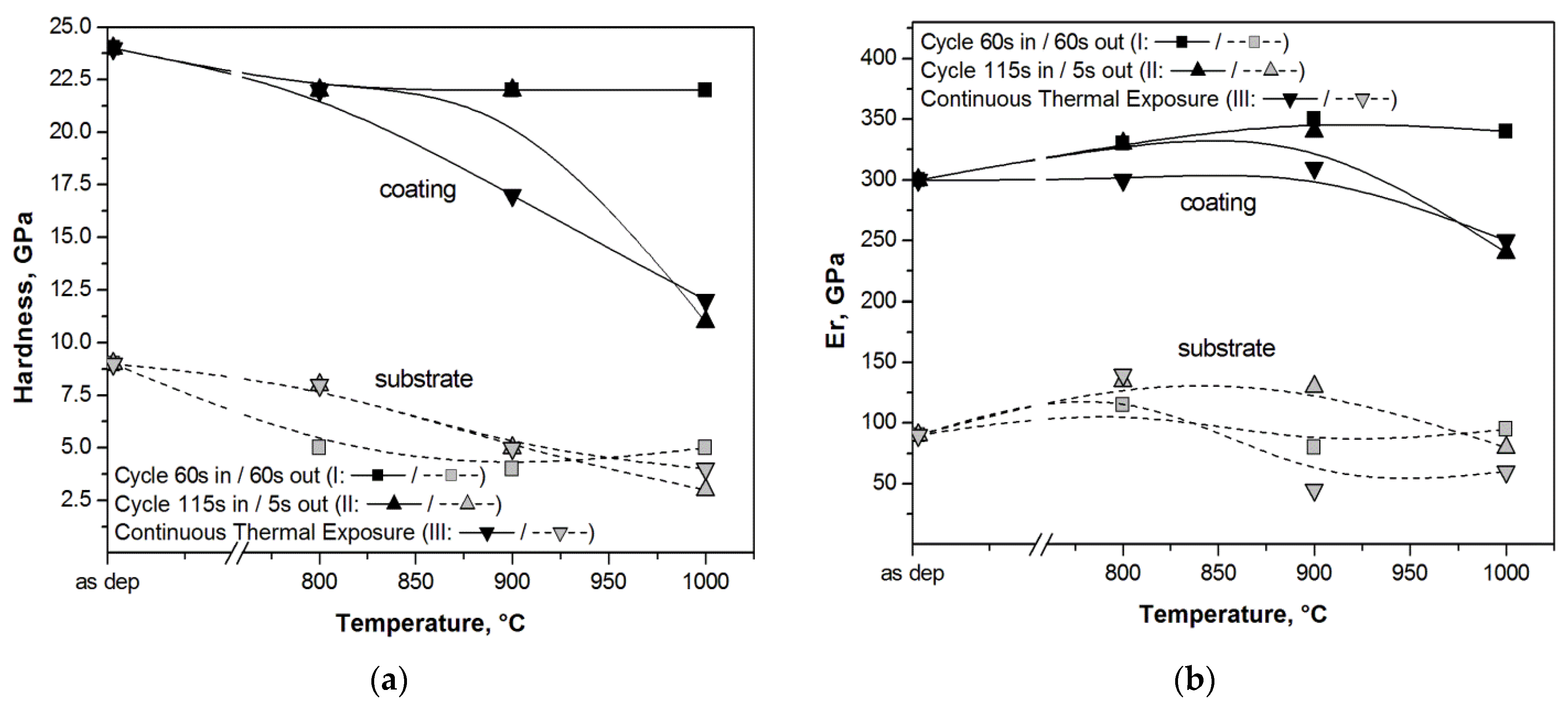




| Materials and Elements | |||
|---|---|---|---|
| Antimony (Sb) | Fluorspar | Magnesium (Mg) | Silicon Metal (Si) |
| Baryte | Gallium (Ga) | Natural Graphite | Tantalum (Ta) |
| Bauxite—N 2020 * | Germanium (Ge) | Natural Rubber | Titanium (Ti)—N 2020 |
| Beryllium (Be) | Hafnium (Hf) | Niobium (Nb) | Vanadium (V) |
| Bismuth (Bi) | HREEs ** | PGMs **** | Tungsten (W) |
| Borates | Indium (In) | Phosphate rock | Strontium (Sr)—N 2020 |
| Cobalt (Co) | Lithium (Li)—N 2020 | Phosphorus (P) | |
| Coking Coal | LREEs *** | Scandium (Sc) | |
| Component | C | Si | Mn | Cr | Mo | V | W | Co | Fe |
|---|---|---|---|---|---|---|---|---|---|
| Mass fraction in % | 2.0 | 0.44 | 0.27 | 3.7 | 2.4 | 5.1 | 14.1 | 11.0 | bal. |
| Material | 0.2% Yield Strength [MPa] | Tensile Strength [MPa] | Elongation [%] | Un-Notched Impact Energy [J] | Hardness HV10 |
|---|---|---|---|---|---|
| DI-F | 314 | 433 | 27.8 | 144 | 161 |
| DI-P | 677 | 880 | 3.2 | 20.5 | 296 |
| ADI-300 | 1395 | 1513 | 3.8 | 68 | 470 |
| ADI-350 | 1071 | 1221 | 8.2 | 108 | 365 |
| ADI-400 | 757 | 1042 | 14.2 | 140 | 306 |
| Load | Paper Grit | DI-F | DI-P | ADI-400 | ADI-350 | ADI-300 |
|---|---|---|---|---|---|---|
| 0.5 kg | P240 | 34.8 | 23.8 | 15.6 | 13.9 | 12.6 |
| P500 | 21.8 | 15.5 | 7.7 | 7.2 | 5.3 | |
| P800 | 6.9 | 5 | 4 | 3.4 | 2.9 | |
| 1.3 kg | P240 | 78.3 | 60 | 35.1 * | 35.6 | 34.7 |
| P500 | 22.7 | 17.3 | 14.3 | 12.7 | 10.1 | |
| P800 | 17.2 | 13.8 | 8.7 | 7.1 | 5.5 | |
| 2 kg | P240 | 108.6 | 81 | 43.4 * | 52 | 41.4 |
| P500 | 57.1 | 36.8 | 18.3 | 16.9 | 14.7 | |
| P800 | 22 | 20 | 15.8 | 13.8 | 10.3 |
| Material | 0.2% Yield Strength [MPa] | Tensile Strength [MPa] | Elongation [%] | Un-Notched Impact Energy [J] | Hardness HV10 |
|---|---|---|---|---|---|
| ADI-275 | - | 1472 | 1 | 23 | 498 |
| ADI-400 | 679 | 914 | 8 | 44 | 300 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novák, P.; Bellezze, T.; Cabibbo, M.; Gamsjäger, E.; Wiessner, M.; Rajnovic, D.; Jaworska, L.; Hanus, P.; Shishkin, A.; Goel, G.; et al. Solutions of Critical Raw Materials Issues Regarding Iron-Based Alloys. Materials 2021, 14, 899. https://doi.org/10.3390/ma14040899
Novák P, Bellezze T, Cabibbo M, Gamsjäger E, Wiessner M, Rajnovic D, Jaworska L, Hanus P, Shishkin A, Goel G, et al. Solutions of Critical Raw Materials Issues Regarding Iron-Based Alloys. Materials. 2021; 14(4):899. https://doi.org/10.3390/ma14040899
Chicago/Turabian StyleNovák, Pavel, Tiziano Bellezze, Marcello Cabibbo, Ernst Gamsjäger, Manfred Wiessner, Dragan Rajnovic, Lucyna Jaworska, Pavel Hanus, Andrei Shishkin, Gaurav Goel, and et al. 2021. "Solutions of Critical Raw Materials Issues Regarding Iron-Based Alloys" Materials 14, no. 4: 899. https://doi.org/10.3390/ma14040899
APA StyleNovák, P., Bellezze, T., Cabibbo, M., Gamsjäger, E., Wiessner, M., Rajnovic, D., Jaworska, L., Hanus, P., Shishkin, A., Goel, G., & Goel, S. (2021). Solutions of Critical Raw Materials Issues Regarding Iron-Based Alloys. Materials, 14(4), 899. https://doi.org/10.3390/ma14040899















