Structural and Material Determinants Influencing the Behavior of Porous Ti and Its Alloys Made by Additive Manufacturing Techniques for Biomedical Applications
Abstract
1. Introduction
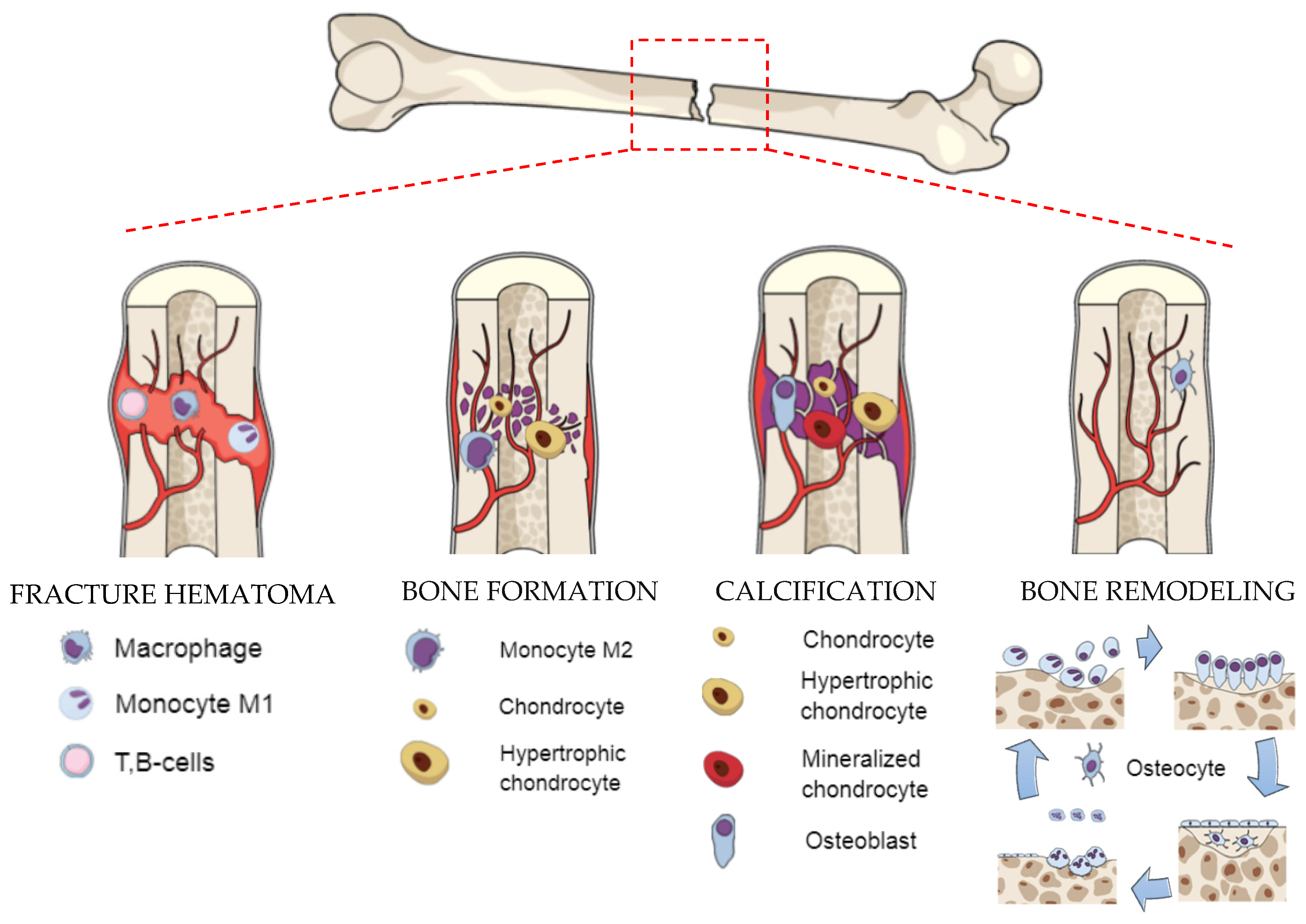
2. Biological Background—Formation and Regeneration Process of Bone
3. The Main Requirements for Bone Scaffolds
3.1. Biomechanical Properties
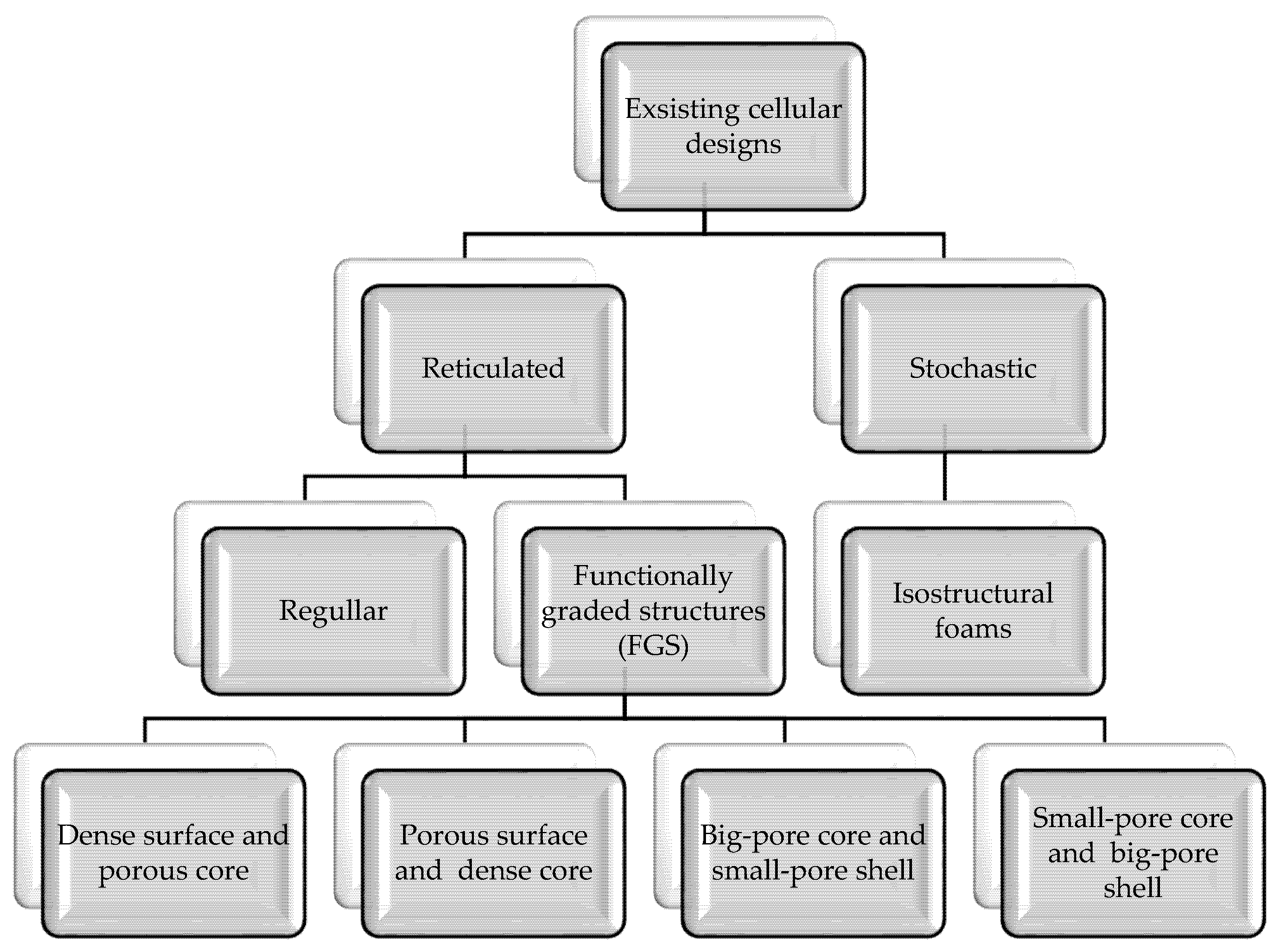
3.2. Design
3.3. Porosity
3.4. Pore and Strut Size
3.5. Pore Shape
4. Fabrication Methods of the Titanium Scaffolds and Effects of Manufacturing Errors
4.1. Conventional Methods
4.1.1. Powder Metallurgy (PM)
4.1.2. Freeze Casting
4.1.3. Polymeric Sponge Replication
4.2. Additive Manufacturing Methods (AM)
4.2.1. Selective Laser Melting (SLM)
4.2.2. Selective Laser Sintering (SLS)
4.2.3. Electron Beam Melting (EBM)
4.2.4. Laser Engineered Net Shaping (LENS)
4.2.5. Fused Deposition Modeling (FDM)
4.2.6. Direct Ink Writing (DIW)
4.2.7. Metal Injection Molding (MIM)
4.2.8. 3D Fiber Deposition (3DF)
4.3. Effects of Manufacturing Errors on Properties of Ti Scaffolds
5. Titanium and Its Alloys for Manufacturing of Scaffolds
6. Structural Factors Influencing the Mechanical Properties
7. Structural Factors Influencing the Biological Properties
7.1. Biocompatibility and Bioactivity
7.2. Osteoconductive and Osteoinductive Properties
7.2.1. In Vitro Studies
7.2.2. In Vivo Studies
7.3. Antibacterial Effects
8. Structural Factors Influencing the Chemical Properties
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| 3DF | 3D fiber deposition |
| AH | alkali–acid–heat treatment |
| ALP | alkaline phosphatase |
| AM | additive manufacturing |
| CAD | computer-assisted design |
| CaP | calcium phosphate |
| CaPNP | calcium phosphate nanoparticles |
| Ch | chitosan |
| CHX | chlorhexidine digluconate |
| CMC | complete mandibular construct |
| CP Ti | commercially pure titanium |
| CT | computer tomography |
| DED | direct energy deposition |
| DIW | direct ink writing |
| DLC | diamond-like carbon (layer) |
| DMLS | direct metal laser sintering |
| EBM | electron beam melting |
| FBCCZ | face- and body-centered cubic unit cell with longitudinal struts) |
| FCCZ | face-centered cubic unit cell with longitudinal struts |
| FDM | fused deposition modeling |
| FG-Ti | functionally graded titanium (FG-Ti) |
| HAp | hydroxyapatite |
| hBMSC | human bone mesenchymal stem cells |
| hMSCs | marrow-derived mesenchymal stem cells |
| HT | hydrothermal treatment |
| ID | interstrut distance |
| LENS | laser engineered net shaping |
| LIPUS | low-intensity pulsed ultrasound |
| MBG | mesoporous bioactive glass |
| MIM | metal injection molding |
| MSCs | mesenchymal stem cells |
| OPG | osteoprotegerin |
| PBS | phosphate-buffered saline |
| PRP | platelet-rich plasma |
| r-BMSCs | rabbit bone marrow mesenchymal stem cells |
| SaOS2 | sarcoma osteogenic cells |
| SBF | simulated body fluid |
| SF | silk fibroin |
| SiHAp | silicon substituted hydroxyapatite |
| SLM | selective laser melting |
| SLS | selective laser sintering |
| Ti-NTs | TiO2 nanotube arrays |
| TMPS | triply periodic minimal surfaces |
| Vanco | vancomycin |
| VEGF | vascular endothelial growth factor |
| βTCP | beta tricalcium phosphate |
References
- Dziaduszewska, M.; Zielinski, A. Titanium Scaffolds-Hopes and Limitations. Am. J. Biomed. Sci. Res. 2019. [Google Scholar] [CrossRef]
- Alvarez, K.; Nakajima, H. Metallic scaffolds for bone regeneration. Materials 2009, 2, 790–832. [Google Scholar] [CrossRef]
- Grimal, Q.; Haupert, S.; Mitton, D.; Vastel, L.; Laugier, P. Assessment of cortical bone elasticity and strength: Mechanical testing and ultrasound provide complementary data. Med. Eng. Phys. 2009, 31, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- De Wild, M.; Ghayor, C.; Zimmermann, S.; Rüegg, J.; Nicholls, F.; Schuler, F.; Chen, T.H.; Weber, F.E. Osteoconductive Lattice Microarchitecture for Optimized Bone Regeneration. 3D Print. Addit. Manuf. 2019, 6, 40–49. [Google Scholar] [CrossRef]
- Maroulakos, M.; Kamperos, G.; Tayebi, L.; Halazonetis, D.; Ren, Y. Applications of 3D printing on craniofacial bone repair: A systematic review. J. Dent. 2019, 80, 1–14. [Google Scholar] [CrossRef]
- Ghorbani, F.; Li, D.; Ni, S.; Zhou, Y.; Yu, B. 3D printing of acellular scaffolds for bone defect regeneration: A review. Mater. Today Commun. 2020, 22, 100979. [Google Scholar] [CrossRef]
- Li, N.; Huang, S.; Zhang, G.; Qin, R.; Liu, W.; Xiong, H.; Shi, G.; Blackburn, J. Progress in additive manufacturing on new materials: A review. J. Mater. Sci. Technol. 2019, 35, 242–269. [Google Scholar] [CrossRef]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262. [Google Scholar] [CrossRef]
- Bari, K.; Arjunan, A. Extra low interstitial titanium based fully porous morphological bone scaffolds manufactured using selective laser melting. J. Mech. Behav. Biomed. Mater. 2019, 95, 1–12. [Google Scholar] [CrossRef]
- Manoj, A.; Kasar, A.K.; Menezes, P.L. Tribocorrosion of Porous Titanium Used in Biomedical Applications. J. Bio-Tribo-Corros. 2019, 5, 1–16. [Google Scholar] [CrossRef]
- Murr, L.E. Metallurgy principles applied to powder bed fusion 3D printing/additive manufacturing of personalized and optimized metal and alloy biomedical implants: An overview. J. Mater. Res. Technol. 2020, 9, 1087–1103. [Google Scholar] [CrossRef]
- Schouman, T.; Schmitt, M.; Adam, C.; Dubois, G.; Rouch, P. Influence of the overall stiffness of a load-bearing porous titanium implant on bone ingrowth in critical-size mandibular bone defects in sheep. J. Mech. Behav. Biomed. Mater. 2016, 59, 484–496. [Google Scholar] [CrossRef] [PubMed]
- Qu, H. Additive manufacturing for bone tissue engineering scaffolds. Mater. Today Commun. 2020, 24, 101024. [Google Scholar] [CrossRef]
- Nover, A.B.; Lee, S.L.; Georgescu, M.S.; Howard, D.R.; Saunders, R.A.; Yu, W.T.; Klein, R.W.; Napolitano, A.P.; Ateshian, G.A.; Hung, C.T. Porous titanium bases for osteochondral tissue engineering. Acta Biomater. 2015, 27, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Ding, S.; Wen, C. Additive manufacturing technology for porous metal implant applications and triple minimal surface structures: A review. Bioact. Mater. 2019, 4, 56–70. [Google Scholar] [CrossRef]
- ISO/ASTM52900–15 Standard Terminology for Additive Manufacturing–General Principles–Terminology. Available online: https://www.astm.org/Standards/ISOASTM52900.htm (accessed on 19 January 2021).
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Alfaify, A.; Saleh, M.; Abdullah, F.M.; Al-Ahmari, A.M. Design for additive manufacturing: A systematic review. Sustainability 2020, 12, 7936. [Google Scholar] [CrossRef]
- Cooke, S.; Ahmadi, K.; Willerth, S.; Herring, R. Metal additive manufacturing: Technology, metallurgy and modelling. J. Manuf. Process. 2020, 57, 978–1003. [Google Scholar] [CrossRef]
- Putra, N.E.; Mirzaali, M.J.; Apachitei, I.; Zhou, J.; Zadpoor, A.A. Multi-material additive manufacturing technologies for Ti-, Mg-, and Fe-based biomaterials for bone substitution. Acta Biomater. 2020, 109, 1–20. [Google Scholar] [CrossRef]
- Bhuvanesh Kumar, M.; Sathiya, P. Methods and materials for additive manufacturing: A critical review on advancements and challenges. Thin-Walled Struct. 2020. [Google Scholar] [CrossRef]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, M.; du Plessis, A.; Ritchie, R.O.; Dallago, M.; Razavi, S.M.J.; Berto, F. Architected cellular materials: A review on their mechanical properties towards fatigue-tolerant design and fabrication. Mater. Sci. Eng. R Rep. 2021, 144, 100606. [Google Scholar] [CrossRef]
- Li, Y.; Jahr, H.; Zhou, J.; Zadpoor, A.A. Additively manufactured biodegradable porous metals. Acta Biomater. 2020, 115, 29–50. [Google Scholar] [CrossRef] [PubMed]
- Azarniya, A.; Colera, X.G.; Mirzaali, M.J.; Sovizi, S.; Bartolomeu, F.; St Weglowski, M.K.; Wits, W.W.; Yap, C.Y.; Ahn, J.; Miranda, G.; et al. Additive manufacturing of Ti–6Al–4V parts through laser metal deposition (LMD): Process, microstructure, and mechanical properties. J. Alloys Compd. 2019, 804, 163–191. [Google Scholar] [CrossRef]
- Ren, D.; Li, S.; Wang, H.; Hou, W.; Hao, Y.; Jin, W.; Yang, R.; Misra, R.D.K.; Murr, L.E. Fatigue behavior of Ti-6Al-4V cellular structures fabricated by additive manufacturing technique. J. Mater. Sci. Technol. 2019, 35, 285–294. [Google Scholar] [CrossRef]
- Liu, S.; Shin, Y.C. Additive manufacturing of Ti6Al4V alloy: A review. Mater. Des. 2019, 164. [Google Scholar] [CrossRef]
- Singh, N.; Hameed, P.; Ummethala, R.; Manivasagam, G.; Prashanth, K.G.; Eckert, J. Selective laser manufacturing of Ti-based alloys and composites: Impact of process parameters, application trends, and future prospects. Mater. Today Adv. 2020, 8, 100097. [Google Scholar] [CrossRef]
- Li, J.; Cui, X.; Hooper, G.J.; Lim, K.S.; Woodfield, T.B.F. Rational design, bio-functionalization and biological performance of hybrid additive manufactured titanium implants for orthopaedic applications: A review. J. Mech. Behav. Biomed. Mater. 2020, 105, 103671. [Google Scholar] [CrossRef]
- Rodriguez-Contreras, A.; Punset, M.; Calero, J.A.; Gil, F.J.; Ruperez, E.; Manero, J.M. Powder metallurgy with space holder for porous titanium implants: A review. J. Mater. Sci. Technol. 2021, 76, 129–149. [Google Scholar] [CrossRef]
- DeLacure, M.D. Physiology of bone healing and bone grafts. Otolaryngol. Clin. N. Am. 1994, 27, 859–874. [Google Scholar] [CrossRef]
- Seeman, E. Bone modeling and remodeling. Crit. Rev. Eukaryot. Gene Expr. 2009, 19, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Spicer, P.P.; Kretlow, J.D.; Young, S.; Jansen, J.A.; Kasper, F.K.; Mikos, A.G. Evaluation of bone regeneration using the rat critical size calvarial defect. Nat. Protoc. 2012, 7, 1918–1929. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Silva, M.; Bahk, W.-J.; McKellop, H.; Lieberman, J.R. Effect of repeated irrigation and debridement on fracture healing in an animal model. J. Orthop. Res. 2002, 20, 1197–1204. [Google Scholar] [CrossRef]
- Bahney, C.S.; Hu, D.P.; Miclau, T.; Marcucio, R.S. The multifaceted role of the vasculature in endochondral fracture repair. Front. Endocrinol. 2015, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Hunt, T.K.; Aslam, R.; Hussain, Z.; Beckert, S. Lactate, with Oxygen, Incites Angiogenesis. In Oxygen Transport to Tissue XXIX; Springer: Boston, MA, USA, 2008; Volume 614, pp. 73–80. [Google Scholar]
- Jensen, J.A.; Hunt, T.K.; Scheuenstuhl, H.; Banda, M.J. Effect of lactate, pyruvate, and pH on secretion of angiogenesis and mitogenesis factors by macrophages. Lab. Investig. 1986, 54, 574–578. [Google Scholar] [PubMed]
- Weiner, S. Biomineralization: A structural perspective. J. Struct. Biol. 2008, 163, 229–234. [Google Scholar] [CrossRef]
- Wang, G.H.; Fu, H.; Zhao, Y.Z.; Zhou, K.C.; Zhu, S.H. Bone integration properties of antibacterial biomimetic porous titanium implants. Trans. Nonferrous Met. Soc. China Engl. Ed. 2017, 27, 2007–2014. [Google Scholar] [CrossRef]
- Frost, H.M. The Skeletal Intermediary Organization. Metab. Bone Dis. Rel. Res. 2002, 290, 281–290. [Google Scholar] [CrossRef]
- Kimmel, D.B. A paradigm for skeletal strength homeostasis. J. Bone Miner. Res. 2009, 8, S515–S522. [Google Scholar] [CrossRef]
- Kalfas, I.H. Principles of bone healing. Neurosurg. Focus 2001, 10, 1–4. [Google Scholar] [CrossRef]
- McKinley, T. Principles of Fracture Healing. Surgery 2003, 21, 209–212. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Fang, G.; Zhou, J. Additively manufactured scaffolds for bone tissue engineering and the prediction of their mechanical behavior: A review. Materials 2017, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Pei, X.; Zhou, C.; Fan, Y.; Jiang, Q.; Ronca, A.; D’Amora, U.; Chen, Y.; Li, H.; Sun, Y.; et al. The biomimetic design and 3D printing of customized mechanical properties porous Ti6Al4V scaffold for load-bearing bone reconstruction. Mater. Des. 2018, 152, 30–39. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Fang, G.; Xing, L.-L.; Liu, W.; Zhou, J. Effect of porosity variation strategy on the performance of functionally graded Ti-6Al-4V scaffolds for bone tissue engineering. Mater. Des. 2018, 157, 523–538. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, C.; Li, C.; Qin, Y.; Zhong, L.; Chen, B.; Li, Z.; Liu, H.; Chang, F.; Wang, J. Analysis of factors influencing bone ingrowth into three-dimensional printed porous metal scaffolds: A review. J. Alloys Compd. 2017, 717, 271–285. [Google Scholar] [CrossRef]
- Zhang, S.; Wei, Q.; Cheng, L.; Li, S.; Shi, Y. Effects of scan line spacing on pore characteristics and mechanical properties of porous Ti6Al4V implants fabricated by selective laser melting. Mater. Des. 2014, 63, 185–193. [Google Scholar] [CrossRef]
- Karpiński, R.; Jaworski, Ł.; Czubacka, P. The structural and mechanical properties of the bone. J. Tech. Exploit. Mech. Eng. 2017, 3, 43–50. [Google Scholar] [CrossRef]
- Cheng, A.; Humayun, A.; Cohen, D.J.; Boyan, B.D.; Schwartz, Z. Additively manufactured 3D porous Ti-6Al-4V constructs mimic trabecular bone structure and regulate osteoblast proliferation, differentiation and local factor production in a porosity and surface roughness dependent manner. Biofabrication 2014, 6, 045007. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef]
- Tan, X.P.; Tan, Y.J.; Chow, C.S.L.; Tor, S.B.; Yeong, W.Y. Metallic powder-bed based 3D printing of cellular scaffolds for orthopaedic implants: A state-of-the-art review on manufacturing, topological design, mechanical properties and biocompatibility. Mater. Sci. Eng. C 2017, 76, 1328–1343. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Nava, E.; Smith, C.J.; Derguti, F.; Tammas-Williams, S.; Léonard, F.; Withers, P.J.; Todd, I.; Goodall, R. The effect of density and feature size on mechanical properties of isostructural metallic foams produced by additive manufacturing. Acta Mater. 2015, 85, 387–395. [Google Scholar] [CrossRef]
- Liu, P.S.; Chen, G.F.; Liu, P.S.; Chen, G.F. Chapter Three—Application of Porous Metals. In Porous Materials; Elsevier: Amsterdam, The Netherlands, 2014; pp. 113–188. ISBN 9780124077881. [Google Scholar]
- Xu, S.; Shen, J.; Zhou, S.; Huang, X.; Xie, Y.M. Design of lattice structures with controlled anisotropy. Mater. Des. 2016, 93, 443–447. [Google Scholar] [CrossRef]
- Fousová, M.; Vojtěch, D.; Kubásek, J.; Jablonská, E.; Fojt, J. Promising characteristics of gradient porosity Ti-6Al-4V alloy prepared by SLM process. J. Mech. Behav. Biomed. Mater. 2017, 69, 368–376. [Google Scholar] [CrossRef]
- Cavo, M.; Scaglione, S. Scaffold microstructure effects on functional and mechanical performance: Integration of theoretical and experimental approaches for bone tissue engineering applications. Mater. Sci. Eng. C 2016, 68, 872–879. [Google Scholar] [CrossRef]
- Ryan, G.E.; Pandit, A.S.; Apatsidis, D.P. Porous titanium scaffolds fabricated using a rapid prototyping and powder metallurgy technique. Biomaterials 2008, 29, 3625–3635. [Google Scholar] [CrossRef]
- Ahmadi, S.; Yavari, S.; Wauthle, R.; Pouran, B.; Schrooten, J.; Weinans, H.; Zadpoor, A.; Ahmadi, S.M.; Yavari, S.A.; Wauthle, R.; et al. Additively Manufactured Open-Cell Porous Biomaterials Made from Six Different Space-Filling Unit Cells: The Mechanical and Morphological Properties. Materials 2015, 8, 1871–1896. [Google Scholar] [CrossRef]
- Markhoff, J.; Wieding, J.; Weissmann, V.; Pasold, J.; Jonitz-Heincke, A.; Bader, R. Influence of different three-dimensional open porous titanium scaffold designs on human osteoblasts behavior in static and dynamic cell investigations. Materials 2015, 8, 5490–5507. [Google Scholar] [CrossRef]
- Li, S.J.; Xu, Q.S.; Wang, Z.; Hou, W.T.; Hao, Y.L.; Yang, R.; Murr, L.E. Influence of cell shape on mechanical properties of Ti–6Al–4V meshes fabricated by electron beam melting method. Acta Biomater. 2014, 10, 4537–4547. [Google Scholar] [CrossRef]
- Di Luca, A.; Ostrowska, B.; Lorenzo-Moldero, I.; Lepedda, A.; Swieszkowski, W.; Van Blitterswijk, C.; Moroni, L. Gradients in pore size enhance the osteogenic differentiation of human mesenchymal stromal cells in three-dimensional scaffolds. Sci. Rep. 2016, 6, 22898. [Google Scholar] [CrossRef]
- Fang, Z.; Starly, B.; Sun, W. Computer-aided characterization for effective mechanical properties of porous tissue scaffolds. Comput. Des. 2005, 37, 65–72. [Google Scholar] [CrossRef]
- Arabnejad, S.; Burnett Johnston, R.; Pura, J.A.; Singh, B.; Tanzer, M.; Pasini, D. High-strength porous biomaterials for bone replacement: A strategy to assess the interplay between cell morphology, mechanical properties, bone ingrowth and manufacturing constraints. Acta Biomater. 2016, 30, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Li, Y.; Shen, M.; Yang, X.; Zhang, L.; Ke, X.; Yang, G.; Gao, C.; Gou, Z.; Xu, S. Bone tissue regeneration: The role of finely tuned pore architecture of bioactive scaffolds before clinical translation. Bioact. Mater. 2021, 6, 1242–1254. [Google Scholar] [CrossRef] [PubMed]
- Deb, P.; Deoghare, A.B.; Borah, A.; Barua, E.; Das Lala, S. Scaffold Development Using Biomaterials: A Review. Mater. Today Proc. 2018, 5, 12909–12919. [Google Scholar] [CrossRef]
- Jones, A.C.; Arns, C.H.; Sheppard, A.P.; Hutmacher, D.W.; Milthorpe, B.K.; Knackstedt, M.A. Assessment of bone ingrowth into porous biomaterials using MICRO-CT. Biomaterials 2007, 28, 2491–2504. [Google Scholar] [CrossRef]
- Otsuki, B.; Takemoto, M.; Fujibayashi, S.; Neo, M.; Kokubo, T.; Nakamura, T. Pore throat size and connectivity determine bone and tissue ingrowth into porous implants: Three-dimensional micro-CT based structural analyses of porous bioactive titanium implants. Biomaterials 2006, 27, 5892–5900. [Google Scholar] [CrossRef]
- Taniguchi, N.; Fujibayashi, S.; Takemoto, M.; Sasaki, K.; Otsuki, B.; Nakamura, T.; Matsushita, T.; Kokubo, T.; Matsuda, S. Effect of pore size on bone ingrowth into porous titanium implants fabricated by additive manufacturing: An in vivo experiment. Mater. Sci. Eng. C 2016, 59, 690–701. [Google Scholar] [CrossRef]
- Zhao, D.; Huang, Y.; Ao, Y.; Han, C.; Wang, Q.; Li, Y.; Liu, J.; Wei, Q.; Zhang, Z. Effect of pore geometry on the fatigue properties and cell affinity of porous titanium scaffolds fabricated by selective laser melting. J. Mech. Behav. Biomed. Mater. 2018, 88, 478–487. [Google Scholar] [CrossRef]
- Van Bael, S.; Chai, Y.C.; Truscello, S.; Moesen, M.; Kerckhofs, G.; Van Oosterwyck, H.; Kruth, J.-P.; Schrooten, J. The effect of pore geometry on the in vitro biological behavior of human periosteum-derived cells seeded on selective laser-melted Ti6Al4V bone scaffolds. Acta Biomater. 2012, 8, 2824–2834. [Google Scholar] [CrossRef]
- Montazerian, H.; Zhianmanesh, M.; Davoodi, E.; Milani, A.S.; Hoorfar, M. Longitudinal and radial permeability analysis of additively manufactured porous scaffolds: Effect of pore shape and porosity. Mater. Des. 2017, 122, 146–156. [Google Scholar] [CrossRef]
- Speirs, M.; Van Humbeeck, J.; Schrooten, J.; Luyten, J.; Kruth, J.P. The effect of pore geometry on the mechanical properties of selective laser melted Ti-13Nb-13Zr scaffolds. Procedia CIRP 2013, 5, 79–82. [Google Scholar] [CrossRef]
- Leong, K.F.; Cheah, C.M.; Chua, C.K. Solid freeform fabrication of three-dimensional scaffolds for engineering replacement tissues and organs. Biomaterials 2003, 24, 2363–2378. [Google Scholar] [CrossRef]
- Qiu, C.; Yue, S.; Adkins, N.J.E.; Ward, M.; Hassanin, H.; Lee, P.D.; Withers, P.J.; Attallah, M.M. Influence of processing conditions on strut structure and compressive properties of cellular lattice structures fabricated by selective laser melting. Mater. Sci. Eng. A 2015, 628, 188–197. [Google Scholar] [CrossRef]
- Amin Yavari, S.; Wauthle, R.; Van Der Stok, J.; Riemslag, A.C.; Janssen, M.; Mulier, M.; Kruth, J.P.; Schrooten, J.; Weinans, H.; Zadpoor, A.A. Fatigue behavior of porous biomaterials manufactured using selective laser melting. Mater. Sci. Eng. C 2013, 33, 4849–4858. [Google Scholar] [CrossRef] [PubMed]
- Karimipour-Fard, P.; Behravesh, A.H.; Jones-Taggart, H.; Pop-Iliev, R.; Rizvi, G. Effects of design, porosity and biodegradation on mechanical and morphological properties of additive-manufactured triply periodic minimal surface scaffolds. J. Mech. Behav. Biomed. Mater. 2020, 112, 104064. [Google Scholar] [CrossRef]
- Huang, Y.-Z.; Xie, H.-Q.; Li, X. Scaffolds in Bone Tissue Engineering: Research Progress and Current Applications. In Encyclopedia of Bone Biology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 204–215. [Google Scholar] [CrossRef]
- García, I.; Gracia-Escosa, E.; Bayod, M.; Conde, A.; Arenas, M.A.; Damborenea, J.; Romero, A.; Rodríguez, G. Sustainable production of titanium foams for biomedical applications by Concentrated Solar Energy sintering. Mater. Lett. 2016, 185, 420–423. [Google Scholar] [CrossRef]
- Aghajanian, A.H.; Bigham, A.; Khodaei, M.; Hossein Kelishadi, S. Porous titanium scaffold coated using forsterite/poly-3-hydroxybutyrate composite for bone tissue engineering. Surf. Coat. Technol. 2019, 378, 124942. [Google Scholar] [CrossRef]
- Caparrós, C.; Ortiz-Hernandez, M.; Molmeneu, M.; Punset, M.; Calero, J.A.; Aparicio, C.; Fernández-Fairén, M.; Perez, R.; Gil, F.J. Bioactive macroporous titanium implants highly interconnected. J. Mater. Sci. Mater. Med. 2016, 27. [Google Scholar] [CrossRef]
- Jakubowicz, J.; Adamek, G.; Dewidar, M. Titanium foam made with saccharose as a space holder. J. Porous Mater. 2013, 20, 1137–1141. [Google Scholar] [CrossRef]
- Chen, Y.; Kent, D.; Bermingham, M.; Dehghan-Manshadi, A.; Wang, G.; Wen, C.; Dargusch, M. Manufacturing of graded titanium scaffolds using a novel space holder technique. Bioact. Mater. 2017, 2, 248–252. [Google Scholar] [CrossRef]
- Chen, Y.; Kent, D.; Bermingham, M.; Dehghan-Manshadi, A.; Dargusch, M. Manufacturing of biocompatible porous titanium scaffolds using a novel spherical sugar pellet space holder. Mater. Lett. 2017, 195, 92–95. [Google Scholar] [CrossRef]
- Hong, T.F.; Guo, Z.X.; Yang, R. Fabrication of porous titanium scaffold materials by a fugitive filler method. J. Mater. Sci. Mater. Med. 2008, 19, 3489–3495. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, S.M.; Arabi, H.; Mirdamadi, S.; Mirsalehi, S.A. Biocompatibility and compressive properties of Ti-6Al-4V scaffolds having Mg element. J. Mech. Behav. Biomed. Mater. 2015, 48, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Frith, J.E.; Dehghan-Manshadi, A.; Attar, H.; Kent, D.; Soro, N.D.M.; Bermingham, M.J.; Dargusch, M.S. Mechanical properties and biocompatibility of porous titanium scaffolds for bone tissue engineering. J. Mech. Behav. Biomed. Mater. 2017, 75, 169–174. [Google Scholar] [CrossRef]
- Xu, W.; Lu, X.; Hayat, M.D.; Tian, J.; Huang, C.; Chen, M.; Qu, X.; Wen, C. Fabrication and properties of newly developed Ti35Zr28Nb scaffolds fabricated by powder metallurgy for bone-tissue engineering. J. Mater. Res. Technol. 2019, 8, 3696–3704. [Google Scholar] [CrossRef]
- Xu, W.; Tian, J.; Liu, Z.; Lu, X.; Hayat, M.D.; Yan, Y.; Li, Z.; Qu, X.; Wen, C. Novel porous Ti35Zr28Nb scaffolds fabricated by powder metallurgy with excellent osteointegration ability for bone-tissue engineering applications. Mater. Sci. Eng. C 2019, 105, 110015. [Google Scholar] [CrossRef]
- Seramak, T.; Zasinska, K.; Mesnard, M.; Bednarz, K.; Fic, P.; Zielinski, A. Determinants of the surface quality, density and dimensional correctness in selective laser melting of the Ti-13Zr-13Nb alloy. Matériaux Tech. 2018, 106, 405. [Google Scholar] [CrossRef]
- Civantos, A.; Domínguez, C.; Pino, R.J.; Setti, G.; Pavón, J.J.; Martínez-Campos, E.; Garcia Garcia, F.J.; Rodríguez, J.A.; Allain, J.P.; Torres, Y. Designing bioactive porous titanium interfaces to balance mechanical properties and in vitro cells behavior towards increased osseointegration. Surf. Coat. Technol. 2019, 368, 162–174. [Google Scholar] [CrossRef]
- Arifvianto, B.; Leeflang, M.A.; Zhou, J. Characterization of the porous structures of the green body and sintered biomedical titanium scaffolds with micro-computed tomography. Mater. Charact. 2016, 121, 48–60. [Google Scholar] [CrossRef]
- Arifvianto, B.; Leeflang, M.A.; Zhou, J. Diametral compression behavior of biomedical titanium scaffolds with open, interconnected pores prepared with the space holder method. J. Mech. Behav. Biomed. Mater. 2017, 68, 144–154. [Google Scholar] [CrossRef]
- Wang, D.; Li, Q.; Xu, M.; Jiang, G.; Zhang, Y.; He, G. A novel approach to fabrication of three-dimensional porous titanium with controllable structure. Mater. Sci. Eng. C 2017, 71, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.K.; Lee, J.B.; Koh, Y.H.; Kim, H.E. Rapid direct deposition of TiH2 paste for porous Ti scaffolds with tailored porous structures and mechanical properties. Mater. Chem. Phys. 2016, 176, 104–109. [Google Scholar] [CrossRef]
- Dezfuli, S.N.; Sadrnezhaad, S.K.; Shokrgozar, M.A.; Bonakdar, S. Fabrication of biocompatible titanium scaffolds using space holder technique. J. Mater. Sci. Mater. Med. 2012, 23, 2483–2488. [Google Scholar] [CrossRef] [PubMed]
- Arifvianto, B.; Zhou, J. Fabrication of Metallic Biomedical Scaffolds with the Space Holder Method: A Review. Materials (Basel) 2014, 7, 3588–3622. [Google Scholar] [CrossRef] [PubMed]
- Nazari, K.A.; Hilditch, T.; Dargusch, M.S.; Nouri, A. Functionally graded porous scaffolds made of Ti-based agglomerates. J. Mech. Behav. Biomed. Mater. 2016, 63, 157–163. [Google Scholar] [CrossRef]
- Fan, X.P.; Feng, B.; Di, Y.L.; Wang, J.X.; Lu, X.; Weng, J. Exchange of experience: Graded porous titanium scaffolds fabricated using powder metallurgy technique. Powder Metall. Met. Ceram. 2012, 51, 372–377. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, B.; Zhu, Y.; Weng, J.; Wang, J.; Lu, X. Preparation and characterization of a novel porous titanium scaffold with 3D hierarchical porous structures. J. Mater. Sci. Mater. Med. 2011, 22, 839–844. [Google Scholar] [CrossRef]
- Mao, M.; Tang, Y.; Zhao, K.; Duan, Z.; Wu, C. Fabrication of porous titanium scaffolds with centrosymmetric pore channels and improved radial fracture loading. J. Mater. Sci. 2019, 54, 3527–3535. [Google Scholar] [CrossRef]
- Mao, M.; Tang, Y.; Zhao, K.; Duan, Z.; Wu, C. Porous Titanium Scaffolds with Aligned Lamellar Pore Channels by Directional Freeze-Casting from Aqueous TiH 2 Slurries. Met. Mater. Int. 2019, 25, 508–515. [Google Scholar] [CrossRef]
- Zhu, W.; Zhao, Y.; Ma, Q.; Wang, Y.; Wu, Z.; Weng, X. 3D-printed porous titanium changed femoral head repair growth patterns: Osteogenesis and vascularisation in porous titanium. J. Mater. Sci. Mater. Med. 2017, 28. [Google Scholar] [CrossRef]
- Lee, H.; Jang, T.S.; Song, J.; Kim, H.E.; Jung, H. Do Multi-scale porous Ti6Al4V scaffolds with enhanced strength and biocompatibility formed via dynamic freeze-casting coupled with micro-arc oxidation. Mater. Lett. 2016, 185, 21–24. [Google Scholar] [CrossRef]
- Chang, J.M.; Liu, G.L.; Tung, H.M. Effects of Sintering Temperature on the Porosity and Mechanical Behavior of Porous Titanium Scaffolds Prepared by Freeze-Casting. J. Mater. Eng. Perform. 2019, 28, 5494–5500. [Google Scholar] [CrossRef]
- Wang, C.; Chen, H.; Zhu, X.; Xiao, Z.; Zhang, K.; Zhang, X. An improved polymeric sponge replication method for biomedical porous titanium scaffolds. Mater. Sci. Eng. C 2017, 70, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Yang, J.; Li, J.; Feng, X.; Chen, Z.; Yuan, Y.; Yong, B.; Chu, C.; Tan, X.; Song, Q. Replication and bioactivation of Ti-based alloy scaffold macroscopically identical to cancellous bone from polymeric template with TiNbZr powders. J. Mech. Behav. Biomed. Mater. 2018, 88, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Li, J.; Feng, X.; Chu, C. Bone-like apatite growth on controllable macroporous titanium scaffolds coated with microporous titania. J. Mech. Behav. Biomed. Mater. 2018, 77, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Cachinho, S.C.P.; Correia, R.N. Titanium scaffolds for osteointegration: Mechanical, in vitro and corrosion behaviour. J. Mater. Sci. Mater. Med. 2008, 19, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, M.D.; Jazayeri, H.E.; Razavi, M.; Masri, R.; Tayebi, L. Three-Dimensional Bioprinting Materials with Potential Application in Preprosthetic Surgery. J. Prosthodont. 2016, 25, 310–318. [Google Scholar] [CrossRef]
- Han, C.; Li, Y.; Wang, Q.; Wen, S.; Wei, Q.; Yan, C.; Hao, L.; Liu, J.; Shi, Y. Continuous functionally graded porous titanium scaffolds manufactured by selective laser melting for bone implants. J. Mech. Behav. Biomed. Mater. 2018, 80, 119–127. [Google Scholar] [CrossRef]
- Li, Y.; Ding, Y.; Munir, K.; Lin, J.; Brandt, M.; Atrens, A.; Xiao, Y.; Kanwar, J.R.; Wen, C. Novel β-Ti35Zr28Nb alloy scaffolds manufactured using selective laser melting for bone implant applications. Acta Biomater. 2019, 87, 273–284. [Google Scholar] [CrossRef]
- Liang, H.; Yang, Y.; Xie, D.; Li, L.; Mao, N.; Wang, C.; Tian, Z.; Jiang, Q.; Shen, L. Trabecular-like Ti-6Al-4V scaffolds for orthopedic: Fabrication by selective laser melting and in vitro biocompatibility. J. Mater. Sci. Technol. 2019, 35, 1284–1297. [Google Scholar] [CrossRef]
- Montufar, E.B.; Tkachenko, S.; Casas-Luna, M.; Škarvada, P.; Slámečka, K.; Diaz-de-la-Torre, S.; Koutný, D.; Paloušek, D.; Koledova, Z.; Hernández-Tapia, L.; et al. Benchmarking of additive manufacturing technologies for commercially-pure-titanium bone-tissue-engineering scaffolds: Processing-microstructure-property relationship. Addit. Manuf. 2020, 36, 101516. [Google Scholar] [CrossRef]
- Sofia, D.; Macrì, D.; Barletta, D.; Lettieri, P.; Poletto, M. Use of titania powders in the laser sintering process: Link between process conditions and product mechanical properties. Powder Technol. 2021, 381, 181–188. [Google Scholar] [CrossRef]
- Wubneh, A.; Tsekoura, E.K.; Ayranci, C.; Uludağ, H. Current state of fabrication technologies and materials for bone tissue engineering. Acta Biomater. 2018, 80, 1–30. [Google Scholar] [CrossRef]
- Liu, F.H.; Lee, R.T.; Lin, W.H.; Liao, Y.S. Selective laser sintering of bio-metal scaffold. Procedia CIRP 2013, 5, 83–87. [Google Scholar] [CrossRef][Green Version]
- Li, J.; Li, Z.; Shi, Y.; Wang, H.; Li, R.; Tu, J.; Jin, G. In vitro and in vivo comparisons of the porous Ti6Al4V alloys fabricated by the selective laser melting technique and a new sintering technique. J. Mech. Behav. Biomed. Mater. 2019, 91, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Shishkovsky, I.; Morozov, Y.; Smurov, I. Nanofractal surface structure under laser sintering of titanium and nitinol for bone tissue engineering. Appl. Surf. Sci. 2007, 254, 1145–1149. [Google Scholar] [CrossRef]
- Nune, K.C.; Li, S.; Misra, R.D.K. Advancements in three-dimensional titanium alloy mesh scaffolds fabricated by electron beam melting for biomedical devices: Mechanical and biological aspects. Sci. China Mater. 2018, 61, 455–474. [Google Scholar] [CrossRef]
- Jia, Z.; Li, M.; Xiu, P.; Xu, X.; Cheng, Y.; Zheng, Y.; Xi, T.; Wei, S.; Liu, Z. A novel cytocompatible, hierarchical porous Ti6Al4V scaffold with immobilized silver nanoparticles. Mater. Lett. 2015, 157, 143–146. [Google Scholar] [CrossRef]
- Surmeneva, M.A.; Surmenev, R.A.; Chudinova, E.A.; Koptioug, A.; Tkachev, M.S.; Gorodzha, S.N.; Rännar, L.-E. Fabrication of multiple-layered gradient cellular metal scaffold via electron beam melting for segmental bone reconstruction. Mater. Des. 2017, 133, 195–204. [Google Scholar] [CrossRef]
- Ataee, A.; Li, Y.; Fraser, D.; Song, G.; Wen, C. Anisotropic Ti-6Al-4V gyroid scaffolds manufactured by electron beam melting (EBM) for bone implant applications. Mater. Des. 2018, 137, 345–354. [Google Scholar] [CrossRef]
- Ataee, A.; Li, Y.; Brandt, M.; Wen, C. Ultrahigh-strength titanium gyroid scaffolds manufactured by selective laser melting (SLM) for bone implant applications. Acta Mater. 2018, 158, 354–368. [Google Scholar] [CrossRef]
- Eldesouky, I.; Harrysson, O.; West, H.; Elhofy, H. Electron beam melted scaffolds for orthopedic applications. Addit. Manuf. 2017, 17, 169–175. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, H.; Qiao, N.; Wang, C.; Hu, M. Corrosion resistance characteristics of a Ti-6Al-4V alloy scaffold that is fabricated by electron beam melting and selective laser melting for implantation in vivo. Mater. Sci. Eng. C 2017, 70, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Ataee, A.; Li, Y.; Wen, C. A comparative study on the nanoindentation behavior, wear resistance and in vitro biocompatibility of SLM manufactured CP–Ti and EBM manufactured Ti64 gyroid scaffolds. Acta Biomater. 2019, 97, 587–596. [Google Scholar] [CrossRef]
- Douglas, T.E.L.; Hempel, U.; Żydek, J.; Vladescu, A.; Pietryga, K.; Kaeswurm, J.A.H.; Buchweitz, M.; Surmenev, R.A.; Surmeneva, M.A.; Cotrut, C.M.; et al. Pectin coatings on titanium alloy scaffolds produced by additive manufacturing: Promotion of human bone marrow stromal cell proliferation. Mater. Lett. 2018, 227, 225–228. [Google Scholar] [CrossRef]
- Harun, W.S.W.; Kamariah, M.S.I.N.; Muhamad, N.; Ghani, S.A.C.; Ahmad, F.; Mohamed, Z. A review of powder additive manufacturing processes for metallic biomaterials. Powder Technol. 2018, 327, 128–151. [Google Scholar] [CrossRef]
- Xue, W.; Krishna, B.V.; Bandyopadhyay, A.; Bose, S. Processing and biocompatibility evaluation of laser processed porous titanium. Acta Biomater. 2007. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Espana, F.; Balla, V.K.; Bose, S.; Ohgami, Y.; Davies, N.M. Influence of porosity on mechanical properties and in vivo response of Ti6Al4V implants. Acta Biomater. 2010, 6, 1640–1648. [Google Scholar] [CrossRef]
- Sterling, A.J.; Torries, B.; Shamsaei, N.; Thompson, S.M.; Seely, D.W. Fatigue behavior and failure mechanisms of direct laser deposited Ti-6Al-4V. Mater. Sci. Eng. A 2016, 655, 100–112. [Google Scholar] [CrossRef]
- Kummailil, J.; Sammarco, C.; Skinner, D.; Brown, C.A.; Rong, K. Effect of select LENSTM processing parameters on the deposition of Ti-6Al-4V. J. Manuf. Process. 2005, 7, 42–50. [Google Scholar] [CrossRef]
- Srivas, P.K.; Kapat, K.; Dadhich, P.; Pal, P.; Dutta, J.; Datta, P.; Dhara, S. Osseointegration assessment of extrusion printed Ti6Al4V scaffold towards accelerated skeletal defect healing via tissue in-growth. Bioprinting 2017, 6, 8–17. [Google Scholar] [CrossRef]
- Ahsan, M.; Student, M.S. 3D Printing and Titanium Alloys: A Paper Review. Eur. Acad. Res. 2016, 3, 11144–11154. [Google Scholar]
- Barui, S.; Chatterjee, S.; Mandal, S.; Kumar, A.; Basu, B. Microstructure and compression properties of 3D powder printed Ti-6Al-4V scaffolds with designed porosity: Experimental and computational analysis. Mater. Sci. Eng. C 2017, 70, 812–823. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, H.; Rebesan, P.; Giacomello, G.; Pasetto, M.; Gardin, C.; Ferroni, L.; Zavan, B.; Biasetto, L. Direct ink writing of porous titanium (Ti6Al4V)lattice structures. Mater. Sci. Eng. C 2019, 103, 109794. [Google Scholar] [CrossRef]
- Chen, Y.; Han, P.; Vandi, L.-J.; Dehghan-Manshadi, A.; Humphry, J.; Kent, D.; Stefani, I.; Lee, P.; Heitzmann, M.; Cooper-White, J.; et al. A biocompatible thermoset polymer binder for Direct Ink Writing of porous titanium scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2019, 95, 160–165. [Google Scholar] [CrossRef]
- Dehghan-Manshadi, A.; Bermingham, M.J.; Dargusch, M.S.; StJohn, D.H.; Qian, M. Metal injection moulding of titanium and titanium alloys: Challenges and recent development. Powder Technol. 2017, 319, 289–301. [Google Scholar] [CrossRef]
- Shbeh, M.; Wally, Z.J.; Elbadawi, M.; Mosalagae, M.; Al-Alak, H.; Reilly, G.C.; Goodall, R. Incorporation of HA into porous titanium to form Ti-HA biocomposite foams. J. Mech. Behav. Biomed. Mater. 2019, 96, 193–203. [Google Scholar] [CrossRef]
- Li, J.P.; Habibovic, P.; van den Doel, M.; Wilson, C.E.; de Wijn, J.R.; van Blitterswijk, C.A.; de Groot, K. Bone ingrowth in porous titanium implants produced by 3D fiber deposition. Biomaterials 2007, 28, 2810–2820. [Google Scholar] [CrossRef]
- Zhao, L.; Pei, X.; Jiang, L.; Hu, C.; Sun, J.; Xing, F.; Zhou, C.; Fan, Y.; Zhang, X. Bionic design and 3D printing of porous titanium alloy scaffolds for bone tissue repair. Compos. Part B Eng. 2019, 162, 154–161. [Google Scholar] [CrossRef]
- Lu, Y.; Cui, Z.; Cheng, L.; Li, J.; Yang, Z.; Zhu, H.; Wu, C. Quantifying the discrepancies in the geometric and mechanical properties of the theoretically designed and additively manufactured scaffolds. J. Mech. Behav. Biomed. Mater. 2020, 112. [Google Scholar] [CrossRef]
- Li, P.; Warner, D.H.; Fatemi, A.; Phan, N. Critical assessment of the fatigue performance of additively manufactured Ti-6Al-4V and perspective for future research. Int. J. Fatigue 2016, 85, 130–143. [Google Scholar] [CrossRef]
- Lei, H.; Yi, T.; Fan, H.; Pei, X.; Wu, L.; Xing, F.; Li, M.; Liu, L.; Zhou, C.; Fan, Y.; et al. Customized additive manufacturing of porous Ti6Al4V scaffold with micro-topological structures to regulate cell behavior in bone tissue engineering. Mater. Sci. Eng. C 2021, 120. [Google Scholar] [CrossRef]
- Sun, D.; Gu, D.; Lin, K.; Ma, J.; Chen, W.; Huang, J.; Sun, X.; Chu, M. Selective laser melting of titanium parts: Influence of laser process parameters on macro- and microstructures and tensile property. Powder Technol. 2019, 342, 371–379. [Google Scholar] [CrossRef]
- Kasperovich, G.; Hausmann, J. Improvement of fatigue resistance and ductility of TiAl6V4 processed by selective laser melting. J. Mater. Process. Technol. 2015, 220, 202–214. [Google Scholar] [CrossRef]
- Xu, W.; Lui, E.W.; Pateras, A.; Qian, M.; Brandt, M. In situ tailoring microstructure in additively manufactured Ti-6Al-4V for superior mechanical performance. Acta Mater. 2017, 125, 390–400. [Google Scholar] [CrossRef]
- Qiu, C.; Adkins, N.J.E.; Attallah, M.M. Microstructure and tensile properties of selectively laser-melted and of HIPed laser-melted Ti-6Al-4V. Mater. Sci. Eng. A 2013, 578, 230–239. [Google Scholar] [CrossRef]
- Krakhmalev, P.; Fredriksson, G.; Yadroitsava, I.; Kazantseva, N.; Du Plessis, A.; Yadroitsev, I. Deformation behavior and microstructure of Ti6Al4V manufactured by SLM. Phys. Procedia 2016, 83, 778–788. [Google Scholar] [CrossRef]
- Rüegg, J.; Schumacher, R.; Weber, F.E.; De Wild, M. Mechanical anisotropy of titanium scaffolds Numerical simulation and biomechanical verification of anisotropic titanium scaffolds. Curr. Dir. Biomed. Eng. 2017, 3, 607–611. [Google Scholar] [CrossRef]
- Barba, D.; Alabort, C.; Tang, Y.T.; Viscasillas, M.J.; Reed, R.C.; Alabort, E. On the size and orientation effect in additive manufactured Ti-6Al-4V. Mater. Des. 2020, 186, 108235. [Google Scholar] [CrossRef]
- Yu, G.; Li, Z.; Li, S.; Zhang, Q.; Hua, Y.; Liu, H.; Zhao, X.; Dhaidhai, D.T.; Li, W.; Wang, X. The select of internal architecture for porous Ti alloy scaffold: A compromise between mechanical properties and permeability. Mater. Des. 2020, 192, 108754. [Google Scholar] [CrossRef]
- Sharma, P.; Pandey, P.M. Corrosion behaviour of the porous iron scaffold in simulated body fluid for biodegradable implant application. Mater. Sci. Eng. C 2019, 99, 838–852. [Google Scholar] [CrossRef] [PubMed]
- Longhitano, G.A.; Conde, A.; Arenas, M.A.; Jardini, A.L.; de Carvalho Zavaglia, C.A.; Maciel Filho, R.; de Damborenea, J.J. Corrosion resistance improvement of additive manufactured scaffolds by anodizing. Electrochim. Acta 2021, 366. [Google Scholar] [CrossRef]
- Trybuś, B.; Zieliński, A.; Beutner, R.; Seramak, T.; Scharnweber, D. Deposition of phosphate coatings on titanium within scaffold structure. Acta Bioeng. Biomech. 2017, 19, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Cui, Y.W.; Zhang, L.C. Recent development in beta titanium alloys for biomedical applications. Metals (Basel) 2020, 10, 1139. [Google Scholar] [CrossRef]
- Haghighi, S.E.; Lu, H.B.; Jian, G.Y.; Cao, G.H.; Habibi, D.; Zhang, L.C. Effect of α″ martensite on the microstructure and mechanical properties of beta-type Ti-Fe-Ta alloys. Mater. Des. 2015, 76, 47–54. [Google Scholar] [CrossRef]
- Kunčická, L.; Kocich, R.; Lowe, T.C. Advances in metals and alloys for joint replacement. Prog. Mater. Sci. 2017, 88, 232–280. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef]
- Huang, S.; Sing, S.L.; de Looze, G.; Wilson, R.; Yeong, W.Y. Laser powder bed fusion of titanium-tantalum alloys: Compositions and designs for biomedical applications. J. Mech. Behav. Biomed. Mater. 2020, 108, 103775. [Google Scholar] [CrossRef]
- Wang, Q.; Han, C.; Choma, T.; Wei, Q.; Yan, C.; Song, B.; Shi, Y. Effect of Nb content on microstructure, property and in vitro apatite-forming capability of Ti-Nb alloys fabricated via selective laser melting. Mater. Des. 2017, 126, 268–277. [Google Scholar] [CrossRef]
- Luo, J.P.; Sun, J.F.; Huang, Y.J.; Zhang, J.H.; Zhao, D.P.; Yan, M.; Zhang, Y.D. Low-modulus biomedical Ti–30Nb–5Ta–3Zr additively manufactured by Selective Laser Melting and its biocompatibility. Mater. Sci. Eng. C 2019, 97, 275–284. [Google Scholar] [CrossRef]
- Lu, J.; Zhao, Y.; Niu, H.; Zhang, Y.; Du, Y.; Zhang, W.; Huo, W. Electrochemical corrosion behavior and elasticity properties of Ti-6Al-xFe alloys for biomedical applications. Mater. Sci. Eng. C 2016. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.D.; Yook, S.W.; Jang, T.S.; Li, Y.; Kim, H.E.; Koh, Y.H. Dynamic freeze casting for the production of porous titanium (Ti) scaffolds. Mater. Sci. Eng. C 2013, 33, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, H.E.; Koh, Y.H. Highly porous titanium (Ti) scaffolds with bioactive microporous hydroxyapatite/TiO2 hybrid coating layer. Mater. Lett. 2009, 63, 1995–1998. [Google Scholar] [CrossRef]
- Morita, A.; Fukui, H.; Tadano, H.; Hayashi, S.; Hasegawa, J.; Niinomi, M. Alloying titanium and tantalum by cold crucible levitation melting (CCLM) furnace. Mater. Sci. Eng. A 2000, 280, 208–213. [Google Scholar] [CrossRef]
- Zhao, D.; Han, C.; Li, Y.; Li, J.; Zhou, K.; Wei, Q.; Liu, J.; Shi, Y. Improvement on mechanical properties and corrosion resistance of titanium-tantalum alloys in-situ fabricated via selective laser melting. J. Alloys Compd. 2019, 804, 288–298. [Google Scholar] [CrossRef]
- Liang, H.; Zhao, D.; Feng, X.; Ma, L.; Deng, X.; Han, C.; Wei, Q.; Yang, C. 3D-printed porous titanium scaffolds incorporating niobium for high bone regeneration capacity. Mater. Des. 2020, 194, 108890. [Google Scholar] [CrossRef]
- Xie, F.; He, X.; Lu, X.; Cao, S.; Qu, X. Preparation and properties of porous Ti–10Mo alloy by selective laser sintering. Mater. Sci. Eng. C 2013, 33, 1085–1090. [Google Scholar] [CrossRef]
- Wang, S.; Liu, L.; Li, K.; Zhu, L.; Chen, J.; Hao, Y. Pore functionally graded Ti6Al4V scaffolds for bone tissue engineering application. Mater. Des. 2019, 168, 107643. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, Y.; Li, T.; Zhou, Y.; Leeflang, S.; Chen, L.; Wu, C.; Zhou, J.; Huan, Z. 3D printed titanium scaffolds with ordered TiO2 nanotubular surface and mesoporous bioactive glass for bone repair. Prog. Nat. Sci. Mater. Int. 2020, 30, 502–509. [Google Scholar] [CrossRef]
- Zhai, C.; Zuo, Q.; Shen, K.; Zhou, J.; Chen, J.; Zhang, X.; Luo, C.; Fei, H.; Zuo, F. Utilizing an integrated tri-layered scaffold with Titanium-Mesh-Cage base to repair cartilage defects of knee in goat model. Mater. Des. 2020, 193, 108766. [Google Scholar] [CrossRef]
- Vlad, M.D.; Fernández Aguado, E.; Gómez González, S.; Ivanov, I.C.; Şindilar, E.V.; Poeată, I.; Iencean, A.Ş.; Butnaru, M.; Avădănei, E.R.; López López, J. Novel titanium-apatite hybrid scaffolds with spongy bone-like micro architecture intended for spinal application: In vitro and in vivo study. Mater. Sci. Eng. C 2020, 110, 110658. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, S.; Hou, W.; Wang, S.; Hao, Y.; Yang, R.; Sercombe, T.B.; Zhang, L.C. Electron Beam Melted Beta-type Ti-24Nb-4Zr-8Sn Porous Structures with High Strength-to-Modulus Ratio. J. Mater. Sci. Technol. 2016, 32, 505–508. [Google Scholar] [CrossRef]
- Liu, Y.J.; Wang, H.L.; Li, S.J.; Wang, S.G.; Wang, W.J.; Hou, W.T.; Hao, Y.L.; Yang, R.; Zhang, L.C. Compressive and fatigue behavior of beta-type titanium porous structures fabricated by electron beam melting. Acta Mater. 2017, 126, 58–66. [Google Scholar] [CrossRef]
- Liu, Y.J.; Li, X.P.; Zhang, L.C.; Sercombe, T.B. Processing and properties of topologically optimised biomedical Ti-24Nb-4Zr-8Sn scaffolds manufactured by selective laser melting. Mater. Sci. Eng. A 2015, 642, 268–278. [Google Scholar] [CrossRef]
- Ehtemam-Haghighi, S.; Attar, H.; Okulov, I.V.; Dargusch, M.S.; Kent, D. Microstructural evolution and mechanical properties of bulk and porous low-cost Ti–Mo–Fe alloys produced by powder metallurgy. J. Alloys Compd. 2021, 853, 156768. [Google Scholar] [CrossRef]
- Kim, W.R.; Bang, G.B.; Kwon, O.; Jung, K.H.; Park, H.K.; Kim, G.H.; Jeong, H.T.; Kim, H.G. Fabrication of porous pure titanium via selective laser melting under low-energy-density process conditions. Mater. Des. 2020, 195, 109035. [Google Scholar] [CrossRef]
- Yan, L.; Wu, J.; Zhang, L.; Liu, X.; Zhou, K.; Su, B. Pore structures and mechanical properties of porous titanium scaffolds by bidirectional freeze casting. Mater. Sci. Eng. C 2017, 75, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; He, X.; Lv, Y.; Wu, M.; He, X.; Qu, X. Selective laser sintered porous Ti-(4-10)Mo alloys for biomedical applications: Structural characteristics, mechanical properties and corrosion behaviour. Corros. Sci. 2015, 95, 117–124. [Google Scholar] [CrossRef]
- Warnke, P.H.; Douglas, T.; Wollny, P.; Sherry, E.; Steiner, M.; Galonska, S.; Becker, S.T.; Springer, I.N.; Wiltfang, J.; Sivananthan, S. Rapid prototyping: Porous titanium alloy scaffolds produced by selective laser melting for bone tissue engineering. Tissue Eng. Part C Methods 2009, 15, 115–124. [Google Scholar] [CrossRef]
- Kelly, C.N.; Francovich, J.; Julmi, S.; Safranski, D.; Guldberg, R.E.; Maier, H.J.; Gall, K. Fatigue behavior of As-built selective laser melted titanium scaffolds with sheet-based gyroid microarchitecture for bone tissue engineering. Acta Biomater. 2019, 94, 610–626. [Google Scholar] [CrossRef]
- Bobbert, F.S.L.; Lietaert, K.; Eftekhari, A.A.; Pouran, B.; Ahmadi, S.M.; Weinans, H.; Zadpoor, A.A. Additively manufactured metallic porous biomaterials based on minimal surfaces: A unique combination of topological, mechanical, and mass transport properties. Acta Biomater. 2017, 53, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xiong, Y.Z.; Zhang, H.; Gao, R.N. Development of functionally graded porous titanium/silk fibroin composite scaffold for bone repair. Mater. Lett. 2021, 282, 128670. [Google Scholar] [CrossRef]
- Soro, N.; Attar, H.; Wu, X.; Dargusch, M.S. Investigation of the structure and mechanical properties of additively manufactured Ti-6Al-4V biomedical scaffolds designed with a Schwartz primitive unit-cell. Mater. Sci. Eng. A 2019, 745, 195–202. [Google Scholar] [CrossRef]
- de Damborenea, J.J.; Larosa, M.A.; Arenas, M.A.; Hernández-López, J.M.; Jardini, A.L.; Ierardi, M.C.F.; Zavaglia, C.A.C.; Filho, R.M.; Conde, A. Functionalization of Ti6Al4V scaffolds produced by direct metal laser for biomedical applications. Mater. Des. 2015, 83, 6–13. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, W.; Gao, R.; Zhang, H.; Dong, L.; Qin, J.; Wang, B.; Jia, W.; Li, X. Fatigue behavior and osseointegration of porous Ti-6Al-4V scaffolds with dense core for dental application. Mater. Des. 2020, 195, 108994. [Google Scholar] [CrossRef]
- Bassous, N.J.; Jones, C.L.; Webster, T.J. 3-D printed Ti-6Al-4V scaffolds for supporting osteoblast and restricting bacterial functions without using drugs: Predictive equations and experiments. Acta Biomater. 2019, 96, 662–673. [Google Scholar] [CrossRef]
- Zhang, L.-C.; Chen, L.-Y.; Wang, L. Surface Modification of Titanium and Titanium Alloys: Technologies, Developments, and Future Interests. Adv. Eng. Mater. 2020, 22, 2070017. [Google Scholar] [CrossRef]
- Dong, Y.P.; Li, Y.L.; Zhou, S.Y.; Zhou, Y.H.; Dargusch, M.S.; Peng, H.X.; Yan, M. Cost-affordable Ti-6Al-4V for additive manufacturing: Powder modification, compositional modulation and laser in-situ alloying. Addit. Manuf. 2021, 37, 101699. [Google Scholar] [CrossRef]
- Fan, B.; Guo, Z.; Li, X.; Li, S.; Gao, P.; Xiao, X.; Wu, J.; Shen, C.; Jiao, Y.; Hou, W. Electroactive barium titanate coated titanium scaffold improves osteogenesis and osseointegration with low-intensity pulsed ultrasound for large segmental bone defects. Bioact. Mater. 2020, 5, 1087–1101. [Google Scholar] [CrossRef]
- Ren, B.; Wan, Y.; Liu, C.; Wang, H.; Yu, M.; Zhang, X.; Huang, Y. Improved osseointegration of 3D printed Ti-6Al-4V implant with a hierarchical micro/nano surface topography: An in vitro and in vivo study. Mater. Sci. Eng. C 2021, 118, 111505. [Google Scholar] [CrossRef]
- Ahmed, N. Direct metal fabrication in rapid prototyping: A review. J. Manuf. Process. 2019, 42, 167–191. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, X.; Shen, T.; Wu, C.; Zhang, L. Template-assisted freeze casting of macroporous Ti6Al4V scaffolds with long-range order lamellar structure. Mater. Lett. 2020, 264, 127374. [Google Scholar] [CrossRef]
- Weaver, J.S.; Kalidindi, S.R.; Wegst, U.G.K. Structure-processing correlations and mechanical properties in freeze-cast Ti-6Al-4V with highly aligned porosity and a lightweight Ti-6Al-4V-PMMA composite with excellent energy absorption capability. Acta Mater. 2017, 132, 182–192. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Xiong, J.; Hodgson, P.D.; Wen, C. Porous TiNbZr alloy scaffolds for biomedical applications. Acta Biomater. 2009, 5, 3616–3624. [Google Scholar] [CrossRef]
- Xu, W.; Xiao, S.; Lu, X.; Chen, G.; Liu, C.; Qu, X. Fabrication of commercial pure Ti by selective laser melting using hydride-dehydride titanium powders treated by ball milling. J. Mater. Sci. Technol. 2019, 35, 322–327. [Google Scholar] [CrossRef]
- Li, J.L.; Wang, S.; Cao, F.; Lin, X.; Wei, X.W.; Zhao, Z.H.; Dou, X.J.; Yu, W.T.; Yang, K.; Zhao, D.W. Fabrication and Characterization of Nanopillar-Like HA Coating on Porous Ti6Al4V Scaffold by a Combination of Alkali–Acid-Heat and Hydrothermal Treatments. Acta Metall. Sin. Engl. Lett. 2019, 32, 1075–1088. [Google Scholar] [CrossRef]
- Dutta, A.; Mukherjee, K.; Dhara, S.; Gupta, S. Design of porous titanium scaffold for complete mandibular reconstruction: The influence of pore architecture parameters. Comput. Biol. Med. 2019, 108, 31–41. [Google Scholar] [CrossRef]
- Pei, X.; Zhang, B.; Fan, Y.; Zhu, X.; Sun, Y.; Wang, Q.; Zhang, X.; Zhou, C. Bionic mechanical design of titanium bone tissue implants and 3D printing manufacture. Mater. Lett. 2017, 208, 133–137. [Google Scholar] [CrossRef]
- Zargarian, A.; Esfahanian, M.; Kadkhodapour, J.; Ziaei-Rad, S. Numerical simulation of the fatigue behavior of additive manufactured titanium porous lattice structures. Mater. Sci. Eng. C 2016, 60, 339–347. [Google Scholar] [CrossRef]
- Luo, D.; Rong, Q.; Chen, Q. Finite-element design and optimization of a three-dimensional tetrahedral porous titanium scaffold for the reconstruction of mandibular defects. Med. Eng. Phys. 2017, 47, 176–183. [Google Scholar] [CrossRef]
- Weißmann, V.; Bader, R.; Hansmann, H.; Laufer, N. Influence of the structural orientation on the mechanical properties of selective laser melted Ti6Al4V open-porous scaffolds. Mater. Des. 2016, 95, 188–197. [Google Scholar] [CrossRef]
- Wysocki, B.; Idaszek, J.; Buhagiar, J.; Szlązak, K.; Brynk, T.; Kurzydłowski, K.J.; Święszkowski, W. The influence of chemical polishing of titanium scaffolds on their mechanical strength and in-vitro cell response. Mater. Sci. Eng. C 2019, 95, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Onal, E.; Frith, J.; Jurg, M.; Wu, X.; Molotnikov, A. Mechanical Properties and In Vitro Behavior of Additively Manufactured and Functionally Graded Ti6Al4V Porous Scaffolds. Metals 2018, 8, 200. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Fang, G.; Leeflang, S.; Zadpoor, A.A.; Zhou, J. Topological design, permeability and mechanical behavior of additively manufactured functionally graded porous metallic biomaterials. Acta Biomater. 2019, 84, 437–452. [Google Scholar] [CrossRef]
- Wysocki, B.; Maj, P.; Krawczyńska, A.; Rożniatowski, K.; Zdunek, J.; Kurzydłowski, K.J.; Święszkowski, W. Microstructure and mechanical properties investigation of CP titanium processed by selective laser melting (SLM). J. Mater. Process. Technol. 2017, 241, 13–23. [Google Scholar] [CrossRef]
- Zafar, M.S.; Ullah, R.; Qamar, Z.; Fareed, M.A.; Amin, F.; Khurshid, Z.; Sefat, F. Properties of dental biomaterials. In Advanced Dental Biomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 7–35. [Google Scholar]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef]
- Baril, E.; Lefebvre, L.P.; Hacking, S.A. Direct visualization and quantification of bone growth into porous titanium implants using micro computed tomography. J. Mater. Sci. Mater. Med. 2011, 22, 1321–1332. [Google Scholar] [CrossRef]
- Khodaei, M.; Valanezhad, A.; Watanabe, I.; Yousefi, R. Surface and mechanical properties of modified porous titanium scaffold. Surf. Coat. Technol. 2017, 315, 61–66. [Google Scholar] [CrossRef]
- Sengottuvelan, A.; Balasubramanian, P.; Will, J.; Boccaccini, A.R. Bioactivation of titanium dioxide scaffolds by ALP-functionalization. Bioact. Mater. 2017, 2, 108–115. [Google Scholar] [CrossRef]
- Domínguez-Trujillo, C.; Ternero, F.; Rodríguez-Ortiz, J.A.; Heise, S.; Boccaccini, A.R.; Lebrato, J.; Torres, Y. Bioactive coatings on porous titanium for biomedical applications. Surf. Coat. Technol. 2018, 349, 584–592. [Google Scholar] [CrossRef]
- Li, D.; Li, K.; Shan, H. Improving biocompatibility of titanium alloy scaffolds by calcium incorporated silicalite-1 coatings. Inorg. Chem. Commun. 2019, 102, 61–65. [Google Scholar] [CrossRef]
- Vidal, E.; Guillem-Marti, J.; Ginebra, M.-P.; Combes, C.; Rupérez, E.; Rodriguez, D. Multifunctional homogeneous calcium phosphate coatings: Toward antibacterial and cell adhesive titanium scaffolds. Surf. Coat. Technol. 2020, 126557. [Google Scholar] [CrossRef]
- Rodríguez-Contreras, A.; Torres, D.; Guillem-Marti, J.; Sereno, P.; Ginebra, M.P.; Calero, J.A.; Manero, J.M.; Rupérez, E. Development of novel dual-action coatings with osteoinductive and antibacterial properties for 3D-printed titanium implants. Surf. Coat. Technol. 2020, 403, 126381. [Google Scholar] [CrossRef]
- Lee, H.; Jung, H.D.; Kang, M.H.; Song, J.; Kim, H.E.; Jang, T.S. Effect of HF/HNO3-treatment on the porous structure and cell penetrability of titanium (Ti) scaffold. Mater. Des. 2018, 145, 65–73. [Google Scholar] [CrossRef]
- Izquierdo-Barba, I.; Santos-Ruiz, L.; Becerra, J.; Feito, M.J.; Fernández-Villa, D.; Serrano, M.C.; Díaz-Güemes, I.; Fernández-Tomé, B.; Enciso, S.; Sánchez-Margallo, F.M.; et al. Synergistic effect of Si-hydroxyapatite coating and VEGF adsorption on Ti6Al4V-ELI scaffolds for bone regeneration in an osteoporotic bone environment. Acta Biomater. 2019, 83, 456–466. [Google Scholar] [CrossRef]
- Li, X.; Ma, X.Y.; Feng, Y.F.; Wang, L.; Wang, C. A novel composite scaffold consisted of porous titanium and chitosan sponge for load-bearing applications: Fabrication, characterization and cellular activity. Compos. Sci. Technol. 2015, 117, 78–84. [Google Scholar] [CrossRef]
- García-Gareta, E.; Hua, J.; Rayan, F.; Blunn, G.W. Stem cell engineered bone with calcium-phosphate coated porous titanium scaffold or silicon hydroxyapatite granules for revision total joint arthroplasty. J. Mater. Sci. Mater. Med. 2014, 25, 1553–1562. [Google Scholar] [CrossRef]
- Brecevich, A.T.; Dowe, C.; Cammisa, F.P.; Abjornson, C. 2. 3D-printed titanium: A surface optimization analysis. Spine J. 2019, 19, S1–S2. [Google Scholar] [CrossRef]
- Chen, H.; Wang, C.; Yang, X.; Xiao, Z.; Zhu, X.; Zhang, K.; Fan, Y.; Zhang, X. Construction of surface HA/TiO2 coating on porous titanium scaffolds and its preliminary biological evaluation. Mater. Sci. Eng. C 2017, 70, 1047–1056. [Google Scholar] [CrossRef]
- Chudinova, E.A.; Surmeneva, M.A.; Timin, A.S.; Karpov, T.E.; Wittmar, A.; Ulbricht, M.; Ivanova, A.; Loza, K.; Prymak, O.; Koptyug, A.; et al. Adhesion, proliferation, and osteogenic differentiation of human mesenchymal stem cells on additively manufactured Ti6Al4V alloy scaffolds modified with calcium phosphate nanoparticles. Colloids Surf. B Biointerfaces 2019, 176, 130–139. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, W.; Qiu, J.; Wang, S.; Yu, X.; Li, L.; Liu, H. Nanostructured titanium foam with metal ions incorporation for promoting osteogenic differentiation of mesenchymal stem cells. J. Alloys Compd. 2017, 729, 816–822. [Google Scholar] [CrossRef]
- Qian, L.; Yu, P.; Zeng, J.; Shi, Z.; Wang, Q.; Tan, G.; Ning, C. Large-scale functionalization of biomedical porous titanium scaffolds surface with TiO2 nanostructures. Sci. China Mater. 2018, 61, 557–564. [Google Scholar] [CrossRef]
- do Prado, R.F.; Rabêlo, S.B.; de Andrade, D.P.; Nascimento, R.D.; Henriques, V.A.R.; Carvalho, Y.R.; Cairo, C.A.A.; de Vasconcellos, L.M.R. Porous titanium and Ti–35Nb alloy: Effects on gene expression of osteoblastic cells derived from human alveolar bone. J. Mater. Sci. Mater. Med. 2015, 26. [Google Scholar] [CrossRef] [PubMed]
- Coffigniez, M.; Gremillard, L.; Balvay, S.; Lachambre, J.; Adrien, J.; Boulnat, X. Direct-ink writing of strong and biocompatible titanium scaffolds with bimodal interconnected porosity. Addit. Manuf. 2021, 39, 101859. [Google Scholar] [CrossRef]
- Ilea, A.; Vrabie, O.G.; Băbțan, A.M.; Miclăuş, V.; Ruxanda, F.; Sárközi, M.; Barbu-Tudoran, L.; Mager, V.; Berce, C.; Boșca, B.A.; et al. Osseointegration of titanium scaffolds manufactured by selective laser melting in rabbit femur defect model. J. Mater. Sci. Mater. Med. 2019, 30. [Google Scholar] [CrossRef] [PubMed]
- Hirota, M.; Hayakawa, T.; Shima, T.; Ametani, A.; Tohnai, I. High porous titanium scaffolds showed higher compatibility than lower porous beta-tricalcium phosphate scaffolds for regulating human osteoblast and osteoclast differentiation. Mater. Sci. Eng. C 2015, 49, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Luan, H.Q.; Wang, L.T.; Ren, W.Y.; Chu, Z.W.; Huang, Y.F.; Lu, C.L.; Fan, Y.B. The effect of pore size and porosity of Ti6Al4V scaffolds on MC3T3-E1 cells and tissue in rabbits. Sci. China Technol. Sci. 2019, 62, 1160–1168. [Google Scholar] [CrossRef]
- De Wild, M.; Schumacher, R.; Mayer, K.; Schkommodau, E.; Thoma, D.; Bredell, M.; Kruse Gujer, A.; Grätz, K.W.; Weber, F.E. Bone regeneration by the osteoconductivity of porous titanium implants manufactured by selective laser melting: A histological and micro computed tomography study in the rabbit. Tissue Eng. Part A 2013, 19, 2645–2654. [Google Scholar] [CrossRef]
- Zhao, G.; Li, S.; Chen, X.; Qu, X.; Chen, R.; Wu, Y.; Liu, Y.; Zou, X.; Lu, X. Porous tantalum scaffold fabricated by gel casting based on 3D printing and electrolysis. Mater. Lett. 2019, 239, 5–8. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Hirota, M.; Shima, T.; Sato, I.; Ozawa, T.; Iwai, T.; Ametani, A.; Sato, M.; Noishiki, Y.; Ogawa, T.; Hayakawa, T.; et al. Development of a biointegrated mandibular reconstruction device consisting of bone compatible titanium fiber mesh scaffold. Biomaterials 2016, 75, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Su, K.; Su, L.; Liang, P.; Ji, P.; Wang, C. Comparison of 3D-printed porous tantalum and titanium scaffolds on osteointegration and osteogenesis. Mater. Sci. Eng. C 2019, 104, 109908. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shi, J.; Zhang, K.; Yang, L.; Yu, F.; Zhu, L.; Liang, H.; Wang, X.; Jiang, Q. Early osteointegration evaluation of porous Ti6Al4V scaffolds designed based on triply periodic minimal surface models. J. Orthop. Transl. 2019, 19, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Song, W.; Han, T.; Yan, J.; Li, F.; Zhao, L.; Kou, H.; Zhang, Y. Influence of pore size of porous titanium fabricated by vacuum diffusion bonding of titanium meshes on cell penetration and bone ingrowth. Acta Biomater. 2016, 33, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Rubshtein, A.P.; Makarova, E.B.; Bliznets, D.G.; Vladimirov, A.B. Properties of biocomposites based on titanium scaffolds with a different porosity. Bull. Mater. Sci. 2017, 40, 453–457. [Google Scholar] [CrossRef]
- Qiao, S.; Sheng, Q.; Li, Z.; Wu, D.; Zhu, Y.; Lai, H.; Gu, Y. 3D-printed Ti6Al4V scaffolds coated with freeze-dried platelet-rich plasma as bioactive interface for enhancing osseointegration in osteoporosis. Mater. Des. 2020, 194, 108825. [Google Scholar] [CrossRef]
- Khodaei, M.; Valanezhad, A.; Watanabe, I. Controlled gentamicin- strontium release as a dual action bone agent: Combination of the porous titanium scaffold and biodegradable polymers. J. Alloys Compd. 2017, 720, 22–28. [Google Scholar] [CrossRef]
- Surmeneva, M.; Lapanje, A.; Chudinova, E.; Ivanova, A.; Koptyug, A.; Loza, K.; Prymak, O.; Epple, M.; Ennen-Roth, F.; Ulbricht, M.; et al. Decreased bacterial colonization of additively manufactured Ti6Al4V metallic scaffolds with immobilized silver and calcium phosphate nanoparticles. Appl. Surf. Sci. 2019, 480, 822–829. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Gao, L.; Sun, Y.; Wang, J.; Qu, S.; Duan, K.; Weng, J.; Feng, B. Porous titanium scaffold surfaces modified with silver loaded gelatin microspheres and their antibacterial behavior. Surf. Coat. Technol. 2016, 286, 140–147. [Google Scholar] [CrossRef]
- Croes, M.; Bakhshandeh, S.; van Hengel, I.A.J.; Lietaert, K.; van Kessel, K.P.M.; Pouran, B.; van der Wal, B.C.H.; Vogely, H.C.; Van Hecke, W.; Fluit, A.C.; et al. Antibacterial and immunogenic behavior of silver coatings on additively manufactured porous titanium. Acta Biomater. 2018, 81, 315–327. [Google Scholar] [CrossRef]
- Vidal, E.; Buxadera-Palomero, J.; Pierre, C.; Manero, J.M.; Ginebra, M.P.; Cazalbou, S.; Combes, C.; Rupérez, E.; Rodríguez, D. Single-step pulsed electrodeposition of calcium phosphate coatings on titanium for drug delivery. Surf. Coat. Technol. 2019, 358, 266–275. [Google Scholar] [CrossRef]
- Dabrowski, B.; Kaminski, J.; Swieszkowski, W.; Kurzydlowski, K.J. Porous titanium scaffolds for biomedical applications: Corrosion resistance and structure investigation. Mater. Sci. Forum 2011, 674, 41–46. [Google Scholar] [CrossRef]
- Chen, X.; Fu, Q.; Jin, Y.; Li, M.; Yang, R.; Cui, X.; Gong, M. In vitro studying corrosion behavior of porous titanium coating in dynamic electrolyte. Mater. Sci. Eng. C 2017, 70, 1071–1075. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.F.; Li, S.J.; Hou, W.T.; Hao, Y.L.; Huang, H.H. Enhancing corrosion resistance and biocompatibility of interconnected porous β-type Ti-24Nb-4Zr-8Sn alloy scaffold through alkaline treatment and type I collagen immobilization. Appl. Surf. Sci. 2019, 476, 325–334. [Google Scholar] [CrossRef]
- Xu, W.; Lu, X.; Zhang, B.; Liu, C.; Lv, S.; Yang, S.; Qu, X. Effects of porosity on mechanical properties and corrosion resistances of PM-fabricated porous Ti-10Mo Alloy. Metals 2018, 8, 188. [Google Scholar] [CrossRef]
| Bone | Trabecular | Cortical | References |
|---|---|---|---|
| Porosity (%) | 50–90 | 3–12 | [46,47,48,49] |
| Density (g/cm3) | 0.30 ± 0.1 | 1.85 ± 0.06 | [47] |
| Stiffness (GPa) | 0.1–2.942 | 14.7–34.3 | [4,50] |
| Tensile strength (MPa) | 10–20 | 50–150 | [34,47] |
| Compressive strength (MPa) | 4–12 | 130–193 | [47,51] |
| Young’s modulus (GPa) | 0.02–0.5 | 3–30 | [46,47] |
| Parameters | Specification | References |
|---|---|---|
| Structural requirements | ||
| Pore shape | Cubic, rhombic dodecahedron, truncated cuboctahedron, rhombicuboctahedron, diamond, truncated cube | [60,73] |
| Optimal pore size | 300–600 µm | [39,51,67] |
| Porosity | 50–90% | [46,67] |
| Mechanical requirements | ||
| Compression strength | 10–200 MPa | [34,78] |
| Young’s modulus | 0.02–30 MPa | [47,78] |
| Material requirements | ||
| 3D architecture Biocompatibility Biodegradability Radiolucent Easily shaped or molded Nonallergic and noncarcinogenic Strong enough to endure trauma Stable over time Osteoconductive | [5,79] | |
| Methods | Advantages | Disadvantages | Resolution (μm) | Costs | Post-Treatment |
|---|---|---|---|---|---|
| SLM | -high-precision complex parts -no support structure required -mechanical properties better than SLS (due to the level of heating during printing) | -worse resolution than SLA, SLS, EBM -unmelted powders may be trapped inside parts -high temperature of the process | 250–700 | $$ | may be necessary due to the presence of partially sintered metal on the struts |
| SLS | -processing speed is high -good mechanical properties, but worse than for SLM and EBM -high utilization of powder materials, -no support structures required, -superior dimensional accuracy, -efficient resource consumption | -high molding principles, high molding conditions, and high cost, -limited part size, particle sizes -the powders are not fully melted | 76–100 μm | $$$ | may be necessary due to the presence of partially sintered metal on the struts |
| EBM | -superior mechanical properties due to the complete melting of the powders -higher resolution than SLM | -the high temperature of the processed areas, -unmelted powders may be trapped inside parts | 50–100 μm | $$$$ | may be necessary due to the presence of partially sintered metal on the struts |
| FDM | -low cost -increased thermal conductivity of parts, -reduced deformation during fabrication | -anisotropic and poor mechanical properties compared with the SLM, EBM -high temperature of the printing has a negative effect on bioactive additives. -possible manufacturing problem with excessively acute angles | 250–370 | $ | poor surface quality, required additional treatment |
| LENS | -excellent mechanical properties -better efficiency, cooling effect, and parts refabricating capability compared to SLM, EBM, SLS -possible integration of different materials, -effective time of the process -highly controlled microstructure | less complex models in comparison with SLM, EBM, SLS | 250 | $$$ | poor surface quality, required additional treatment |
| MIM | -high printing speed -high manufacturing precision compare to SLS -suited to mass production -low cost -low melting temperature | -low dimensional stability and reproducibility | - | $ | Required additional treatment |
| DIW | -low temperature of the process -flexible manufacturing -high storage modulus and excellent shape retention capacity, -good level of resolution -low cost | requires support structures when manufacturing complex architectures | <200 μm | $ | May be required additional treatment |
| 3DF | -preparation time is reduced -each layer may have a different fiber diameter, thickness, fiber space, and fiber orientation -parametric analyses are possible | -low resolution | - | $$ | High surface quality |
| Material | Modulus (GPa) | Tensile Strength (MPa) | Alloy Type | Mechanical Properties | Biocompatibility | References |
|---|---|---|---|---|---|---|
| CP-Ti | 100–120 | 240–550 | α | [160,161] | ||
| Ti-Ta | 200 | - | α | Modulus much higher compare to cortical bone The increasing of Ta addition increases tensile strength and decrease modulus | +, Elements such titanium, niobium, tantalum after implantation in rats exhibited good biocompatibility | [160,161,162] |
| Ti-35Nb | 80 | 382 | β | Nb element reduces the elastic modulus | +, The addition of Nb to Ti promoted apatite-formation | [160,161,163] |
| Ti-7.5Mo | 80 | 665 | α + β | Better corrosion resistance compared to CP-Ti, Ti-6Al-4V | + | [160] |
| Ti-6Al-4V | 112 | 895–930 | α + β | Modulus much higher compared to cortical bone | +, contains toxic elements V, Al | |
| Ti-13Nb-13Zr | 79–84 | 973–1037 | Metastable β | Nb and Zr addition enhance mechanical properties, corrosion, and wear resistance, Nb elements reduce the elastic modulus | ++, better compared to α and α + β alloys, the addition of Nb to Ti promoted apatite-formation | [160,161] |
| Ti-24Nb-4Zr-8Sn | 42 | - | β | [160,161] | ||
| Ti–10Nb-3Mo | - | - | β | Nb and Mo enhance mechanical properties, Nb element reduces the elastic modulus | [160,161] | |
| Ti-20Nb-15Zr | - | - | β | Nb and Zr addition enhance mechanical properties, corrosion, and wear resistance, Nb element reduces the elastic modulus | [159] | |
| Ti-35Zr-28Nb | - | - | β | [159] | ||
| Ti-30Nb-5Ta-3Zr | 90 | 700 | β | [164] | ||
| Ti-10Mo-xFe | 91 | - | α + β | addition of Fe and Mo to Ti alloys enhanced their mechanical strength and reduced elastic modulus | [158,165] |
| Material | Manufacturing Method | References |
|---|---|---|
| CP-Ti | SLM/robocasting | [115] |
| SLM | [71,128,162,180] | |
| Freeze-casting | [102,103,166,181] | |
| Sponge replication process | [107,109,110,167] | |
| Powder metallurgy | [80,81,83,84,85,93,96,97,98,100,101,106] | |
| Injection molding | [140,141] | |
| Direct ink writing | [139] | |
| LENS | [131] | |
| Ti-xTa | SLM | [162,169] |
| Ti-xNb | SLM | [170] |
| Ti-xMo | SLS | [171,182] |
| Ti-6Al-4V | SLM | [9,112,172,173,174,183,184,185,186,187,188,189] |
| SLS | [119,175,190] | |
| EBM | [27,122,125,126,127,191,192,193,194] | |
| LENS | [133,134,195] | |
| Direct ink writing | [137,138] | |
| 3DF deposition | [142] | |
| (Dynamic) freeze-casting | [105,196,197] | |
| FDM (customized) | [135] | |
| Injection molding | [140] | |
| Ti-13Nb-13Zr | SLM | [74] |
| Ti-24Nb-4Zr-8Sn (Ti2448) | EBM | [176,177] |
| SLM | [178] | |
| Ti–10Mo-xFe | Powder metallurgy | [179] |
| Ti–10Nb-3Mo | Powder metallurgy | [99] |
| Ti-20Nb-15Zr | Sponge replication process | [108] |
| Ti-35Zr-28Nb | Powder metallurgy | [198,199] |
| SLM | [113] | |
| Ti-30Nb-5Ta-3Zr | SLM | [164] |
| Material | Pore Shape | Pore Size 1 (µm) | Strut Size 1 (µm) | Porosity 2 (%) | Mechanical Properties 3 | References | ||
|---|---|---|---|---|---|---|---|---|
| Young’s Modulus (GPa) | Compressive Stiffness (MPa) | Ultimate Compressive Strength (MPa) | ||||||
| Fully dense | ||||||||
| Ti-6Al-4V (SLM) | - | - | - | 0.8 | 118.9 | 1040 | 1842 | [57] |
| Ti-6Al-4V (hot-rolled) | - | - | - | 0 | 117.2 | 879 | 1835 | |
| Fully porous | ||||||||
| Triangular | 500 | 200 | 31.63 | N/A | 2840 | N/A | [72] | |
| 1000 | 200 | 19.17 | N/A | 453 | N/A | |||
| Ti6-Al-4V | Hexagonal | 500 | 200 | 57.66 | N/A | 11,256 | N/A | |
| 1000 | 200 | 29.75 | N/A | 3881 | N/A | |||
| Rectangular | 500 | 200 | 33.35 | N/A | 2038 | N/A | ||
| 1000 | 200 | 16.95 | N/A | 1300 | N/A | |||
| 650 | 200 | 79.5 | 1.22 | N/A | 36.45 | [45] | ||
| 650 | 250 | 76.3 | 2.00 | N/A | 56.63 | |||
| Ti-6Al-4V | Diamond | 650 | 300 | 72.6 | 3.02 | N/A | 85.81 | |
| 650 | 350 | 67.9 | 3.79 | N/A | 109.20 | |||
| 650 | 400 | 66.1 | 5.15 | N/a | 140.26 | |||
| Ti-6Al-4V | Rhombic dodecahedron | 200 | 300 | 79.2 | N/A | 19.0 | 21.5 | [57] |
| Triangular | 1000 | 200 | 34.88 | N/A | 5426 | 102.87 | [74] | |
| 750 | 200 | 52.32 | N/A | 3418 | 198.81 | |||
| Hexagonal | 1000 | 200 | 33.03 | N/A | 1623 | 55.38 | ||
| Ti-13Nb-13Zr | 750 | 200 | 34.86 | N/A | 3256 | 112.59 | ||
| Diamond | 1000 | 200 | 25.81 | N/A | 868 | 21.12 | ||
| 750 | 200 | 34.98 | N/A | 1912 | 59.87 | |||
| 177 | 628 | 15.0 | 3.69 | N/A | N/A | [51] | ||
| Ti-6Al-4V | - | 383 | 454 | 37.9 | 3.52 | N/A | N/A | |
| 653 | 305 | 70.0 | 2.58 | N/A | N/A | |||
| Primitive | 679 | 260 | 65 | 6.4 | 295.4 | N/A | [154] | |
| Ti-6Al-4V | Gyroid | 574 | 220 | 65 | 7.6 | 392.1 | N/A | |
| Body-centered cubic | 882 | 600 | 65 | 4.7 | 216.0 | N/A | ||
| Face centered cubic | 2000 | 300 | 87.3 (83.2) | 1.1 | N/A | 27 | [113] | |
| Ti-35Zr-28Nb | Face and body-centered cubic | 2000 | 300 | 78.9 (49.9) | 1.3 | N/A | 58 | |
| CP-Ti | Cubic | 54.9 | 7.22 | 75.04 | [115] | |||
| Functionally graded structure –bimodal pore size | ||||||||
| CP-Ti | Diamond | Core 200 Shell 500 | Core 100 Shell 200 | 56–67 | 42.7 | N/A | 447 | [209] |
| Porous shell + Dense core | ||||||||
| Ti-6Al-4V | Rhombic dodecahedron | 200 (porous shell: 1 mm) | 300 | 37.9 | 65.1 | 578 | 1072 | [57] |
| 200 (porous shell: 2 mm) | 300 | 62.1 | 30.05 | 257 | 393 | |||
| Dense shell + Porous core | ||||||||
| Ti-6Al-4V | Rhombic dodecahedron | 200 (porous core: | 300 | 48.4 | 47.6 | 422 | 579 | [57] |
| Material and Manufacturing Methods | Surface Treatment | Apatite Forming Ability | Antibacterial | In-Vitro Assay | In-Vivo Assay | References | ||
|---|---|---|---|---|---|---|---|---|
| Cells | Results | Model | Results | |||||
| Ti-6Al-4V, SLM | AH HT/AH | The highest for HT/AH treatment | - | MSCs | The best adhesion and differentiation after HT/AH treatment | - | - | [200] |
| CP-Ti, Space holder technique | Heat treatment for various time | Increasing with the rising heat treatment time up to 240 min | - | - | - | - | - | [213] |
| TiO2, foam replica method | ALP using self-polymerization of dopamine | An increased HAp formation for ALP- coated titania | - | - | - | - | - | [214] |
| Ti-6Al-4V, hydrothermal synthesis | Zeolite silicalite-1 coatings by secondary growth method | Formation of mineralized nodules noticed | - | Rabbit bone marrow mesenchymal stem cells (r-BMSCs) | Significantly enhanced the attachment and proliferation of r-BMSCs | - | - | [216] |
| Ti-6Al-4V, SLM | - | - | - | MG63 cells | Enhanced osteoblasts’ proliferation and differentiation for trabecular-like scaffolds with the full irregularity (0.5) and higher porosity (63 or 74%) | - | - | [114] |
| CP-Ti, freeze-casting | HF/HNO3 acid treatment with various time condition | - | - | Preosteoblast cell line (MC3T3-E1) | high number of cells attached to the pore surface after 12 min of treatment | - | - | [219] |
| Ti-6A-4V, EBM | Ti/Ti+SiHAp+VEGF obtained by dip-coating method | - | - | Murine preosteoblastic MC3T3-E1/mature endothelial cells | VEGF stimulated the proliferation of endothelial cells on the surface. The stimulated proliferation of preosteoblasts on SiHAp coated scaffolds | Osteoporotic sheep model | SiHAp+ VEGF: a significant increase in ossification and angiogenesis degree | [220] |
| CP-Ti, EBM | Chitosan/HAp sponge by freeze-drying | - | - | Rat osteoblasts | Improved osteoblast adhesion, proliferation and alkaline phosphatase (ALP) activity | - | - | [221] |
| CP-Ti, SLM | Various structures | - | - | Marrow-derived mesenchymal stem cells (hMSCs) | The strongest cell adhesion for porosities 50–70%, at lower porosities the increased levels of DNA and ALP | x | x | [223] |
| CP-Ti, Sintering | HAp/TiO2 subject to AA treatment | - | - | MC3T3-E1 osteoblasts | HAp/TiO2 improved adsorption of serum proteins and enhanced the ALP activity | - | - | [224] |
| Ti-6Al-4V, EBM | Calcium phosphate nanoparticles (CaPNPs) by electrophoretic deposition | - | - | hMSCs | Improved cell attachment, proliferation, and differentiation, increase of ALP activity | - | - | [225] |
| CP-Ti, freeze-casting | Thermal oxidation | - | - | MG63 osteosarcoma cells | With increasing coculture time from 1 to 5 days, cell proliferation increased with co-culture time from 1 to 5 days. Significant increase in cell proliferation and differentiation after thermal treatment. | Rabbits | No loosening or bone resorption, and bone ingrowth and osteogenesis were found for modified and unmodified scaffolds. Thermal modification improved the differentiation of osteoblasts in the pores. | [235] |
| CP-Ti, SLM | TiO2 obtained by HT method | - | - | BMSCs | Enhanced cell adhesion and spreading on the nanowire-functionalized scaffold. | - | - | [227] |
| Ti6Al4V, SLM | Gradient porous structures | - | - | - | - | Mini pigs | Stimulated bone ingrowth achieving a stable interface after 5 weeks after implantation (the push-out force 1100 N–1300 N). | [238] |
| CP-Ti, SLM | Various pore sizes: 300, 600, 900 μm | - | - | - | - | Rabbits (fixation ability for the cortical bone of the rabbit tibia/bone ingrowth for cancellous bone in the rabbit femur) | At 600 μm, a significantly higher fixation ability in 2 weeks than the other implants. After 4 weeks, sufficiently high fixation ability for all porosities. | [70] |
| CP-Ti, SLM | nano-SiHAp 0.8 and 1 mm cell size | - | - | - | - | Femur bone defects of White Californian male rabbits | Better osseointegration of nano SiHAp coated specimens higher osseointegration at 0.8 mm cell size | [230] |
| Ti-6Al-4V, EBM | Various pore sizes (low, middle, and high) and porosities | - | - | MC3T3-E1 | No differences were observed in cell adhesion and morphological characteristic. ALP activity significantly higher after 7 and 14 days for middle and high pore size | Rabbits with distal femoral defects | New bone formation higher for middle and high pore size after 12 weeks after implantation | [232] |
| Ti-35Zr-28Nb, SLM | FCCZ and FBCCZ structures | - | - | Human osteoblastlike cells (SaOS2) | No significant difference in cell adhesion, proliferation, and viability. Good cell adhesion after 14 days. Cell adhesion density in order: control > FBCCZ > FCCZ. | - | - | [113] |
| Ti-6Al-4V, SLM | Varying irregularities (0.05–0.5) and porosities (48.83–74.28%) | - | - | MG63 | Cells number higher in specimens with smaller irregularities and lower porosities. Good cytocompatibility in all groups Higher cell density at lower porosities and for higher irregularities Higher ALP activity for high irregularities and high porosities | - | - | [114] |
| CP Ti, direct metal printing | Chitosan gel/chitosan gel+Ag/chitosan gel+ vancomycin | - | Ch + 50 mM Ag and Ch + 100 mM Ag reduced the number of S. aureus both at 24 h and 7th day in 99.9%. Ch + vancomycin completely killed bacteria. | MG-63 | Ch + Ag coatings reduced the number of attached MG-63 cells after 24 h | Rat tibia | Ch + vancomycin coatings reduced the infection rate more as compared to chitosan-only coatings. Ch + Ag coatings did not indicate the antibacterial effects. | [245] |
| Ti-6Al-4V, SLM | Silk fibroin | - | - | Rat osteoblast | Cell attachment, growth, and proliferation on the FG-Ti scaffold improved by adding ECM-like SF sponge in the porous scaffold | - | - | [186] |
| Ta, Gel casting | - | - | - | L929 | Uniformly attached to the scaffolds and the significant cell proliferation observed after 4 days | - | - | [234] |
| Ti-6A-l4V, DIW | Sintering | - | - | Human fibroblast | Fibroblasts well attached and spread on the surface. The best results after the 14 days | - | - | [138] |
| Ti-6Al-4V, EBM | BaTiO3 deposition LIPUS treatment | - | - | MSCs Rabbit primary BMSCs | Cells adhesion, proliferation, and gene expression significantly higher after surface treatment. | Rabbits | Osteogenesis and osseointegration in 6 and 12 weeks improved after implantation for surface-treated scaffolds. | [193] |
| CP Ti, SLM/robocasting | - | - | - | SAOS-2 osteogenic cell line | The high cytocompatibility of SLM-made, and Rob-scaffolds. Higher ALP activity in Rob-scaffolds. | - | - | [115] |
| Ti-6Al-4V SLM | Ti-NTs and Ti-NTs-MBG | - | - | hBMSCs | Improved adhesion and proliferation rate of Ti-NTs and Ti-NTs-MBG compared to Ti scaffolds. No significant difference in biological activity between Ti-NTs and Ti-NTs-MBG. | - | - | [173] |
| Ti-6Al-4V, SLS | HAp bioactive matrix | - | - | Human osteoblasts | Cell adhesion, proliferation, and viability are not negatively affected with time by compositional factors. Ability to promote and sustain osteogenic differentiation, matrix maturation, and mineralization in vitro. | Transverse and spinous processes of sheep’s | Vertebrae hybrid scaffolds had greater infiltration, with the fully mineralized bone after 6 months than those without bioactive matrix. | [175] |
| Ti-6Al-4V, EBM | PRP-coated porous Ti | - | - | BMSCs | Significant promotion of BMSCs attachment, proliferation, migration, and osteogenic differentiation | Osteoporosis models | Enhanced bone regeneration and osseointegration | [241] |
| CP Ti, DIW | CaP coating loaded with CHX | Surface covered by platelike and whisker-like CaP crystal (mainly octacalcium phosphate and brushite) | Reduced bacteria adhesion (73% for S. aureus and 70% for E. coli). 52% of CHX released during the first 12 h | Sarcoma osteogenic cells (SaOS2) | Adhesion and spreading of cells on coated surfaces. CaP + 1.5 m MCHX considered optimal for reaching a compromise between cell adhesion and antibacterial response | - | - | [217] |
| CP Ti, DIW | Gallium deposited by thermochemical treatment | Ga improved the nucleation of an apatite layer Ca/P = 1.7 after 5 days | Ga improved an antibacterial effect against Gram-negative bacteria during the first hours, correlated with high initial release of Ga ions | SaOS-2 osteoblast-like cells | Ga improved cells adhesion, proliferation, differentiation, and mineralization. | - | - | [218] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziaduszewska, M.; Zieliński, A. Structural and Material Determinants Influencing the Behavior of Porous Ti and Its Alloys Made by Additive Manufacturing Techniques for Biomedical Applications. Materials 2021, 14, 712. https://doi.org/10.3390/ma14040712
Dziaduszewska M, Zieliński A. Structural and Material Determinants Influencing the Behavior of Porous Ti and Its Alloys Made by Additive Manufacturing Techniques for Biomedical Applications. Materials. 2021; 14(4):712. https://doi.org/10.3390/ma14040712
Chicago/Turabian StyleDziaduszewska, Magda, and Andrzej Zieliński. 2021. "Structural and Material Determinants Influencing the Behavior of Porous Ti and Its Alloys Made by Additive Manufacturing Techniques for Biomedical Applications" Materials 14, no. 4: 712. https://doi.org/10.3390/ma14040712
APA StyleDziaduszewska, M., & Zieliński, A. (2021). Structural and Material Determinants Influencing the Behavior of Porous Ti and Its Alloys Made by Additive Manufacturing Techniques for Biomedical Applications. Materials, 14(4), 712. https://doi.org/10.3390/ma14040712