Copper–Calcium Hydroxide and Permanent Electrophoretic Current for Treatment of Apical Periodontitis
Abstract
1. Introduction
2. Materials and Methods
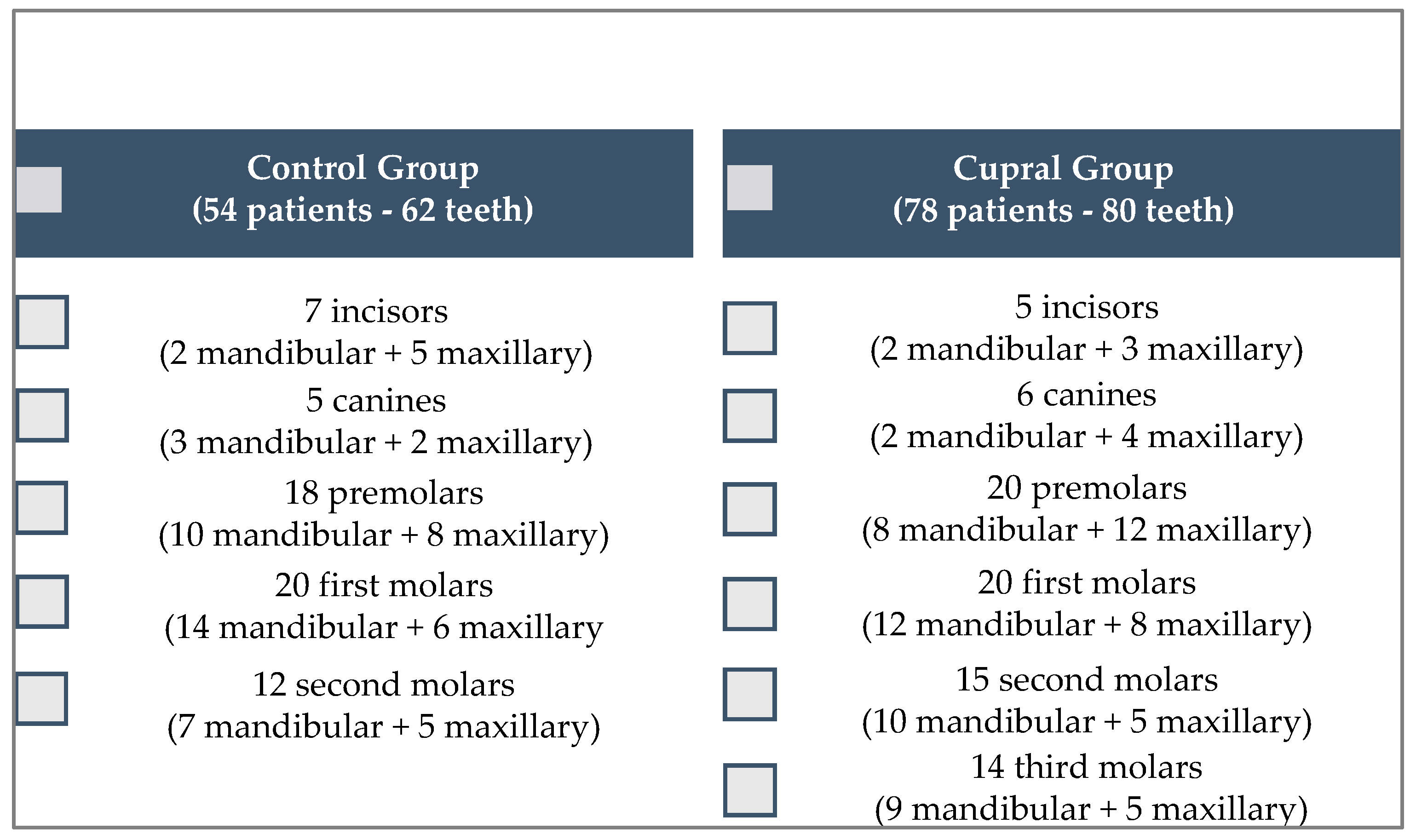
2.1. Patients’ Selection
2.2. Criteria of Inclusion and Exclusion of the Cases
2.3. Endodontic Traditional Treatment of Control Group
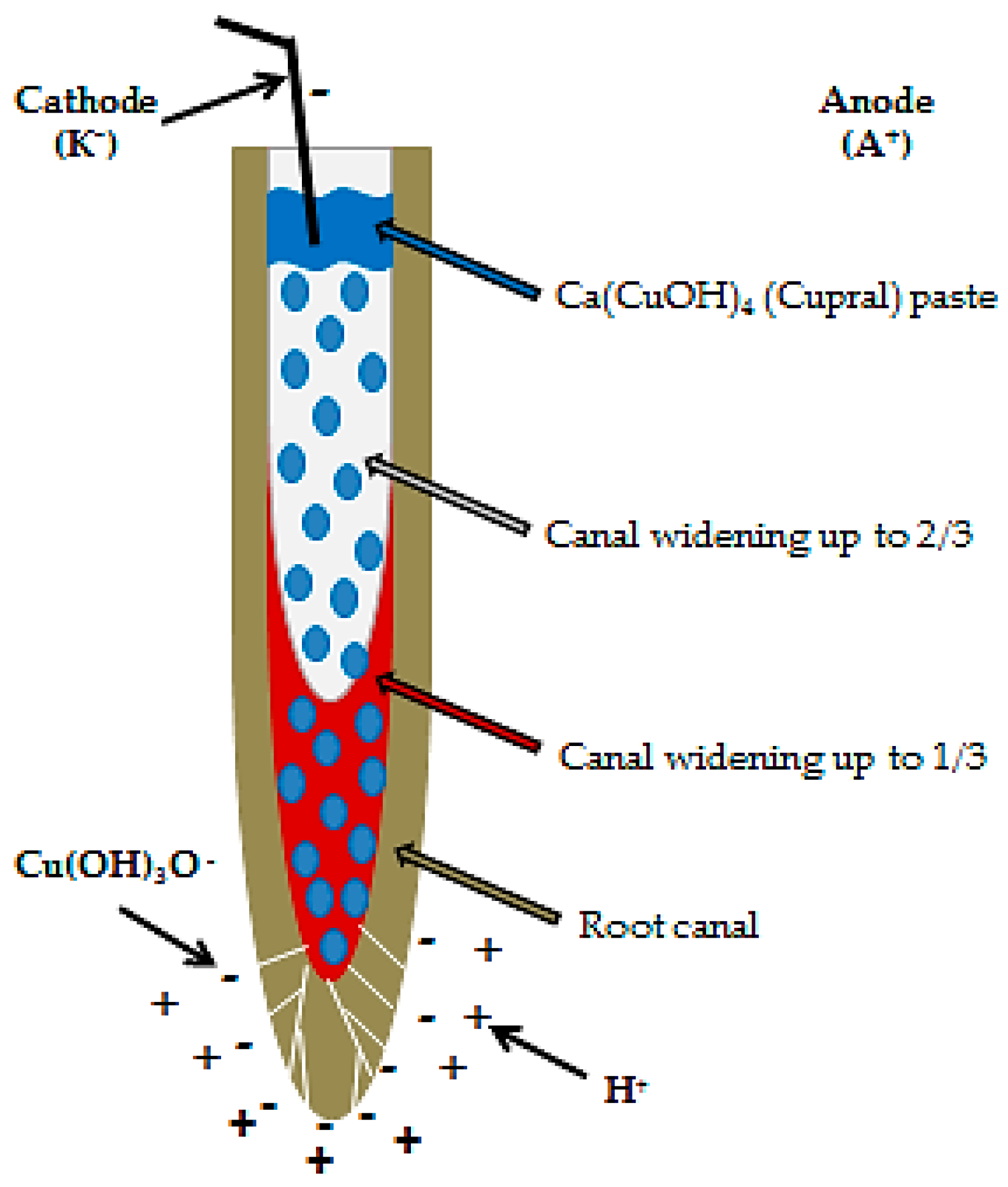
2.4. Electrophoresis-Based Endodontic Treatment in Cupral Group
2.5. Measurement of Apical Bone-Destruction
2.6. Histological Evaluation
2.7. Statistical Analysis
3. Results
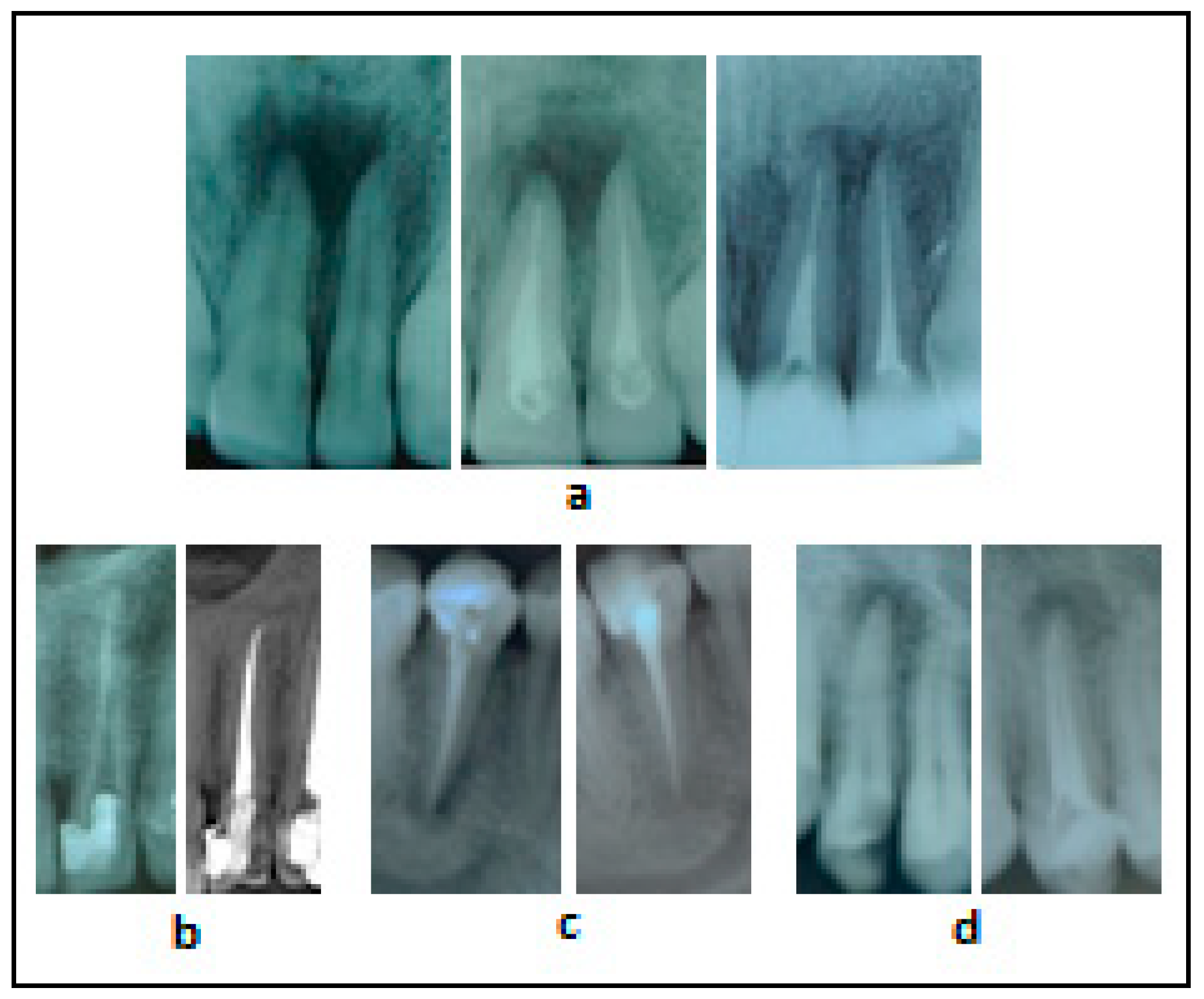
3.1. Radiographs of Clinical Cases Treated with the Traditional Methodology
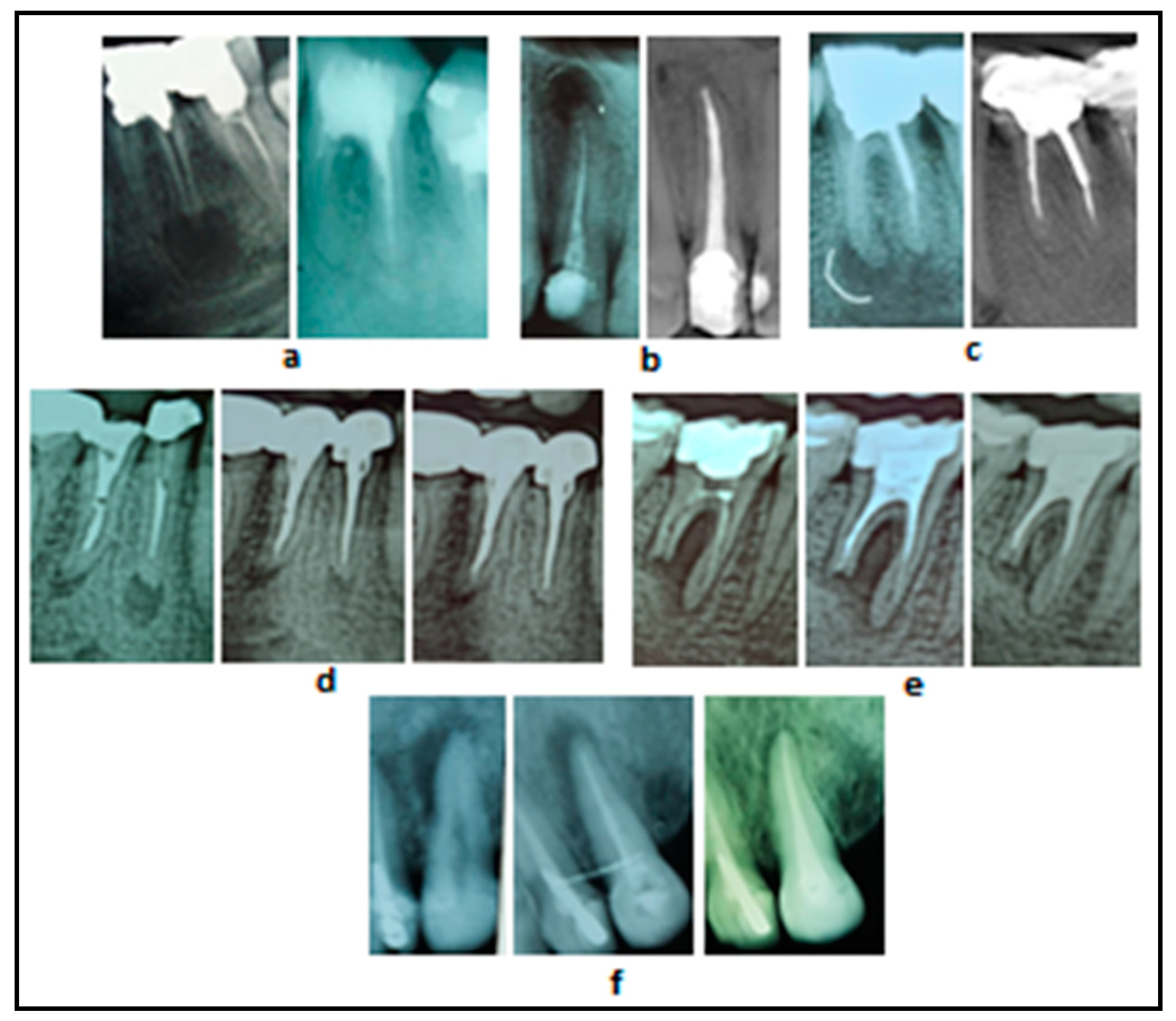
3.2. Radiographs of Clinical Cases Treated with Cupral Associated with Electrophoresis
3.3. Clinical Outcomes with Respect to Patients’ Age
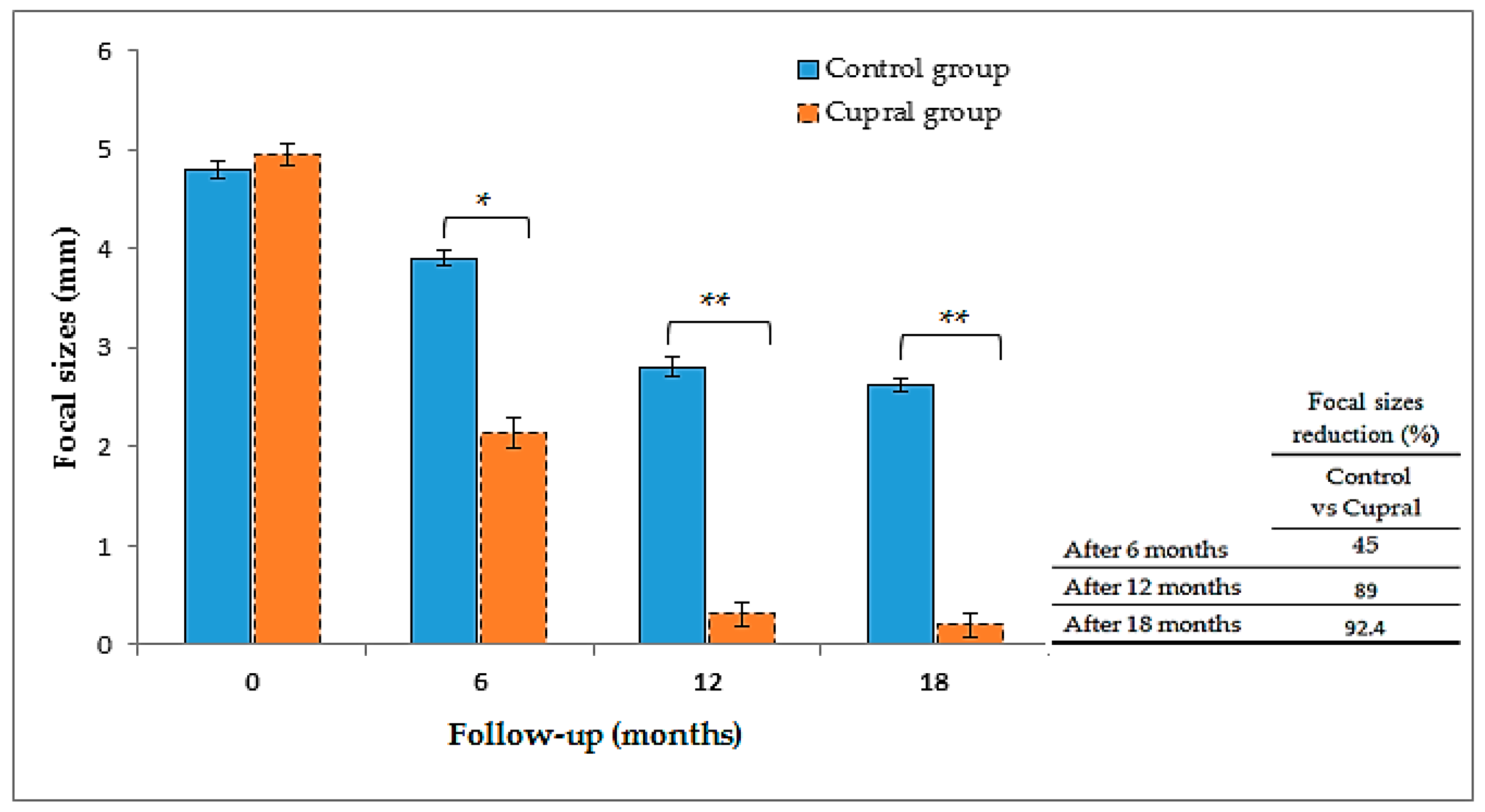
3.4. Measurements of the Focal Sizes
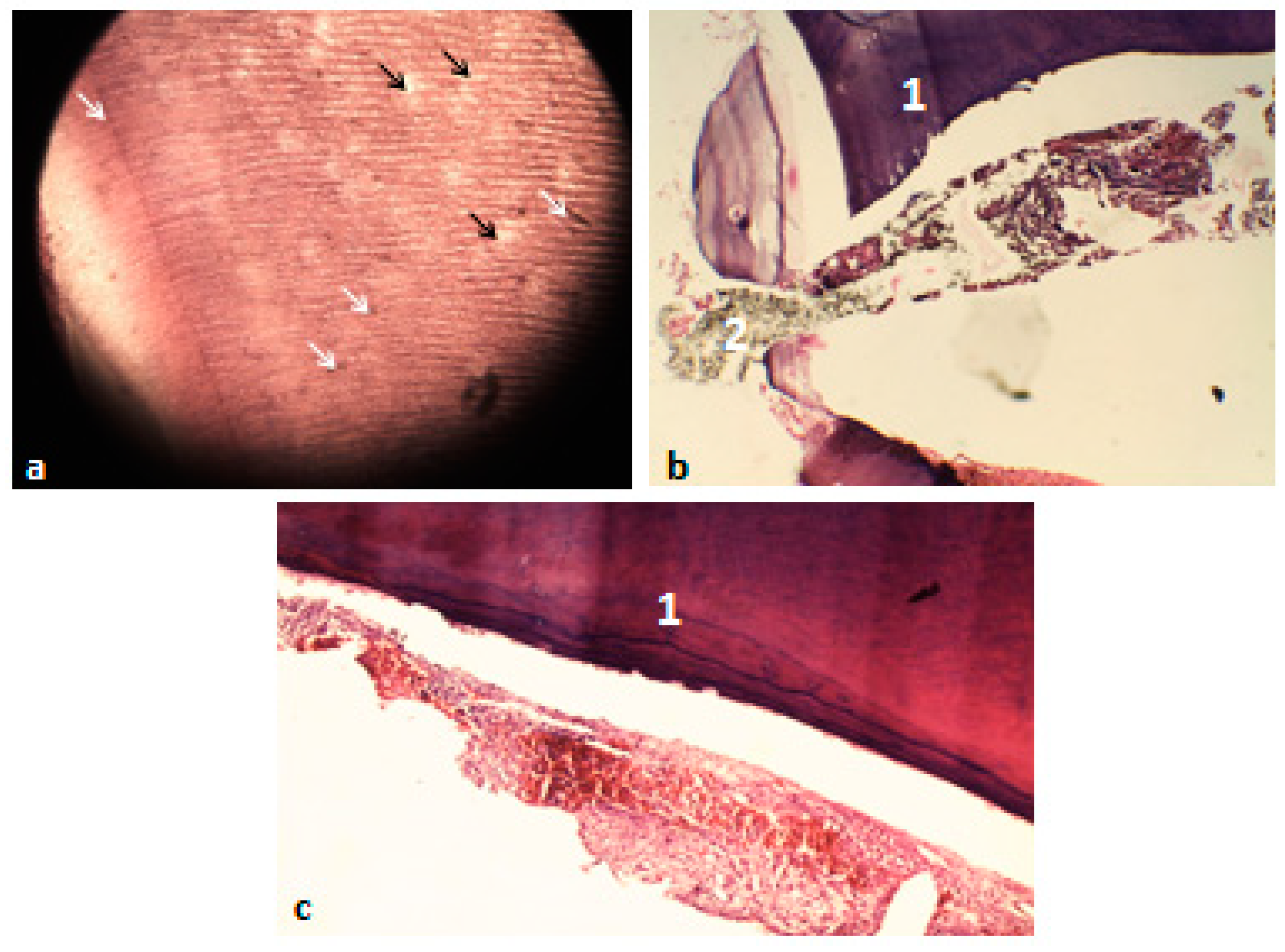
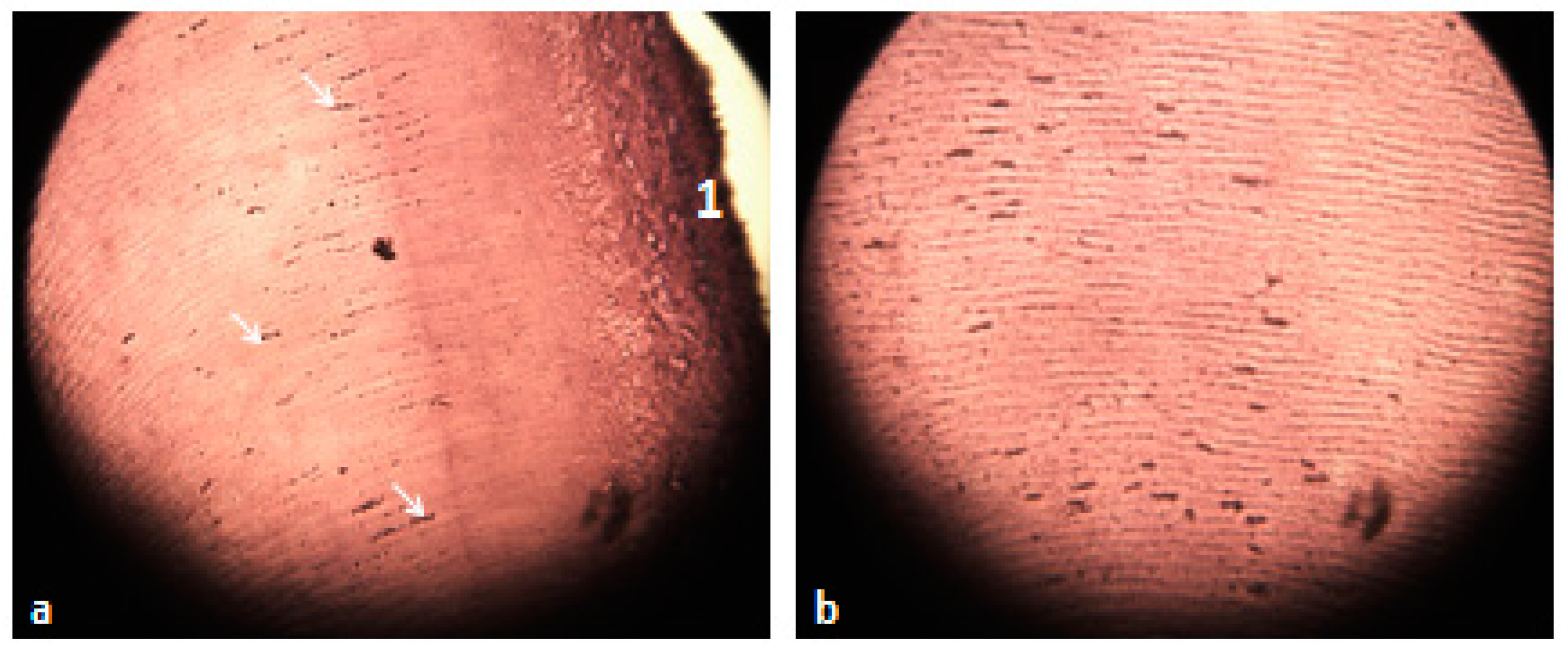
3.5. Histological Analysis of the Extracted Teeth
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antonova, I.N.; Goncharov, V.D.; Kipchuk, A.V.; Bobrova, E.A. Evaluation of dental hard tissues by means of atomic force microscopy. Stomatologiia 2014, 93, 11–14. [Google Scholar] [PubMed]
- Komabayashi, T.; Nonomura, G.; Watanabe, L.G.; Marshall, J.G.W.; Marshall, S.J. Dentin tubule numerical density variations below the CEJ. J. Dent. 2008, 36, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F., Jr.; Rôças, I.N.; Ricucci, D.; Hulsmann, M. Causes and management of post-treatment apical periodontitis. Br. Dent. J. 2014, 216, 305. [Google Scholar] [CrossRef] [PubMed]
- Palma, P.J.; Martins, J.; Diogo, P.; Sequeira, D.; Ramos, J.C.; Diogenes, A.; Santos, J.M. Does Apical Papilla Survive and Develop in Apical Periodontitis Presence after Regenerative Endodontic Procedures? Appl. Sci. 2019, 9, 3942. [Google Scholar] [CrossRef]
- Palma, P.J.; Ramos, J.C.; Martins, J.B.; Diogenes, A.; Figueiredo, M.H.; Ferreira, P.; Viegas, C.; Santos, J.M. Histologic Evaluation of Regenerative Endodontic Procedures with the Use of Chitosan Scaffolds in Immature Dog Teeth with Apical Periodontitis. J. Endod. 2017, 43, 1279–1287. [Google Scholar] [CrossRef]
- Stambolsky, C.; Rodríguez-Benítez, S.; Gutiérrez-Pérez, J.L.; Torres-Lagares, D.; Martín-González, J.; Segura-Egea, J.J. Histologic characterization of regenerated tissues after pulp revascularization of immature dog teeth with apical periodontitis using tri-antibiotic paste and platelet-rich plasma. Arch. Oral. Biol. 2016, 71, 122–128. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr.; Rôças, I.N. Clinical implications and microbiology of bacterial persistence after treatment procedures. J. Endod. 2008, 34, 1291–1301. [Google Scholar] [CrossRef]
- Pinheiro, E.T.; Gomes, B.P.; Ferraz, C.C.; Sousa, E.L.; Teixeira, F.B.; Souza-Filho, F.J. Microorganisms from canals of root-filled teeth with periapical lesions. Int. Endod. J. 2003, 36, 1–11. [Google Scholar] [CrossRef]
- Gazzaneo, I.; Amoroso-Silva, P.; Pacheco-Yanes, J.; Alves, F.R.F.; Marceliano-Alves, M.; Olivares, P.; Meto, A.; Mdala, I.; Siqueira, J.F., Jr.; Rôças, I.N. Disinfecting and shaping type I C-shaped root canals: A correlative micro-computed tomographic and molecular microbiology study. J. Endod. 2020. [Google Scholar] [CrossRef]
- Torabinejad, M.; Khademi, A.A.; Babagoli, J.; Cho, Y.; Johnson, W.B.; Bozhilov, K.; Kim, J.; Shabahang, S. A new solution for the removal of the smear layer. J. Endod. 2003, 29, 170–175. [Google Scholar] [CrossRef]
- Montero-Miralles, P.; Torres-Lagares, D.; Segura-Egea, J.J.; Serrera-Figallo, M.Á.; Gutierrez-Perez, J.L.; Castillo-Dali, G. Comparative study of debris and smear layer removal with EDTA and Er,Cr:YSGG laser. J. Clin. Exp. Dent. 2018, 10, e598–e602. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Lopez, S.; Camejo-Aguilar, D.; Sanchez-Sanchez, P.; Bolanos-Carmona, V. Effect of CHX on the decalcifying effect of 10% Citric Acid, 20% Citric Acid or 17% EDTA. J. Endod. 2006, 32, 781–784. [Google Scholar] [CrossRef] [PubMed]
- Siqueria Junior, J.F.; Machado, A.G.; Silviera, R.M.; Lopes, H.P.; de Uzeda, M. Evaluation of the effectiveness of sodium hypochlorite used with three irrigation methods in the elimination of Enterococcus faecalis from the root canal in vitro. Int. Endod. J. 1997, 30, 279–282. [Google Scholar] [CrossRef]
- Neglia, R.; Ardizzoni, A.; Giardino, L.; Ambu, E.; Grazi, S.; Calignano, S.; Rimoldi, C.; Righi, E.; Blasi, E. Comparative in vitro and ex vivo studies on the bactericidal activity of Tetraclean, a new generation endodontic irrigant, and sodium hypochlorite. New Microbiol. 2008, 31, 57–65. [Google Scholar]
- Azhar, I. Antimicrobial irrigants in the endodontic therapy. Int. J. Health Sci. 2012, 6, 186–192. [Google Scholar]
- Santos, J.M.; Palma, P.J.; Ramos, J.C.; Cabrita, A.S.; Friedman, S. Periapical inflammation subsequent to coronal inoculation of dog teeth root filled with resilon/epiphany in 1 or 2 treatment sessions with chlorhexidine medication. J. Endod. 2014, 40, 837–841. [Google Scholar] [CrossRef]
- Fava, L.R.; Saunders, W.P. Calcium hydroxide pastes: Classification and clinical indications. Int. Endod. J. 1999, 32, 257–282. [Google Scholar] [CrossRef]
- Peters, E.; Lau, M. Histopathologic examination to confirm diagnosis of periapical lesions. J. Can. Dent. Assoc. 2003, 69, 598–600. [Google Scholar]
- Kovác, J.; Kovác, D. Histopathology and etiopathogenesis of chronic apical periodontitis-periapical granuloma. Epidemiol. Mikrobiol. Imunol. 2011, 60, 77–86. [Google Scholar]
- Nair, P.N. On the causes of persistent apical periodontitis: A review. Int. Endod. J. 2006, 39, 249–281. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr.; Alves, F.R.; Rôças, I.N. Pyrosequencing analysis of the apical root canal microbiota. J. Endod. 2011, 37, 1499–1503. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Sanhueza, G.; Alcántara-Dufeu, R.; Carrillo, L.; Mansilla, H.; Novoa, C.; Bello-Toledo, H. Ex vivo effect of copper sulfate on Enterococcus faecalis in root canal. Int. Odont. J. 2015, 9, 505–510. [Google Scholar] [CrossRef]
- Knappwost, A. Das Depotphorese-Verfahren mit Kupfer-Calciumhydroxid, die zur systematischen Ausheilung führende Alternative in der Endodontie. ZWR—Der Zahnarzt. Das Deutsche Zahnärzteblatt 1993, 102, 618–628. [Google Scholar]
- Meto, A.; Colombari, B.; Sala, A.; Pericolini, E.; Meto, A.; Peppoloni, S.; Blasi, E. Antimicrobial and antibiofilm efficacy of a copper/calcium hydroxide-based endodontic paste against Staphylococcus aureus, Pseudomonas aeruginosa and Candida albicans. Dent. Mater. J. 2019, 38, 591–603. [Google Scholar] [CrossRef]
- Lin, S.; Tsesis, I.; Zukerman, O.; Weiss, E.I.; Fuss, Z. Effect of electrophoretically activated calcium hydroxide on bacterial viability in dentinal tubules in vitro. Dent. Traumatol. 2005, 21, 42–45. [Google Scholar] [CrossRef]
- Meto, A.; Colombari, B.; Castagnoli, A.; Sarti, M.; Denti, L.; Blasi, E. Efficacy of a Copper-Calcium-Hydroxide Solution in Reducing Microbial Plaque on Orthodontic Clear Aligners: A Case Report. Eur. J. Dent. 2019, 13, 478–484. [Google Scholar] [CrossRef]
- Available online: https://www.aae.org/specialty/wp-content/uploads/sites/2/2017/07/endodonticdiagnosisfall2013.pdf (accessed on 29 January 2021).
- Estrela, C.; Bueno, M.R.; Sousa-Neto, M.D.; Pécora, J.D. Method for determination of root curvature radius using cone-beam computed tomography images. Braz. Dent. J. 2008, 19, 114–118. [Google Scholar] [CrossRef]
- Groves, R.M.; Fowler, F.J., Jr.; Couper, M.P.; Lepkowski, J.M.; Singer, E.; Tourangeau, R. Survey Methodology; Wiley: New York, NY, USA, 2004; pp. 43–198. [Google Scholar]
- Portenier, I.; Haapasalo, H.; Rye, A.; Waltimo, T.; Ørstavik, D.; Haapasalo, M. Inactivation of root canal medicaments by dentine, hydroxylapatite and bovine serum albumin. Int. Endod. J. 2001, 34, 184–188. [Google Scholar] [CrossRef]
- Weiger, R.; de Lucena, J.; Decker, H.E.; Lost, C. Vitality status of microorganisms in infected human root dentine. Int. Endod. J. 2002, 35, 166–171. [Google Scholar] [CrossRef]
- Peters, L.B.; Wesselink, P.R. Periapical healing of endodontically treated teeth in one and two visits obturated in the presence or absence of detectable microorganisms. Int. Endod. J. 2002, 35, 660–667. [Google Scholar] [CrossRef]
- Sakamoto, M.; Siqueira, J.F., Jr.; Rôças, I.N.; Benno, Y. Molecular analysis of the root canal microbiota associated with endodontic treatment failures. Oral Microbiol. Immunol. 2008, 23, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Martinho, F.C.; Leite, F.R.; Nascimento, G.G.; Cirelli, J.A.; Gomes, B.P. Clinical investigation of bacterial species and endotoxin in endodontic infection and evaluation of root canal content activity against macrophages by cytokine production. Clin. Oral Investig. 2014, 18, 2095–2102. [Google Scholar] [CrossRef] [PubMed]
- Meto, A.; Meto, A. Histological Observation of Copper Sulfide in vitro of Extracted Teeth. Asian Acad. Res. Assoc. 2016, 3, 103–111. [Google Scholar]
- Ahmed, H.M.; Abbott, P.V. Discolouration potential of endodontic procedures and materials: A review. Int. Endod. J. 2012, 45, 883–897. [Google Scholar] [CrossRef] [PubMed]
- Kahler, B.; Rossi-Fedele, G. A Review of Tooth Discoloration after Regenerative Endodontic Therapy. J. Endod. 2016, 42, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Lo Giudice, G.; Lipari, F.; Lizio, A.; Cervino, G.; Cicciù, M. Tooth fragment reattachment technique on a pluri traumatized tooth. J Conserv. Dent. 2012, 15, 80–83. [Google Scholar]
- Palma, P.J.; Marques, J.A.; Falacho, R.I.; Correia, E.; Vinagre, A.; Santos, J.M.; Ramos, J.C. Six-Month Color Stability Assessment of Two Calcium Silicate-Based Cements Used in Regenerative Endodontic Procedures. J. Funct. Biomater. 2019, 10, 14. [Google Scholar] [CrossRef]
- Ramos, J.C.; Palma, P.J.; Nascimento, R.; Caramelo, F.; Messias, A.; Vinagre, A.; Santos, J.M. 1-year in vitro evaluation of tooth discoloration induced by 2 calcium silicate-based cements. J. Endod. 2016, 42, 1403–1407. [Google Scholar] [CrossRef]
- Prado, J.V.; Vidal, A.R.; Durán, T.C. Application of copper bactericidal properties in medical practice. Rev. Med. Chile 2012, 140, 1325–1332. [Google Scholar]
- Grass, G.; Rensing, C.; Solioz, M. Metallic copper as an antimicrobial surface. Appl. Environ. Microbiol. 2011, 77, 1541–1547. [Google Scholar] [CrossRef]
- Fiorillo, L.; D’Amico, C.; Turkina, A.Y.; Nicita, F.; Amoroso, G.; Risitano, G. Endo and Exoskeleton: New Technologies on Composite Materials. Prosthesis 2020, 2, 1–9. [Google Scholar] [CrossRef]
- Cicciù, M. New Technological Opportunities and Innovative Biomedical Devices. Prosthesis 2019, 1, 1–2. [Google Scholar] [CrossRef]
| Control Group | ||||
|---|---|---|---|---|
| Age Groups (Years) | No of Treated Teeth | Success (%) | Failure (%) | Extracted Teeth |
| 19–21 | 11 | 100 | - | - |
| 22–35 | 16 | 87.5 | 12.5 | 2 |
| 36–50 | 15 | 86.7 | 13.3 | 2 |
| 51–65 | 20 | 85 | 15 | 3 |
| n = 62 | ||||
| Cupral Group | ||||
| 19–21 | 8 | 100 | - | - |
| 22–35 | 22 | 100 | - | - |
| 36–50 | 30 | 96.7 | 3.3 | 1 |
| 51–65 | 20 | 90 | 10 | 2 |
| n = 80 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meto, A.; Droboniku, E.; Blasi, E.; Colombari, B.; Tragaj, E.; Cervino, G.; Fiorillo, L.; Meto, A. Copper–Calcium Hydroxide and Permanent Electrophoretic Current for Treatment of Apical Periodontitis. Materials 2021, 14, 678. https://doi.org/10.3390/ma14030678
Meto A, Droboniku E, Blasi E, Colombari B, Tragaj E, Cervino G, Fiorillo L, Meto A. Copper–Calcium Hydroxide and Permanent Electrophoretic Current for Treatment of Apical Periodontitis. Materials. 2021; 14(3):678. https://doi.org/10.3390/ma14030678
Chicago/Turabian StyleMeto, Agron, Etleva Droboniku, Elisabetta Blasi, Bruna Colombari, Emiljano Tragaj, Gabriele Cervino, Luca Fiorillo, and Aida Meto. 2021. "Copper–Calcium Hydroxide and Permanent Electrophoretic Current for Treatment of Apical Periodontitis" Materials 14, no. 3: 678. https://doi.org/10.3390/ma14030678
APA StyleMeto, A., Droboniku, E., Blasi, E., Colombari, B., Tragaj, E., Cervino, G., Fiorillo, L., & Meto, A. (2021). Copper–Calcium Hydroxide and Permanent Electrophoretic Current for Treatment of Apical Periodontitis. Materials, 14(3), 678. https://doi.org/10.3390/ma14030678












