Ultrafine-Grained Ti-31Mo-Type Composites with HA and Ag, Ta2O5 or CeO2 Addition for Implant Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
- -
- ultrafine-grained Ti31Mo alloy—Ti31Mo
- -
- ultrafine-grained Ti31Mo alloy after NaOH 60 °C/24 h oxidation—Ti31Mo (Ox)
- -
- ultrafine-grained Ti31Mo alloy after hydrothermal treatment—Ti31Mo (HT)
- -
- ultrafine-grained Ti31Mo-5 wt. % HA composite—Ti31Mo5HA
- -
- ultrafine-grained Ti31Mo-5 wt. % HA-1 wt. % Ag composite—Ti31Mo5HA1Ag
- -
- ultrafine-grained Ti31Mo-5 wt. % HA-2 wt. % CeO2 composite—Ti31Mo5HA2CeO2
- -
- ultrafine-grained Ti31Mo-5wt. % HA-2 wt. % Ta2O5 composite—Ti31Mo5HA2Ta2O5
2.2. Materials Characterization
2.3. In Vitro Evaluation
3. Results
3.1. Crystal Structure, Phase Contents, the Morphology
3.2. Surface Properties
3.3. Corrosion and Surface Wetting Properties
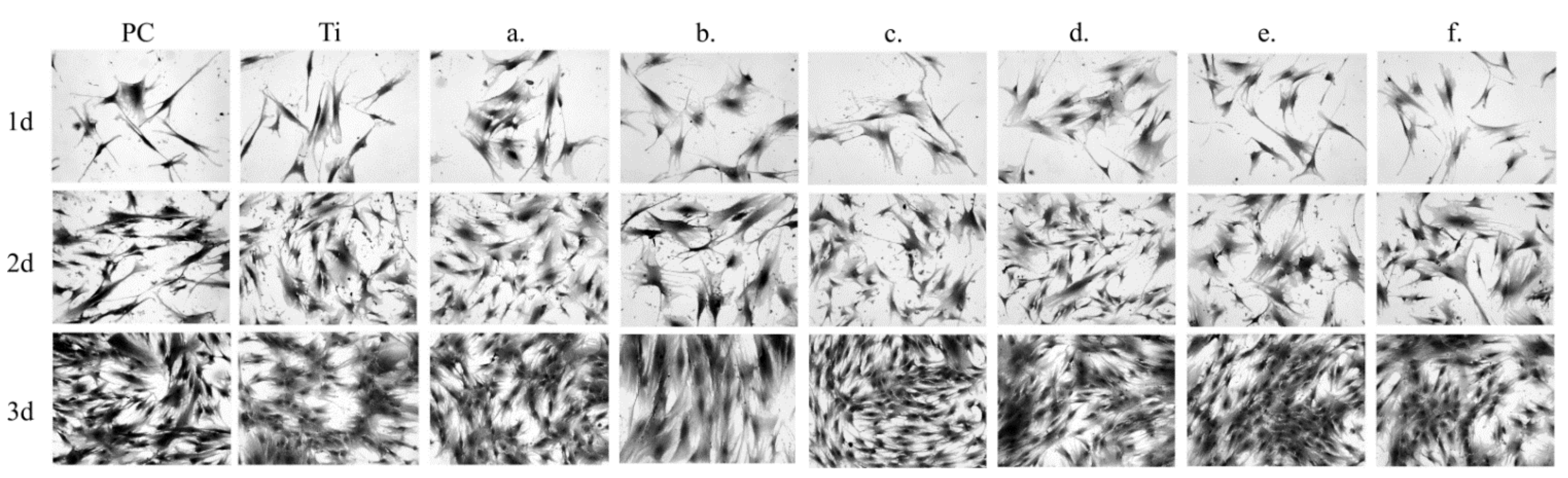
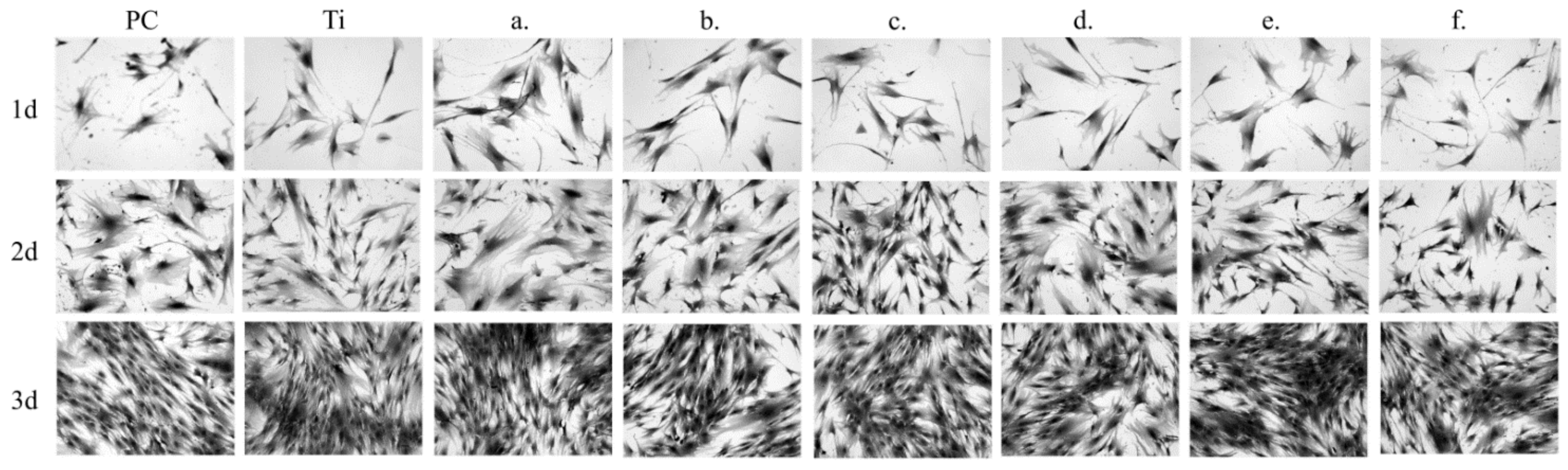
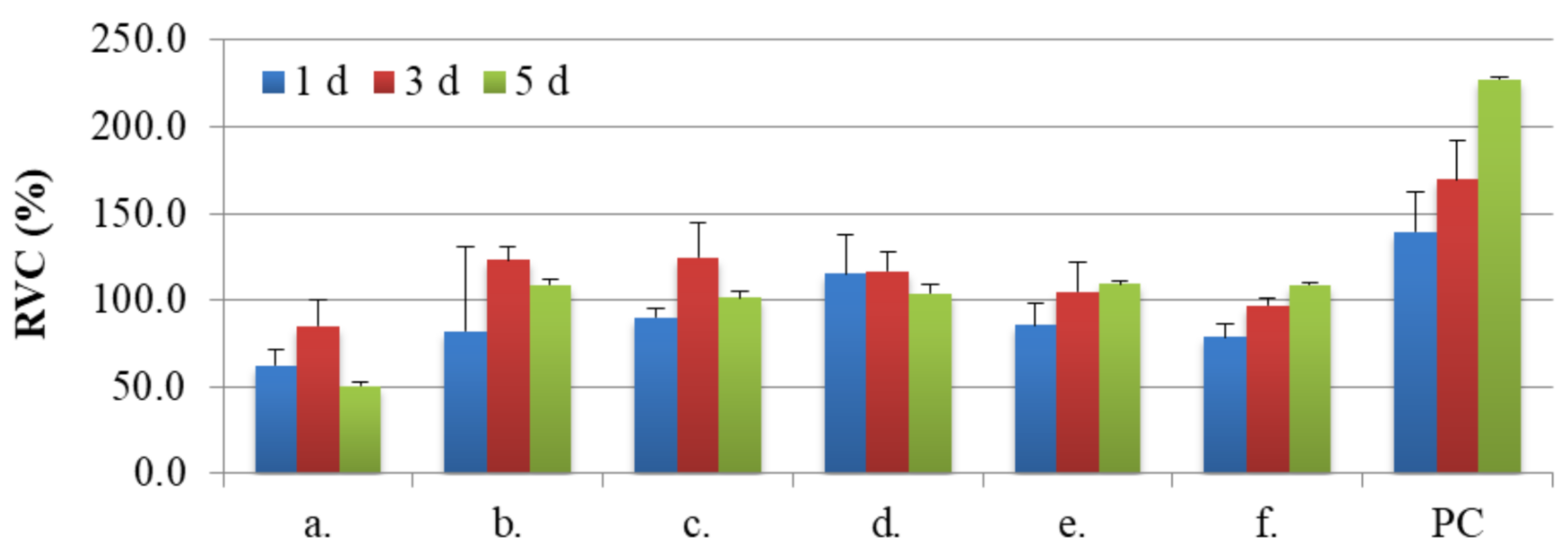
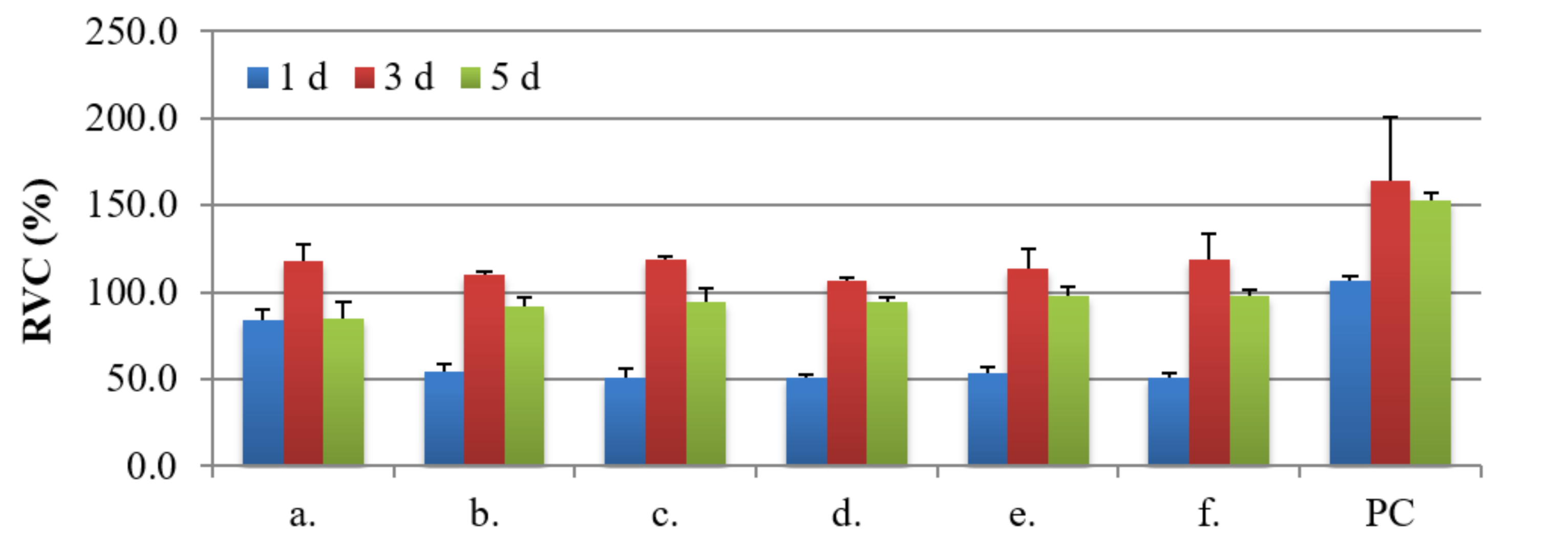
3.4. Biocompatibility Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Zaman, H.K.; Sharif, S.; Idris, M.H.; Kamarudin, A. Metallic Biomaterials for Medical Implant Applications: A Review. Appl. Mech. Mater. 2015, 735, 19–28. [Google Scholar] [CrossRef]
- Rack, H.J.; Qazi, J.I. Titanium alloys for biomedical applications. Mater. Sci. Eng. C 2006, 26, 1269–1277. [Google Scholar] [CrossRef]
- Bodunrin, M.O.; Chown, L.H.; van der Merwe, J.W.; Alaneme, K.K.; Oganbule, C.; Klenam, D.E.F.; Mphasha, N.P. Corrosion behavior of titanium alloys in acidic and saline media: Role of alloy design, passivation integrity, and electrolyte modification. Corros. Rev. 2020, 38, 25–47. [Google Scholar] [CrossRef]
- Elias, C.N.; Lima, J.H.C.; Valiev, R.; Meyers, M.A. Biomedical applications of titanium and its alloys. JOM 2008, 60, 46–49. [Google Scholar] [CrossRef]
- Dragan-Raileanu, L.A.; Cotrutz, C.E.; Munteanu, C.; Strugaru, S.; Avram, P.; Istrate, B.; Petreus, T. In vitro study regarding the cytotoxicity of some TiNbZr alloys. Ann. Rom. Soc. Cell Biol. 2013, 18, 186–191. [Google Scholar]
- Sidambe, A.T. Biocompatibility of advanced manufactured titanium implants-A review. Materials 2014, 7, 8168–8188. [Google Scholar] [CrossRef] [Green Version]
- Cojocaru, V.D.; Nocivin, A.; Trisca-Rusu, C.; Dan, A.; Irimescu, R.; Raducanu, D.; Galbinasu, B.M. Improving the Mechanical Properties of a β-type Ti-Nb-Zr-Fe-O Alloy. Metals 2020, 10, 1491. [Google Scholar] [CrossRef]
- Godarzi, R.; Rasmusson, L.; Dasmah, A.; Albrektsson, T. Effects of implant design and surface on osseointegration. An experimental study in the dog mandible. Appl. Osseointegr. Res. 2008, 7, 58–60. [Google Scholar]
- Eisenbarth, E.; Velten, D.; Müller, M.; Thull, R.; Breme, J. Biocompatibility of β-stabilizing elements of titanium alloys. Biomaterials 2004, 25, 5705–5713. [Google Scholar] [CrossRef]
- Jurczyk, K.; Niespodziana, K.; Jurczyk, M.U.; Jurczyk, M. Synthesis and characterization of titanium-45S5 bioglass nanocomposites. Mater. Des. 2011, 32, 2554–2560. [Google Scholar] [CrossRef]
- Miklaszewski, A.; Jurczyk, M.U.; Jurczyk, M. Microstructural development of Ti-β alloyed layer for hard tissue applications. J. Mater. Sci. Technol. 2013, 29, 565–572. [Google Scholar] [CrossRef]
- Miklaszewski, A.; Jurczyk, M.; Kaczmarek, M.; Paszel-Jaworska, A.; Romaniuk, A.; Lipińska, N.; Żurawski, J.; Urbaniak, P.; Jurczyk, M. Nanoscale size effect in in situ titanium based composites with cell viability and cytocompatibility studies. Mater. Sci. Eng. C 2017, 73, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Zhang, J.; Huo, Y.; Sui, Y.; Zhang, J.; Guo, S.; Zhao, X. Design of low modulus β-type titanium alloys by tuning shear modulus C44. J. Alloys Compd. 2018, 745, 579–585. [Google Scholar] [CrossRef]
- Frutos, E.; Karlík, M.; Jiménez, J.A.; Langhansová, H.; Lieskovská, J.; Polcar, T. Development of new β/α″-Ti-Nb-Zr biocompatible coating with low Young’s modulus and high toughness for medical applications. Mater. Des. 2018, 142, 44–55. [Google Scholar] [CrossRef] [Green Version]
- Marczewski, M.; Miklaszewski, A.; Jurczyk, M. Structure evolution analysis in ultrafine-grained Zr and Nb-based beta titanium alloys. J. Alloys Compd. 2018, 765, 459–469. [Google Scholar] [CrossRef]
- Sochacka, P.; Miklaszewski, A.; Jurczyk, M. Development of β-type Ti-x at. % Mo alloys by mechanical alloying and powder metallurgy: Phase evolution and mechanical properties (10 ≤ x ≤ 35). J. Alloys Compd. 2019, 776, 370–378. [Google Scholar] [CrossRef]
- Sochacka, P.; Miklaszewski, A.; Kowalski, K.; Jurczyk, M. Influence of the processing method on the properties of Ti23 at.% Mo alloy. Metals 2019, 9, 931–947. [Google Scholar] [CrossRef] [Green Version]
- Sochacka, P.; Miklaszewski, A.; Jurczyk, M.; Pecyna, P.; Ratajczak, M.; Gajecka, M.; Jurczyk, M.U. Effect of hydroxyapatite and Ag, Ta2O5 or CeO2 addition on the properties of ultrafine-grained Ti31Mo alloy. J. Alloys Compd. 2020, 823, 153749. [Google Scholar] [CrossRef]
- Marczewski, M.; Jurczyk, M.U.; Kowalski, K.; Miklaszewski, A.; Wirstlein, P.K.; Jurczyk, M. Composite and surface functionalization of ultrafine-grained Ti23Zr25Nb alloy for medical applications. Materials 2020, 13, 5252. [Google Scholar] [CrossRef]
- Guillemot, F.; Prima, F.; Bareille, R.; Gordin, D.-M.; Gloriant, T.; Durrieu, M.; Ansel, D.; Baquey, C. Design of new titanium alloys for orthopaedic applications. Med. Biol. Eng. Comput. 2004, 42, 137–141. [Google Scholar] [CrossRef]
- Bai, Y.; Deng, Y.; Zheng, Y.; Li, Y.; Zhang, R.; Lv, Y.; Zhao, Q.; Wei, S. Characterization, corrosion behavior, cellular response and in vivo bone tissue compatibility of titanium–niobium alloy with low Young’s modulus. Mater. Sci. Eng. C 2016, 59, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, P.; Singh, S.B.; Dhara, S.; Chakraborty, M. Influence of boron addition to Ti–13Zr–13Nb alloy on MG63 osteoblast cell viability and protein adsorption. Mater. Sci. Eng. C 2015, 46, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.L.; Li, L.; Zheng, Y.F. In vitro cytotoxicity and hemocompatibility studies of Ti-Nb, Ti-Nb-Zr and Ti-Nb-Hf biomedical shape memory alloys. Biomed. Mater. 2010, 5, 044102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bărbînţă, A.C.; Earar, K.; Crimu, C.I.; DrĂgan, L.A.; Munteanu, C. In Vitro Evaluation of the Cytotoxicity of Some New Titanium Alloys. Key Eng. Mater. 2014, 587, 303–308. [Google Scholar] [CrossRef]
- Kopova, I.; Stráský, J.; Harcuba, P.; Landa, M.; Janeček, M.; Bačákova, L. Newly developed Ti–Nb–Zr–Ta–Si–Fe biomedical beta titanium alloys with increased strength and enhanced biocompatibility. Mater. Sci. Eng. C 2016, 60, 230–238. [Google Scholar] [CrossRef]
- Park, Y.-J.; Song, Y.-H.; An, J.-H.; Song, H.-J.; Anusavice, K.J. Cytocompatibility of pure metals and experimental binary titanium alloys for implant materials. J. Dent. 2013, 41, 1251–1258. [Google Scholar] [CrossRef]
- Murray, J.L. The Mo-Ti (Molybdenum-Titanium) System. Bull. Alloy Phase Diagrams 1981, 2, 185–192. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Semenova, I.P.; Jakushina, E.; Latysh, V.V.; Rack, H.; Lowe, R.C.; Petruzelka, J.; Dluhos, L.; Hrusak, D.; Sochova, J. Nanostructured SPD processed titanium for medical implants. Mater. Sci. Forum 2008, 584, 49–54. [Google Scholar] [CrossRef]
- Estrin, Y.; Kasper, C.; Diederichs, S.; Lapovok, R. Accelerated growth of preosteoblastic cells on ultrafine grained titanium. J. Biomed. Mater. Res. Part A 2009, 90, 1239–1242. [Google Scholar] [CrossRef]
- Zhang, L.; Webster, T.J. Nanotechnology and nanomaterials: Promises for improved tissue regeneration. Nano. Today 2009, 4, 66–80. [Google Scholar] [CrossRef]
- Webster, T.J.; Ejiofor, J.U. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials 2004, 25, 4731–4739. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, M.; Jurczyk, K.; Koper, J.K.; Paszel-Jaworska, A.; Romaniuk, A.; Lipińska, N.; Żurawski, J.; Urbaniak, P.; Jakubowicz, J.; Jurczyk, M.U. In vitro biocompatibility of anodized titanium with deposited silver nanodendrites. J. Mater. Sci. 2016, 51, 5259–5270. [Google Scholar] [CrossRef] [Green Version]
- Cao, W.P.; Hench, L. Bioactive materials. Ceramics Int. 1966, 22, 493–507. [Google Scholar] [CrossRef]
- Türk, S.; Altınsoy, I.; Efe, G.Ç.; Ipek, M.; Özacar, M.; Bindal, C. Biomimetic synthesis of Ag, Zn or Co doped HA and coating of Ag, Zn or Co doped HA/fMWCNT composite on functionalized Ti. Mater. Sci. Eng. C 2019, 99, 986–998. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, M.; Jurczyk, M.U.; Rubis, B.; Banaszak, A.; Kolecka, A.; Paszel, A.; Jurczyk, K.; Murias, M.; Sikora, J.; Jurczyk, M. In vitro biocompatibility of Ti-45S5 bioglass nanocomposites and their scaffolds. J. Biomed. Mater. Res. Part A 2014, 102, 1316–1324. [Google Scholar] [CrossRef]
- Tulinski, M.; Jurczyk, M. Nanostructured nickel-free austenitic stainless steel composites with different content of hydroxyapatite. Appl. Surf. Sci. 2012, 260, 80–83. [Google Scholar] [CrossRef]
- Fitzgibbon, A.; Pilu, M.; Fisher, R.B. Direct least square fitting of ellipses. IEEE Trans. Pattern. Anal. Mach. Intell. 1999, 21, 476–480. [Google Scholar] [CrossRef] [Green Version]
- Kaelble, D.H. Dispersion-polar surface tension properties of organic solids. J. Adhes. 1970, 2, 66–81. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Kim, C.; Kendall, M.R.; Miller, M.A.; Long, C.L.; Larson, P.R.; Humphrey, M.B.; Madden, A.S.; Tas, A.C. Comparison of titanium soaked in 5 M NaOH or 5 M KOH solutions. Mater. Sci. Eng. C 2013, 33, 327–339. [Google Scholar] [CrossRef] [Green Version]
- Ward, B.C.; Webster, T.J. Increased functions of osteoblasts on nanophase metals. Mater. Sci. Eng. C 2007, 27, 575–578. [Google Scholar] [CrossRef]
- Webster, T.J.; Schadler, L.S.; Siegel, R.W.; Bizios, R. Mechanisms of enhanced osteoblast adhesion on nanophase alumina involve vitronectin. Tissue Eng. 2001, 7, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. Bioceramics: From concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Burgers, R.; Hahnel, S.; Reichert, T.E.; Rosentritt, M.; Behr, M.; Gerlach, T.; Handel, G.; Gosau, M. Adhesion of Candida albicans to various dental implant surfaces and the influence of salivary pellicle proteins. Acta Biomater. 2010, 6, 2307–2313. [Google Scholar] [CrossRef]
- Wong, S.Y.; Moskowitz, J.S.; Veselinovic, J.; Rosario, R.A.; Timachova, K.; Blaisse, M.R.; Fuller, R.C.; Klibanov, A.M.; Hammond, P.T. Dual functional polyelectrolyte multilayer coatings for implants: Permanent microbicidal base with controlled release of therapeutic agents. J. Am. Chem. Soc. 2010, 132, 17840–17848. [Google Scholar] [CrossRef] [Green Version]
- Guillem-Marti, J.; Delgado, L.; Godoy-Gallardo, M.; Pegueroles, M.; Herrero, M.; Gil, F.J. Fibroblast adhesion and activation onto micro-machined titanium surfaces. Clin. Oral Implants Res. 2013, 24, 770–780. [Google Scholar] [CrossRef]
- Yamazoe, M. Study of corrosion of combinations of titanium/Ti-6Al-4V implants and dental alloys. Dent. Mater. J. 2010, 29, 542–553. [Google Scholar] [CrossRef] [Green Version]
- Adamek, G.; Jurczyk, M.U.; Jakubowicz, J. Biocompatibility of the electrochemically modified surface of the ti-6Zr4Nb alloy. J. Biomater. Tissue Eng. 2011, 1, 101–109. [Google Scholar] [CrossRef]
- Sun, L.; Berndt, C.C.; Gross, K.A.; Kucuk, A. Material fundamentals and clinical performance of plasma-sprayed hydroxyapatite coatings: A review. J. Biomed. Mater. Res. 2001, 58, 570–592. [Google Scholar] [CrossRef]
- Wirsching, K.; Lehle, K.; Jacob, P.; Gleich, O.; Strutz, J.; Kwok, P. Influence of surface processing on the biocompatibility of titanium. Materials 2011, 4, 1238–1248. [Google Scholar] [CrossRef]
- Niespodziana, K.; Jurczyk, K.; Jakubowicz, J.; Jurczyk, M. Fabrication and properties of titanium—hydroxyapatite nanocomposites. Mater. Chem. Phys. 2010, 123, 160–165. [Google Scholar] [CrossRef]
- Upadhyay, D.; Panchal, M.A.; Dubey, R.S.; Srivastava, V.K. Corrosion of alloys used in dentistry: A review. Mater. Sci. Eng. A 2006, 432, 1–11. [Google Scholar] [CrossRef]
- Zhang, B.B.; Zheng, Y.F.; Liu, Y. Effect of Ag on the corrosion behavior of Ti–Ag alloys in artificial saliva solutions. Dent. Mater. 2009, 25, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [Green Version]
- Unabia, R.B.; Candidato, R.T.; Pawłowski, L.; Salvatori, R.; Bellucci, D.; Cannillo, V. In vitro studies of solution precursor plasma-sprayed copper-doped hydroxyapatite coatings with increasing copper content. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 2579–2589. [Google Scholar] [CrossRef]
- Pelletier, D.A.; Suresh, A.K.; Holton, G.A.; McKeown, C.K.; Wang, W.; Gu, B.; Mortensen, N.P.; Allison, D.P.; Joy, D.C.; Allison, M.R.; et al. Effects of engineered cerium oxide nanoparticles on bacterial growth and viability. Appl. Environ. Microbiol. 2010, 76, 7981–7989. [Google Scholar] [CrossRef] [Green Version]
- Jakubowicz, J.; Adamek, G.; Jurczyk, M.U.; Jurczyk, M. 3D topography study of the biofunctionalizednanocrystalline Ti-6Zr-4Nb/Ca-P. Mater. Charact. 2012, 70, 55–62. [Google Scholar] [CrossRef]
- Fan, X.P.; Feng, B.; Weng, J.; Wang, J.X.; Lu, X. Processing and properties of porous titanium with high porosity coated by bioactive titania nanotubes. Mater. Lett. 2011, 65, 2899–2901. [Google Scholar] [CrossRef]
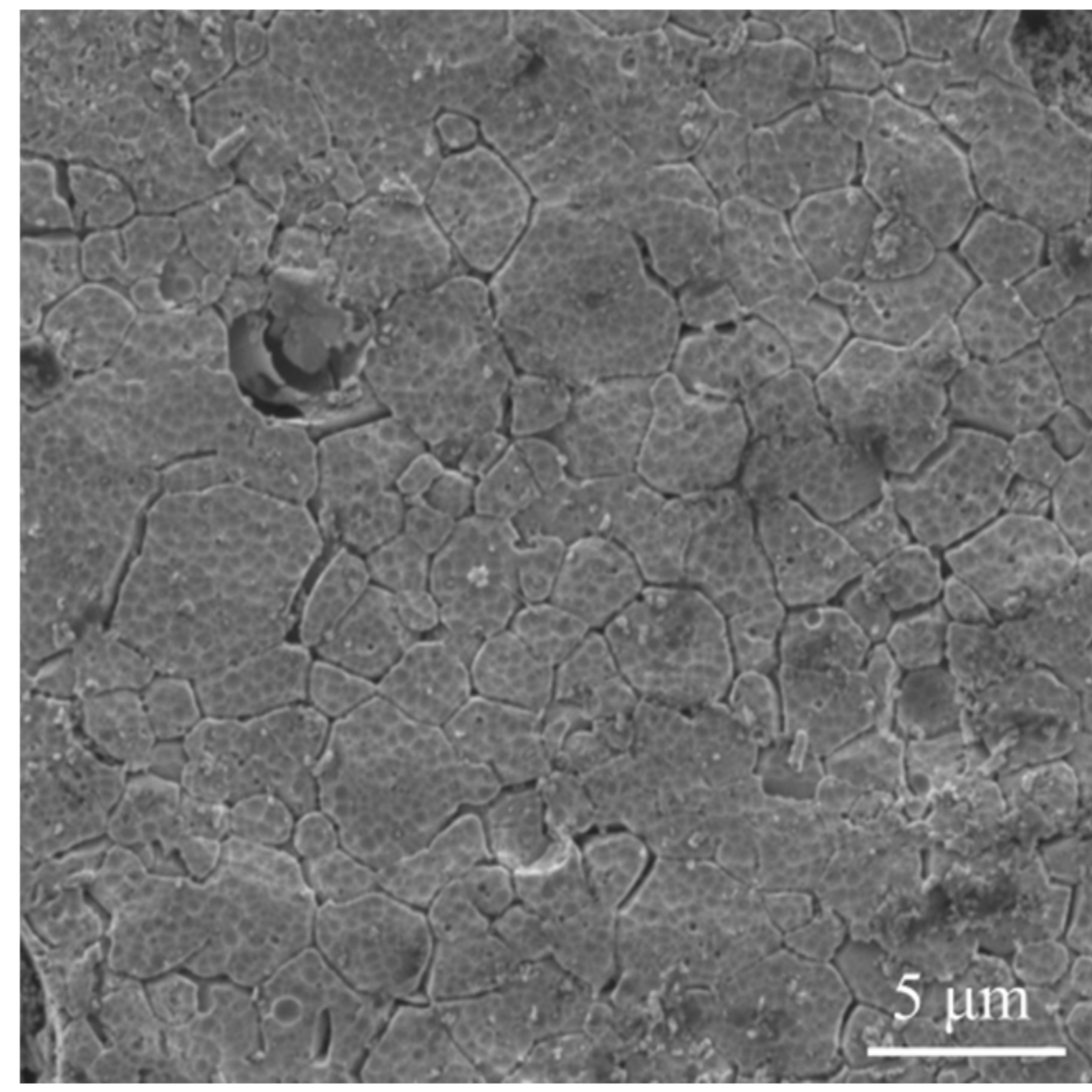
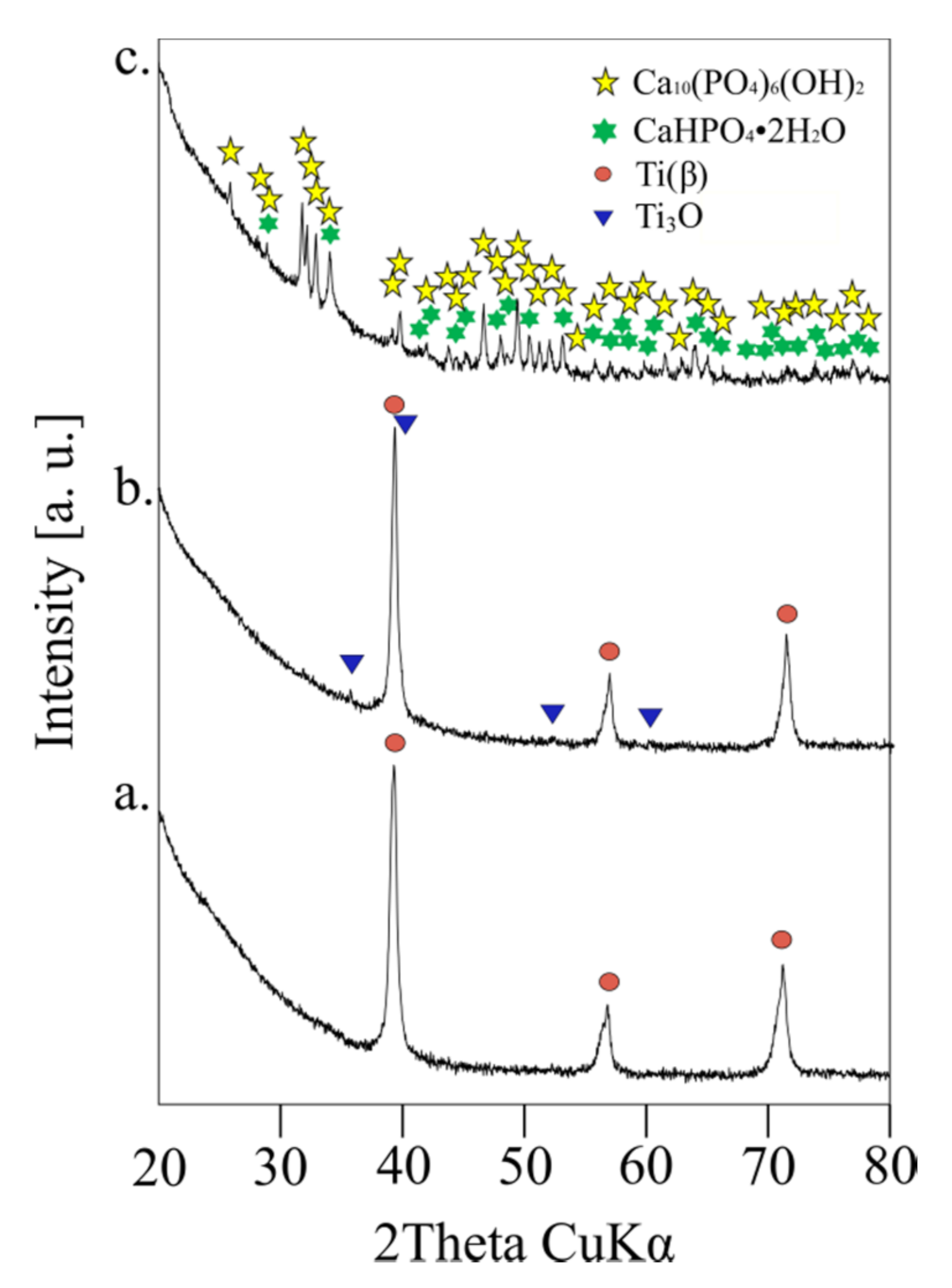
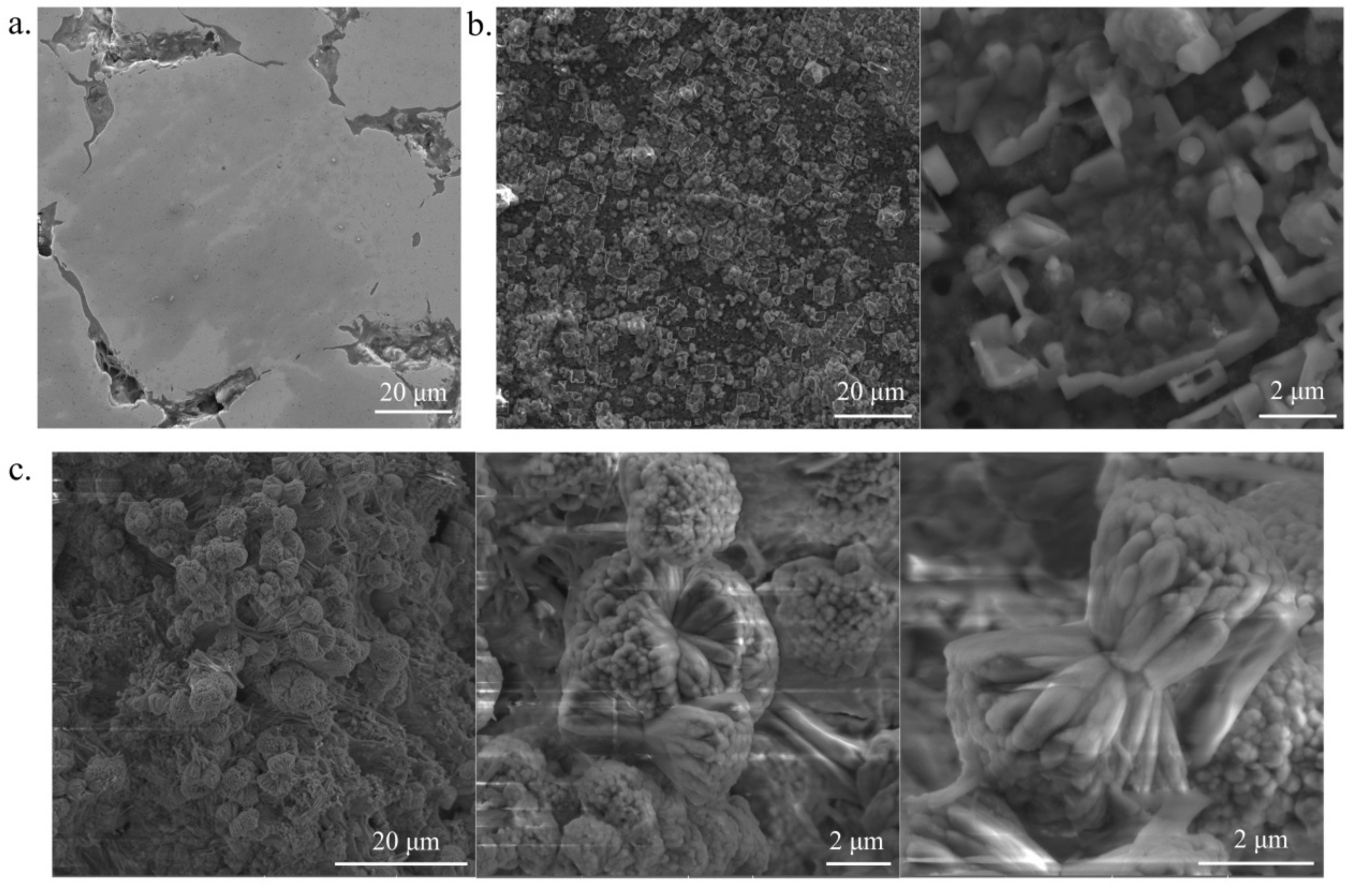

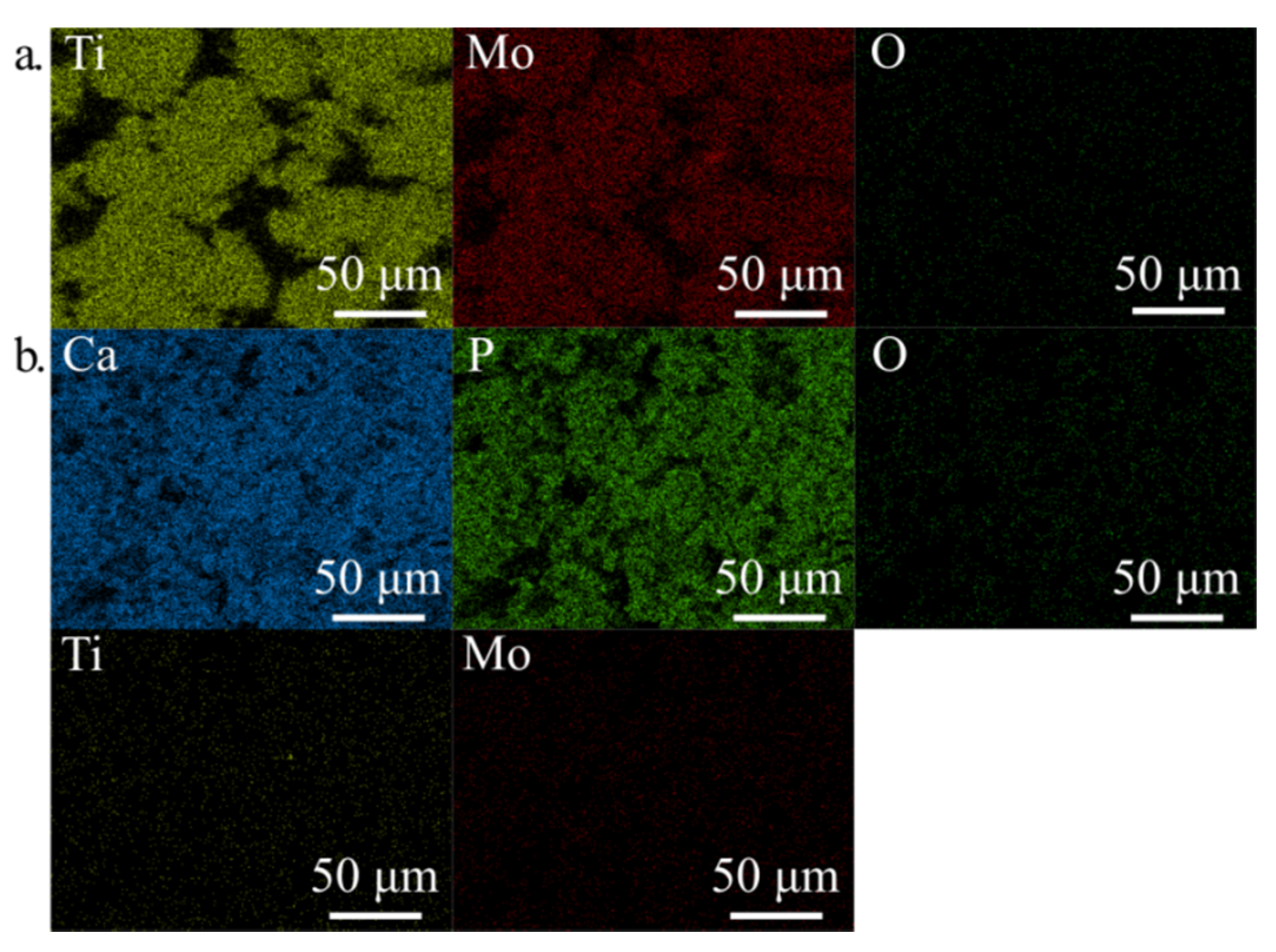
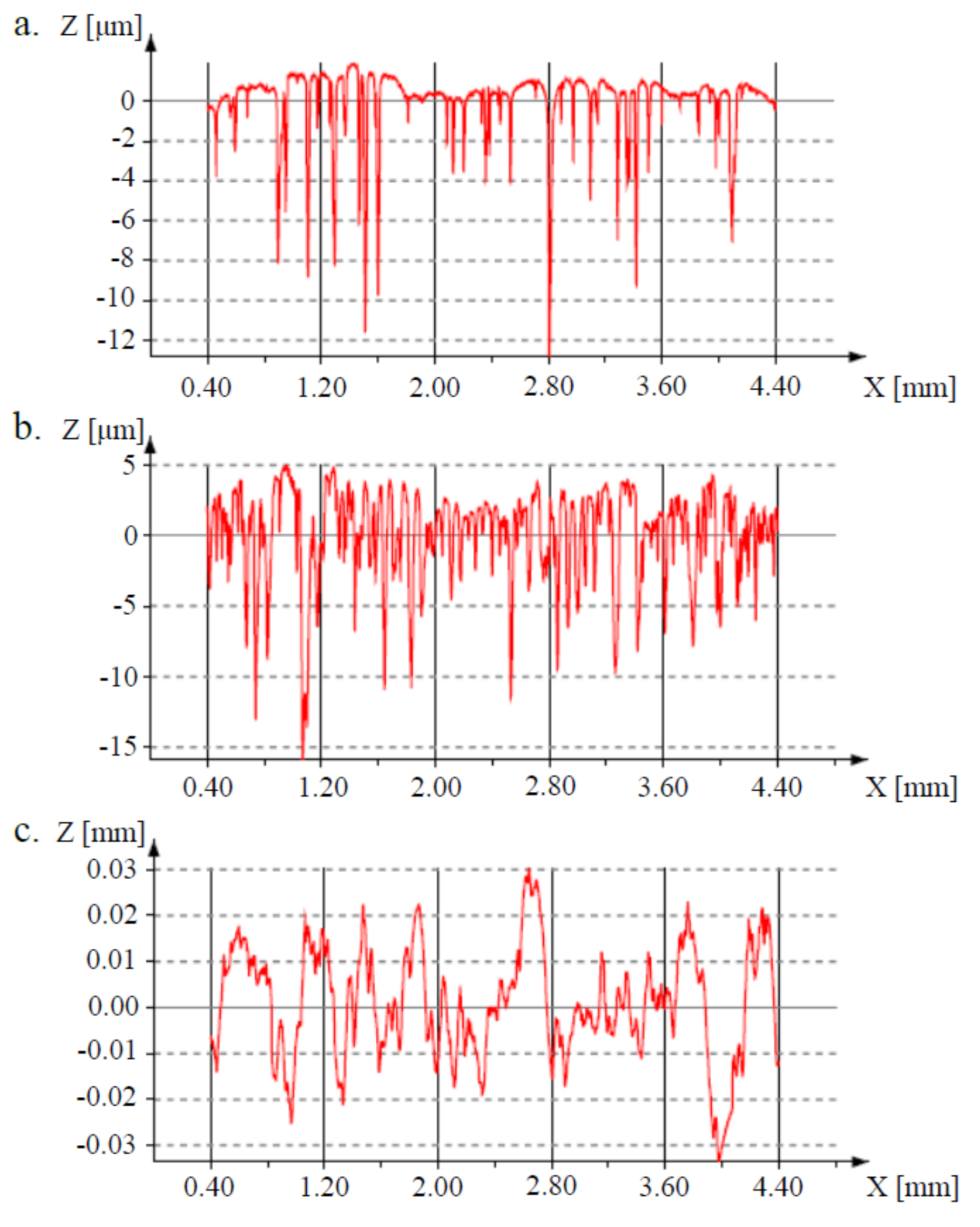
| Structure Parameters | Ti31Mo | sTi31Mo (Ox) | Ti31Mo (HT) | ||
|---|---|---|---|---|---|
| sig | - | 1.4629564 | 1.5791782 | 1.405559 | |
| Rwp | [%] | 6.0970262 | 6.329574 | 5.535124 | |
| Rexp | [%] | 4.167606 | 4.0081444 | 3.9381716 | |
| Ti(β) | A | [%] | 100.00 | 95.07 | - |
| a | [Å] | 3.2433(1) | 3.2426(1) | - | |
| V | [Å3] | 34.1(0) | 33.5(0) | - | |
| Ti3O | A | [%] | - | 4.93 | - |
| a | [Å] | - | 5.1361(41) | - | |
| c | [Å] | - | 9.5623(164) | - | |
| V | [Å3] | - | 36.6(0) | - | |
| Ca10(PO4)6(OH)2 | A | [%] | - | - | 81.23 |
| a | [Å] | - | - | 9.4252(6) | |
| c | [Å] | - | - | 6.8894(6) | |
| V | [Å3] | - | - | 530.1(1) | |
| CaHPO4·2H2O | A | [%] | - | - | 18.77 |
| a | [Å] | - | - | 6.3748(32) | |
| b | [Å] | - | - | 15.2564(58) | |
| c | [Å] | - | - | 5.7910(31) | |
| V | [Å3] | - | - | 487.8(7) | |
| Element | Line | Ti31Mo(Ox) | Ti31Mo(HT) |
|---|---|---|---|
| wt. % | wt. % | ||
| Ti | Kα1 | 50.07 | 0.00 |
| Mo | Lα1 | 41.63 | 0.00 |
| Ca | Kα1 | 0.00 | 45.07 |
| P | Kα1 | 0.00 | 24.28 |
| O | Kα1 | 6.07 | 16.56 |
| Na | Kα1 | 2.22 | 6.81 |
| K | Kα1 | 0.00 | 7.28 |
| Total | - | 100.00 | 100.00 |
| 2D Parameters | Ti31Mo | Ti31Mo (Ox) | Ti31Mo (HT) |
|---|---|---|---|
| Ra | 1.04 ± 0.13 | 2.40 ± 0.36 | 9.22 ± 1.93 |
| Rt | 14.55 ± 0.78 | 22.18 ± 1.85 | 62.52 ± 10.85 |
| Rz | 10.59 ± 0.77 | 15.70 ± 1.69 | 45.39 ± 5.95 |
| Parameter | Unit | Ti31Mo | Ti31Mo (Ox) | Ti31Mo (HT) |
|---|---|---|---|---|
| W | (mg/day) | 0.0496(16) | 0.0357(22) | 0.0150(25) |
| Diiodomethane CA | (°) | 58.76 ± 4.54 | 46.16 ± 5.71 | impossible to measure |
| Glycerol CA | (°) | 50.12 ± 1.39 | 55.97 ± 5.31 | 31.28 ± 2.78 |
| Surface free energy | (mN/m) | 43.16 ± 0.37 | 42.54 ± 3.07 | - |
| Disperse | (mN/m) | 29.29 ± 2.61 | 36.37 ± 3.10 | - |
| Polar | (mN/m) | 13.87 ± 2.87 | 6.17 ± 1.41 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sochacka, P.; Jurczyk, M.U.; Kowalski, K.; Wirstlein, P.K.; Jurczyk, M. Ultrafine-Grained Ti-31Mo-Type Composites with HA and Ag, Ta2O5 or CeO2 Addition for Implant Applications. Materials 2021, 14, 644. https://doi.org/10.3390/ma14030644
Sochacka P, Jurczyk MU, Kowalski K, Wirstlein PK, Jurczyk M. Ultrafine-Grained Ti-31Mo-Type Composites with HA and Ag, Ta2O5 or CeO2 Addition for Implant Applications. Materials. 2021; 14(3):644. https://doi.org/10.3390/ma14030644
Chicago/Turabian StyleSochacka, Patrycja, Mieczyslawa U. Jurczyk, Kamil Kowalski, Przemyslaw K. Wirstlein, and Mieczyslaw Jurczyk. 2021. "Ultrafine-Grained Ti-31Mo-Type Composites with HA and Ag, Ta2O5 or CeO2 Addition for Implant Applications" Materials 14, no. 3: 644. https://doi.org/10.3390/ma14030644
APA StyleSochacka, P., Jurczyk, M. U., Kowalski, K., Wirstlein, P. K., & Jurczyk, M. (2021). Ultrafine-Grained Ti-31Mo-Type Composites with HA and Ag, Ta2O5 or CeO2 Addition for Implant Applications. Materials, 14(3), 644. https://doi.org/10.3390/ma14030644








