The Role of Electrospun Nanomaterials in the Future of Energy and Environment
Abstract
1. Introduction
2. Energy Conversion
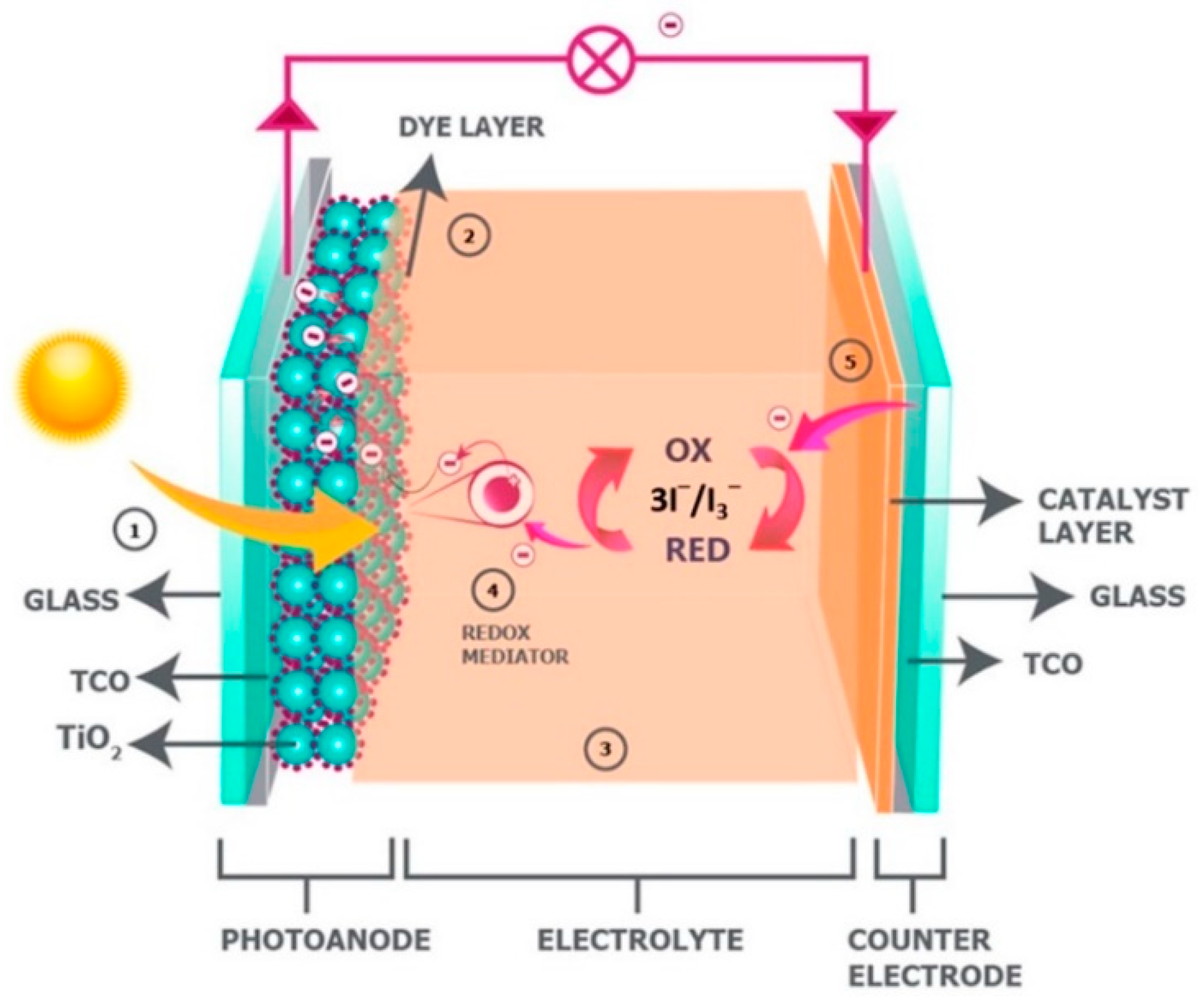
2.1. Dye-Sensitized Solar Cells
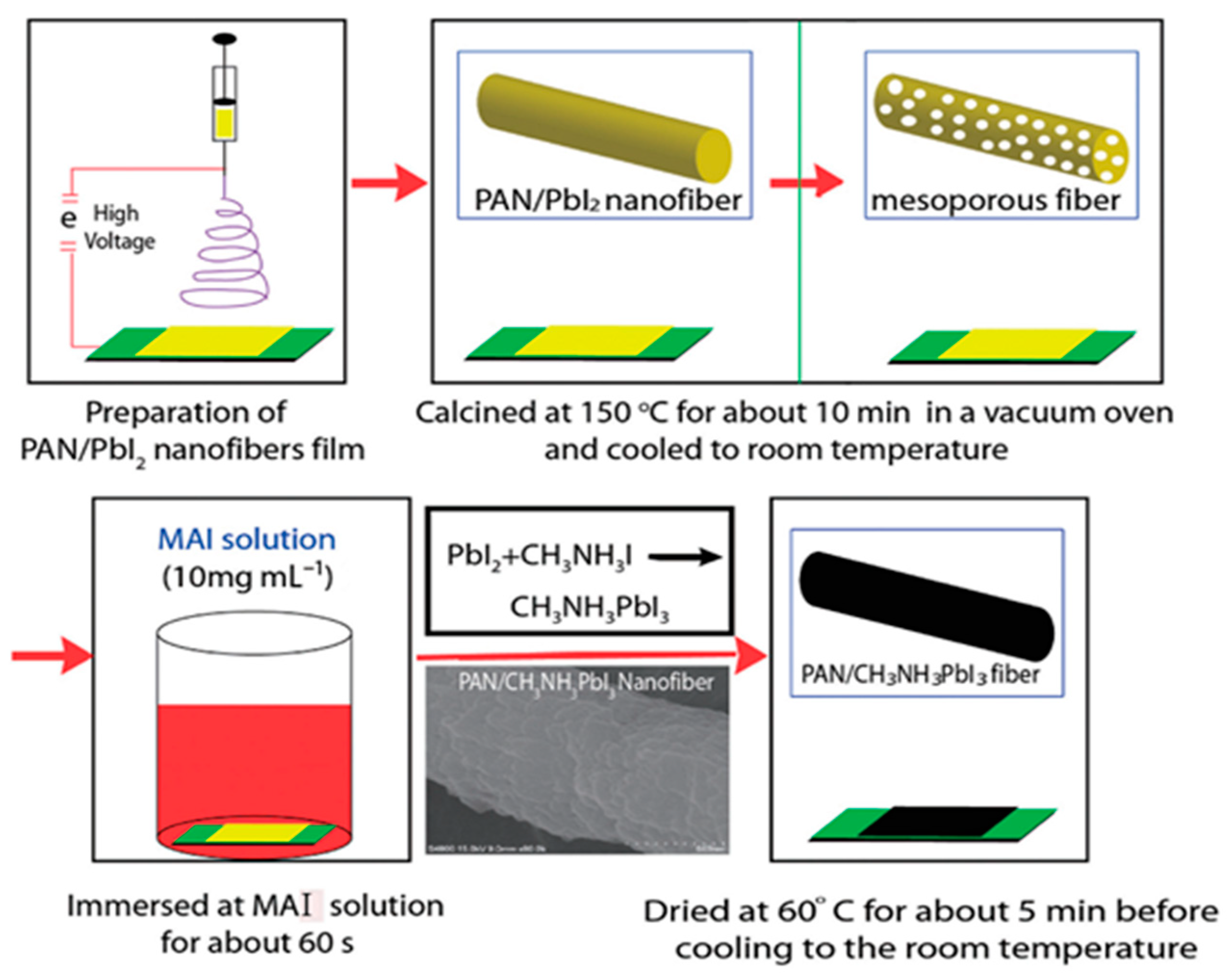
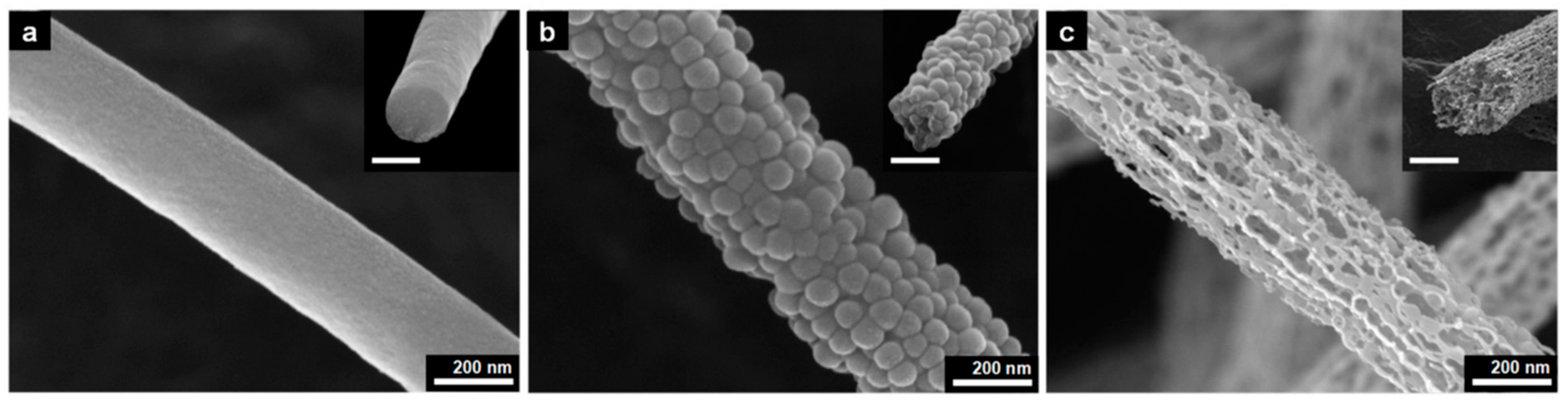
2.2. Perovskite Solar Cells
2.3. Fuel Cells
2.3.1. Electrocatalysts and Supports by Electrospinning
2.3.2. Electrospun Electrolyte Membrane
2.3.3. Microbial Fuel Cells and Biobatteries
3. Energy Storage
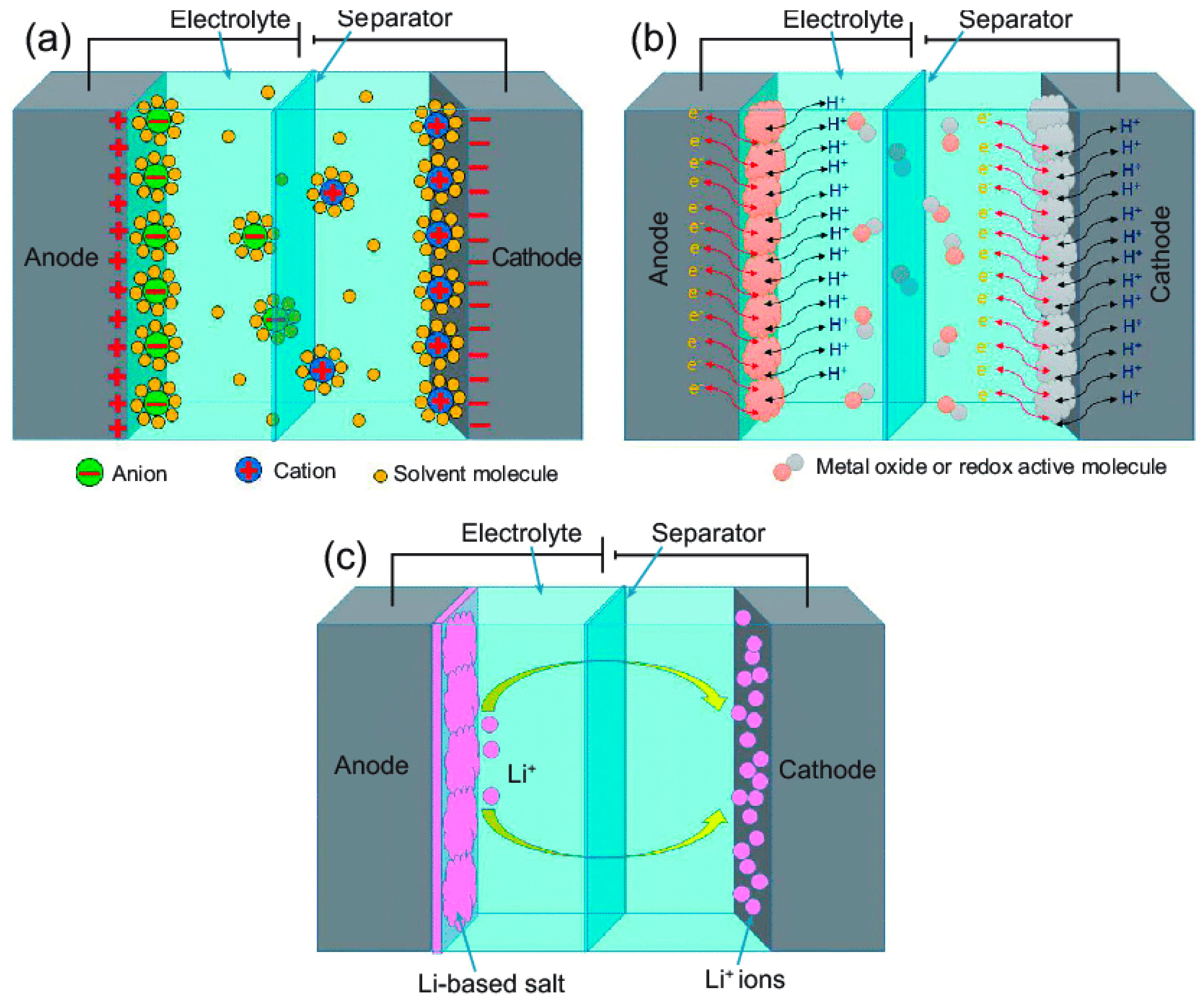
3.1. Lithium-Ion Batteries
3.1.1. Anodes
3.1.2. Cathodes
3.2. Lithium-Sulfur Batteries
3.3. Lithium-O2 or Lithium-Air Batteries
3.4. Supercapacitors
4. Environmental Application
4.1. Oil Sorption
4.1.1. Hydrophobic-Oleophilic Polymeric Nanofibers
4.1.2. Composite Nanofibers for Oil Sorption
4.1.3. Carbon-Based Nanofibers CNFs
4.2. Oil/Water Separation
4.3. Heavy Metal Ion Adsorptions (Metal Ion Removal)
4.4. Adsorption of Organic Compounds
4.5. Photocatalysis
4.6. Nanofibrous Membranes in Water Treatment
4.6.1. Microfiltration
4.6.2. Ultrafiltration (UF)
4.6.3. Nanofiltration
4.6.4. Reverse Osmosis
4.6.5. Forward Osmosis
4.6.6. Membrane Distillation
4.6.7. Membrane Bioreactor
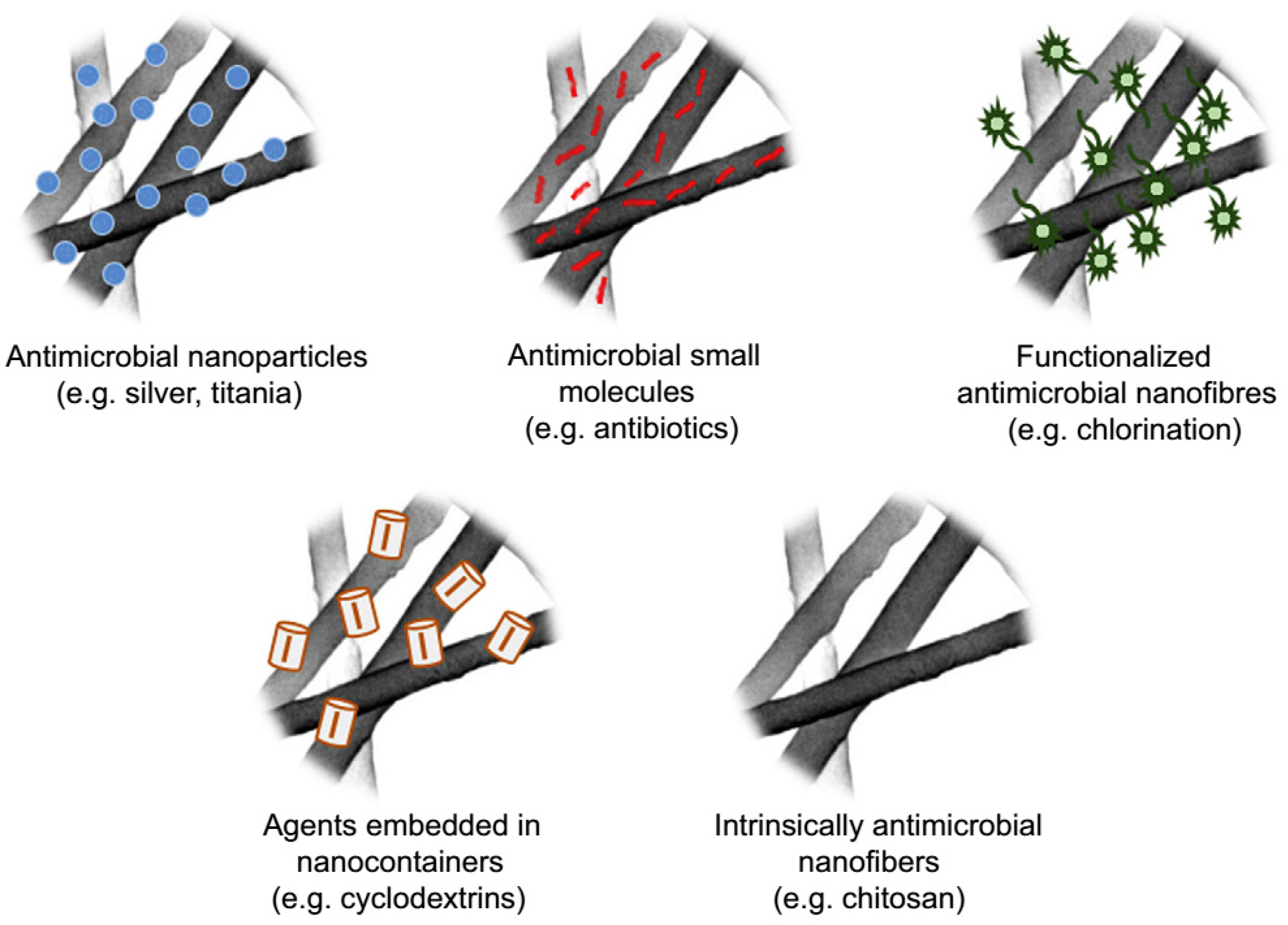
4.6.8. Antimicrobial Treatment
4.7. Gas Sensor
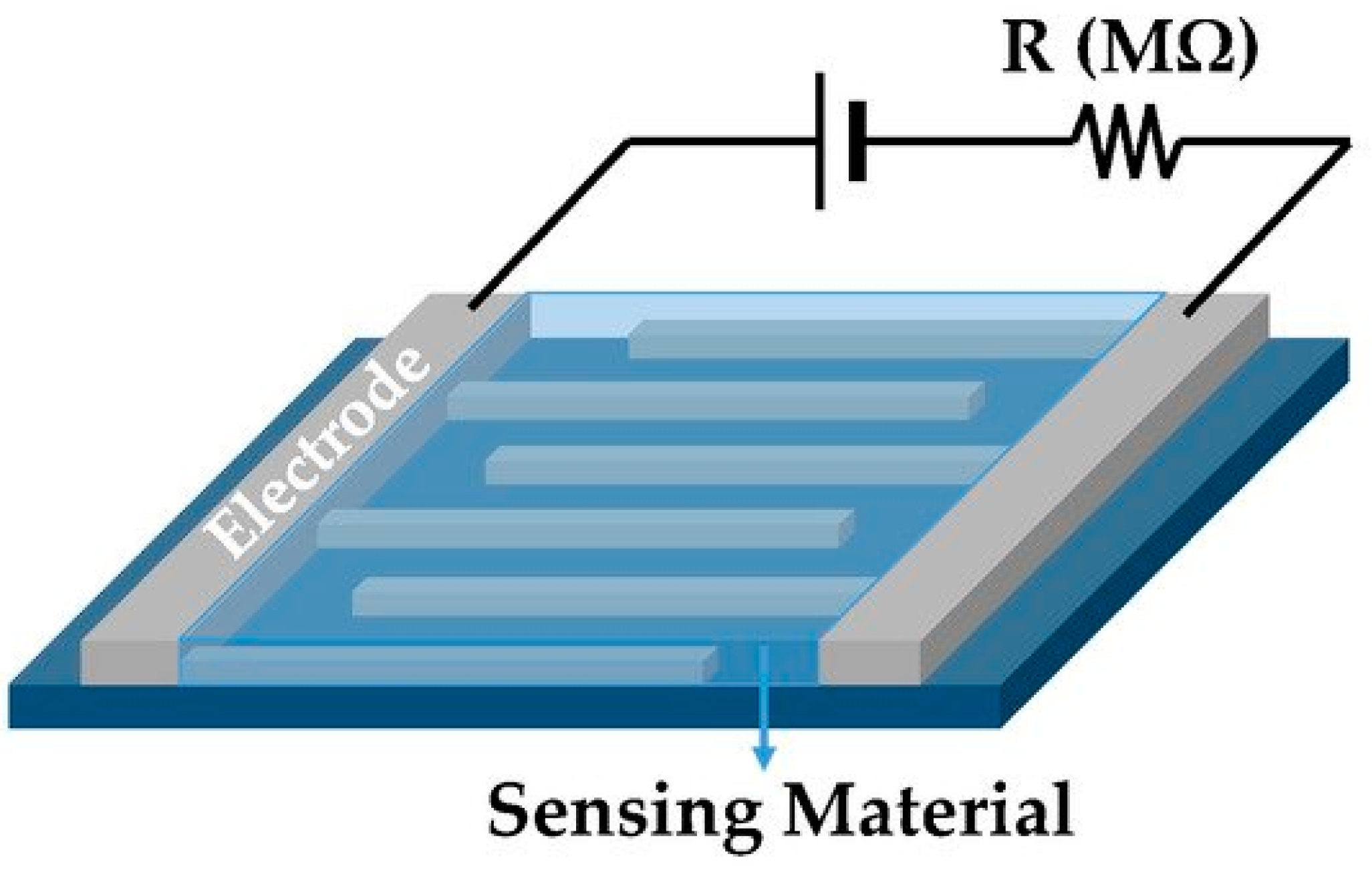
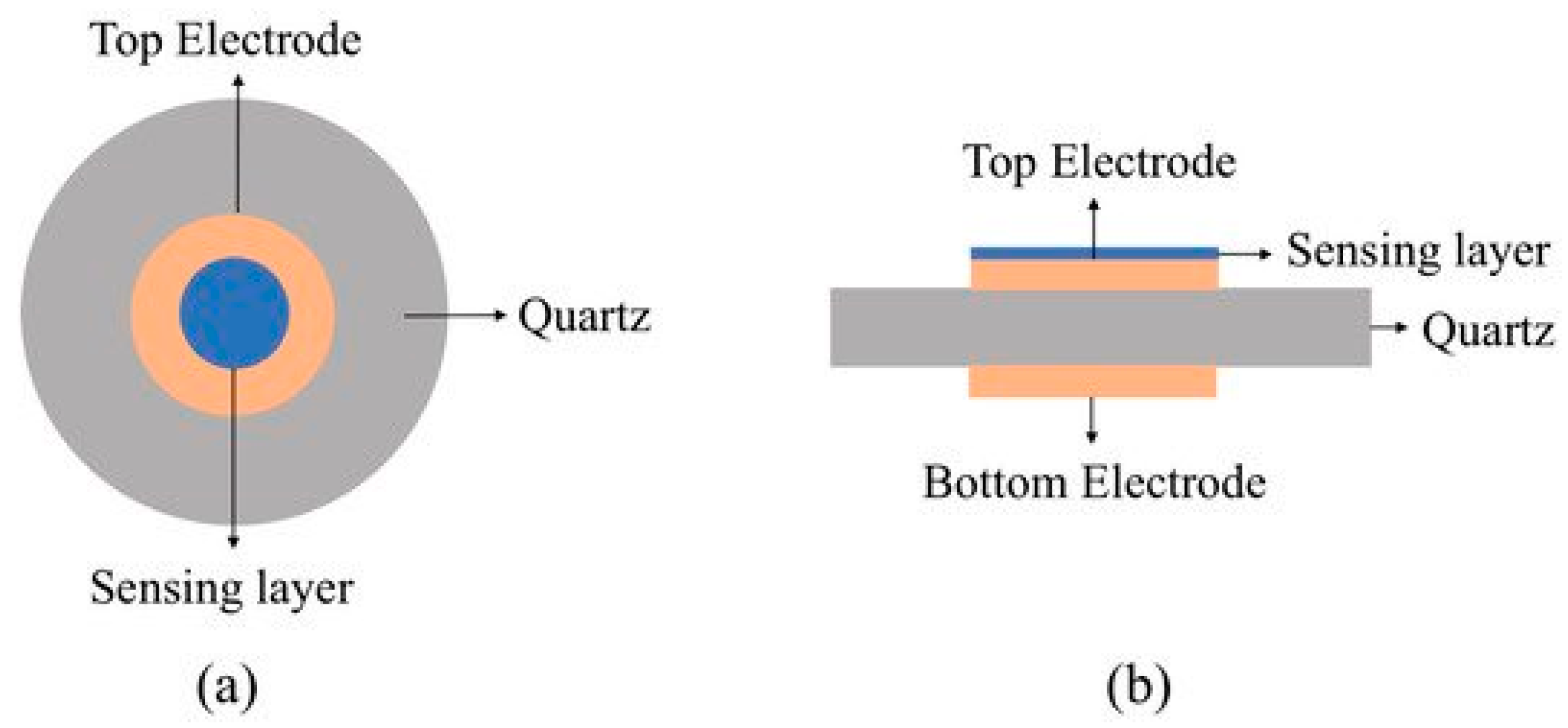
4.8. Liquid Sensor
4.9. Air Filtration
4.10. Catalyst Supports
4.11. EMI Shielding
4.12. Carbon Dioxide Capture
4.13. Ion Exchange
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jamshidi, M.; Askarzadeh, A. Techno-economic analysis and size optimization of an off-grid hybrid photovoltaic, fuel cell and diesel generator system. Sustain. Cities Soc. 2019, 44, 310–320. [Google Scholar] [CrossRef]
- Zerta, M.; Schmidt, P.R.; Stiller, C.; Landinger, H. Alternative World Energy Outlook (AWEO) and the role of hydrogen in a changing energy landscape. Int. J. Hydrogen Energy 2008, 33, 3021–3025. [Google Scholar] [CrossRef]
- Zhang, X.; Estoque, R.C.; Murayama, Y. An urban heat island study in Nanchang City, China based on land surface temperature and social-ecological variables. Sustain. Cities Soc. 2017, 32, 557–568. [Google Scholar] [CrossRef]
- Ahmad, T.; Zhang, D. A critical review of comparative global historical energy consumption and future demand: The story told so far. Energy Rep. 2020, 6, 1973–1991. [Google Scholar] [CrossRef]
- Wagner, L.; Ross, I.; Foster, J.; Hankamer, B. Trading Off Global Fuel Supply, CO2 Emissions and Sustainable Development. PLoS ONE 2016, 11, e0149406. [Google Scholar] [CrossRef]
- Lu, X.; Wang, C.; Favier, F.; Pinna, N. Electrospun nanomaterials for supercapacitor electrodes: Designed architectures and electrochemical performance. Adv. Energy Mater. 2017, 7, 1601301. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Zhang, Q. Toward Safe Lithium Metal Anode in Rechargeable Batteries: A Review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef]
- Li, L.; Peng, S.; Lee, J.K.Y.; Ji, D.; Srinivasan, M.; Ramakrishna, S. Electrospun hollow nanofibers for advanced secondary batteries. Nano Energy 2017, 39, 111–139. [Google Scholar] [CrossRef]
- Kumar, P.S.; Sundaramurthy, J.; Sundarrajan, S.; Babu, V.J.; Singh, G.; Allakhverdiev, S.I.; Ramakrishna, S. Hierarchical electrospun nanofibers for energy harvesting, production and environmental remediation. Energy Environ. Sci. 2014, 7, 3192–3222. [Google Scholar] [CrossRef]
- Khalid, B.; Bai, X.; Wei, H.; Huang, Y.; Wu, H.; Cui, Y. Direct Blow-Spinning of Nanofibers on a Window Screen for Highly Efficient PM2.5 Removal. Nano Lett. 2017, 17, 1140–1148. [Google Scholar] [CrossRef]
- Kang, G.-D.; Cao, Y.-M. Application and modification of poly(vinylidene fluoride) (PVDF) membranes—A review. J. Membr. Sci. 2014, 463, 145–165. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Elbahri, M. Nanocomposite Electrospun Nanofiber Membranes for Environmental Remediation. Materials 2014, 7, 1017–1045. [Google Scholar] [CrossRef] [PubMed]
- Gasparotto, A.; Barreca, D.; Maccato, C.; Tondello, E. Manufacturing of inorganic nanomaterials: Concepts and perspectives. Nanoscale 2012, 4, 2813–2825. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Aboagye, A.; Kelkar, A.D.; Lai, C.; Fong, H. A review: Carbon nanofibers from electrospun polyacrylonitrile and their applications. J. Mater. Sci. 2014, 49, 463–480. [Google Scholar] [CrossRef]
- Zhang, B.; Kang, F.; Tarascon, J.-M.; Kim, J.-K. Recent advances in electrospun carbon nanofibers and their application in electrochemical energy storage. Prog. Mater. Sci. 2016, 76, 319–380. [Google Scholar] [CrossRef]
- Dong, Z.; Kennedy, S.J.; Wu, Y. Electrospinning materials for energy-related applications and devices. J. Power Sources 2011, 196, 4886–4904. [Google Scholar] [CrossRef]
- Doyle, J.J.; Choudhari, S.K.; Ramakrishna, S.; Babu, R.P. Electrospun Nanomaterials: Biotechnology, Food, Water, Environment, and Energy. In Proceedings of the Conference Papers in Materials Science, Benghazi, Libya, 26–28 March 2013; Volume 2013, pp. 1–14. [Google Scholar]
- Renugopalakrishnan, E.; Kannan, A.M.; Srinivasan, S.; Thavasi, V.; Ramakrishna, P.S.; Li, A.M.; Filipek, A.S.; Kumar, J.D. Nanomaterials for Energy Conversion Applications; American Scientific Publishers: Stevenson Ranch, CA, USA, 2009. [Google Scholar]
- Santangelo, S. Electrospun Nanomaterials for Energy Applications: Recent Advances. Appl. Sci. 2019, 9, 1049. [Google Scholar] [CrossRef]
- World Energy Outlook 2019. Available online: https://www.iea.org/reports/world-energy-outlook-2019 (accessed on 5 November 2020).
- O’Regan, B.; Grätzel, M.; Gr, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Kakiage, K.; Aoyama, Y.; Yano, T.; Oya, K.; Fujisawa, J.-I.; Hanaya, M. Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes. Chem. Commun. 2015, 51, 15894–15897. [Google Scholar] [CrossRef]
- Zhang, Q.; Cao, G. Nanostructured photoelectrodes for dye-sensitized solar cells. Nano Today 2011, 6, 91–109. [Google Scholar] [CrossRef]
- Iftikhar, H.; Sonai, G.G.; Hashmi, S.G.; Nogueira, A.F.; Lund, P.D. Progress on Electrolytes Development in Dye-Sensitized Solar Cells. Materials 2019, 12, 1998. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Dai, S.; Weng, J.; Xiao, S.; Sui, Y.; Huang, Y.; Chen, S.; Kong, F.; Pan, X.; Liang, L.; et al. Microstructure Design of Nanoporous TiO2Photoelectrodes for Dye-Sensitized Solar Cell Modules. J. Phys. Chem. B 2007, 111, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Y.; Kuang, D.-B.; Su, C.-Y. Hierarchically micro/nanostructured photoanode materials for dye-sensitized solar cells. J. Mater. Chem. 2012, 22, 15475–15489. [Google Scholar] [CrossRef]
- Chen, T.; Qiu, L.; Cai, Z.; Gong, F.; Yang, Z.; Wang, Z.; Peng, H. Intertwined Aligned Carbon Nanotube Fiber Based Dye-Sensitized Solar Cells. Nano Lett. 2012, 12, 2568–2572. [Google Scholar] [CrossRef]
- Zhang, Q.; Christopher, S.; Zhou, X.; Cao, G. ZnO Nanostructures for Dye-Sensitized Solar Cells. Adv. Mater. 2009, 21, 4087–4108. [Google Scholar] [CrossRef]
- Feng, H.-L.; Wu, W.-Q.; Rao, H.-S.; Wan, Q.; Li, L.-B.; Kuang, D.; Su, C.-Y. Three-Dimensional TiO2/ZnO Hybrid Array as a Heterostructured Anode for Efficient Quantum-Dot-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 5199–5205. [Google Scholar] [CrossRef]
- Mukherjee, K.; Teng, T.-H.; Jose, R.; Ramakrishna, S. Electron transport in electrospun TiO2 nanofiber dye-sensitized solar cells. Appl. Phys. Lett. 2009, 95, 012101. [Google Scholar] [CrossRef]
- Yang, L.; Leung, W.W.-F. Electrospun TiO2 Nanorods with Carbon Nanotubes for Efficient Electron Collection in Dye-Sensitized Solar Cells. Adv. Mater. 2013, 25, 1792–1795. [Google Scholar] [CrossRef]
- Francis, L.; Nair, A.S.; Jose, R.; Ramakrishna, S.; Thavasi, V.; Marsano, E. Fabrication and characterization of dye-sensitized solar cells from rutile nanofibers and nanorods. Energy 2011, 36, 627–632. [Google Scholar] [CrossRef]
- Song, M.Y.; Kim, D.K.; Jo, S.M.; Kim, D.Y. Enhancement of the photocurrent generation in dye-sensitized solar cell based on electrospun TiO2 electrode by surface treatment. Synth. Met. 2005, 155, 635–638. [Google Scholar] [CrossRef]
- Krishnamoorthy, T.; Thavasi, V.G.; Ramakrishna, S. A first report on the fabrication of vertically aligned anatase TiO2 nanowires by electrospinning: Preferred architecture for nanostructured solar cells. Energy Environ. Sci. 2011, 4, 2807–2812. [Google Scholar] [CrossRef]
- Elumalai, N.K.; Jin, T.M.; Chellappan, V.; Jose, R.; Palaniswamy, S.K.; Jayaraman, S.; Raut, H.K.; Ramakrishna, S. Electrospun ZnO Nanowire Plantations in the Electron Transport Layer for High-Efficiency Inverted Organic Solar Cells. ACS Appl. Mater. Interfaces 2013, 5, 9396–9404. [Google Scholar] [CrossRef] [PubMed]
- Hieu, N.T.; Baik, S.J.; Jun, Y.; Lee, M.; Chung, O.H.; Park, J.S. Electrospun coaxial titanium dioxide/carbon nanofibers for use in anodes of dye-sensitized solar cells. Electrochim. Acta 2014, 142, 144–151. [Google Scholar] [CrossRef]
- Joshi, P.; Zhang, L.; Davoux, D.; Zhu, Z.; Galipeau, D.; Fong, H.; Qiao, Q. Composite of TiO2 nanofibers and nanoparticles for dye-sensitized solar cells with significantly improved efficiency. Energy Environ. Sci. 2010, 3, 1507–1510. [Google Scholar] [CrossRef]
- Jean, J.; Chang, S.; Brown, P.R.; Cheng, J.J.; Rekemeyer, P.H.; Bawendi, M.G.; Gradečak, S.; Bulović, V. ZnO Nanowire Arrays for Enhanced Photocurrent in PbS Quantum Dot Solar Cells. Adv. Mater. 2013, 25, 2790–2796. [Google Scholar] [CrossRef]
- Krishnamoorthy, T.; Tang, M.Z.; Verma, A.; Nair, A.S.; Pliszka, D.; Subodh, G.; Ramakrishna, S. A facile route to vertically aligned electrospun SnO2 nanowires on a transparent conducting oxide substrate for dye-sensitized solar cells. J. Mater. Chem. 2012, 22, 2166–2172. [Google Scholar] [CrossRef]
- Song, M.Y.; Kim, D.K.; Ihn, K.J.; Jo, S.M.; Kim, D.Y. Electrospun TiO2 electrodes for dye-sensitized solar cells. Nanotechnology 2004, 15, 1861–1865. [Google Scholar] [CrossRef]
- Mali, S.S.; Shim, C.-S.; Kim, H.; Patil, J.V.; Ahn, D.H.; Patil, P.; Hong, C.K. Evaluation of various diameters of titanium oxide nanofibers for efficient dye sensitized solar cells synthesized by electrospinning technique: A systematic study and their application. Electrochim. Acta 2015, 166, 356–366. [Google Scholar] [CrossRef]
- Motlak, M.; Hamza, A.; Hammed, M.G.; Barakat, N.A.M. Cd-doped TiO2 nanofibers as effective working electrode for the dye sensitized solar cells. Mater. Lett. 2019, 246, 206–209. [Google Scholar] [CrossRef]
- Yuan, H.; Jiao, Q.; Liu, J.; Liu, X.; Yang, H.; Zhao, Y.; Wu, Q.; Shi, D.; Li, H. Ultrathin-walled Co9S8 nanotube/reduced graphene oxide composite as an efficient electrocatalyst for the reduction of triiodide. J. Power Sources 2016, 336, 132–142. [Google Scholar] [CrossRef]
- Chen, M.; Shao, L.-L. Review on the recent progress of carbon counter electrodes for dye-sensitized solar cells. Chem. Eng. J. 2016, 304, 629–645. [Google Scholar] [CrossRef]
- Cho, S.; Hwang, S.H.; Kim, C.; Jang, J. Polyaniline porous counter-electrodes for high performance dye-sensitized solar cells. J. Mater. Chem. 2012, 22, 12164–12171. [Google Scholar] [CrossRef]
- Trevisan, R.; Döbbelin, M.; Boix, P.P.; Barea, E.M.; Tena-Zaera, R.; Mora-Seró, I.; Bisquert, J. PEDOT Nanotube Arrays as High Performing Counter Electrodes for Dye Sensitized Solar Cells. Study of the Interactions Among Electrolytes and Counter Electrodes. Adv. Energy Mater. 2011, 1, 781–784. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, Q.; Xiao, J.; Ma, C.; Lin, X.; He, Y.; Gao, Y.; Hagfeldt, A.; Ma, T.; Miao, C. Two flexible counter electrodes based on molybdenum and tungsten nitrides for dye-sensitized solar cells. J. Mater. Chem. 2011, 21, 10761–10766. [Google Scholar] [CrossRef]
- Jiang, Q.W.; Li, G.R.; Gao, X. Highly ordered TiN nanotube arrays as counter electrodes for dye-sensitized solar cells. Chem. Commun. 2009, 6720–6722. [Google Scholar] [CrossRef] [PubMed]
- Li, G.-R.; Wang, F.; Jiang, Q.-W.; Gao, X.; Shen, P.-W. Carbon Nanotubes with Titanium Nitride as a Low-Cost Counter-Electrode Material for Dye-Sensitized Solar Cells. Angew. Chem. Int. Ed. 2010, 49, 3653–3656. [Google Scholar] [CrossRef]
- Huang, X.; Huang, S.; Zhang, Q.; Guo, X.; Li, N.; Luo, Y.; Shen, Q.; Toyoda, T.; Meng, Q. A flexible photoelectrode for CdS/CdSe quantum dot-sensitized solar cells (QDSSCs). Chem. Commun. 2011, 47, 2664–2666. [Google Scholar] [CrossRef]
- Wu, M.; Lin, X.; Wang, Y.; Wang, L.; Guo, W.; Qi, D.; Peng, X.; Hagfeldt, A.; Grätzel, M.; Ma, T. Economical Pt-Free Catalysts for Counter Electrodes of Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2012, 134, 3419–3428. [Google Scholar] [CrossRef]
- Xin, X.; He, M.; Han, W.; Jung, J.; Lin, Z. Low-Cost Copper Zinc Tin Sulfide Counter Electrodes for High-Efficiency Dye-Sensitized Solar Cells. Angew. Chem. Int. Ed. 2011, 50, 11739–11742. [Google Scholar] [CrossRef]
- Sun, H.; Qin, D.; Huang, S.; Guo, X.; Li, D.; Luo, Y.; Meng, Q. Dye-sensitized solar cells with NiS counter electrodes electrodeposited by a potential reversal technique. Energy Environ. Sci. 2011, 4, 2630–2637. [Google Scholar] [CrossRef]
- Thomas, S.; Deepak, T.G.; Anjusree, G.S.; Arun, T.A.; Nair, S.V.; Nair, A.S. A review on counter electrode materials in dye-sensitized solar cells. J. Mater. Chem. A 2014, 2, 4474–4490. [Google Scholar] [CrossRef]
- Malara, F.; Manca, M.; De Marco, L.; Pareo, P.; Gigli, G. Flexible Carbon Nanotube-Based Composite Plates as Efficient Monolithic Counter Electrodes for Dye Solar Cells. ACS Appl. Mater. Interfaces 2011, 3, 3625–3632. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Pint, C.L.; Hainey, M.; Mirri, F.; Zhan, Y.; Zhang, J.; Pasquali, M.; Hauge, R.H.; Verduzco, R.; Jiang, M.; et al. Vertically Aligned Single-Walled Carbon Nanotubes as Low-cost and High Electrocatalytic Counter Electrode for Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2011, 3, 3157–3161. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.; Zhang, L.; Chen, Q.; Galipeau, D.; Fong, H.; Qiao, Q. Electrospun Carbon Nanofibers as Low-Cost Counter Electrode for Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2010, 2, 3572–3577. [Google Scholar] [CrossRef]
- Park, S.-H.; Kim, B.-K.; Lee, W.-J. Electrospun activated carbon nanofibers with hollow core/highly mesoporous shell structure as counter electrodes for dye-sensitized solar cells. J. Power Sources 2013, 239, 122–127. [Google Scholar] [CrossRef]
- Song, L.; Chen, P.; Li, Z.; Du, P.; Yang, Y.; Li, N.; Xiong, J. Flexible carbon nanotubes/TiO2/C nanofibrous film as counter electrode of flexible quasi-solid dye-sensitized solar cells. Thin Solid Films 2020, 711, 138307. [Google Scholar] [CrossRef]
- Yousef, A.; Brooks, R.M.; El-Newehy, M.H.; Al-Deyab, S.S.; Kim, H.Y. Electrospun Co-TiC nanoparticles embedded on carbon nanofibers: Active and chemically stable counter electrode for methanol fuel cells and dye-sensitized solar cells. Int. J. Hydrogen Energy 2017, 42, 10407–10415. [Google Scholar] [CrossRef]
- Best Research-Cell Efficiency Chart—Photovoltaic Research—NREL. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 13 November 2020).
- Petridis, C.; Kakavelakis, G.; Kymakis, E. Renaissance of graphene-related materials in photovoltaics due to the emergence of metal halide perovskite solar cells. Energy Environ. Sci. 2018, 11, 1030–1061. [Google Scholar] [CrossRef]
- Yang, W.S.; Noh, J.H.; Jeon, N.J.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 2015, 348, 1234–1237. [Google Scholar] [CrossRef]
- Bi, D.; Tress, W.; Dar, M.I.; Gao, P.; Luo, J.; Renevier, C.; Schenk, K.; Abate, A.; Giordano, F.; Baena, J.-P.C.; et al. Efficient luminescent solar cells based on tailored mixed-cation perovskites. Sci. Adv. 2016, 2, e1501170. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Mora-Sero, I.; Gonzalez-Pedro, V.; Fabregat-Santiago, F.; Pérez, E.J.J.; Park, N.-G.; Bisquert, J. Mechanism of carrier accumulation in perovskite thin-absorber solar cells. Nat. Commun. 2013, 4, 2242. [Google Scholar] [CrossRef] [PubMed]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.P.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-Hole Diffusion Lengths Exceeding 1 Micrometer in an Organometal Trihalide Perovskite Absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, C.R.; Im, J.H.; Lee, K.B.; Moehl, T.; Marchioro, A.; Moon, S.J.; Humphry-Baker, R.; Yum, J.H.; Moser, J.E.; et al. Lead iodide perovskite sensitized all-solidstate submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Sci. Rep. 2012, 2, 591–597. [Google Scholar] [CrossRef]
- Dharani, S.; Mulmudi, H.K.; Yantara, N.; Trang, P.T.T.; Park, N.G.; Graetzel, M.; Mhaisalkar, S.; Mathews, N.; Boix, P.P. High efficiency electrospun TiO2nanofiber based hybrid organic–inorganic perovskite solar cell. Nanoscale 2014, 6, 1675–1679. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, L.; Peng, C.; Rao, L.; Wan, M. Preparation and Characterization of CH3NH3PbI3 Perovskite Deposited onto Polyacrylonitrile (PAN) Nanofiber Substrates. Chem. Lett. 2016, 45, 312–314. [Google Scholar] [CrossRef]
- Patil, J.V.; Mali, S.S.; Patil, A.P.; Patil, P.S.; Hong, C.K. Highly efficient mixed-halide mixed-cation perovskite solar cells based on rGO-TiO2 composite nanofibers. Energy 2019, 189, 116396. [Google Scholar] [CrossRef]
- Li, Q.; Balilonda, A.; Ali, A.; Jose, R.; Zabihi, F.; Yang, S.; Ramakrishna, S.; Zhu, M. Flexible Solar Yarns with 15.7% Power Conversion Efficiency, Based on Electrospun Perovskite Composite Nanofibers. Sol. RRL 2020, 4, 2000269. [Google Scholar] [CrossRef]
- Kabir, S.; Medina, S.; Wang, G.; Bender, G.; Pylypenko, S.; Neyerlin, K.C. Improving the bulk gas transport of Fe-N-C platinum group metal-free nanofiber electrodes via electrospinning for fuel cell applications. Nano Energy 2020, 73, 104791. [Google Scholar] [CrossRef]
- He, Y.; Guo, H.; Hwang, S.; Yang, X.; He, Z.; Braaten, J.; Karakalos, S.; Shan, W.; Wang, M.; Zhou, H.; et al. Single Cobalt Sites Dispersed in Hierarchically Porous Nanofiber Networks for Durable and High-Power PGM-Free Cathodes in Fuel Cells. Adv. Mater. 2020, 32, e2003577. [Google Scholar] [CrossRef] [PubMed]
- Ponomarev, I.I.; Skupov, K.M.; Zhigalina, O.M.; Naumkin, A.V.; Modestov, A.D.; Basu, V.G.; Sufiyanova, A.E.; Razorenov, D.Y.; Ponomarev, I.I. New Carbon Nanofiber Composite Materials Containing Lanthanides and Transition Metals Based on Electrospun Polyacrylonitrile for High Temperature Polymer Electrolyte Membrane Fuel Cell Cathodes. Polymers 2020, 12, 1340. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, D.; Li, Q.; Huang, L.; Bao, H. Composite Polymer Electrolyte Membranes based on Nafion and Modified PVDF Electrospun Nanofiber Mats. J. Wuhan Univ. Technol. Sci. Ed. 2020, 35, 677–681. [Google Scholar] [CrossRef]
- Liu, G.; Tsen, W.-C.; Jang, S.-C.; Hu, F.; Zhong, F.; Zhang, B.; Wang, J.; Liu, H.; Wang, G.; Wen, S.; et al. Composite membranes from quaternized chitosan reinforced with surface-functionalized PVDF electrospun nanofibers for alkaline direct methanol fuel cells. J. Membr. Sci. 2020, 611, 118242. [Google Scholar] [CrossRef]
- Ikram, S.; Ahmed, S.; Ali, S.W.; Agarwal, H. Chitosan-Based Polymer Electrolyte Membranes for Fuel Cell Applications. In Organic-Inorganic Composite Polymer Electrolyte Membranes; Springer: New York, NY, USA, 2017; Volume 377, pp. 381–398. [Google Scholar]
- Slate, A.J.; Whitehead, K.A.; Brownson, D.A.; Banks, C.E. Microbial fuel cells: An overview of current technology. Renew. Sustain. Energy Rev. 2019, 101, 60–81. [Google Scholar] [CrossRef]
- Gao, Y.; Mohammadifar, M.; Choi, S. From Microbial Fuel Cells to Biobatteries: Moving toward On-Demand Micropower Generation for Small-Scale Single-Use Applications. Adv. Mater. Technol. 2019, 4, 1900079. [Google Scholar] [CrossRef]
- Li, S.; Chen, G. Factors Affecting the Effectiveness of Bioelectrochemical System Applications: Data Synthesis and Meta-Analysis. Batteries 2018, 4, 34. [Google Scholar] [CrossRef]
- Baptista, A.C.; Martins, J.I.; Fortunato, E.; Martins, R.; Borges, J.P.; Ferreira, I. Thin and flexible bio-batteries made of electrospun cellulose-based membranes. Biosens. Bioelectron 2010, 26, 2742–2745. [Google Scholar] [CrossRef]
- Massaglia, G.; Frascella, F.; Chiadò, A.; Sacco, A.; Marasso, S.L.; Cocuzza, M.; Pirri, C.F.; Quaglio, M. Electrospun Nanofibers: From Food to Energy by Engineered Electrodes in Microbial Fuel Cells. Nanomaterials 2020, 10, 523. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Wolverton, C.M.; Isaacs, E.D. Electrical energy storage for transportation—Approaching the limits of, and going beyond, lithium-ion batteries. Energy Environ. Sci. 2012, 5, 7854–7863. [Google Scholar] [CrossRef]
- Aravindan, V.; Gnanaraj, J.; Lee, Y.-S.; Madhavi, S. LiMnPO4—A next generation cathode material for lithium-ion batteries. J. Mater. Chem. A 2013, 1, 3518–3539. [Google Scholar] [CrossRef]
- Obrovac, M.N.; Chevrier, V.L. Alloy Negative Electrodes for Li-Ion Batteries. Chem. Rev. 2014, 114, 11444–11502. [Google Scholar] [CrossRef] [PubMed]
- Kavan, L. Electrochemistry of titanium dioxide: Some aspects and highlights. Chem. Rec. 2011, 12, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Yang, K.-S.; Kojima, M.; Yoshida, K.; Kim, Y.J.; Endo, M. Fabrication of Electrospinning-Derived Carbon Nanofiber Webs for the Anode Material of Lithium-Ion Secondary Batteries. Adv. Funct. Mater. 2006, 16, 2393–2397. [Google Scholar] [CrossRef]
- Zhang, B.; Yu, Y.; Xu, Z.L.; Abouali, S.; Akbari, M.; He, Y.B.; Kang, F.; Kim, J.K. Correlation between atomic structure and electrochemical performance of anodes made from electrospun carbon nanofiber films. Adv. Energy Mater 2014, 4, 1301448. [Google Scholar] [CrossRef]
- Han, W.; Xiao, Y.; Yin, J.; Gong, Y.; Tuo, X.; Cao, J. Fe3O4@Carbon Nanofibers Synthesized from Cellulose Acetate and Application in Lithium-Ion Battery. Langmuir 2020, 36, 11237–11244. [Google Scholar] [CrossRef]
- Hu, J.; Wang, H.; Qin, C.; Li, Y.; Yang, Y. Fabrication of TiO2@C/N composite nanofibers and application as stable lithium-ion battery anode. Mater. Lett. 2020, 279, 128491. [Google Scholar] [CrossRef]
- Xu, G.-L.; Wang, Q.; Fang, J.-C.; Xu, Y.-F.; Li, J.-T.; Huang, L.; Sun, S.-G. Tuning the structure and property of nanostructured cathode materials of lithium ion and lithium sulfur batteries. J. Mater. Chem. A 2014, 2, 19941–19962. [Google Scholar] [CrossRef]
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, D.; Jiao, X. Synthesis and Electrochemical Properties of Nanostructured LiCoO2Fibers as Cathode Materials for Lithium-Ion Batteries. J. Phys. Chem. B 2005, 109, 17901–17906. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, D.; Jiao, X.; Liu, F. LiCoO2–MgO coaxial fibers: Co-electrospun fabrication, characterization and electrochemical properties. J. Mater. Chem. 2007, 17, 1769–1776. [Google Scholar] [CrossRef]
- Liu, Y.; Taya, M. Electrospinning fabrication and electrochemical properties of lithium cobalt nanofibers for lithium battery cathode. In Proceedings of the SPIE Smart Structures and Materials + Nondestructive Evaluation and Health Monitoring, San Diego, CA, USA, 8–12 March 2009; Volume 7288, p. 728806. [Google Scholar]
- Wang, Z.; Xu, K.; Zhang, Y.; Wu, J.; Lin, X.; Liu, C.; Hua, J. Study on electro-spin performance of different types of cellulose by activation in the solvent of LiCl/DMAc. J. For. Eng. 2020, 5, 108–113. [Google Scholar]
- Zhu, C.; Yu, Y.; Gu, L.; Weichert, K.; Maier, J. Electrospinning of Highly Electroactive Carbon-Coated Single-Crystalline LiFePO4 Nanowires. Angew. Chem. 2011, 123, 6402–6406. [Google Scholar] [CrossRef]
- Cheah, Y.L.; Gupta, N.; Pramana, S.S.; Aravindan, V.; Wee, G.; Madhavi, S. Morphology, structure and electrochemical properties of single phase electrospun vanadium pentoxide nanofibers for lithium ion batteries. J. Power Sources 2011, 196, 6465–6472. [Google Scholar] [CrossRef]
- Toprakci, O.; Ji, L.; Lin, Z.; Toprakci, H.A.; Zhang, X. Fabrication and electrochemical characteristics of electrospun LiFePO4/carbon composite fibers for lithium-ion batteries. J. Power Sources 2011, 196, 7692–7699. [Google Scholar] [CrossRef]
- Mai, L.-Q.; Xu, L.; Han, C.; Xu, X.; Luo, Y.; Zhao, S.; Zhao, Y. Electrospun Ultralong Hierarchical Vanadium Oxide Nanowires with High Performance for Lithium Ion Batteries. Nano Lett. 2010, 10, 4750–4755. [Google Scholar] [CrossRef]
- Zheng, D.; Zhang, X.; Wang, J.; Qu, D.; Yang, X.; Qu, D. Reduction mechanism of sulfur in lithium–sulfur battery: From elemental sulfur to polysulfide. J. Power Sources 2016, 301, 312–316. [Google Scholar] [CrossRef]
- Pope, M.A.; Aksay, I.A. Structural Design of Cathodes for Li-S Batteries. Adv. Energy Mater. 2015, 5, 1500124–1500146. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Y.; Kang, F. Porous carbon nanofiber paper as an effective interlayer for high-performance lithium-sulfur batteries. Electrochim. Acta 2015, 168, 271–276. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, C.; Chen, Z.; Liu, H.K.; Guo, Z. Large-scale synthesis of ordered mesoporous carbon fiber and its application as cathode material for lithium–sulfur batteries. Carbon 2015, 81, 782–787. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, M.; Li, X.; Liu, Y.-F.; Pan, H. Preparation of mesohollow and microporous carbon nanofiber and its application in cathode material for lithium–sulfur batteries. J. Alloys Compd. 2014, 608, 220–228. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Wu, Z.-Z.; Pan, G.-L.; Liu, S.; Gao, X. Microporous Carbon Polyhedrons Encapsulated Polyacrylonitrile Nanofibers as Sulfur Immobilizer for Lithium–Sulfur Battery. ACS Appl. Mater. Interfaces 2017, 9, 12436–12444. [Google Scholar] [CrossRef] [PubMed]
- Rauloa, A.; Bandyopadhyaya, S.; Ahamadb, S.; Gupta, A.; Srivastavaa, R.; Formanekc, P.; Nandan, B. Bio-inspired poly(3,4-ethylenedioxythiophene): Poly(styrene sulfonate)-sulfur@polyacrylonitrile electrospun nanofibers for lithium-sulfur batteries. J. Power Sources 2019, 431, 250–258. [Google Scholar] [CrossRef]
- Tong, Z.; Huang, L.; Lei, W.; Zhang, H.; Zhang, S. Carbon-containing electrospun nanofibers for lithium–sulfur battery: Current status and future directions. J. Energy Chem. 2021, 54, 254–273. [Google Scholar] [CrossRef]
- Peng, S.; Ilango, P.R. Electrospinning of Nanofibers for Li-Air Battery. In Electrospinning of Nanofibers for Battery Applications; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Perathoner, S.; Centi, G. Chapter 9—Advanced Nanocarbon Materials for Future Energy Applications. In Emerging Materials for Energy Conversion and Storage; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Tsou, Y.-H.; Chuang, Y.-Y.; Chen, J.-S. Effect of surface bonding of FePC with electrospun carbon nanofiber on electrocatalytic performance for aprotic Li-O2 batteries. J. Colloid Interface Sci. 2020, 562, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Zhaoa, L.; Xingb, Y.; Chena, N.; Laia, J.; Lia, L.; Wua, F.; Chena, R. A robust cathode of RuO2 nH2O clusters anchored on the carbon nanofibers for ultralong-life lithium-oxygen batteries. J. Power Sources 2020, 463, 228161. [Google Scholar] [CrossRef]
- Sun, M.; Guo, S.; Wang, Z.; Zou, L.; Chi, B.; Pu, J.; Li, J. Novel and highly efficient catalyst for Li–O2 battery: Porous LaCo0.6Ni0.4O3 nanofibers decorated with ultrafine Co3O4 nanoparticles. Electrochim. Acta 2020, 363, 137235. [Google Scholar] [CrossRef]
- Chen, X.; Paul, R.; Dai, L. Carbon-based supercapacitors for efficient energy storage. Natl. Sci. Rev. 2017, 4, 453–489. [Google Scholar] [CrossRef]
- Wei, K.; Kim, I.S. Application of Nanofibers in Supercapacitors. Surf. Eff. Magn. Nanopart. 2014, 163–181. [Google Scholar] [CrossRef]
- Liang, J.; Zhao, H.; Yue, L.; Fan, G.; Li, T.S.; Lu, S.; Chen, G.; Gao, S.; Asiri, A.M.; Sun, X. Recent advances in electrospun nanofibers for supercapacitors. J. Mater. Chem. A 2020, 8, 16747–16789. [Google Scholar] [CrossRef]
- Pant, B.; Pant, H.R.; Park, M. Fe1−xS Modified TiO2 NPs Embedded Carbon Nanofiber Composite via Electrospinning: A Potential Electrode Material for Supercapacitors. Molecules 2020, 25, 1075. [Google Scholar] [CrossRef]
- Kim, J.; Heo, Y.-J.; Hong, J.-Y.; Kim, S.-K. Preparation of Porous Carbon Nanofibers with Tailored Porosity for Electrochemical Capacitor Electrodes. Materials 2020, 13, 729. [Google Scholar] [CrossRef]
- Jeon, S.; Jeong, J.H.; Yoo, H.; Yu, H.K.; Kim, B.-H.; Kim, M.H. RuO2 Nanorods on Electrospun Carbon Nanofibers for Supercapacitors. ACS Appl. Nano Mater. 2020, 3, 3847–3858. [Google Scholar] [CrossRef]
- Yang, S.; Ai, J.; Han, Z.; Zhang, L.; Zhao, D.; Wang, J.; Yang, C.; Cao, B. Electrospun ZnFe2O4/carbon nanofibers as high-rate supercapacitor electrodes. J. Power Sources 2020, 469, 228416. [Google Scholar] [CrossRef]
- Wang, X.; Yu, J.; Sun, G.; Ding, B. Electrospun nanofibrous materials: A versatile medium for effective oil/water separation. Mater. Today 2016, 19, 403–414. [Google Scholar] [CrossRef]
- Lin, J.; Shang, Y.; Ding, B.; Yang, J.; Yu, J.; Al-Deyab, S.S. Nanoporous polystyrene fibers for oil spill cleanup. Mar. Pollut. Bull. 2012, 64, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Sarbatly, R.; Duduku, K.; Kamin, Z. A review of polymer nanofibres by electrospinning and their application in oil–water separation for cleaning up marine oil spills. Mar. Pollut. Bull. 2016, 106, 8–16. [Google Scholar] [CrossRef]
- Liu, L.; Lin, Z.; Niu, J.; Tian, D.; He, J. Electrospun polysulfone/poly(lactic acid) nanoporous fibrous mats for oil removal from water. Adsorpt. Sci. Technol. 2019, 37, 438–450. [Google Scholar] [CrossRef]
- Blinovskaya, Y. Efficiency Sorbents Comparative Analysis for Heavy Oil Products in the Conditions of Low Temperatures. IOP Conf. Ser. Earth Environ. Sci. 2019, 272, 032116. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, Q.; Hua, D.; Xiong, R.; Zhao, J.; Rao, W.; Huang, S.; Zhan, X.; Chen, F.; Huang, C. Electrospun fibers for oil–water separation. RSC Adv. 2016, 6, 12868–12884. [Google Scholar] [CrossRef]
- Lee, M.W.; An, S.; Latthe, S.S.; Lee, C.; Hong, S.; Yoon, S.S. Electrospun Polystyrene Nanofiber Membrane with Superhydrophobicity and Superoleophilicity for Selective Separation of Water and Low Viscous Oil. ACS Appl. Mater. Interfaces 2013, 5, 10597–10604. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Zhang, Z.; Mai, Z.; Ma, Y.; Liu, B.; Jiang, L.; Zhu, D. A Super-Hydrophobic and Super-Oleophilic Coating Mesh Film for the Separation of Oil and Water. Angew. Chem. Int. Ed. 2004, 43, 2012–2014. [Google Scholar] [CrossRef] [PubMed]
- Alnaqbi, M.A.; Al Blooshi, A.G.; Greish, Y.E. Polyethylene and Polyvinyl Chloride-Blended Polystyrene Nanofibrous Sorbents and Their Application in the Removal of Various Oil Spills. Adv. Polym. Technol. 2020, 2020, 1–12. [Google Scholar] [CrossRef]
- Sundaran, S.P.; Sujith, A. Fabrication of superhydrophobic polycaprolactone/beeswax electrospun membranes for high-efficiency oil/water separation. RSC Adv. 2017, 7, 2092–2102. [Google Scholar] [CrossRef]
- Jiang, Z.; Tijing, L.D.; Amarjargal, A.; Park, C.H.; An, K.-J.; Shon, H.K.; Kim, C. Removal of oil from water using magnetic bicomponent composite nanofibers fabricated by electrospinning. Compos. Part. B Eng. 2015, 77, 311–318. [Google Scholar] [CrossRef]
- Behera, A.; Sahu, P.; Mohapatra, S.; Ghadei, S. Characterization of carbon based nanofibers in nanocomposites and their applications. Mater. Today: Proc. 2020, 33, 5714–5719. [Google Scholar] [CrossRef]
- Noamani, S.; Niroomand, S.; Shakeri, A.; Sadrzadeh, M. Carbon-based polymer nanocomposite membranes for oily wastewater treatment. NPJ Clean Water 2019, 2, 1–14. [Google Scholar] [CrossRef]
- Liu, H.; Cao, C.-Y.; Wei, F.-F.; Huang, P.-P.; Sun, Y.; Jiang, L.; Song, W.-G. Flexible macroporous carbon nanofiber film with high oil adsorption capacity. J. Mater. Chem. A 2014, 2, 3557–3562. [Google Scholar] [CrossRef]
- Lin, Y.-Z.; Zhong, L.-B.; Dou, S.; Shao, Z.-D.; Liu, Q.; Zheng, Y.-M. Facile synthesis of electrospun carbon nanofiber/graphene oxide composite aerogels for high efficiency oils absorption. Environ. Int. 2019, 128, 37–45. [Google Scholar] [CrossRef]
- Kwon, G.; Kota, A.K.; Li, Y.; Sohani, A.; Mabry, J.M.; Tuteja, A. On-Demand Separation of Oil-Water Mixtures. Adv. Mater. 2012, 24, 3666–3671. [Google Scholar] [CrossRef]
- Focarete, M.L.; Gualandi, C.; Ramakrishna, S. Filtering Media by Electrospinning: Next Generation Membranes for Separation Applications; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Ma, W.; Zhang, M.; Liu, Z.; Kang, M.; Huang, C.; Fu, G. Fabrication of highly durable and robust superhydrophobic-superoleophilic nanofibrous membranes based on a fluorine-free system for efficient oil/water separation. J. Membr. Sci. 2019, 570, 303–313. [Google Scholar] [CrossRef]
- Ma, W.; Li, Y.; Zhang, M.; Gao, S.; Cui, J.; Huang, C.; Fu, G. Biomimetic Durable Multifunctional Self-Cleaning Nanofibrous Membrane with Outstanding Oil/Water Separation, Photodegradation of Organic Contaminants, and Antibacterial Performances. ACS Appl. Mater. Interfaces 2020, 12, 34999–35010. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Dunderdale, G.J.; England, M.W.; Hozumi, A. Oil/water separation techniques: A review of recent progresses and future directions. J. Mater. Chem. A 2017, 5, 16025–16058. [Google Scholar] [CrossRef]
- Ding, B.; Wang, X.; Yu, J. Electrospinning: Nanofabrication and Applications; William Andrew Publishing: Norwich, NY, USA, 2019. [Google Scholar]
- Xue, Z.; Cao, Y.; Liu, N.; Feng, L.; Jiang, L. Special wettable materials for oil/water separation. J. Mater. Chem. A 2014, 2, 2445–2460. [Google Scholar] [CrossRef]
- Huang, M.; Si, Y.; Tang, X.; Zhu, Z.; Ding, B.; Liu, L.; Zheng, G.; Luo, W.; Yu, J. Gravity driven separation of emulsified oil–water mixtures utilizing in situ polymerized superhydrophobic and superoleophilic nanofibrous membranes. J. Mater. Chem. A 2013, 1, 14071–14074. [Google Scholar] [CrossRef]
- Moatmed, S.M.; Khedr, M.H.; El-Dek, S.; Kim, H.Y.; El-Deen, A.G. Highly efficient and reusable superhydrophobic/superoleophilic polystyrene@ Fe3O4 nanofiber membrane for high-performance oil/water separation. J. Environ. Chem. Eng. 2019, 7, 103508. [Google Scholar] [CrossRef]
- Wang, J.-C.; Lou, H.; Cui, Z.-H.; Hou, Y.; Li, Y.; Zhang, Y.; Jiang, K.; Shi, W.; Qu, L. Fabrication of porous polyacrylamide/polystyrene fibrous membranes for efficient oil-water separation. Sep. Purif. Technol. 2019, 222, 278–283. [Google Scholar] [CrossRef]
- World Health Organization. Chemicals of Major Public Health Concern. Available online: https://www.who.int/ipcs/features/chemicals_concern/en/ (accessed on 10 November 2020).
- Wang, M.; Hossain, F.; Sulaiman, R.; Ren, X. Exposure to Inorganic Arsenic and Lead and Autism Spectrum Disorder in Children: A Systematic Review and Meta-Analysis. Chem. Res. Toxicol. 2019, 32, 1904–1919. [Google Scholar] [CrossRef]
- Nurchi, V.M.; Djordjevic, A.B.; Crisponi, G.; Alexander, J.; Bjørklund, G.; Aaseth, J. Arsenic Toxicity: Molecular Targets and Therapeutic Agents. Biomolecules 2020, 10, 235. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Cariccio, V.L.; Samà, A.; Bramanti, P.; Mazzon, E. Mercury Involvement in Neuronal Damage and in Neurodegenerative Diseases. Biol. Trace Elem. Res. 2018, 187, 341–356. [Google Scholar] [CrossRef] [PubMed]
- Masindi, V.; Muedi, K.L. Environmental Contamination by Heavy Metals. Heavy Met. 2018, 10, 115–132. [Google Scholar] [CrossRef]
- Zang, L.; Lin, R.; Dou, T.; Wang, L.W.L.; Ma, J.; Sun, L. Electrospun superhydrophilic membranes for effective removal of Pb(ii) from water. Nanoscale Adv. 2019, 1, 389–394. [Google Scholar] [CrossRef]
- Peng, L.; Zhang, X.; Sun, Y.; Xing, Y.; Li, C. Heavy metal elimination based on metal organic framework highly loaded on flexible nanofibers. Environ. Res. 2020, 188, 109742. [Google Scholar] [CrossRef]
- Atashgahi, S.; Shetty, S.A.; Smidt, H.; De Vos, W.M. Flux, Impact, and Fate of Halogenated Xenobiotic Compounds in the Gut. Front. Physiol. 2018, 9, 888. [Google Scholar] [CrossRef]
- El-Shahawi, M.; Hamza, A.; Bashammakh, A.; Al-Saggaf, W. An overview on the accumulation, distribution, transformations, toxicity and analytical methods for the monitoring of persistent organic pollutants. Talanta 2010, 80, 1587–1597. [Google Scholar] [CrossRef]
- Genuis, S.K.; Birkholz, D.; Genuis, S.J. Human Excretion of Polybrominated Diphenyl Ether Flame Retardants: Blood, Urine, and Sweat Study. BioMed Res. Int. 2017, 2017, 1–14. [Google Scholar] [CrossRef]
- Senthamizhan, A.; Balusamy, B.; Uyar, T. Electrospun Filters for Organic Pollutants Removal; Springer Nature: London, UK, 2018; pp. 115–150. [Google Scholar]
- Ligneris, E.D.; Dumée, L.F.; Kong, L. Nanofiber-Based Materials for Persistent Organic Pollutants in Water Remediation by Adsorption. Appl. Sci. 2018, 8, 166. [Google Scholar] [CrossRef]
- Najafi, M.; Frey, M.W. Electrospun Nanofibers for Chemical Separation. Nanomaterials 2020, 10, 982. [Google Scholar] [CrossRef]
- Ehteshami, S.; Feizbakhsh, A.; Sarrafi, A.H.M.; Panahi, H.A.; Roostaie, A.; Feiabakhsh, A. An electrospun polyamide/graphene oxide nanocomposite as a novel fiber coating. Anal. Methods 2018, 10, 2123–2128. [Google Scholar] [CrossRef]
- Celebioglu, A.; Topuz, F.; Yildiz, Z.I.; Uyar, T. Efficient Removal of Polycyclic Aromatic Hydrocarbons and Heavy Metals from Water by Electrospun Nanofibrous Polycyclodextrin Membranes. ACS Omega 2019, 4, 7850–7860. [Google Scholar] [CrossRef] [PubMed]
- Ardekani, R.; Borhani, S.; Rezaei, B. Selective molecularly imprinted polymer nanofiber sorbent for the extraction of bisphenol A in a water sample. Polym. Int. 2020, 69, 780–793. [Google Scholar] [CrossRef]
- Yu, D.; Bai, J.; Liang, H.; Ma, T.; Li, C. AgI-modified TiO2 supported by PAN nanofibers: A heterostructured composite with enhanced visible-light catalytic activity in degrading MO. Dyes Pigm. 2016, 133, 51–59. [Google Scholar] [CrossRef]
- Xu, T.; Wu, F.; Gu, Y.; Chen, Y.; Cai, J.; Lu, W.; Hu, H.; Zhu, Z.; Chen, W. Visible-light responsive electrospun nanofibers based on polyacrylonitrile-dispersed graphitic carbon nitride. RSC Adv. 2015, 5, 86505–86512. [Google Scholar] [CrossRef]
- Panthi, G.; Gyawali, K.R.; Park, M. Towards the Enhancement in Photocatalytic Performance of Ag3PO4 Nanoparticles through Sulfate Doping and Anchoring on Electrospun Nanofibers. Nanomaterials 2020, 10, 929. [Google Scholar] [CrossRef]
- Liang, H.; Bai, J.; Xu, T.; Li, C. Enhancing photocatalytic performance of heterostructure MoS2/g-C3N4 embeded in PAN frameworks by electrospining process. Mater. Sci. Semicond. Process. 2021, 121, 105414. [Google Scholar] [CrossRef]
- Sundaran, S.P.; Reshmi, C.R.; Sagitha, P.; Sujith, A. Polyurethane nanofibrous membranes decorated with reduced graphene oxide–TiO2 for photocatalytic templates in water purification. J. Mater. Sci. 2020, 55, 5892–5907. [Google Scholar] [CrossRef]
- Lakshmi, K.; Kadirvelu, K.; Mohan, P.S. Chemically modified electrospun nanofiber for high adsorption and effective photocatalytic decontamination of organophosphorus compounds. J. Chem. Technol. Biotechnol. 2019, 94, 3190–3200. [Google Scholar] [CrossRef]
- Kumar, R.; Ahmed, M.; Bhadrachari, G.; Thomas, J.P. Desalination for agriculture: Water quality and plant chemistry, technologies and challenges. Water Supply 2018, 18, 1505–1517. [Google Scholar] [CrossRef]
- Wang, Z.; Sahadevan, R.; Crandall, C.; Menkhaus, T.J.; Fong, H. Hot-pressed PAN/PVDF hybrid electrospun nanofiber membranes for ultrafiltration. J. Membr. Sci. 2020, 611, 118327. [Google Scholar] [CrossRef]
- Al Aani, S.; Haroutounian, A.; Wright, C.; Hilal, N. Thin Film Nanocomposite (TFN) membranes modified with polydopamine coated metals/carbon-nanostructures for desalination applications. Desalination 2018, 427, 60–74. [Google Scholar] [CrossRef]
- Shahmirzadi, M.A.A.; Kargari, A. Nanocomposite membranes. In Emerging Technologies for Sustainable Desalination Handbook; Butterworth-Heinemann: Waltham, MA, USA, 2018. [Google Scholar]
- Gonzales, R.R.; Park, M.J.; Tijing, L.D.; Han, D.S.; Phuntsho, S.; Shon, H. Modification of Nanofiber Support Layer for Thin Film Composite forward Osmosis Membranes via Layer-by-Layer Polyelectrolyte Deposition. Membranes 2018, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Idarraga-Mora, J.A.; Childress, A.S.; Friedel, P.S.; Ladner, D.A.; Rao, A.M.; Husson, S.M. Role of Nanocomposite Support Stiffness on TFC Membrane Water Permeance. Membranes 2018, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Huang, M.; Liu, Y.; Meng, L.; Ma, M. Functionalized electrospun nanofiber membranes for water treatment: A review. Sci. Total Environ. 2020, 739, 139944. [Google Scholar] [CrossRef] [PubMed]
- Van Der Bruggen, B.; Vandecasteele, C.; Van Gestel, T.; Doyen, W.; Leysen, R. A review of pressure-driven membrane processes in wastewater treatment and drinking water production. Environ. Prog. 2003, 22, 46–56. [Google Scholar] [CrossRef]
- Shirazi, M.M.A.; Bazgir, S.; Meshkani, F. Electrospun Nanofibrous Membranes for Water Treatment. In Advances in Membrane Technologies; Abdelrasoul, A., Ed.; Intechopen: Rijeka, Croatia, 2020. [Google Scholar] [CrossRef]
- Wang, Z.; Crandall, C.; Sahadevan, R.; Menkhaus, T.J.; Fong, H. Microfiltration performance of electrospun nanofiber membranes with varied fiber diameters and different membrane porosities and thicknesses. Polymers 2017, 114, 64–72. [Google Scholar] [CrossRef]
- Tang, N.; Si, Y.; Yu, J.; Ding, B. Leaf vein-inspired microfiltration membrane based on ultrathin nanonetworks. Environ. Sci. Nano 2020, 7, 2644–2653. [Google Scholar] [CrossRef]
- Li, M.; Li, J.; Zhou, M.; Xian, Y.; Shui, Y.; Wu, M.; Yao, Y. Super-hydrophilic electrospun PVDF/PVA-blended nanofiber membrane for microfiltration with ultrahigh water flux. J. Appl. Polym. Sci. 2020, 137, 48416. [Google Scholar] [CrossRef]
- Bolto, B.; Zhang, J.; Wu, X.; Xie, Z. A Review on Current Development of Membranes for Oil Removal from Wastewaters. Membranes 2020, 10, 65. [Google Scholar] [CrossRef]
- Dobosz, K.M.; Kuo-Leblanc, C.A.; Martin, T.J.; Schiffman, J.D. Ultrafiltration Membranes Enhanced with Electrospun Nanofibers Exhibit Improved Flux and Fouling Resistance. Ind. Eng. Chem. Res. 2017, 56, 5724–5733. [Google Scholar] [CrossRef]
- Bahmani, P.; Maleki, A.; Daraei, H.; Khamforoush, M.; Rezaee, R.; Gharibi, F.; Tkachev, A.G.; Burakov, A.E.; Agarwal, S.; Gupta, V.K. High-flux ultrafiltration membrane based on electrospun polyacrylonitrile nanofibrous scaffolds for arsenate removal from aqueous solutions. J. Colloid Interface Sci. 2017, 506, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Modesti, M.; Boaretti, C.; Roso, M. Electrospun Nanofibers for Water and Wastewater Treatment Applications. In Encyclopedia of Membranes; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Singh, R.; Bhadouria, R.; Singh, P.; Kumar, A.; Pandey, S.; Singh, V.K. Nanofiltration technology for removal of pathogens present in drinking water. Waterborne Pathog. 2020, 463–489. [Google Scholar] [CrossRef]
- Abdel-Fatah, M.A. Nanofiltration systems and applications in wastewater treatment: Review article. AIN Shams Eng. J. 2018, 9, 3077–3092. [Google Scholar] [CrossRef]
- Kaur, S.; Sundarrajan, S.; Rana, D.; Matsuura, T.; Ramakrishna, S. Influence of electrospun fiber size on the separation efficiency of thin film nanofiltration composite membrane. J. Membr. Sci. 2012, 101–111. [Google Scholar] [CrossRef]
- Liu, F.; Wang, L.; Li, D.; Liu, Q.; Deng, B. Preparation and characterization of novel thin film composite nanofiltration membrane with PVDF tree-like nanofiber membrane as composite scaffold. Mater. Des. 2020, 196, 109101. [Google Scholar] [CrossRef]
- Nakhowong, R. Fabrication of PVDF/PVP nanofiltration membrane containing chitosan/activated carbon/Ag nanoparticles by electrospinning and their antibacterial activity. SNRUJST 2020, 12, 137–145. [Google Scholar]
- Zhijiang, C.; Cong, Z.; Ping, X.; Jie, G.; Kongyin, Z. Calcium alginate-coated electrospun polyhydroxybutyrate/carbon nanotubes composite nanofibers as nanofiltration membrane for dye removal. J. Mater. Sci. 2018, 53, 14801–14820. [Google Scholar] [CrossRef]
- Ziolkowska, J.R. Desalination leaders in the global market—Current trends and future perspectives. Water Supply 2015, 16, 563–578. [Google Scholar] [CrossRef]
- Jones, E.; Qadir, M.; Van Vliet, M.T.; Smakhtin, V.; Kang, S.-M. The state of desalination and brine production: A global outlook. Sci. Total Environ. 2019, 657, 1343–1356. [Google Scholar] [CrossRef]
- Berenguel, F.; Galera, A.L. Requirements for the Construction of New Desalination Plants into a Framework of Sustainability. Sustainability 2020, 12, 5124. [Google Scholar] [CrossRef]
- Lehmann, O.; Eckhaus, O.; Lahav, O.; Birnhack, L. Replenishing Mg(II) to desalinated water by seawater nanofiltration followed by magnetic separation of Mg(OH)2(s)Fe3O4 particles. Desalination Water Treat. 2015, 57, 19903–19916. [Google Scholar] [CrossRef]
- Wang, X.-N.; Liu, Y.; Pan, X.-H.; Han, J.-X.; Hao, J. Parameters for Seawater Reverse Osmosis Product Water: A Review. Expo. Health 2016, 9, 157–168. [Google Scholar] [CrossRef]
- Raza, M.A.; Islam, A.; Sabir, A.; Gull, N.; Ali, I.; Mehmood, R.; Bae, J.; Hassan, G.; Khan, M.U. PVA/TEOS crosslinked membranes incorporating zinc oxide nanoparticles and sodium alginate to improve reverse osmosis performance for desalination. J. Appl. Polym. Sci. 2019, 136, 47559. [Google Scholar] [CrossRef]
- Wang, X.; Ma, H.; Chu, B.; Hsiao, B.S. Thin-film nanofibrous composite reverse osmosis membranes for desalination. Desalination 2017, 420, 91–98. [Google Scholar] [CrossRef]
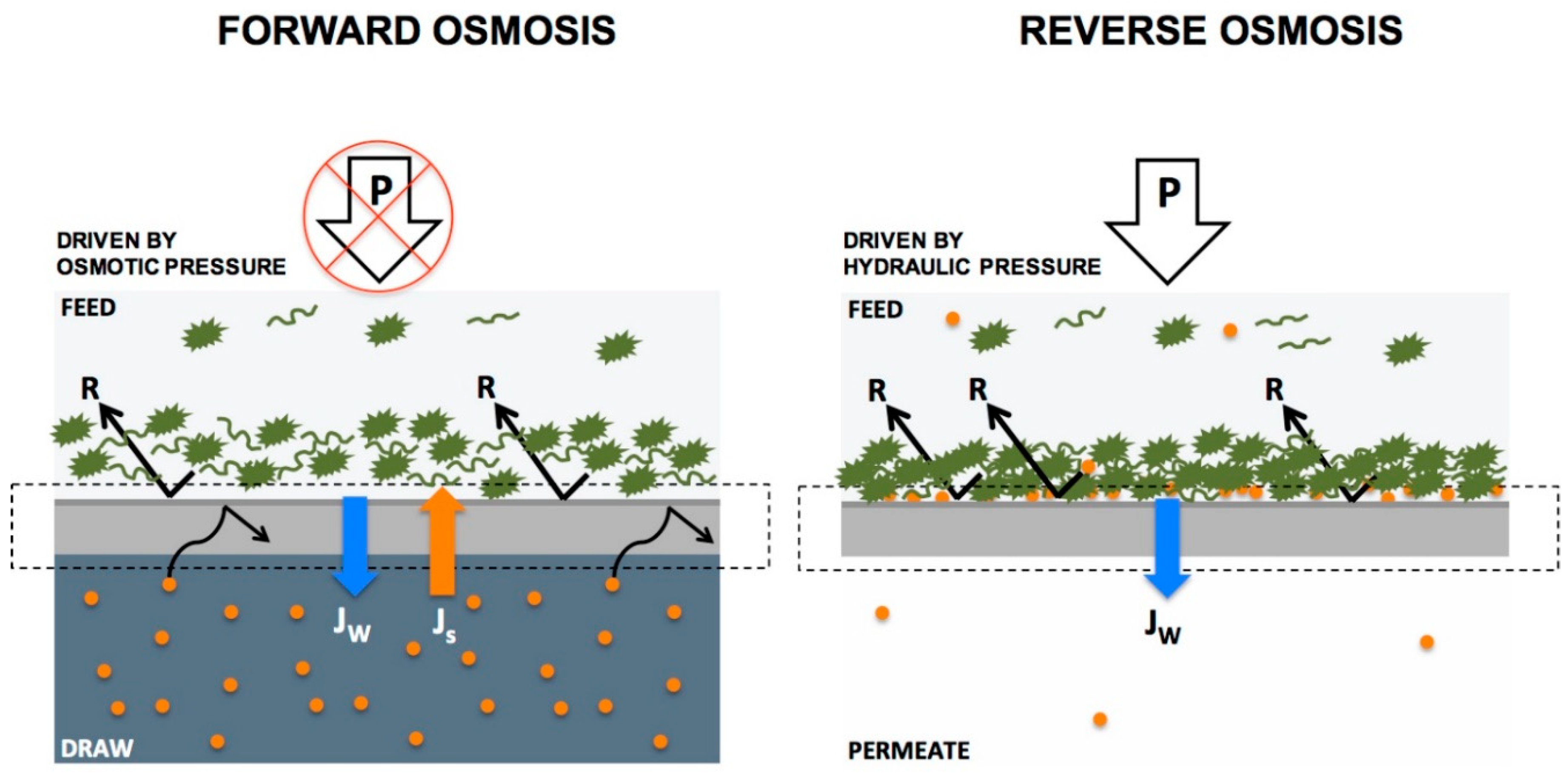
- Mohammadifakhr, M.; De Grooth, J.; Roesink, H.D.W.; Kemperman, A.J. Forward Osmosis: A Critical Review. Processes 2020, 8, 404. [Google Scholar] [CrossRef]
- Johnson, D.J.; Suwaileh, W.A.; Mohammed, A.W.; Hilal, N. Osmotic’s potential: An overview of draw solutes for forward osmosis. Desalination 2018, 434, 100–120. [Google Scholar] [CrossRef]
- Mark, P. General intro to Forward Osmosis Membranes and Processes—Forward OsmosisTech. 2013. Available online: https://www.forwardosmosistech.com/forward-osmosis-membranes-and-membrane-processes/ (accessed on 13 November 2020).
- Haupt, A.; Lerch, A. Forward Osmosis Application in Manufacturing Industries: A Short Review. Membranes 2018, 8, 47. [Google Scholar] [CrossRef]
- Lee, S.; Kim, Y.; Park, J.; Shon, H.; Hong, S. Treatment of medical radioactive liquid waste using Forward Osmosis (FO) membrane process. J. Membr. Sci. 2018, 556, 238–247. [Google Scholar] [CrossRef]
- Dou, P.; Zhao, S.; Xu, S.; Li, X.-M.; He, T. Feasibility of osmotic dilution for recycling spent dialysate: Process performance, scaling, and economic evaluation. Water Res. 2020, 168, 115157. [Google Scholar] [CrossRef]
- Soo, K.W.; Wong, K.C.; Goh, P.S.; Ismail, A.F.; Othman, N. Efficient heavy metal removal by thin film nanocomposite forward osmosis membrane modified with geometrically different bimetallic oxide. J. Water Process. Eng. 2020, 38, 101591. [Google Scholar] [CrossRef]
- Khulbe, K.; Matsuura, T. Art to use electrospun nanofibers/nanofiber based membrane in waste water treatment, chiral separation and desalination. J. Membr. Sci. Res. 2019, 5, 100–125. [Google Scholar]
- Pan, S.-F.; Dong, Y.; Zheng, Y.-M.; Zhong, L.-B.; Yuan, Z.-H. Self-sustained hydrophilic nanofiber thin film composite forward osmosis membranes: Preparation, characterization and application for simulated antibiotic wastewater treatment. J. Membr. Sci. 2017, 523, 205–215. [Google Scholar] [CrossRef]
- Al-Furaiji, M.; Kadhom, M.; Kalash, K.; Waisi, B.; Albayati, N. Preparation of TFC Membranes Supported with Electrospun Nanofibers for Desalination by Forward Osmosis. Drink. Water Eng. Sci. Discuss. 2020, 13, 51–57. [Google Scholar] [CrossRef]
- Karanasiou, A.; Kostoglou, M.; Karabelas, A.J. An Experimental and Theoretical Study on Separations by Vacuum Membrane Distillation Employing Hollow-Fiber Modules. Water 2018, 10, 947. [Google Scholar] [CrossRef]
- Said, I.A.; Fuentes, N.; He, Z.; Xin, R.; Zuo, K.; Li, Q.; Abdallah, I. Low-cost desalination of seawater and hypersaline brine using nanophotonics enhanced solar energy membrane distillation. Environ. Sci. Water Res. Technol. 2020, 6, 2180–2196. [Google Scholar] [CrossRef]
- He, J.; Zhang, L.; Zhang, K.; Qin, Y.; Liu, L. Concentrating aqueous urea solution by using continuous-effect membrane distillation. Chem. Eng. Res. Des. 2015, 104, 589–604. [Google Scholar] [CrossRef]
- Criscuoli, A.; Capuano, A.; Andreucci, M.; Drioli, E. Low-Temperature Direct Contact Membrane Distillation for the Treatment of Aqueous Solutions Containing Urea. Membranes 2020, 10, 176. [Google Scholar] [CrossRef]
- Amaya-Vías, D.; López-Ramírez, J.A. Techno-Economic Assessment of Air and Water Gap Membrane Distillation for Seawater Desalination under Different Heat Source Scenarios. Water 2019, 11, 2117. [Google Scholar] [CrossRef]
- Membrane Distillation—EMIS. Available online: https://emis.vito.be/en/bat/tools-overview/sheets/membrane-distillation (accessed on 15 November 2020).
- Teoh, M.M.; Chung, T.-S.; Yeo, Y.S. Dual-layer PVDF/PTFE composite hollow fibers with a thin macrovoid-free selective layer for water production via membrane distillation. Chem. Eng. J. 2011, 171, 684–691. [Google Scholar] [CrossRef]
- Zuo, J.; Chung, T.-S.; O’Brien, G.S.; Kosar, W. Hydrophobic/hydrophilic PVDF/Ultem® dual-layer hollow fiber membranes with enhanced mechanical properties for vacuum membrane distillation. J. Membr. Sci. 2017, 523, 103–110. [Google Scholar] [CrossRef]
- Khayet, M.C.; García-Payo, L. García-Fernández, J. Contreras-Martínez. Dual-layered electrospun nanofibrous membranes for membrane distillation. Desalination 2018, 426, 174–184. [Google Scholar] [CrossRef]
- Woo, Y.C.; Tijing, L.D.; Park, M.J.; Yao, M.; Choi, J.-S.; Lee, S.; Kim, S.-H.; An, K.-J.; Shon, H. Electrospun dual-layer nonwoven membrane for desalination by air gap membrane distillation. Desalination 2017, 403, 187–198. [Google Scholar] [CrossRef]
- Attia, H.; Johnson, D.J.; Wright, C.J.; Hilal, N. Robust superhydrophobic electrospun membrane fabricated by combination of electrospinning and electrospraying techniques for air gap membrane distillation. Desalination 2018, 446, 70–82. [Google Scholar] [CrossRef]
- Deka, B.J.; Lee, E.-J.; Guo, J.; Kharraz, J.; An, A.K. Electrospun Nanofiber Membranes Incorporating PDMS-Aerogel Superhydrophobic Coating with Enhanced Flux and Improved Antiwettability in Membrane Distillation. Environ. Sci. Technol. 2019, 53, 4948–4958. [Google Scholar] [CrossRef]
- Matsubara, M.E.; Helwig, K.; Hunter, C.; Roberts, J.; Subtil, E.L.; Coelho, L.H.G. Amoxicillin removal by pre-denitrification membrane bioreactor (A/O-MBR): Performance evaluation, degradation by-products, and antibiotic resistant bacteria. Ecotoxicol. Environ. Saf. 2020, 192, 110258. [Google Scholar] [CrossRef]
- Pathak, N.; Tran, V.H.; Merenda, A.; Johir, M.; Phuntsho, S.; Shon, H. Removal of Organic Micro-Pollutants by Conventional Membrane Bioreactors and High-Retention Membrane Bioreactors. Appl. Sci. 2020, 10, 2969. [Google Scholar] [CrossRef]
- Mao, X.; Myavagh, P.H.; Lotfikatouli, S.; Hsiao, B.S.; Walker, H.W. Membrane Bioreactors for Nitrogen Removal from Wastewater: A Review. J. Environ. Eng. 2020, 146, 03120002. [Google Scholar] [CrossRef]
- Yang, J.; Gou, Y.; Fang, F.; Guo, J.; Lu, L.; Zhou, Y.; Ma, H. Potential of wastewater treatment using a concentrated and suspended algal-bacterial consortium in a photo membrane bioreactor. Chem. Eng. J. 2018, 335, 154–160. [Google Scholar] [CrossRef]
- Basile, A.; Cassano, A.; Rastogi, N. Advances in Membrane Technologies for Water Treatment; Woodhead Publishing: Cambridge, UK, 2015. [Google Scholar]
- Aslam, M.; Charfi, A.; Lesage, G.; Heran, M.; Kim, J. Membrane bioreactors for wastewater treatment: A review of mechanical cleaning by scouring agents to control membrane fouling. Chem. Eng. J. 2017, 307, 897–913. [Google Scholar] [CrossRef]
- Di Trapani, D.; Di Bella, G.; Mannina, G.; Torregrossa, M.; Viviani, G. Comparison between moving bed-membrane bioreactor (MB-MBR) and membrane bioreactor (MBR) systems: Influence of wastewater salinity variation. Bioresour. Technol. 2014, 162, 60–69. [Google Scholar] [CrossRef]
- Subtil, E.L.; Mierzwa, J.C.; Hespanhol, I. Comparison between a conventional membrane bioreactor (C-MBR) and a biofilm membrane bioreactor (BF-MBR) for domestic wastewater treatment. Braz. J. Chem. Eng. 2014, 31, 683–691. [Google Scholar] [CrossRef]
- Yang, Y.; Shao, Z.; Du, J.; He, Q.; Chai, H. Enhancement of Organic Matter Removal in an Integrated Biofilm-Membrane Bioreactor Treating High-Salinity Wastewater. Archaea 2018, 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pathak, N.; Tran, V.; Phuntsho, S.; Shon, H.Y. Chapter 10—Membrane bioreactors for the removal of micro-pollutants. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Bilad, M.R.P.; Westbroek, I.V. Assessment and optimization of electrospun nanofiber-membranes in a membrane bioreactor (MBR). J. Membr. Sci. 2011, 380, 181–191. [Google Scholar] [CrossRef]
- Ren, L.-F.; Ngo, H.H.; Bu, C.; Ge, C.; Ni, S.-Q.; Shao, J.; He, Y. Novel external extractive membrane bioreactor (EMBR) using electrospun polydimethylsiloxane/polymethyl methacrylate membrane for phenol-laden saline wastewater. Chem. Eng. J. 2020, 383, 123179. [Google Scholar] [CrossRef]
- Livingston, A.G. Extractive membrane bioreactors: A new process technology for detoxifying chemical industry wastewaters. J. Chem. Technol. Biotechnol. 1994, 60, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Wenten, I.G.; Friatnasary, D.L.; Khoiruddin, K.; Setiadi, T.; Boopathy, R. Extractive membrane bioreactor (EMBR): Recent advances and applications. Bioresour. Technol. 2019, 297, 122424. [Google Scholar] [CrossRef]
- Moradi, G.; Zinadini, S.; Rajabi, L.; Dadari, S. Fabrication of high flux and antifouling mixed matrix fumarate-alumoxane/PAN membranes via electrospinning for application in membrane bioreactors. Appl. Surf. Sci. 2018, 427, 830–842. [Google Scholar] [CrossRef]
- Arslan, S.; Eyvaz, M.; Güçlü, S.; Yüksekdağ, A.; Koyuncu, I.; Yuksel, E. Investigation of water and salt flux performances of polyamide coated tubular electrospun nanofiber membrane under pressure. J. Environ. Sci. Health 2020, 55, 606–614. [Google Scholar] [CrossRef]
- Fahimirad, S.; Fahimirad, Z.; Sillanpää, M. Efficient removal of water bacteria and viruses using electrospun nanofibers. Sci. Total Environ. 2021, 751, 141673. [Google Scholar] [CrossRef]
- Botes, M.; Cloete, T.E. The potential of nanofibers and nanobiocides in water purification. Crit. Rev. Microbiol. 2010, 36, 68–81. [Google Scholar] [CrossRef]
- Rodríguez-Tobías, H.; Morales, G.; Grande, D. Comprehensive review on electrospinning techniques as versatile approaches toward antimicrobial biopolymeric composite fibers. Mater. Sci. Eng. C 2019, 101, 306–322. [Google Scholar] [CrossRef]
- Vineis, C.; Varesano, A. Natural polymer-based electrospun fibers for antibacterial uses. Electrofluidodyn. Technol. Biomater. Med. Devices 2018, 275–294. [Google Scholar] [CrossRef]
- Schabikowski, M.; Cichoń, A.; Németh, Z.; Kubiak, W.; Kata, D.; Graule, T. Electrospun iron and copper oxide fibers for virus retention applications. Text. Res. J. 2019, 89, 4373–4382. [Google Scholar] [CrossRef]
- Ren, H.; Du, Y.; Su, Y.; Guo, Y.; Zhu, Z.; Dong, A. A Review on Recent Achievements and Current Challenges in Antibacterial Electrospun N-halamines. Colloid Interface Sci. Commun. 2018, 24, 24–34. [Google Scholar] [CrossRef]
- Kwon, H.-J.; Cha, J.-R.; Lee, D.R.; Chin, B.D.; Kim, O.Y.; Hwang, S.-H. Preparation and Characterization of Antimicrobial Bilayer Electrospun Nanofiber Membrane for Oily Wastewater Treatment. J. Korean Phys. Soc. 2020, 76, 34–43. [Google Scholar] [CrossRef]
- Taheran, M.; Kumar, P.; Naghdi, M.; Brar, S.K.; Knystautas, E.J.; Verma, M.; Surampalli, R.Y. Development of an advanced multifunctional portable water purifier. Nanotechnol. Environ. Eng. 2019, 4, 7. [Google Scholar] [CrossRef]
- Lan, S.; Lu, Y.; Zhang, J.; Guo, Y.; Li, C.; Zhao, S.; Sheng, X.; Dong, A. Electrospun Sesbania Gum-Based Polymeric N-Halamines for Antibacterial Applications. Polymers 2019, 11, 1117. [Google Scholar] [CrossRef]
- Abideen, Z.U.; Kim, J.H.; Lee, J.-H.; Kim, J.-Y.; Mirzaei, A.; WooKimab, H.; Kim, S.S. Electrospun Metal Oxide Composite Nanofibers Gas Sensors: A Review. J. Korean Ceram. Soc. 2017, 54, 366–379. [Google Scholar] [CrossRef]
- Nazemi, H.; Joseph, A.; Park, J.; Emadi, A. Advanced Micro- and Nano-Gas Sensor Technology: A Review. Sensors 2019, 19, 1285. [Google Scholar] [CrossRef]
- Manea, L.R.; Bertea, A.-P. Sensors from Electrospun Nanostructures. In Nanostructures in Energy Generation, Transmission and Storage; Fedorenko, Y., Ed.; Intechopen: Rijeka, Croatia, 2019. [Google Scholar] [CrossRef]
- Ding, B.; Wang, M.; Yu, J.; Sun, G. Gas Sensors Based on Electrospun Nanofibers. Sensors 2009, 9, 1609–1624. [Google Scholar] [CrossRef]
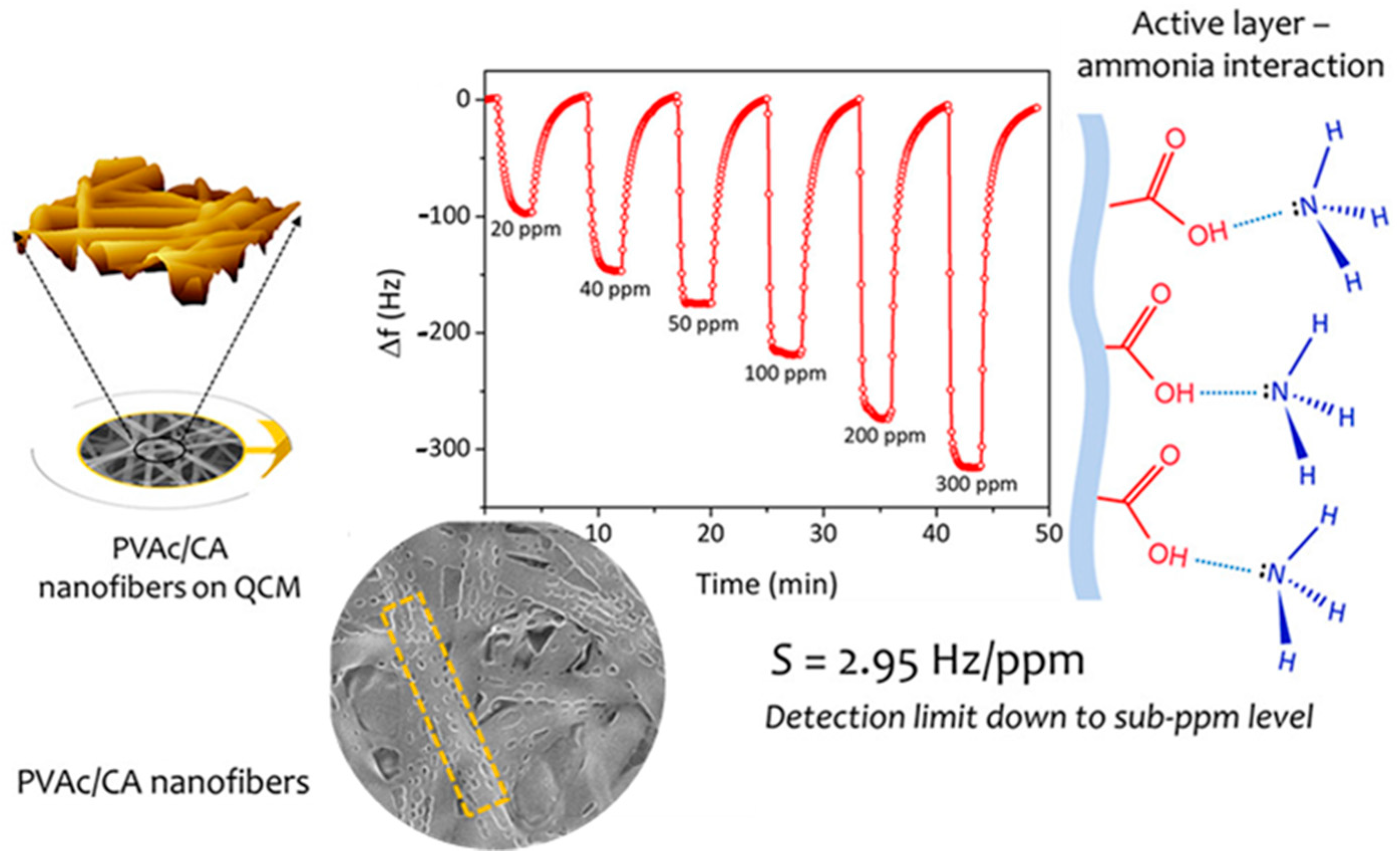
- Roto, R.; Rianjanu, A.; Rahmawati, A.; Fatyadi, I.A.; Yulianto, N.; Majid, N.; Syamsu, I.; Wasisto, H.S.; Triyana, K. Quartz Crystal Microbalances Functionalized with Citric Acid-Doped Polyvinyl Acetate Nanofibers for Ammonia Sensing. ACS Appl. Nano Mater. 2020, 3, 5687–5697. [Google Scholar] [CrossRef]
- Al-Hazeem, N.Z.A.; Ahmed, N.M.; MatJafri, M.Z.; Bououdina, M. Hydrogen gas sensor based on nanofibers TiO2-PVP thin film at room temperature prepared by electrospinning. Microsyst. Technol. 2020, 1–7. [Google Scholar] [CrossRef]
- Schoolaert, E.; Steyaert, I.; Vancoillie, G.; Geltmeyer, J.; Lava, K.; Hoogenboom, R.; de Clerck, K. Blend electrospinning of dye-functionalized chitosan and poly (ε-caprolactone): Towards biocompatible pH-sensors. J. Mater. Chem. B 2016, 4, 4507–4516. [Google Scholar] [CrossRef] [PubMed]
- Teli, M.; Nadathur, G.T. Reversible colourimetric sensing of volatile phase by dye doped electrospun silica based nanofibers. J. Environ. Chem. Eng. 2020, 8, 103920. [Google Scholar] [CrossRef]
- van den Broek, S.; Abegg, S.E.; Pratsinis, A.T. Güntner. Highly selective detection of methanol over ethanol by a handheld gas sensor. Nat. Commun. 2019, 10, 4220. [Google Scholar] [CrossRef]
- Liu, W.; Xu, L.; Sheng, K.; Zhou, X.; Dong, B.; Lu, G.; Song, H. A highly sensitive and moisture-resistant gas sensor for diabetes diagnosis with Pt@In2O3 nanowires and a molecular sieve for protection. NPG Asia Mater. 2018, 10, 293–308. [Google Scholar] [CrossRef]
- Horne, J.; McLoughlin, L.; Bridgers, B.; Wujcik, E.K. Recent developments in nanofiber-based sensors for disease detection, immunosensing, and monitoring. Sens. Actuators Rep. 2020, 2, 100005. [Google Scholar] [CrossRef]
- Al-Dhahebi, A.M.; Gopinath, S.C.B.; Saheed, M.S.M. Graphene impregnated electrospun nanofiber sensing materials: A comprehensive overview on bridging laboratory set-up to industry. Nano Converg. 2020, 7, 1–23. [Google Scholar] [CrossRef]
- Krishnan, S.K.; Singh, E.; Singh, P.; Meyyappan, M.; Nalwa, H.S. A review on graphene-based nanocomposites for electrochemical and fluorescent biosensors. RSC Adv. 2019, 9, 8778–8881. [Google Scholar] [CrossRef]
- Cabral, T.S.; Sgobbi, L.F.; Delezuk, J.; Pessoa, R.S.; Lobo, A.O.; Rodrigues, B.V. Glucose sensing via a green and low-cost platform from electrospun poly (vinyl alcohol)/graphene quantum dots fibers. Mater. Today: Proc. 2019, 14, 694–699. [Google Scholar] [CrossRef]
- Guan, H.; Zhang, J.; Liu, Y.; Zhao, Y.; Zhang, B. Rapid quantitative determination of hydrogen peroxide using an electrochemical sensor based on PtNi alloy/CeO2 plates embedded in N-doped carbon nanofibers. Electrochim. Acta 2019, 295, 997–1005. [Google Scholar] [CrossRef]
- Zhu, M.; Xiong, R.; Huang, C. Bio-based and photocrosslinked electrospun antibacterial nanofibrous membranes for air filtration. Carbohydr. Polym. 2019, 205, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Wang, R.; Tang, G.; Mou, Z.; Lei, J.; Han, J.; De Smedt, S.C.; Xiong, R.; Huang, C. Ecofriendly Electrospun Membranes Loaded with Visible-Light-Responding Nanoparticles for Multifunctional Usages: Highly Efficient Air Filtration, Dye Scavenging, and Bactericidal Activity. ACS Appl. Mater. Interfaces 2019, 11, 12880–12889. [Google Scholar] [CrossRef] [PubMed]
- Tebyetekerwa, M.; Xu, Z.; Yang, S.; Ramakrishna, S. Electrospun Nanofibers-Based Face Masks. Adv. Fiber Mater. 2020, 2, 161–166. [Google Scholar] [CrossRef]
- He, H.; Gao, M.; Illés, B.; Molnar, K. 3D Printed and Electrospun, Transparent, Hierarchical Polylactic Acid Mask Nanoporous Filter. Int. J. Bioprint. 2020, 6, 278. [Google Scholar] [CrossRef]
- Das, O.; Neisiany, R.E.; Capezza, A.J.; Hedenqvist, M.S.; Försth, M.; Xu, Q.; Jiang, L.; Ji, D.; Ramakrishna, S. The need for fully bio-based facemasks to counter coronavirus outbreaks: A perspective. Sci. Total Environ. 2020, 736, 139611. [Google Scholar] [CrossRef]
- Wang, X.; Ding, B.; Sun, G.; Wang, M.; Yu, J. Electro-spinning/netting: A strategy for the fabrication of three-dimensional polymer nano-fiber/nets. Prog. Mater. Sci. 2013, 58, 1173–1243. [Google Scholar] [CrossRef]
- Hu, J.; Wang, X.; Ding, B.; Lin, J.; Yu, J.; Sun, G. One-step Electro-spinning/netting Technique for Controllably Preparing Polyurethane Nano-fiber/net. Macromol. Rapid Commun. 2011, 32, 1729–1734. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, S.; Liu, L.; Yu, J.; Ding, B. High-Performance PM0.3 Air Filters Using Self-Polarized Electret Nanofiber/Nets. Adv. Funct. Mater 2020, 30, 1909554. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, S.; Liu, L.; Yu, J.; Ding, B. High-performance filters from biomimetic wet-adhesive nanoarchitectured networks. J. Mater. Chem. A 2020, 8, 18955–18962. [Google Scholar] [CrossRef]
- Maddah, B.; Yavaripour, A.; Ramedani, S.H.; Hosseni, H.; Hasanzadeh, M. Fabrication of electrospun PU nanofiber composites based on carbon nanotubes decorated with nickel-zinc ferrite particles as an adsorbent for removal of hydrogen sulfide from air. Environ. Sci. Pollut. Res. 2020, 27, 35515–35525. [Google Scholar] [CrossRef] [PubMed]
- Bortolassi, A.C.C.; Nagarajan, S.; Lima, B.D.A.; Guerra, V.G.; Aguiar, M.L.; Huon, V.; Soussan, L.; Cornu, D.; Miele, P.; Bechelany, M. Efficient nanoparticles removal and bactericidal action of electrospun nanofibers membranes for air filtration. Mater. Sci. Eng. C 2019, 102, 718–729. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Murray, S.; Zhu, M. Chapter 23—Electrospun Nanofibers for Catalysts. In Electrospinning: Nanofabrication and Applications; Micro and Nano Technologie; William Andrew Publishing: Norwich, NY, USA, 2019. [Google Scholar]
- Ogunlaja, A.S.; Kleyi, P.; Walmsley, R.S.; Tshentu, Z.R. Nanofiber-supported metal-based catalysts. In Catalysis; Royal Society of Chemistry (RSC): London, UK, 2016; Volume 28, pp. 144–174. [Google Scholar]
- Niu, Q.; Guo, J.; Chen, B.; Nie, J.; Guo, X.; Ma, G. Bimetal-organic frameworks/polymer core-shell nanofibers derived heteroatom-doped carbon materials as electrocatalysts for oxygen reduction reaction. Carbon 2017, 114, 250–260. [Google Scholar] [CrossRef]
- Misson, M.; Jin, B.; Dai, S.; Zhang, H. Interfacial Biocatalytic Performance of Nanofiber-Supported β-Galactosidase for Production of Galacto-Oligosaccharides. Catalysts 2020, 10, 81. [Google Scholar] [CrossRef]
- Liu, G.; Bonakdarpour, A.; Wang, X.; Bi, X.; Wilkinson, D.P. Antimony-Doped Tin Oxide Nanofibers as Catalyst Support Structures for the Methanol Oxidation Reaction in Direct Methanol Fuel Cells. Electrocatalysis 2019, 10, 262–271. [Google Scholar] [CrossRef]
- Zhong, S. Preparation of chitosan/poly(methacrylic acid) supported palladium nanofibers as an efficient and stable catalyst for Heck reaction. J. Chem. Sci. 2020, 132, 1–9. [Google Scholar] [CrossRef]
- Kamaci, U.D.; Peksel, A. Enhanced Catalytic Activity of Immobilized Phytase into Polyvinyl Alcohol-Sodium Alginate Based Electrospun Nanofibers. Catal. Lett. 2020, 164, 3315–3322. [Google Scholar]
- Li, X.; Qi, T.; Wang, J.; She, W.; Mao, G.; Yan, P.; Li, W.; Li, G. Enhanced catalytic performance of nitrogen-doped carbon supported FeOx-based catalyst derived from electrospun nanofiber crosslinked N, Fe-containing MOFs for efficient hydrogenation of nitroarenes. Mol. Catal. 2019, 477, 110544. [Google Scholar] [CrossRef]
- Jia, Z.; Zhang, M.; Liu, B.; Wang, F.; Wei, G.; Su, Z. Graphene Foams for Electromagnetic Interference Shielding: A Review. ACS Appl. Nano Mater. 2020, 3, 6140–6155. [Google Scholar] [CrossRef]
- Yang, X.; Fan, S.; Li, Y.; Guo, Y.; Li, Y.; Ruan, K.; Zhang, S.; Zhang, J.; Kong, J.; Gu, J. Synchronously improved electromagnetic interference shielding and thermal conductivity for epoxy nanocomposites by constructing 3D copper nanowires/thermally annealed graphene aerogel framework. Compos. Part A Appl. Sci. Manuf. 2020, 128, 105670. [Google Scholar] [CrossRef]
- Wanasinghe, D.; Aslani, F.; Ma, G.; Habibi, D. Review of Polymer Composites with Diverse Nanofillers for Electromagnetic Interference Shielding. Nanomaterials 2020, 10, 541. [Google Scholar] [CrossRef] [PubMed]
- Sancak, E.; Ozen, M.S.; Erdem, R.; Yilmaz, A.C.; Yuksek, M.; Soin, N.; Shah, T. PA6/silver blends: Investigation of mechanical and electromagnetic shielding behaviour of electrospun nanofibers. Tekst. Konfeksiyon 2018, 28, 229–235. [Google Scholar]
- Shukla, V. Review of electromagnetic interference shielding materials fabricated by iron ingredients. Nanoscale Adv. 2019, 1, 1640–1671. [Google Scholar] [CrossRef]
- Ji, H.; Zhao, R.; Zhang, N.; Jin, C.; Lu, X.; Wang, C. Lightweight and flexible electrospun polymer nanofiber/metal nanoparticle hybrid membrane for high-performance electromagnetic interference shielding. NPG Asia Mater. 2018, 10, 749–760. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.; Kim, C.; Park, Y.; Kim, M.-H.; Seol, J.H. Electromagnetic Interference Shield of Highly Thermal-Conducting, Light-Weight, and Flexible Electrospun Nylon 66 Nanofiber-Silver Multi-Layer Film. Polymers 2020, 12, 1805. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, J.; Li, Y.; He, X.; Yuan, Y. Lightweight and flexible hybrid film based on delicate design of electrospun nanofibers for high-performance electromagnetic interference shielding. Nanoscale 2019, 11, 8616–8625. [Google Scholar] [CrossRef]
- IEA Online Data Services. CO2 Emissions from Fuel Combustion. Available online: http://data.iea.org/payment/products/115-co2-emissions-from-fuelcombustion.aspx (accessed on 15 November 2020).
- Styring, P. Carbon Dioxide Capture Agents and Processes; Elsevier BV: Amsterdam, The Netherlands, 2015; pp. 19–32. [Google Scholar]
- Knapik, E.; Kosowski, P.; Stopa, J. Cryogenic liquefaction and separation of CO2 using nitrogen removal unit cold energy. Chem. Eng. Res. Des. 2018, 131, 66–79. [Google Scholar] [CrossRef]
- Iqbal, N.; Babar, A.A.; Zainab, G.; Ding, B. Chapter 20—Electrospun Nanofibers for Carbon Dioxide Capture. In Micro and Nano Technologies, Electrospinning: Nanofabrication and Applications; William Andrew Publishing: Norwich, NY, USA, 2019. [Google Scholar]
- Abbasi, A.; Nasef, M.M.; Babadi, F.E.; Faridi-Majidi, R.; Takeshi, M.; Abouzari-Lotf, E.; Choong, T.; Somwangthanaroj, A.; Kheawhom, S. Carbon Dioxide Adsorption on Grafted Nanofibrous Adsorbents Functionalized Using Different Amines. Front. Energy Res. 2019, 7, 145. [Google Scholar] [CrossRef]
- Heo, Y.-J.; Zhang, Y.; Rhee, K.Y.; Park, S.J. Synthesis of PAN/PVDF nanofiber composites-based carbon adsorbents for CO2 capture. Compos. Part B Eng. 2019, 156, 95–99. [Google Scholar] [CrossRef]
- Othman, F.E.C.; Yusof, N.; González-Benito, J.; Fan, X.; Ismail, A.F. Electrospun Composites Made of Reduced Graphene Oxide and Polyacrylonitrile-Based Activated Carbon Nanofibers (rGO/ACNF) for Enhanced CO2 Adsorption. Polymers 2020, 12, 2117. [Google Scholar] [CrossRef]
- Choi, C.; Kadam, R.L.; Gailwad, S.; Hwang, K.-S.; Han, S. Metal organic frameworks immobilized polyacrylonitrile fiber mats with polyethyleneimine impregnation for CO2 capture. Microporous Mesoporous Mater. 2020, 296, 110006. [Google Scholar] [CrossRef]
- Tran, D.N.; Marti, A.M.; Balkus, K.J. Electrospun Zeolite/Cellulose Acetate Fibers for Ion Exchange of Pb2+. Fibers 2014, 2, 308–317. [Google Scholar] [CrossRef]
- Jaime-Ferrer, J.S.; Mosqueda-Quintero, M.; Toriello, V.A.S.; Anderson, S.M.; González-Vargas, O.A.; Villafaña-López, L. Heterogeneous PVC cation-exchange membrane synthesis by electrospinning for reverse electrodialysis. Int. J. Chem. React. Eng. 2020, 18, 20200020. [Google Scholar] [CrossRef]
- Kallem, P.; Yanar, N.; Choi, H. Nanofiber-Based Proton Exchange Membranes: Development of Aligned Electrospun Nanofibers for Polymer Electrolyte Fuel Cell Applications. ACS Sustain. Chem. Eng. 2018, 7, 1808–1825. [Google Scholar] [CrossRef]
- Zhang, S.; Tanioka, A.; Matsumoto, H. Nanofibers as novel platform for high-functional ion exchangers. J. Chem. Technol. Biotechnol. 2018, 93, 2791–2803. [Google Scholar] [CrossRef]
- Jalal, N.M.; Jabur, A.R.; Hamza, M.S.; Allami, S. Sulfonated electrospun polystyrene as cation exchange membranes for fuel cells. Energy Rep. 2020, 6, 287–298. [Google Scholar] [CrossRef]
- Alnaqbi, M.A.; Samson, J.A.; Greish, Y.E. Electrospun Polystyrene/LDH Fibrous Membranes for the Removal of Cd2+ Ions. J. Nanomater. 2020, 2020, 1–12. [Google Scholar] [CrossRef]
- Johns, A.; Qian, J.; Carolan, M.E.; Shaikh, N.; Peroutka, A.; Seeger, A.; Cerrato, J.M.; Forbes, T.Z.; Cwiertny, D.M. Functionalized electrospun polymer nanofibers for treatment of water contaminated with uranium. Environ. Sci. Water Res. Technol. 2020, 6, 622–634. [Google Scholar] [CrossRef]





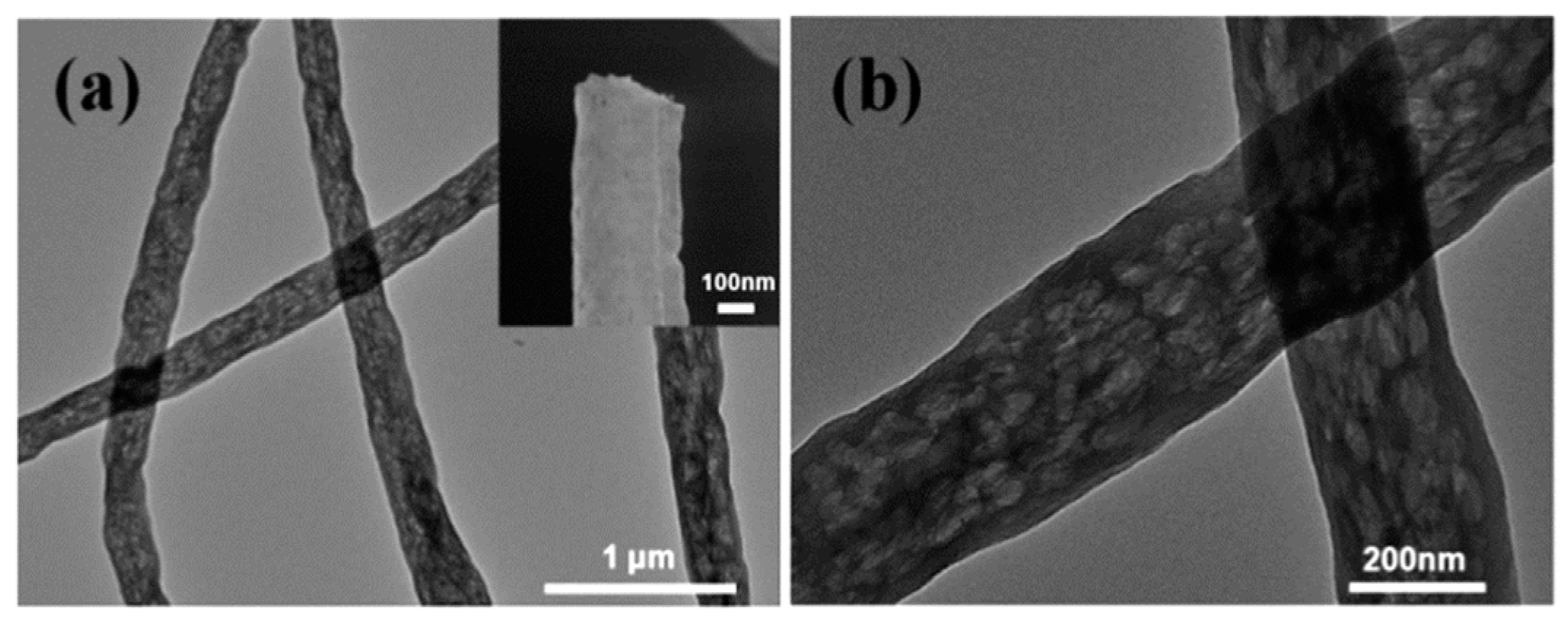
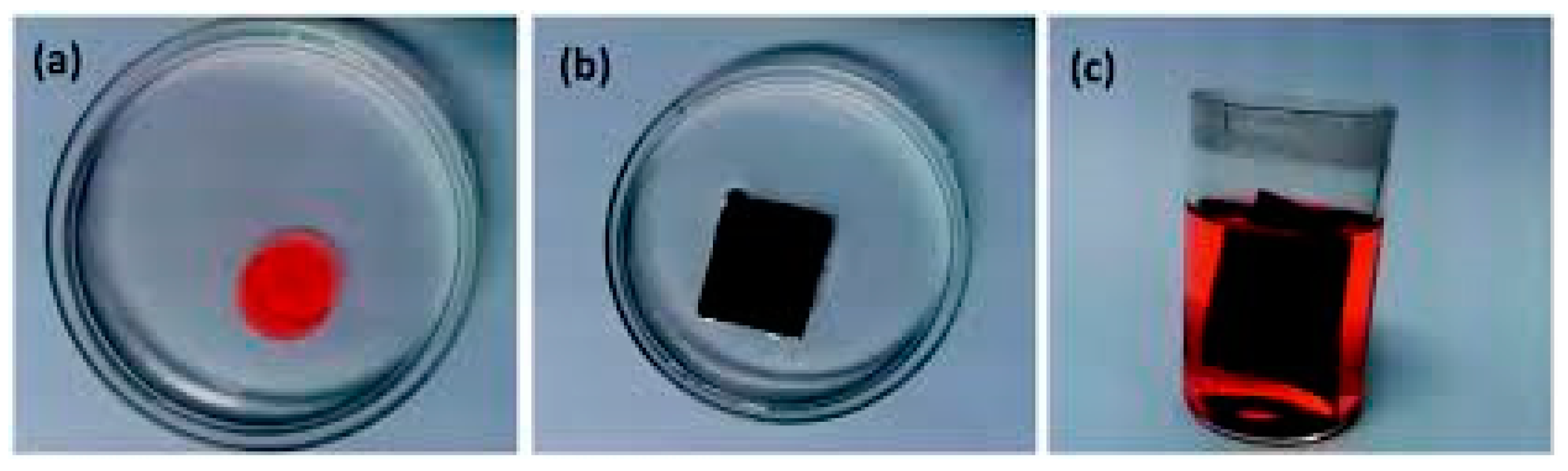

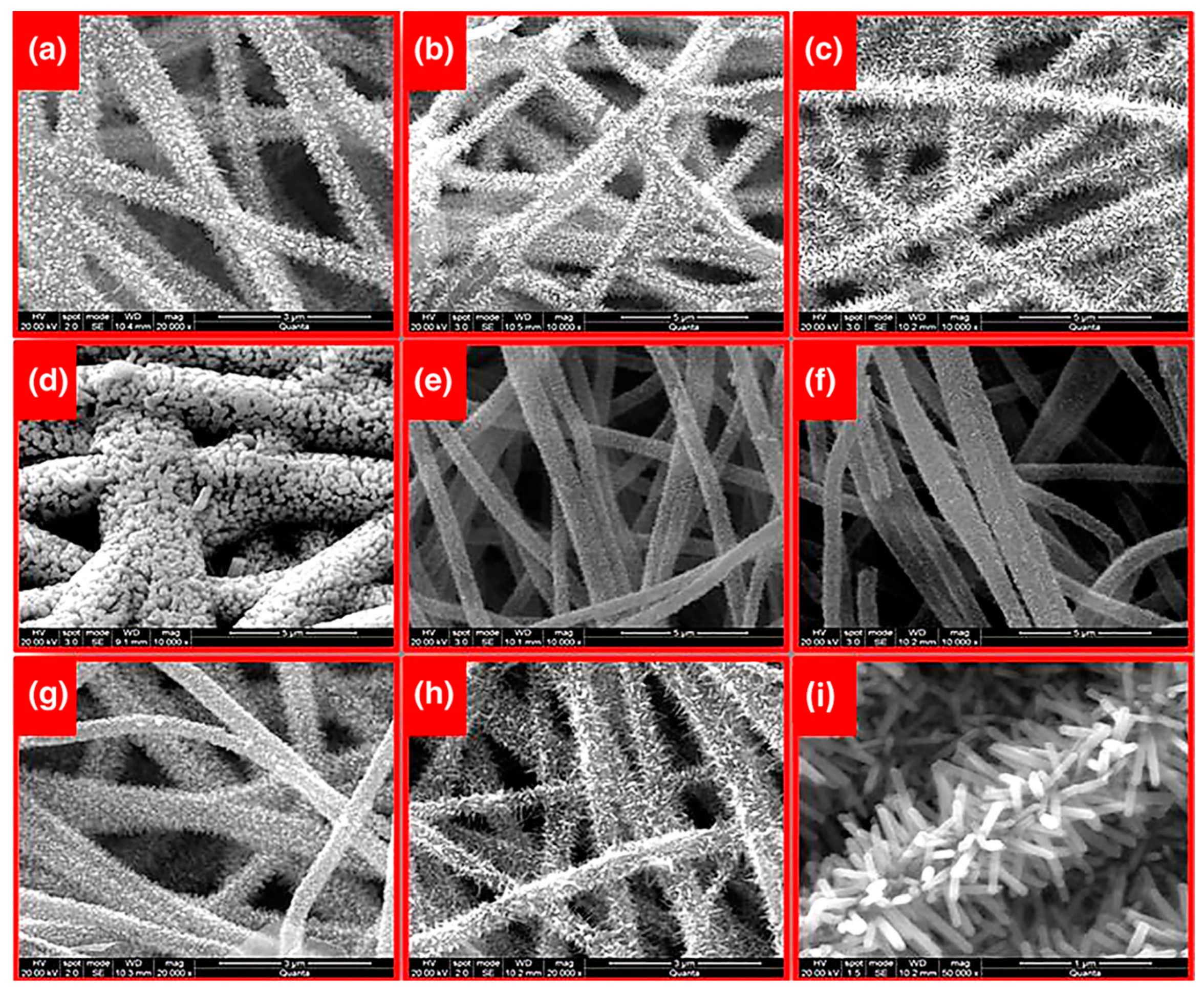
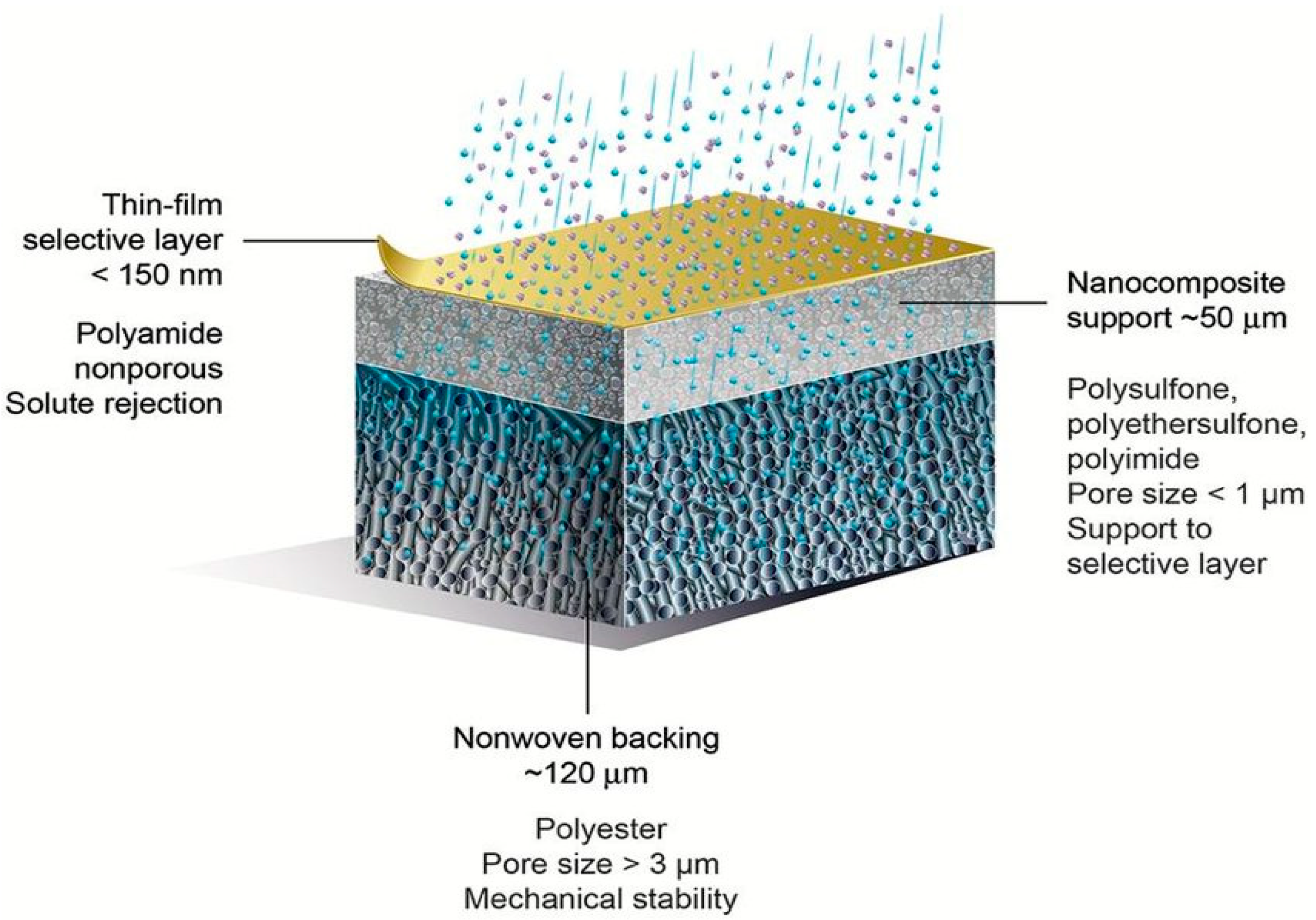

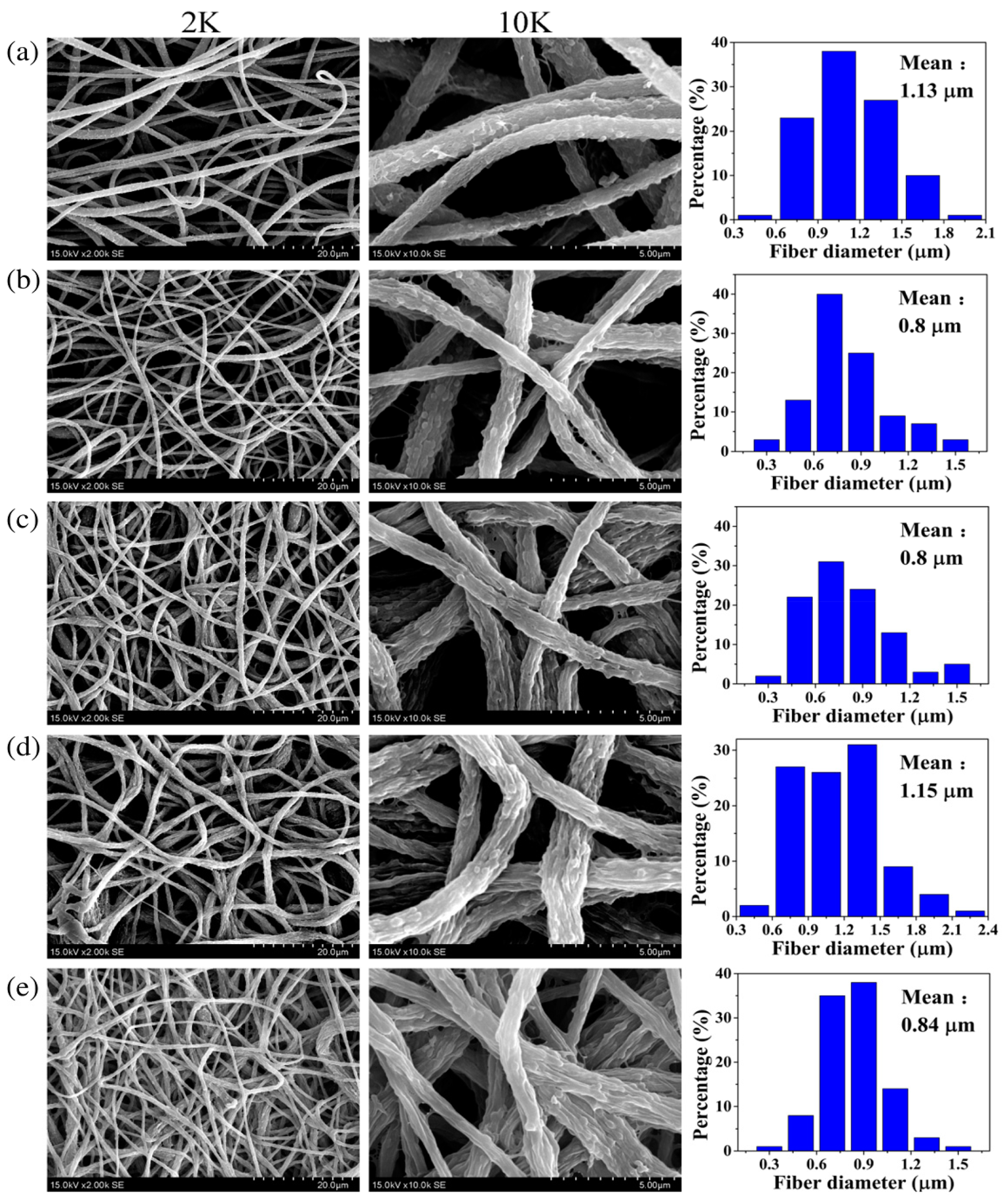
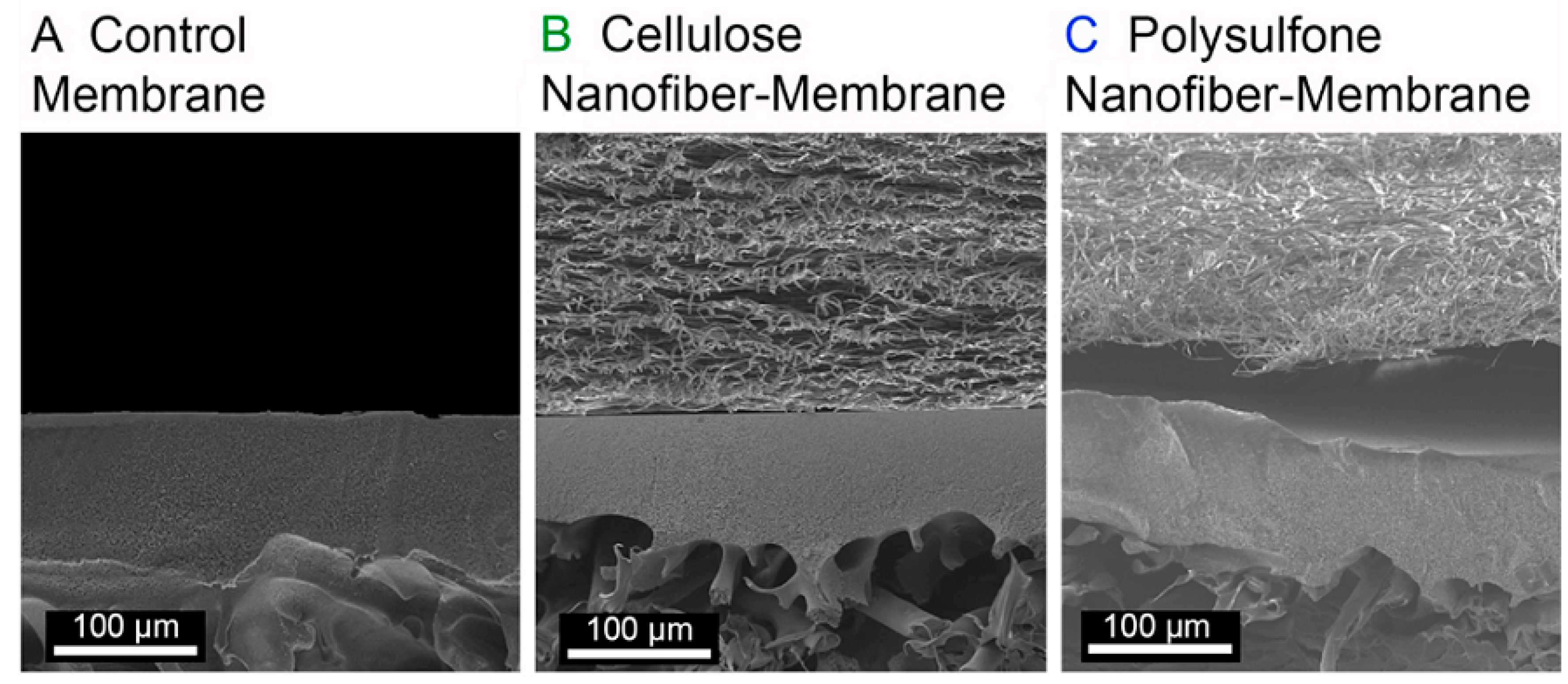
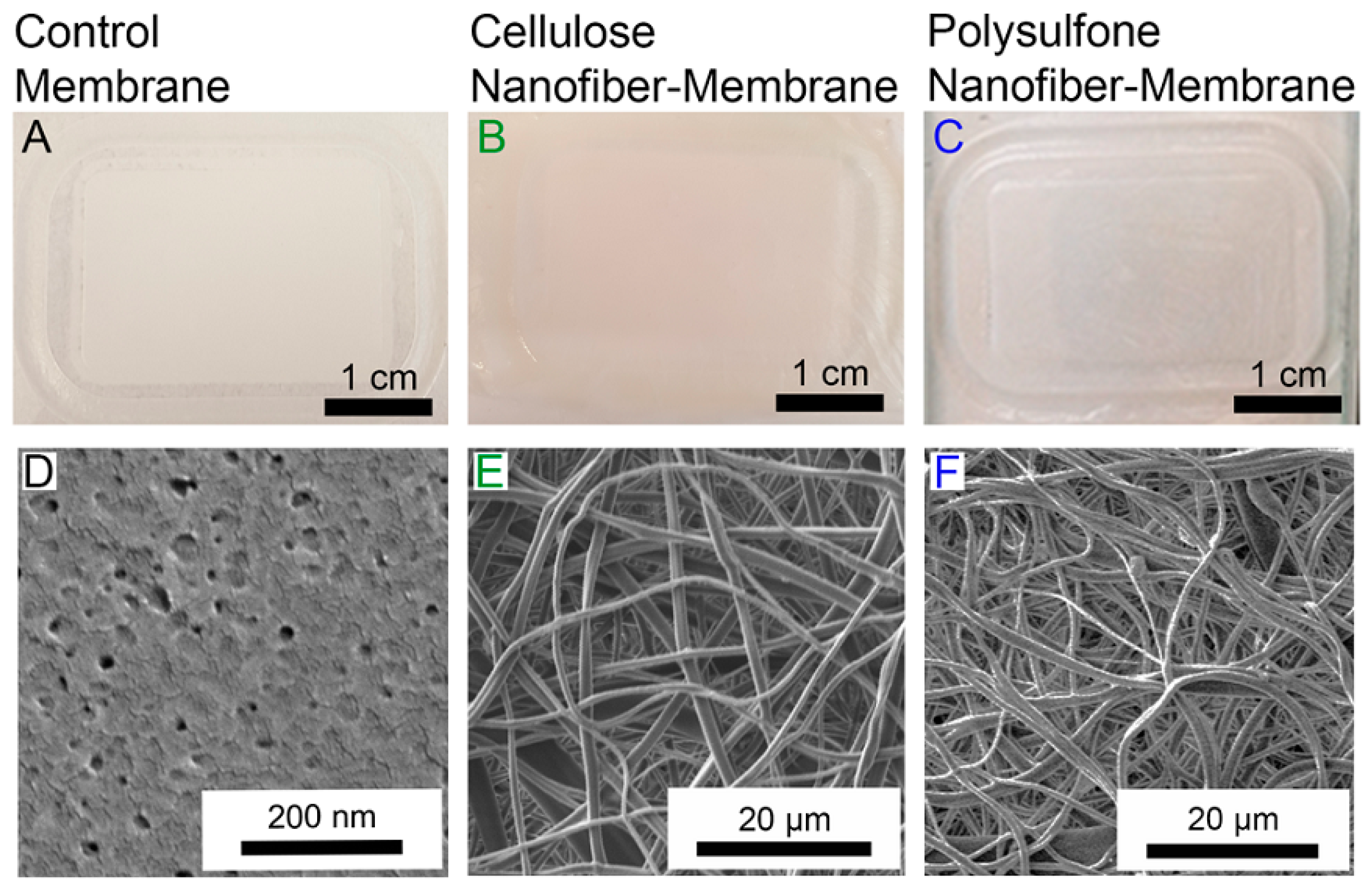



| Catalyst | Support Material | Catalytic Process | Catalytic Performance | Ref. |
|---|---|---|---|---|
| β-galactosidase immobilized on electrospun polystyrene nanofibers | Electrospun polystyrene nanofibers (PSNF) | Lactose conversion to galacto-oligosaccharides (GOS) in a disc reactor | High bioconversion performance | [276] |
| Pt nanoparticles supported on porous nanofibers of antimony-doped tin oxide | Antimony-doped tin oxide nanofibers | Electrooxidation of methanol in the anode of a direct methanol fuel cells | Lower loss of electrochemical surface area and higher stability of the supported Pt catalyst compared to conventional Pt/C catalysts was observed | [277] |
| Pd species incorporated crosslinked chitosan/poly(methacrylic acid) composite nanofibers | Crosslinked chitosan/poly(methacrylic acid) composite nanofibers | Heck reaction of aromatic iodides with acrylates | Facile separation from the reaction mixture for 10 subsequent runs without loss of activity despite 5.8% leaching of the palladium in each cycle | [278] |
| Phytase immobilized polyvinyl alcohol-sodium alginate (PVA-SA) electrospun nanofibers | Polyvinyl alcohol-sodium alginate (PVA-SA) nanofibers | Hydrolysis of phosphate moieties | Higher thermal and pH stability, enhanced catalytic activity of phytase after immobilization into PVA-SA based nanofibers compared to the free phytase | [279] |
| Nitrogen-doped carbon nanofibers supported FeOx-based nano catalyst | N-doped carbon nanofiber produced via pyrolysis of electrospun Fe-based MOF containing polyacrylonitrile and polymethylmethacrylate | Hydrogenation of nitroarenes to arylamines | Ease of recovery, reusability, higher activity, and chemoselectivity in reduction of functionalized nitroarenes compared to non-supported catalyst | [280] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baghali, M.; Jayathilaka, W.A.D.M.; Ramakrishna, S. The Role of Electrospun Nanomaterials in the Future of Energy and Environment. Materials 2021, 14, 558. https://doi.org/10.3390/ma14030558
Baghali M, Jayathilaka WADM, Ramakrishna S. The Role of Electrospun Nanomaterials in the Future of Energy and Environment. Materials. 2021; 14(3):558. https://doi.org/10.3390/ma14030558
Chicago/Turabian StyleBaghali, Mitra, W.A.D.M. Jayathilaka, and Seeram Ramakrishna. 2021. "The Role of Electrospun Nanomaterials in the Future of Energy and Environment" Materials 14, no. 3: 558. https://doi.org/10.3390/ma14030558
APA StyleBaghali, M., Jayathilaka, W. A. D. M., & Ramakrishna, S. (2021). The Role of Electrospun Nanomaterials in the Future of Energy and Environment. Materials, 14(3), 558. https://doi.org/10.3390/ma14030558








